Coating of Nanolipid Structures by a Novel Simil-Microfluidic Technique: Experimental and Theoretical Approaches
Abstract
1. Introduction
2. Materials and Methods
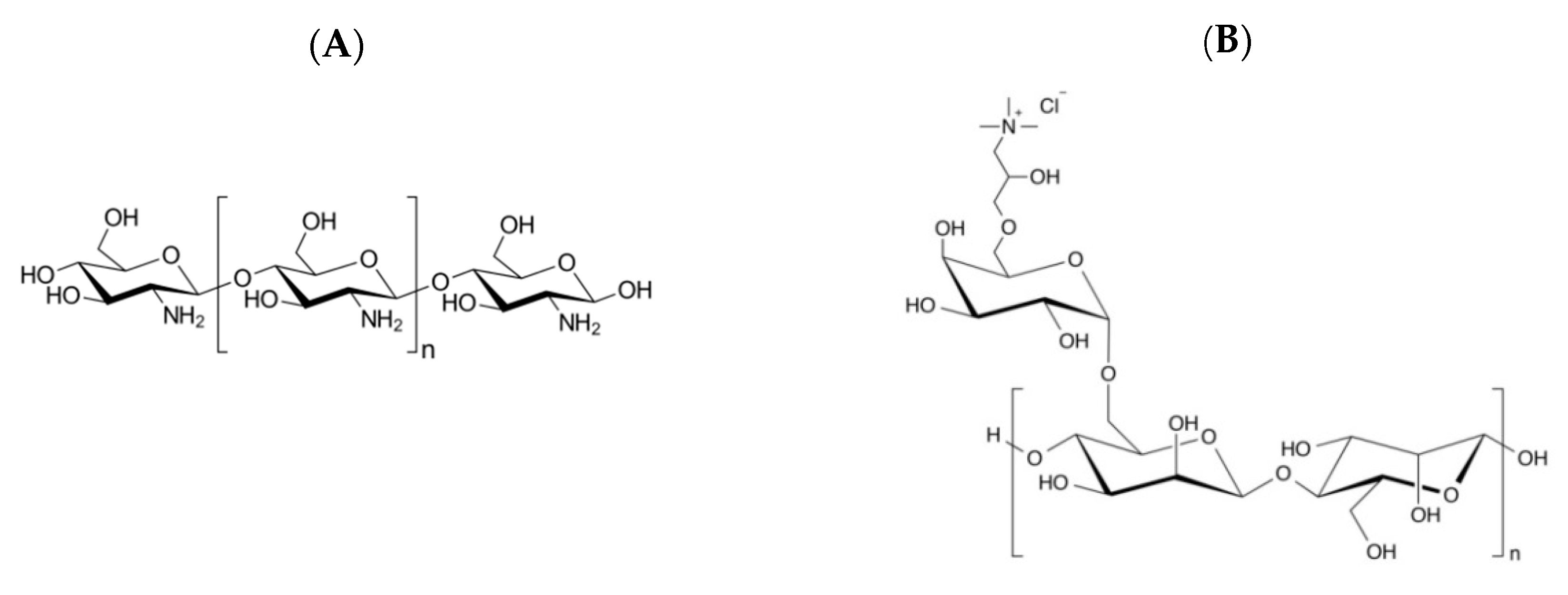
2.1. Materials
2.2. Methods
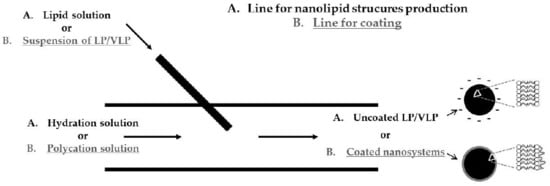
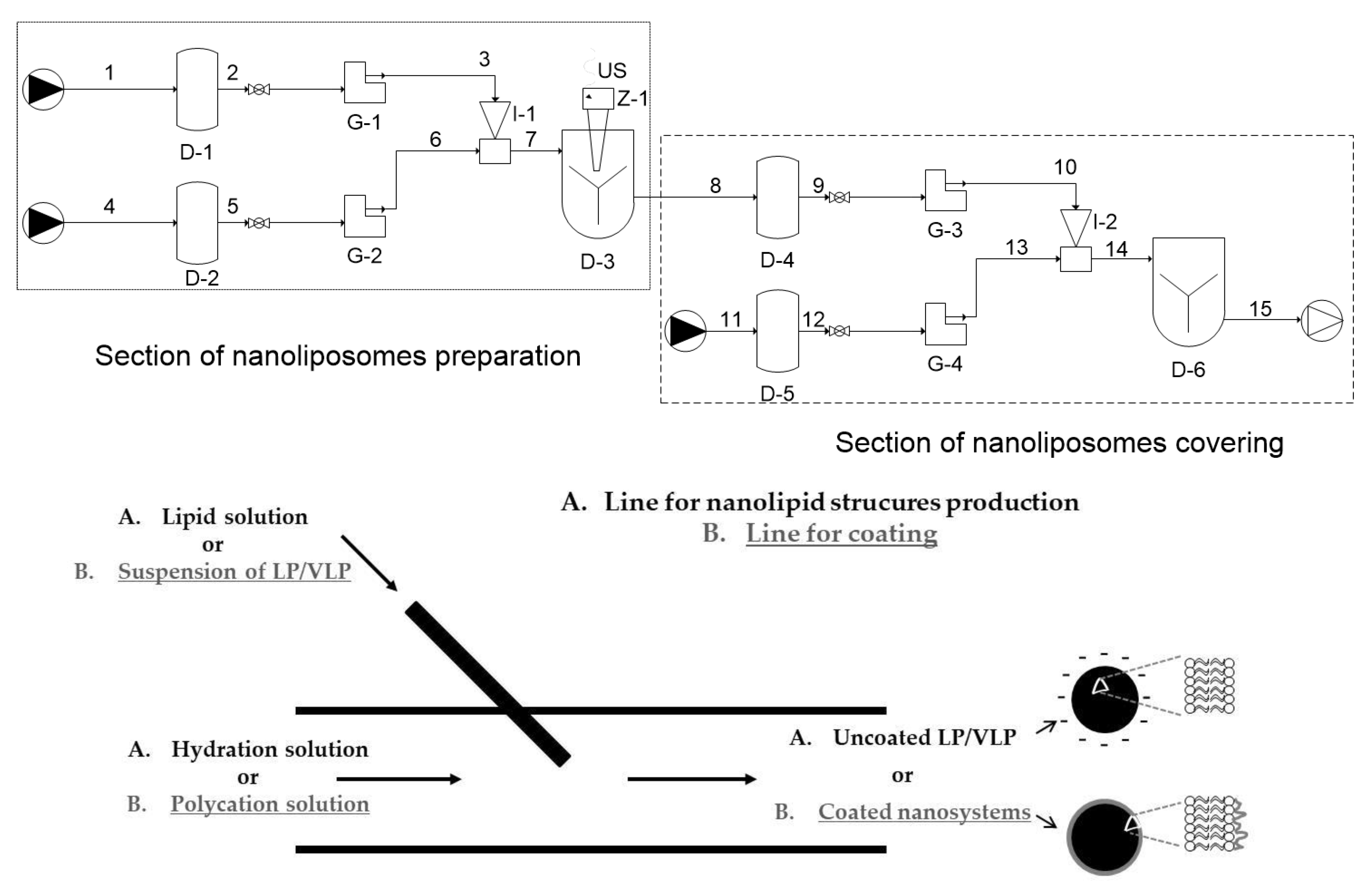
2.2.1. Production of Uncoated and Polycation-Coated Nanolipid Structures
2.2.2. Z-potential and Size of Nanolipid Structures
2.2.3. Transmission Electron Microscopy
2.2.4. Mucoadhesiveness by Mucin Binding Assay
2.2.5. Turbidimetry
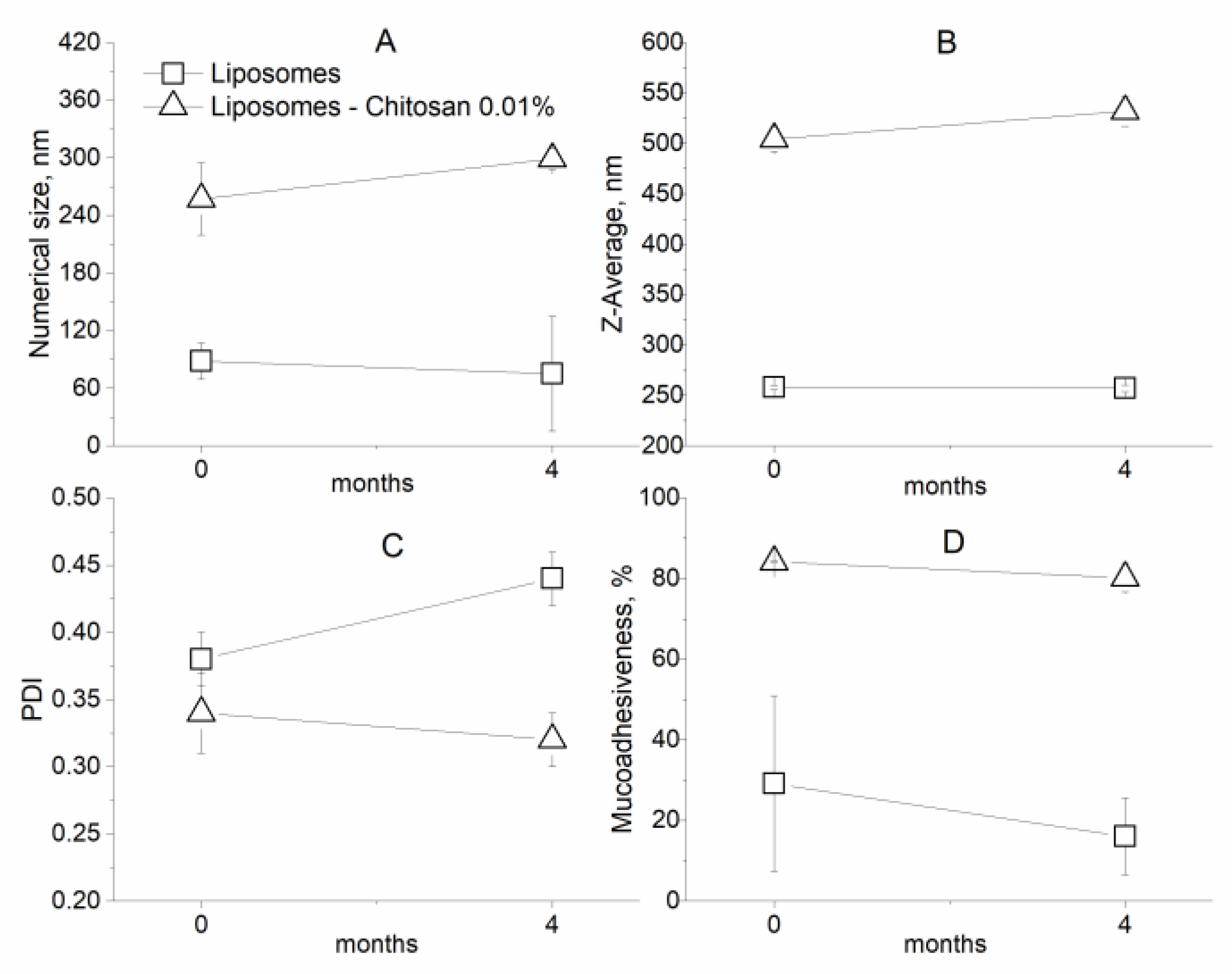
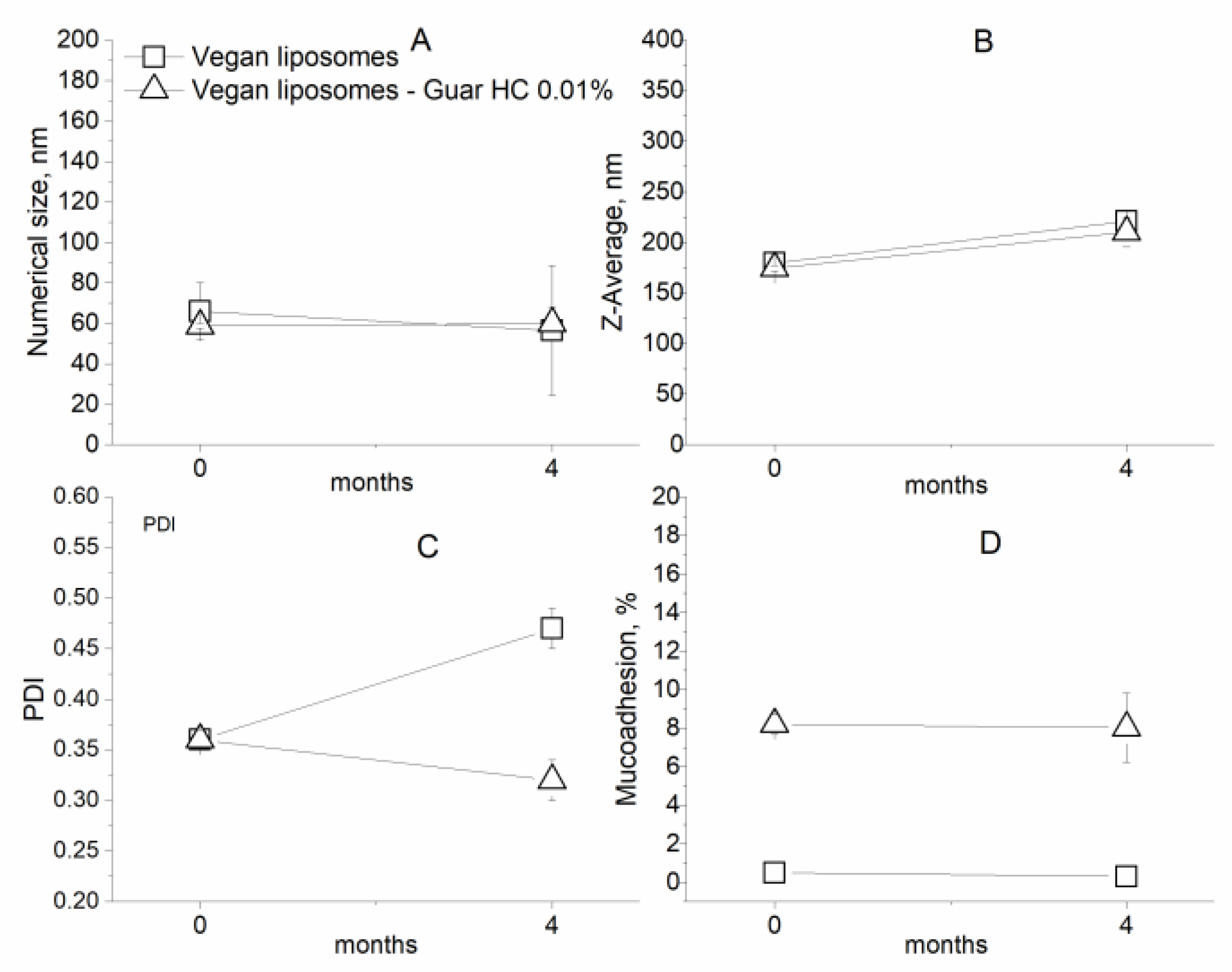
2.2.6. Stability during Storage
3. Results
3.1. Adsorption of Polycations on a Nanolipid Structure Surface: Combining Experimental and Theoretical Approaches
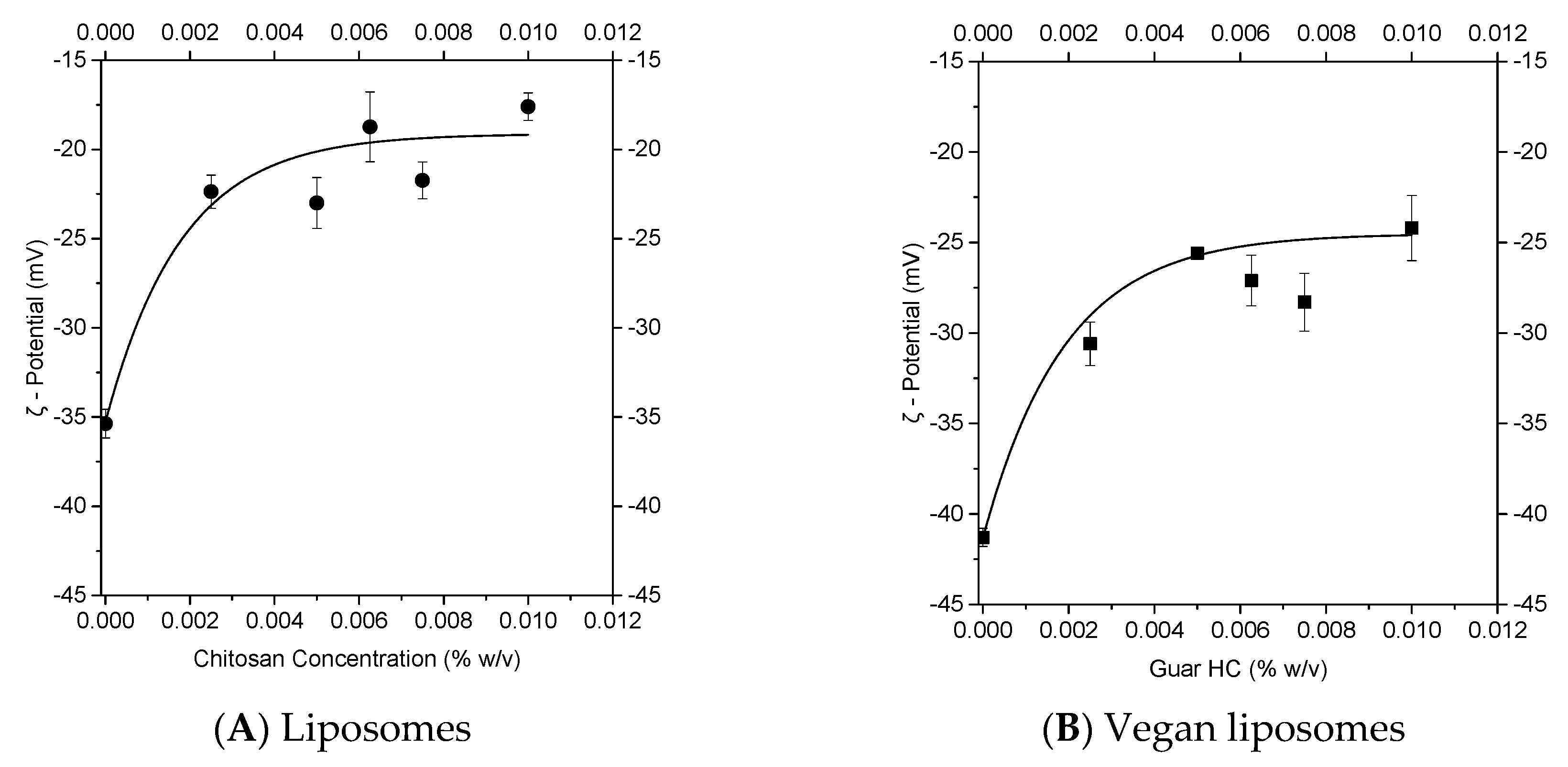
3.1.1. Experimental Approach to Nanolipid Structure Coating with a Polycation: Z-potential Evolution
3.1.2. Theoretical Approach to a Nanolipid Structure Coating with a Polycation: Concepts of Saturation
3.1.3. Combination of Experimental and Theoretical Approaches to Nanolipid Structure Coating with a Polycation
3.2. Characterization of Coated Nanolipid Structures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bochicchio, S.; Dalmoro, A.; Barba, A.; d’Amore, M.; Lamberti, G. New Preparative Approaches for Micro and Nano Drug Delivery Carriers. Curr. Drug Deliv. 2017, 14, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, S.; Dalmoro, A.; Bertoncin, P.; Lamberti, G.; Moustafine, R.I.; Barba, A.A. Design and production of hybrid nanoparticles with polymeric-lipid shell–core structures: Conventional and next-generation approaches. RSC Adv. 2018, 8, 34614–34624. [Google Scholar] [CrossRef]
- Volodkin, D.; Mohwald, H.; Voegel, J.C.; Ball, V. Coating of negatively charged liposomes by polylysine: Drug release study. J. Control. Release 2007, 117, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.; Ball, V.; Schaaf, P.; Voegel, J.C.; Mohwald, H. Complexation of phosphocholine liposomes with polylysine. Stabilization by surface coverage versus aggregation. Biochim. Et Biophys. Acta (BBA) Biomembr. 2007, 1768, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Sybachin, A.; Efimova, A.; Litmanovich, E.; Menger, F.; Yaroslavov, A. Complexation of polycations to anionic liposomes: Composition and structure of the interfacial complexes. Langmuir 2007, 23, 10034–10039. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavov, A.A.; Sybachin, A.V.; Kesselman, E.; Schmidt, J.; Talmon, Y.; Rizvi, S.A.; Menger, F.M. Liposome fusion rates depend upon the conformation of polycation catalysts. J. Am. Chem. Soc. 2011, 133, 2881–2883. [Google Scholar] [CrossRef] [PubMed]
- Horner, I.J.; Kraut, N.D.; Hurst, J.J.; Rook, A.M.; Collado, C.M.; Atilla-Gokcumen, G.E.; Maziarz, E.P.; Liu, X.M.; Merchea, M.M.; Bright, F.V. Effects of polyhexamethylene biguanide and polyquaternium-1 on phospholipid bilayer structure and dynamics. J. Phys. Chem. B 2015, 119, 10531–10542. [Google Scholar] [CrossRef] [PubMed]
- Refai, H.; Hassan, D.; Abdelmonem, R. Development and characterization of polymer-coated liposomes for vaginal delivery of sildenafil citrate. Drug Deliv. 2017, 24, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Mengoni, T.; Adrian, M.; Pereira, S.; Santos-Carballal, B.; Kaiser, M.; Goycoolea, F. A Chitosan—Based Liposome Formulation Enhances the In Vitro Wound Healing Efficacy of Substance P Neuropeptide. Pharmaceutics 2017, 9, 56. [Google Scholar] [CrossRef]
- Dalmoro, A.; Bochicchio, S.; Lamberti, G.; Bertoncin, P.; Janssens, B.; Barba, A.A. Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology. RSC Adv. 2019, 9, 19800–19812. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128, 227–248. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Theoretical analysis of factors affecting the formation and stability of multilayered colloidal dispersions. Langmuir 2005, 21, 9777–9785. [Google Scholar] [CrossRef] [PubMed]
- Laye, C.; McClements, D.; Weiss, J. Formation of biopolymer-coated liposomes by electrostatic deposition of chitosan. J. Food Sci. 2008, 73, N7–N15. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, C.; Deb, T.K.; Shivakumar, H. Cationic Guar Gum Poly Electrolytecomplex-Microparticles. J. Young Pharm. 2014, 6, 11. [Google Scholar] [CrossRef]
- Erazo-Majewicz, P.; Modi, J.J.; Xu, Z.F. Cationic, oxidized Polysaccharides in Conditioning Applications. U.S. Patent 7,589,051, 25 September 2009. [Google Scholar]
- Dalmoro, A.; Bochicchio, S.; Nasibullin, S.F.; Bertoncin, P.; Lamberti, G.; Barba, A.A.; Moustafine, R.I. Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems. Eur. J. Pharm. Sci. 2018, 121, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, S.; Dalmoro, A.; Recupido, F.; Lamberti, G.; Barba, A.A. Nanoliposomes Production by a Protocol Based on a Simil-Microfluidic Approach. In Advances in Bionanomaterials; Springer: Berlin, Germany, 2018; pp. 3–10. [Google Scholar]
- Barba, A.A.; Lamberti, G.; d’Amore, M.; Bochicchio, S.; Dalmoro, A. Process for Preparing Nanoliposomes Comprising Micronutrients and Food Products Comprising Said Nanoliposomes. WO 2019/049186 A1, 6 september 2017. Patent WO 2019/049186 A1, 6 September 2017. [Google Scholar]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S.; Chang, D. Insights into chitosan multiple functional properties: The role of chitosan conformation in the behavior of liposomal membrane. Food Funct. 2015, 6, 3702–3711. [Google Scholar] [CrossRef]
- Magarkar, A.; Dhawan, V.; Kallinteri, P.; Viitala, T.; Elmowafy, M.; Róg, T.; Bunker, A. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci. Rep. 2014, 4, 5005. [Google Scholar] [CrossRef]
- Mady, M.M.; Darwish, M.M. Effect of chitosan coating on the characteristics of DPPC liposomes. J. Adv. Res. 2010, 1, 187–191. [Google Scholar] [CrossRef]
- Risica, D.; Dentini, M.; Crescenzi, V. Guar gum methyl ethers. Part I. Synthesis and macromolecular characterization. Polymer 2005, 46, 12247–12255. [Google Scholar] [CrossRef]
- Efimova, A.; Sybachin, A.; Yaroslavov, A. Effect of anionic-lipid-molecule geometry on the structure and properties of liposome-polycation complexes. Polym. Sci. Ser. C 2011, 53, 89. [Google Scholar] [CrossRef]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. Macromolecular conformation of chitosan in dilute solution: A new global hydrodynamic approach. Carbohydr. Polym. 2009, 76, 616–621. [Google Scholar] [CrossRef]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Li, P.; Niu, Y. Interactions between fluorinated cationic guar gum and surfactants in the dilute and semi-dilute solutions. Carbohydr. Polym. 2014, 99, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Haldar, S. Interactions between cholesterol and lipids in bilayer membranes. Role of lipid headgroup and hydrocarbon chain–backbone linkage. Biochim. Et Biophys. Acta (BBA) Biomembr. 2000, 1467, 39–53. [Google Scholar] [CrossRef]
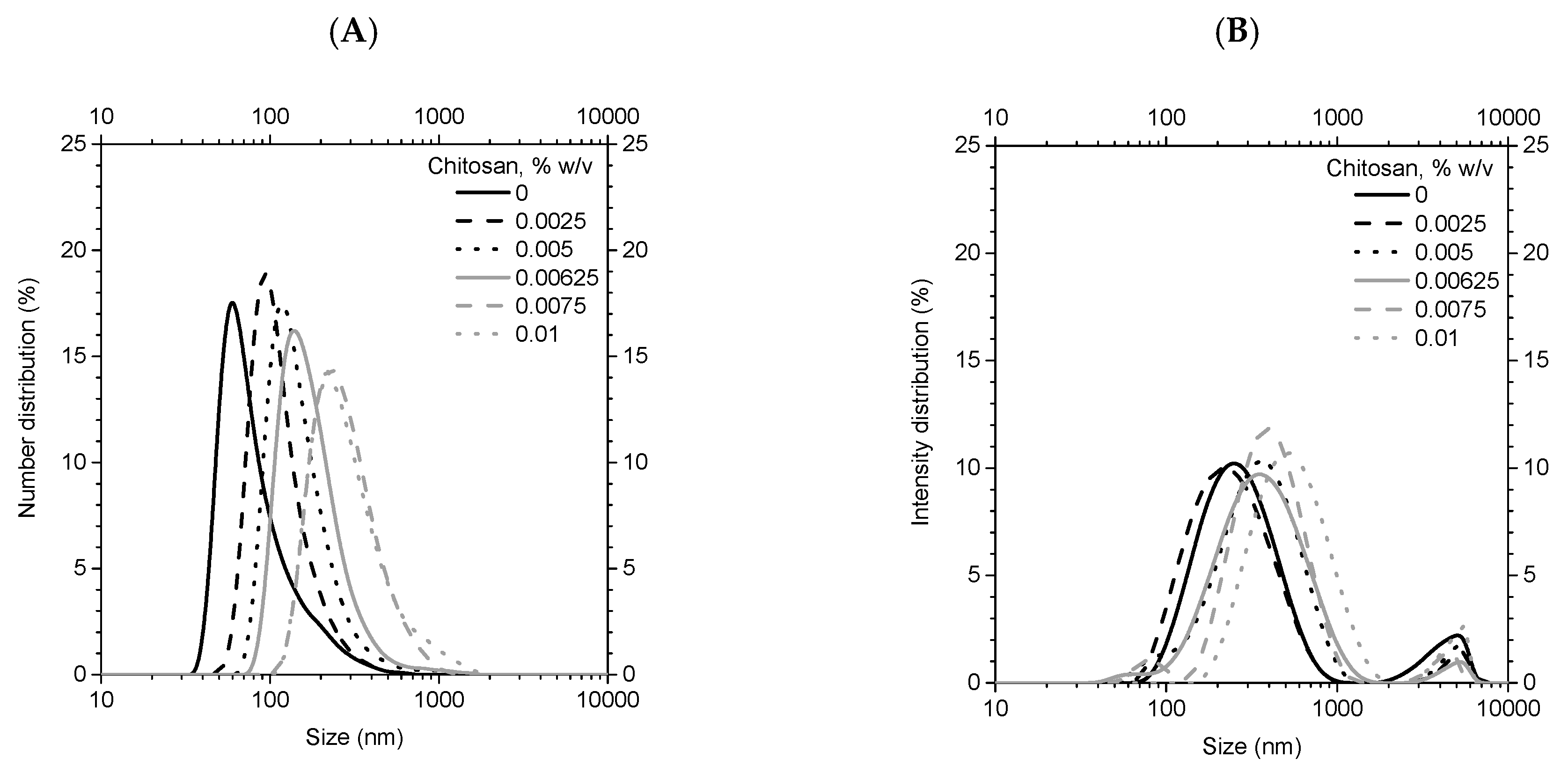

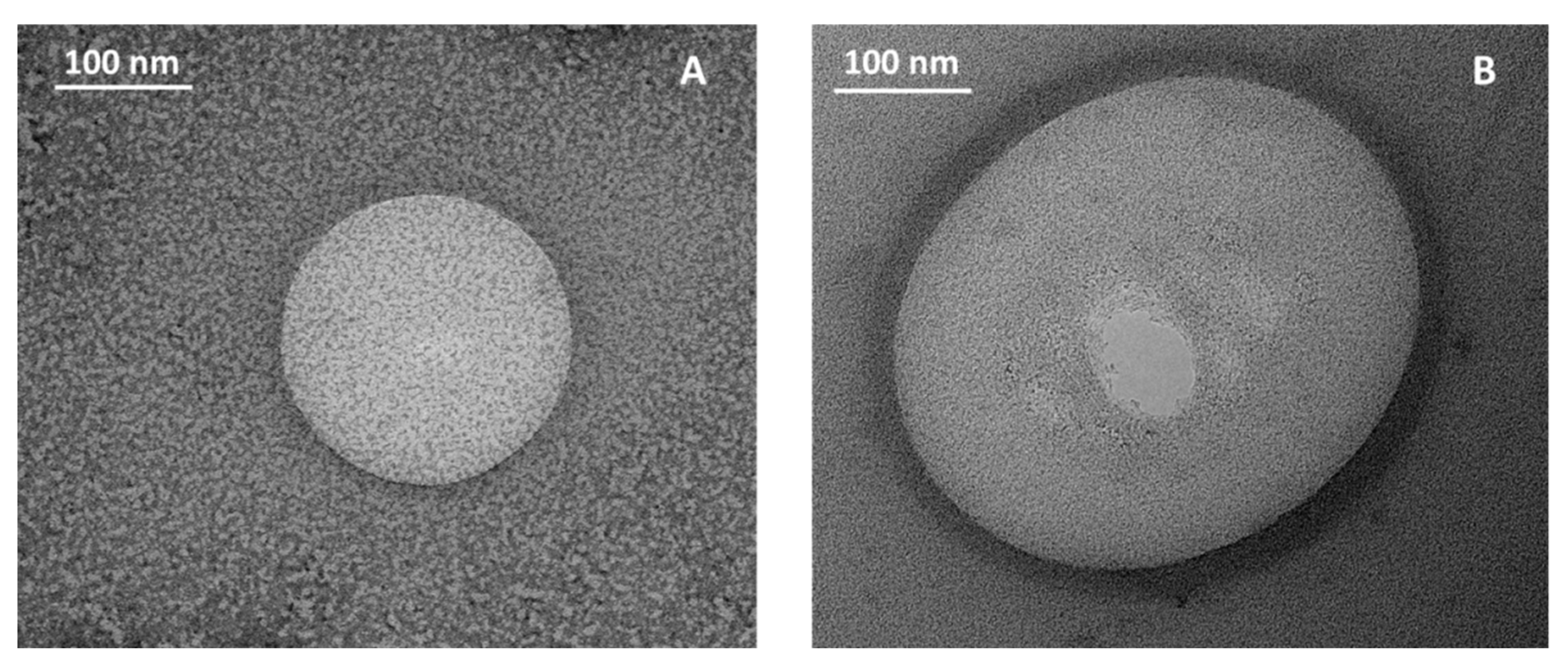


| ZSat, mV | CSat, % w/v | R2 | ΓSat, kg/m2 | |
|---|---|---|---|---|
| Liposomes | −19.1 | 0.00537 | 0.948 | 1.01 × 10−7 |
| Vegan liposomes | −24.5 | 0.00570 | 0.990 | 1.06 × 10−7 |
| M, kg/mol | rPE, m | CDep*, % w/v | CAds, % w/v | |
|---|---|---|---|---|
| Liposomes–Chitosan | 310 | 4 × 10−9 [26] | 0.0484 | 0.0057 |
| Vegan liposomes–Guar HC | 660 | 3 × 10−9 [27] | 0.2282 | 0.0086 |
| Numerical Size (nm) ± SD | Z-Average (nm) ± SD | PDI ± SD | |
|---|---|---|---|
| Liposomes | 88.3 ± 19.0 | 252.8 ± 2.1 | 0.38 ± 0.02 |
| Liposomes–Chitosan 0.01% | 257 ± 37.6 | 504.4 ± 12.1 | 0.34 ± 0.03 |
| Vegan liposomes | 65.9 ± 14.2 | 179.5 ± 2.3 | 0.36 ± 0.00 |
| Vegan liposomes–Guar HC 0.01% | 58.8 ± 1.3 | 174.5 ± 2.7 | 0.36 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba, A.A.; Bochicchio, S.; Bertoncin, P.; Lamberti, G.; Dalmoro, A. Coating of Nanolipid Structures by a Novel Simil-Microfluidic Technique: Experimental and Theoretical Approaches. Coatings 2019, 9, 491. https://doi.org/10.3390/coatings9080491
Barba AA, Bochicchio S, Bertoncin P, Lamberti G, Dalmoro A. Coating of Nanolipid Structures by a Novel Simil-Microfluidic Technique: Experimental and Theoretical Approaches. Coatings. 2019; 9(8):491. https://doi.org/10.3390/coatings9080491
Chicago/Turabian StyleBarba, Anna Angela, Sabrina Bochicchio, Paolo Bertoncin, Gaetano Lamberti, and Annalisa Dalmoro. 2019. "Coating of Nanolipid Structures by a Novel Simil-Microfluidic Technique: Experimental and Theoretical Approaches" Coatings 9, no. 8: 491. https://doi.org/10.3390/coatings9080491
APA StyleBarba, A. A., Bochicchio, S., Bertoncin, P., Lamberti, G., & Dalmoro, A. (2019). Coating of Nanolipid Structures by a Novel Simil-Microfluidic Technique: Experimental and Theoretical Approaches. Coatings, 9(8), 491. https://doi.org/10.3390/coatings9080491