Starch/Poly (Glycerol-Adipate) Nanocomposite Film as Novel Biocompatible Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
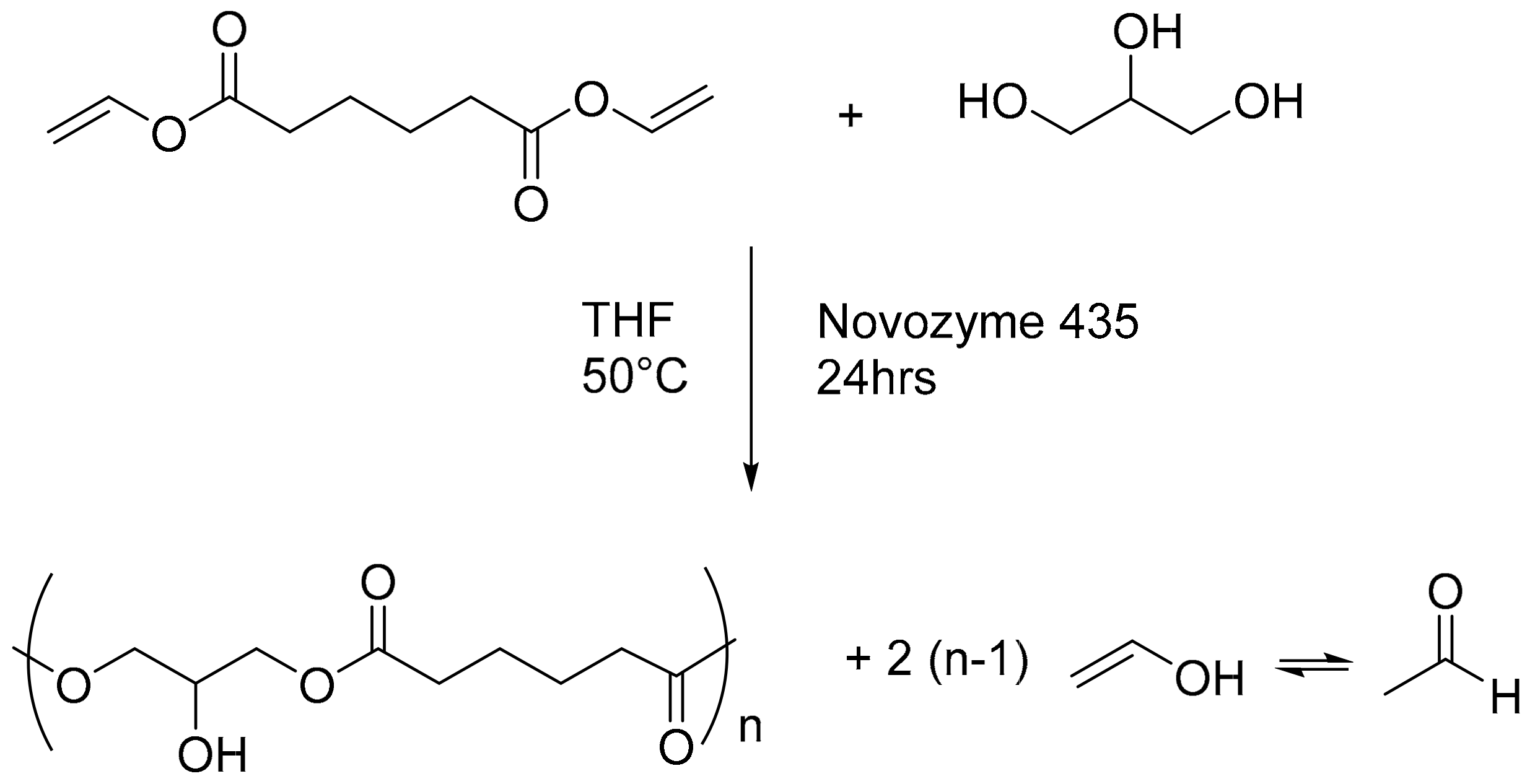
2.2.1. Poly-(Glycerol Adipate)(PGA) Synthesis
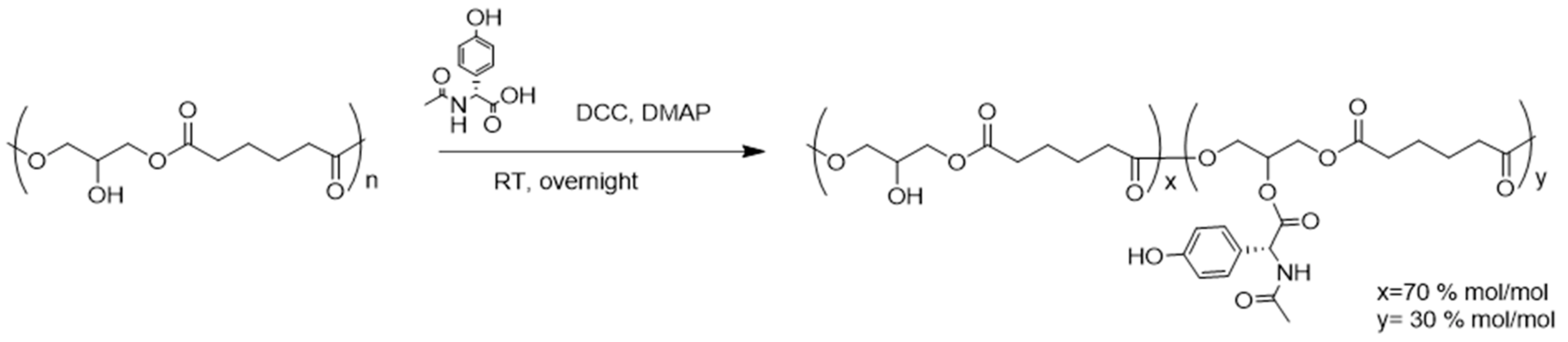
2.2.2. PGA N-Acyl-Tyrosine (Pgatyr) Coupling Reaction
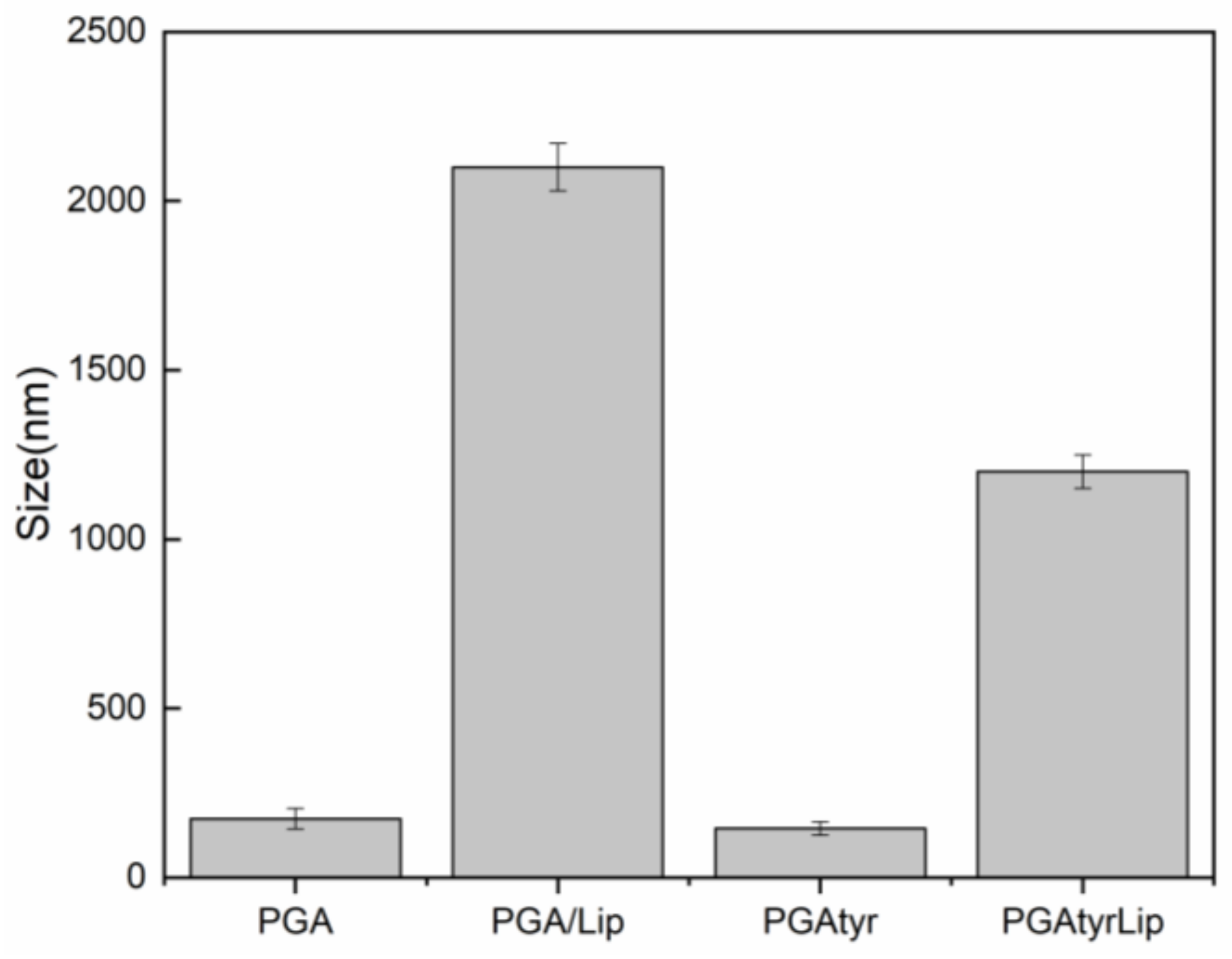
2.2.3. Particle Size of PGA and PGATyr Nanoparticles (NPs)
2.2.4. Enzymatic Degradation Assay
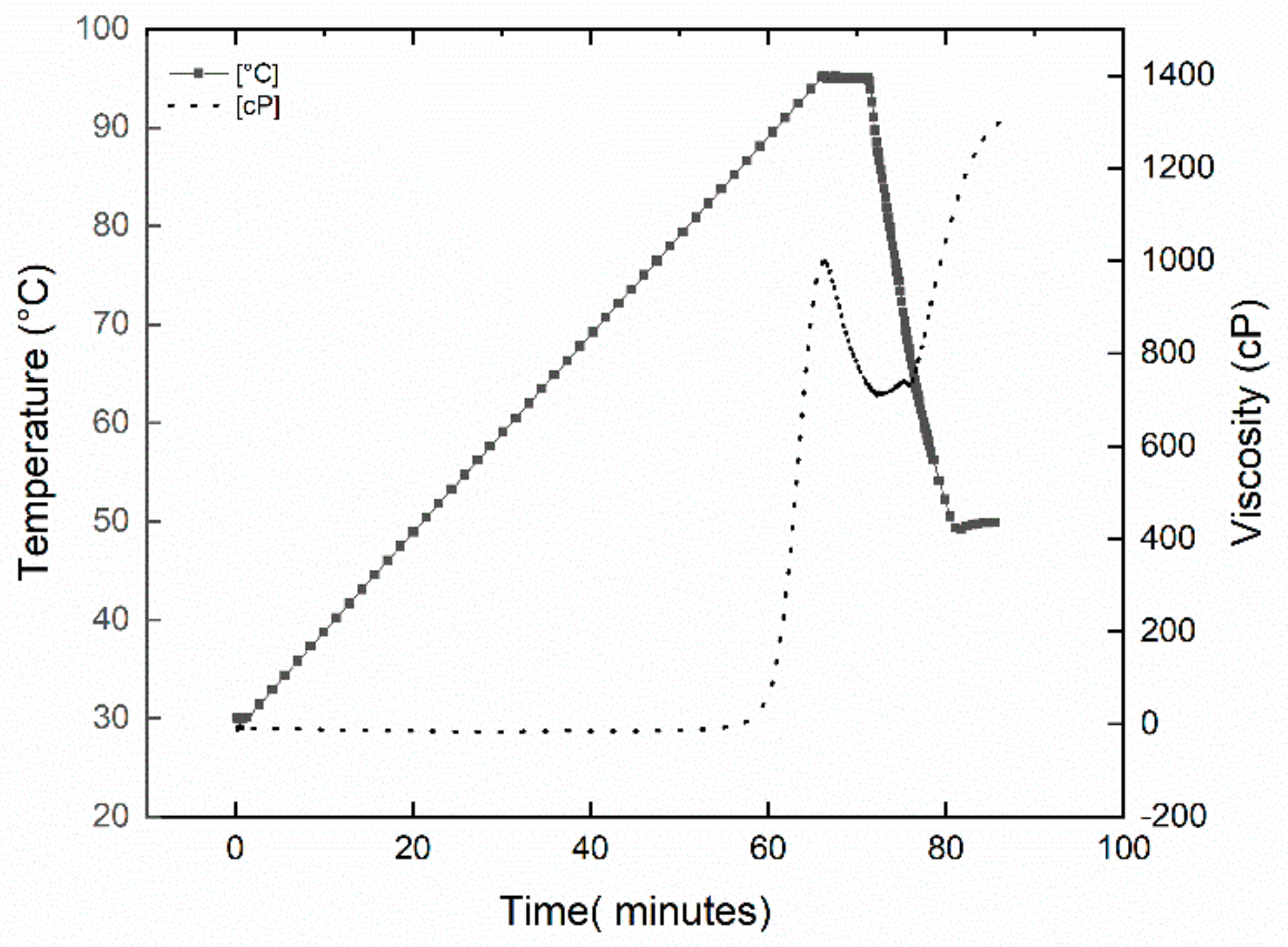
2.2.5. Melt Behavior Measurements
2.2.6. Water Contact Angle (Θw)
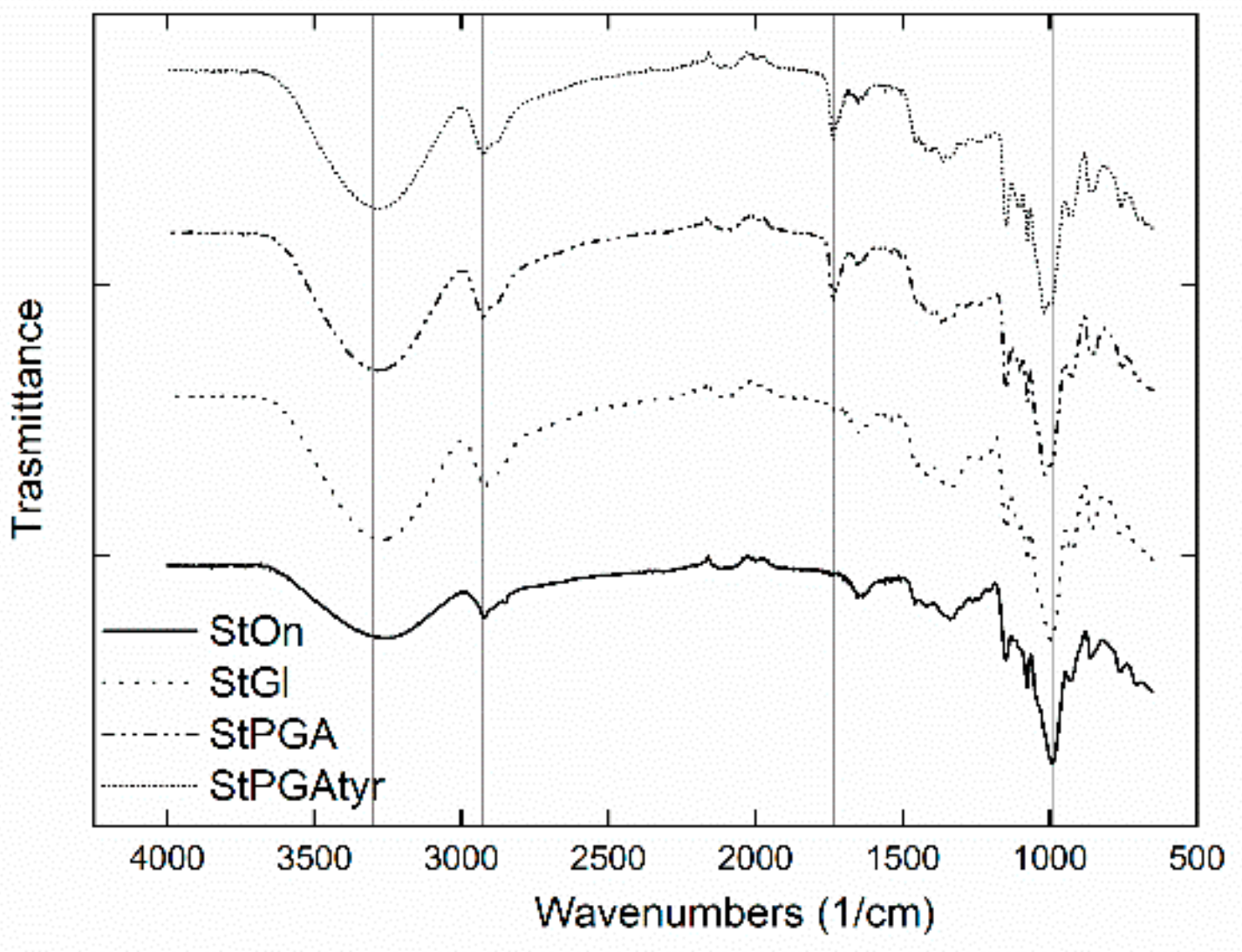
2.2.7. ATR FT-IR
2.2.8. Casting of Films
2.2.9. X-Ray Scattering
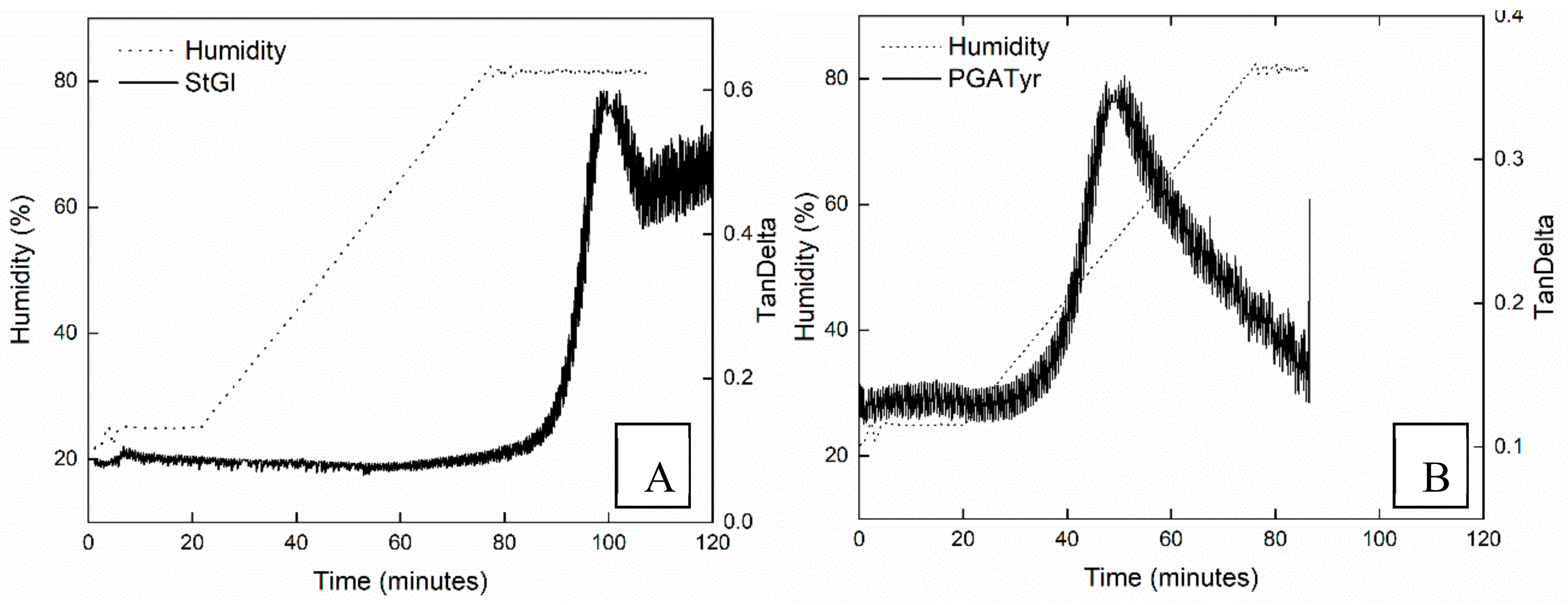
2.2.10. Dynamic Mechanical Analysis
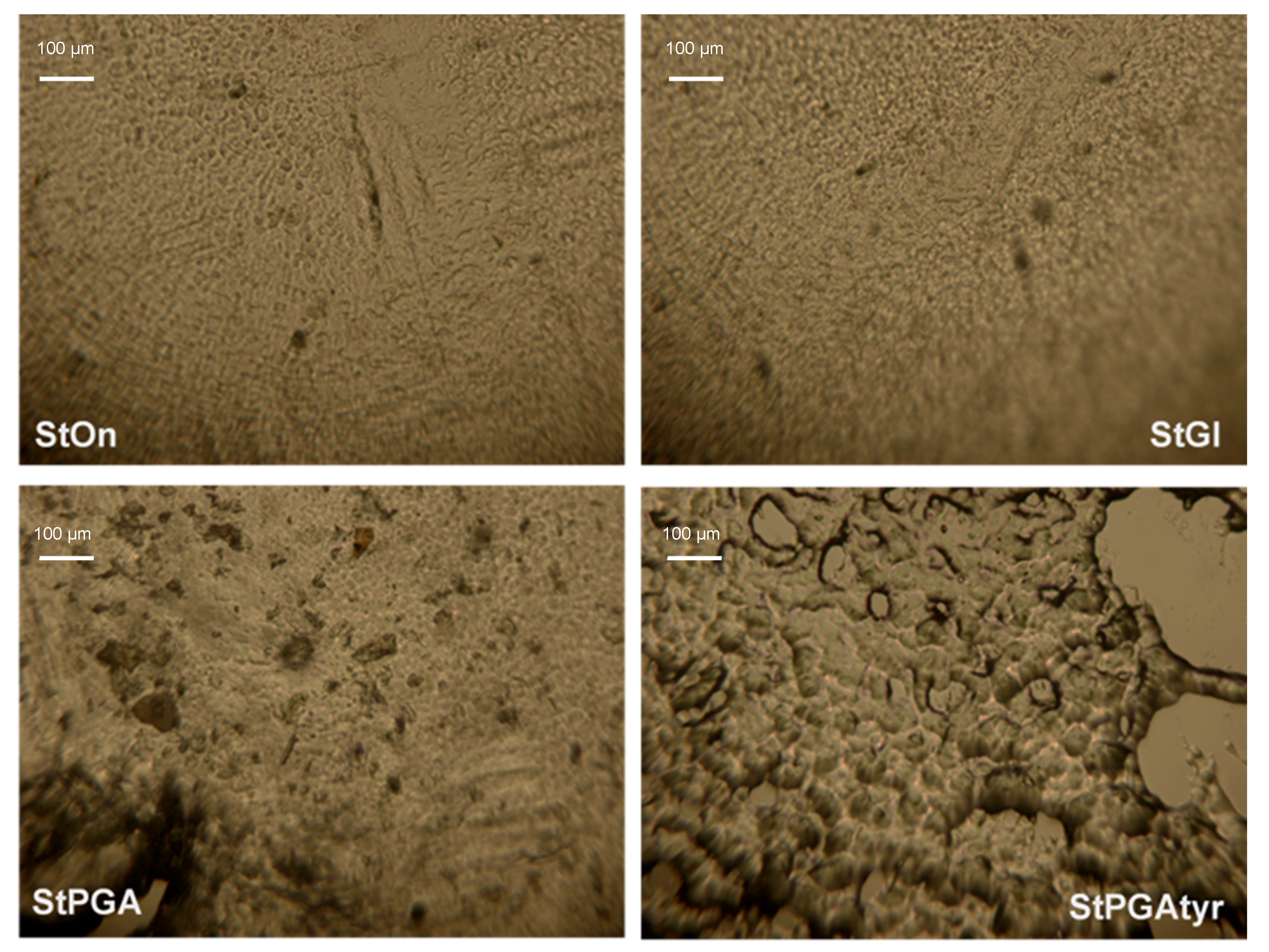
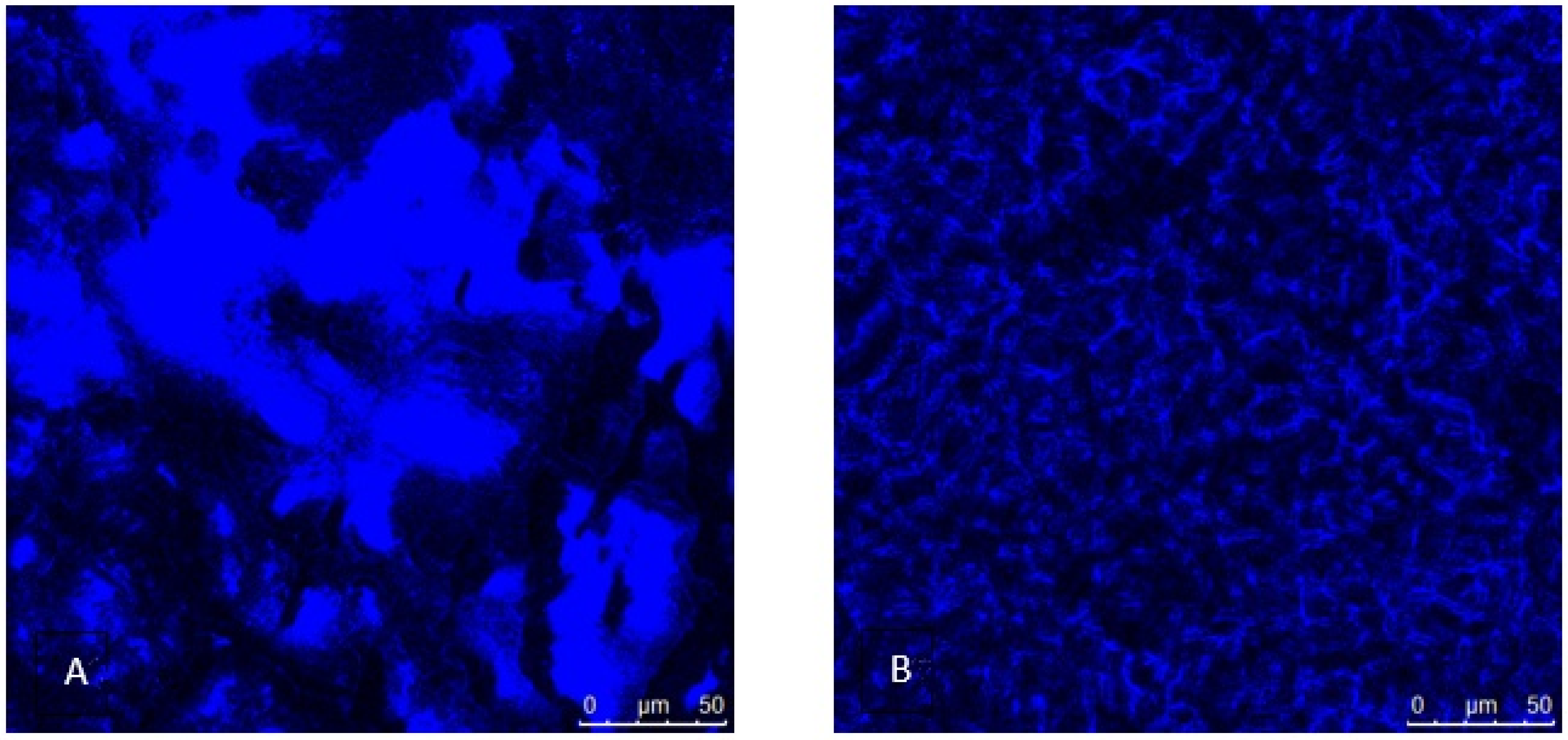
2.2.11. Confocal Microscopy
2.2.12. Cytotoxicity Test
3. Results and Discussion
3.1. Native Starch Melt and Crystallinity Characterization
3.2. Synthesis and Characterization of Nanoparticles
3.3. Crystallinity, Contact Angle, and Microstructures of Films
3.4. Thermo-Mechanical Properties
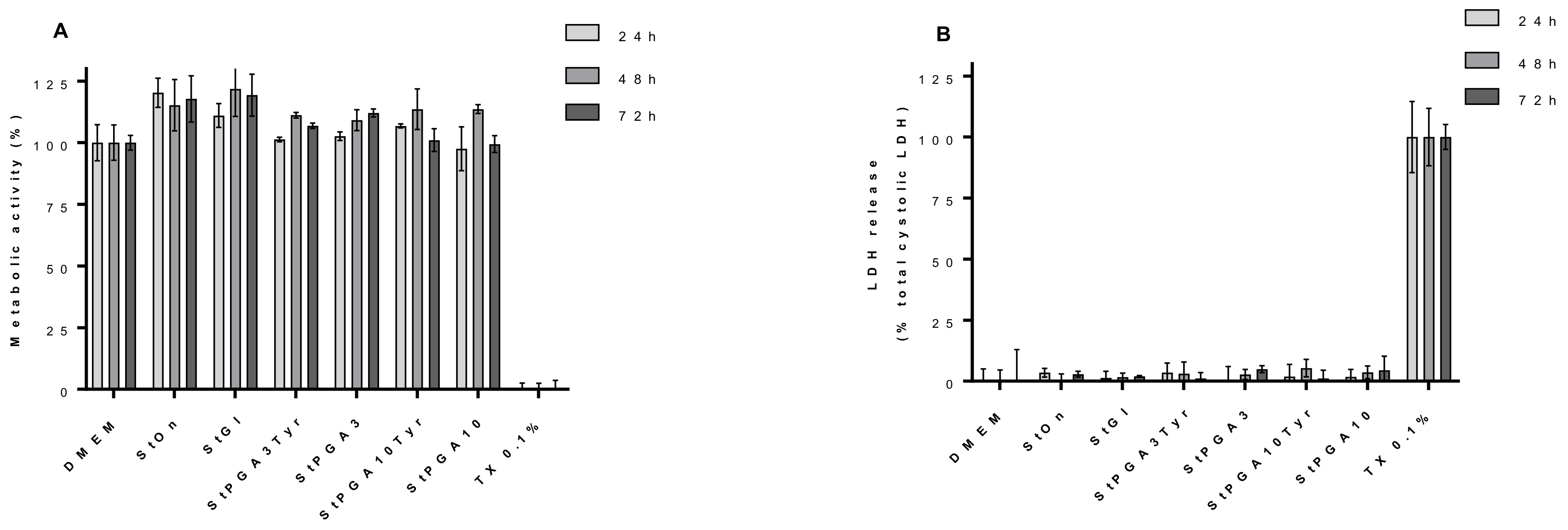
3.5. Biocompatibility Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch/Staerke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Blennow, A.; Engelsen, S.B.; Nielsen, T.H.; Baunsgaard, L.; Mikkelsen, R. Starch phosphorylation: A new front line in starch research. Trends Plant Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Blennow, A.; Mette Bay-Smidt, A.; Bauer, R. Amylopectin aggregation as a function of starch phosphate content studied by size exclusion chromatography and on-line refractive index and light scattering. Int. J. Biol. Macromol. 2001, 28, 409–420. [Google Scholar] [CrossRef]
- Sagnelli, D.; Kirkensgaard, J.J.K.; Giosafatto, C.V.L.; Ogrodowicz, N.; Kruczał, K.; Mikkelsen, M.S.; Maigret, J.E.; Lourdin, D.; Mortensen, K.; Blennow, A. All-natural bio-plastics using starch-betaglucan composites. Carbohydr. Polym. 2017, 172, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, D.; Hebelstrup, K.H.; Leroy, E.; Rolland-Sabaté, A.; Guilois, S.; Kirkensgaard, J.J.K.; Mortensen, K.; Lourdin, D.; Blennow, A. Plant-crafted starches for bioplastics production. Carbohydr. Polym. 2016, 152, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, D.; Hooshmand, K.; Kemmer, G.C.; Kirkensgaard, J.J.K.; Mortensen, K.; Giosafatto, C.V.L.; Holse, M.; Hebelstrup, K.H.; Bao, J.; Stelte, W.; et al. Cross-Linked Amylose Bio-Plastic: A Transgenic-Based Compostable. Int. J. Mol. Sci. 2017, 18, 2075. [Google Scholar]
- Lourdin, D.; Coignard, L.; Bizot, H.; Colonna, P. Influence of equilibrium relative humidity and plasticizer concentration on the water content and glass transition of starch materials. Polymer (Guildf) 1997, 38, 5401–5406. [Google Scholar] [CrossRef]
- Follain, N.; Joly, C.; Dole, P.; Bliard, C. Mechanical properties of starch-based materials. I. Short review and complementary experimental analysis. J. Appl. Polym. Sci. 2005, 97, 1783–1794. [Google Scholar] [CrossRef]
- Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Effect of nanocellulose as a filler on biodegradable thermoplastic starch films from tuber, cereal legume. Carbohydr. Polym. 2017, 157, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Jantanasakulwong, K.; Leksawasdi, N.; Seesuriyachan, P.; Wongsuriyasak, S.; Techapun, C.; Ougizawa, T. Reactive blending of thermoplastic starch, epoxidized natural rubber and chitosan. Eur. Polym. J. 2016, 84, 292–299. [Google Scholar] [CrossRef]
- Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; St. Pourçain, C.B.; Garnett, M.C. Novel functionalized biodegradable polymers for nanoparticle drug delivery systems. Biomacromolecules 2005, 6, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Taresco, V.; Creasey, R.G.; Kennon, J.; Mantovani, G.; Alexander, C.; Burley, J.C.; Garnett, M.C. Variation in structure and properties of poly(glycerol adipate) via control of chain branching during enzymatic synthesis. Polymer (Guildf) 2016, 89, 41–49. [Google Scholar] [CrossRef]
- Taresco, V.; Suksiriworapong, J.; Styliari, I.D.; Argent, R.H.; Swainson, S.E.; Booth, J.; Turpin, E.; Laughton, C.A.; Burley, J.C.; Alexander, C.; et al. New N-acyl amino acid-functionalized biodegradable polyesters for pharmaceutical and biomedical applications. RSC Adv. 2016, 6, 109401–109405. [Google Scholar] [CrossRef]
- Bilal, M.H.; Hussain, H.; Prehm, M.; Baumeister, U.; Meister, A.; Hause, G.; Busse, K.; Mäder, K.; Kressler, J. Synthesis of poly(glycerol adipate)-g-oleate and its ternary phase diagram with glycerol monooleate and water. Eur. Polym. J. 2017, 91, 162–175. [Google Scholar] [CrossRef]
- Wersig, T.; Krombholz, R.; Janich, C.; Meister, A.; Kressler, J.; Mäder, K. Indomethacin functionalised poly(glycerol adipate) nanospheres as promising candidates for modified drug release. Eur. J. Pharm. Sci. 2018, 123, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Taresco, V.; Suksiriworapong, J.; Creasey, R.; Burley, J.C.; Mantovani, G.; Alexander, C.; Treacher, K.; Booth, J.; Garnett, M.C. Properties of acyl modified poly(glycerol-adipate) comb-like polymers and their self-assembly into nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Suksiriworapong, J.; Taresco, V.; Ivanov, D.P.; Styliari, I.D.; Sakchaisri, K.; Junyaprasert, V.B.; Garnett, M.C. Synthesis and properties of a biodegradable polymer-drug conjugate: Methotrexate-poly(glycerol adipate). Colloids Surf. B Biointerfaces 2018, 167, 115–125. [Google Scholar] [CrossRef]
- Wang, M.O.; Etheridge, J.M.; Thompson, J.A.; Vorwald, C.E.; Dean, D.; Fisher, J.P. Evaluation of the in vitro cytotoxicity of cross-linked biomaterials. Biomacromolecules 2013, 14, 1321–1329. [Google Scholar] [CrossRef]
- Vasanthan, T.; Hoover, R. Barley Starch: Production, Properties, Modification and Uses. In Starch; Elsevier: Amsterdam, The Netherlands, 2009; pp. 601–628. [Google Scholar]
- Marin, E.; Briceño, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar] [PubMed]
- Colombo, C.; Dragoni, L.; Gatti, S.; Pesce, R.M.; Rooney, T.R.; Mavroudakis, E.; Ferrari, R.; Moscatelli, D. Tunable degradation behavior of PEGylated polyester-based nanoparticles obtained through emulsion free radical polymerization. Ind. Eng. Chem. Res. 2014, 53, 9128–9135. [Google Scholar] [CrossRef]
| Sample | Cryst. (%) | Vh (%) | B (%) | A (%) | Contact Angle (°) |
|---|---|---|---|---|---|
| StNative | 20 | 5 | 18 | 77 | |
| StOn | 6 | 37 | 63 | 0 | 102 ± 1 |
| StGl | 6 | 20 | 80 | 0 | 88 ± 2 |
| StPGA3 | 7 | 52 | 48 | 0 | nd |
| StPGA10 | 7 | 47 | 53 | 0 | 57 ± 0.5 |
| StPGAtyr3 | 5 | 57 | 39 | 0 | nd |
| StPGAtyr10 | 4 | 50 | 50 | 0 | 32 ± 1 |
| Film | Tg | Tg-RH |
|---|---|---|
| starch | 66 | 37 °C – 80% |
| StPGA3 | 38/- | |
| StPGA3tyr | -/80 | 37 °C – 57% |
| StPGA10 | 37/80 | |
| StPGA10tyr | 37/80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagnelli, D.; Cavanagh, R.; Xu, J.; Swainson, S.M.E.; Blennow, A.; Duncan, J.; Taresco, V.; Howdle, S. Starch/Poly (Glycerol-Adipate) Nanocomposite Film as Novel Biocompatible Materials. Coatings 2019, 9, 482. https://doi.org/10.3390/coatings9080482
Sagnelli D, Cavanagh R, Xu J, Swainson SME, Blennow A, Duncan J, Taresco V, Howdle S. Starch/Poly (Glycerol-Adipate) Nanocomposite Film as Novel Biocompatible Materials. Coatings. 2019; 9(8):482. https://doi.org/10.3390/coatings9080482
Chicago/Turabian StyleSagnelli, Domenico, Robert Cavanagh, Jinchuan Xu, Sadie M. E. Swainson, Andreas Blennow, John Duncan, Vincenzo Taresco, and Steve Howdle. 2019. "Starch/Poly (Glycerol-Adipate) Nanocomposite Film as Novel Biocompatible Materials" Coatings 9, no. 8: 482. https://doi.org/10.3390/coatings9080482
APA StyleSagnelli, D., Cavanagh, R., Xu, J., Swainson, S. M. E., Blennow, A., Duncan, J., Taresco, V., & Howdle, S. (2019). Starch/Poly (Glycerol-Adipate) Nanocomposite Film as Novel Biocompatible Materials. Coatings, 9(8), 482. https://doi.org/10.3390/coatings9080482






