Predictability of Microbial Adhesion to Dental Materials by Roughness Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Sample Treatment and Roughness Measurements
2.3. Surface Free Energy
2.4. Microbial Culture
Microbial Adhesion via Luminescence Assay
2.5. Statistical Analysis
2.5.1. Analyses of SFE
2.5.2. Analyses of Adhesion
2.5.3. Analyses of Roughness Parameters
2.5.4. Combined Analyses
3. Results
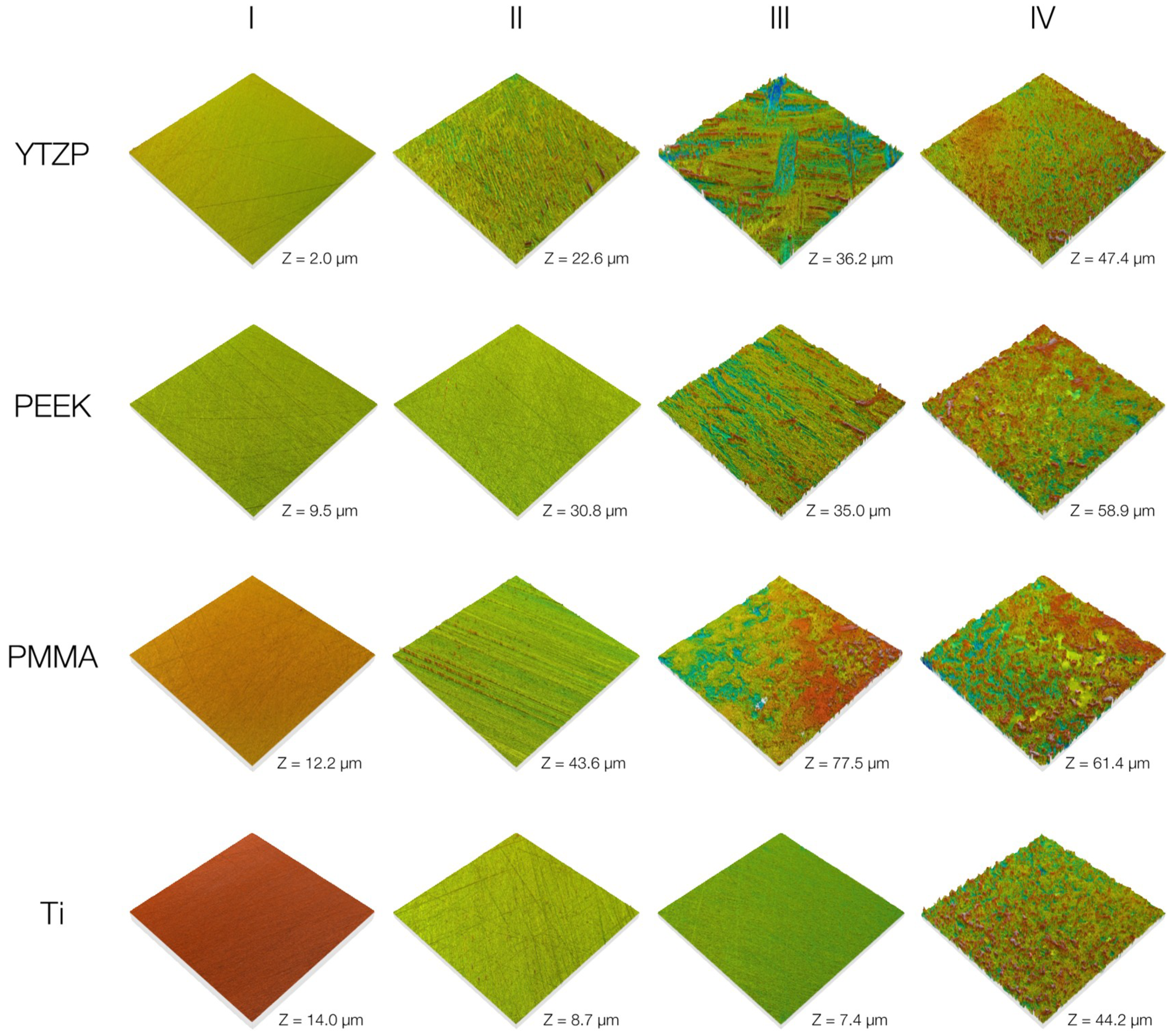
3.1. Surface Roughness
3.2. Surface Free Energy
3.3. Microbial Adhesion
3.4. Association of the Roughness Parameters with Microbial Adhesion
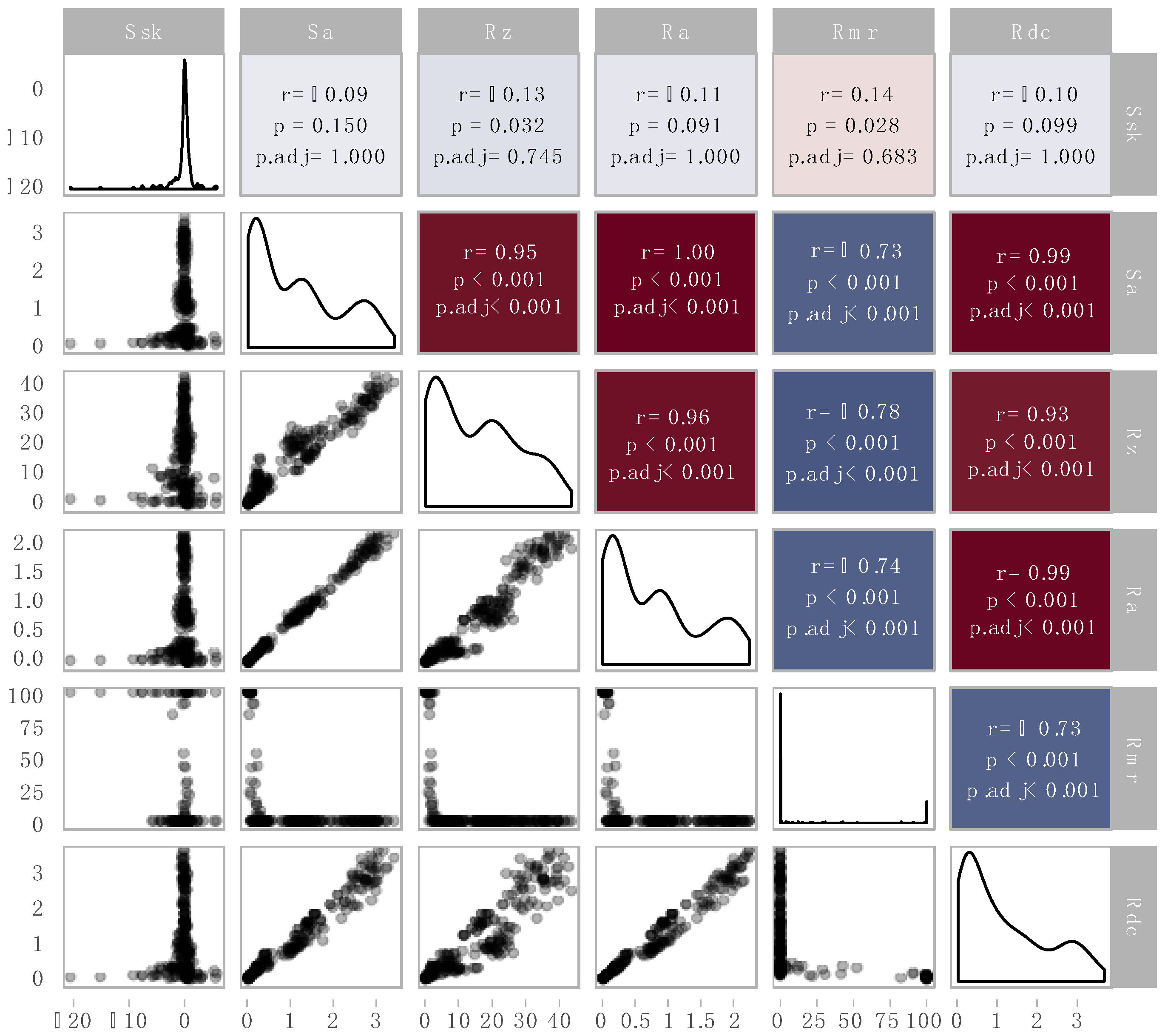
3.5. Correlation Analysis of the Roughness Parameters
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Verma, R.; Murari, A.; Agrawal, A. Oral candidiasis: An overview. JOMFP 2014, 18, S81–S85. [Google Scholar] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implan. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincon, M.V.; Gomez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and microbial biofilm profiles of peri-implantitis: A systematic review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Moter, A.; Devine, D.A. Dental plaque biofilms: communities, conflict and control. Periodontology 2000 2011, 55, 16–35. [Google Scholar] [CrossRef]
- Li, J.; Helmerhorst, E.J.; Leone, C.W.; Troxler, R.F.; Yaskell, T.; Haffajee, A.D.; Socransky, S.S.; Oppenheim, F.G. Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 2004, 97, 1311–1318. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. MMBR 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Aguayo, S.; Marshall, H.; Pratten, J.; Bradshaw, D.; Brown, J.S.; Porter, S.R.; Spratt, D.; Bozec, L. Early adhesion of candida albicans onto dental acrylic surfaces. J. Dent. Res. 2017, 96, 917–923. [Google Scholar] [CrossRef]
- Aslanimehr, M.; Rezvani, S.; Mahmoudi, A.; Moosavi, N. Comparison of candida albicans adherence to conventional acrylic denture base materials and injection molding acrylic materials. J. Dent. 2017, 18, 61–64. [Google Scholar]
- Budtz-Jorgensen, E. The significance of Candida albicans in denture stomatitis. Scand. J. Dent. Res. 1974, 82, 151–190. [Google Scholar] [CrossRef]
- Burgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Rosentritt, M.; Handel, G.; Burgers, R. In vitro evaluation of artificial ageing on surface properties and early Candida albicans adhesion to prosthetic resins. J. Mater. Sci. Mater. Med. 2009, 20, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hannah, V.E.; O’Donnell, L.; Robertson, D.; Ramage, G. Denture stomatitis: Causes, cures and prevention. Prim. Dent. J. 2017, 6, 46–51. [Google Scholar] [PubMed]
- Rzhepishevska, O.; Hakobyan, S.; Ruhal, R.; Gautrot, J.; Barbero, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 2013, 1, 589–602. [Google Scholar] [CrossRef]
- Terada, A.; Okuyama, K.; Nishikawa, M.; Tsuneda, S.; Hosomi, M. The effect of surface charge property on Escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 2012, 109, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M. The clinical meaning of the surface roughness and the surface free energy of intra-oral hard substrata on the microbiology of the supra- and subgingival plaque: Results of in vitro and in vivo experiments. J. Dent. 1994, 22, S13–S16. [Google Scholar] [CrossRef]
- Suh, A.Y.; Polycarpou, A.A.; Conry, T.F. Detailed surface roughness characterization of engineering surfaces undergoing tribological testing leading to scuffing. Wear 2003, 255, 556–568. [Google Scholar] [CrossRef]
- Jarnstrom, J.; Ihalainen, P.; Backfolk, K.; Peltonen, J. Roughness of pigment coatings and its influence on gloss. Appl. Surf. Sci. 2008, 254, 5741–5749. [Google Scholar] [CrossRef]
- Etxeberria, M.; Escuin, T.; Vinas, M.; Ascaso, C. Useful surface parameters for biomaterial discrimination. Scanning 2015, 37, 429–437. [Google Scholar] [CrossRef]
- Whitehouse, D.J. The parameter rash—Is there a cure? Wear 1982, 83, 75–78. [Google Scholar] [CrossRef]
- Moda, M.D.; Godas, A.G.L.; Fernandes, J.C.; Suzuki, T.Y.U.; Guedes, A.P.A.; Briso, A.L.F.; Bedran-Russo, A.K.; Dos Santos, P.H. Comparison of different polishing methods on the surface roughness of microhybrid, microfill, and nanofill composite resins. JICD 2018, 9, e12287. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Gurdogan, E.B.; Ozdemir-Ozenen, D.; Sandalli, N. Evaluation of surface roughness characteristics using atomic force microscopy and inspection of microhardness following resin infiltration with icon®. J. Esthet. Restor. Dent. 2017, 29, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Folwaczny, M.; Merkel, U.; Mehl, A.; Hickel, R. Influence of parameters on root surface roughness following treatment with a magnetostrictive ultrasonic scaler: An in vitro study. J. Periodontol. 2004, 75, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Schafer, L.; Felthaus, O.; Allerdings, J.; Hahnel, S.; Behr, M.; Burgers, R. The bacterial adhesion on and the cytotoxicity of various dental cements used for implant-supported fixed restorations. Acta Odontol. Scand. 2014, 72, 241–250. [Google Scholar] [CrossRef]
- Burgers, R.; Cariaga, T.; Muller, R.; Rosentritt, M.; Reischl, U.; Handel, G.; Hahnel, S. Effects of aging on surface properties and adhesion of Streptococcus mutans on various fissure sealants. Clin. Oral Invest. 2009, 13, 419–426. [Google Scholar] [CrossRef]
- DIN-Deutsches Institut für Normung, DIN EN ISO 4287. Available online: https://www.din.de/de/mitwirken/normenausschuesse/natg/normen/wdc-beuth:din21:129356592 (accessed on 19 July 2019).
- DIN-Deutsches Institut für Normung. DIN EN ISO 25178-600. Available online: https://www.din.de/de/mitwirken/normenausschuesse/natg/entwuerfe/wdc-beuth:din21:269554610 (accessed on 19 July 2019).
- Owens, D.K.; Wendt, R.G. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Wojciechowski, L.; Kubiak, K.J.; Mathia, T.G. Roughness and wettability of surfaces in boundary lubricated scuffing wear. Tribol Int. 2016, 93, 593–601. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Coll. Interface Sci. 2012, 179, 142–149. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Eriksson, B.; Lekholm, U.; Branemark, P.I.; Jemt, T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. JOMI 1990, 5, 347–359. [Google Scholar] [PubMed]
- Jemt, T.; Chai, J.; Harnett, J.; Heath, M.R.; Hutton, J.E.; Johns, R.B.; McKenna, S.; McNamara, D.C.; van Steenberghe, D.; Taylor, R.; et al. A 5-year prospective multicenter follow-up report on overdentures supported by osseointegrated implants. JOMI 1996, 11, 291–298. [Google Scholar] [PubMed]
- Vojdani, M.; Giti, R. Polyamide as a denture base material: A literature review. J. Dent. (Shiraz) 2015, 16, 1–9. [Google Scholar]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Invest. 2016, 20, 1403–1417. [Google Scholar] [CrossRef]
- Kohal, R.J.; Att, W.; Bachle, M.; Butz, F. Ceramic abutments and ceramic oral implants. An update. Periodontology 2000 2008, 47, 224–243. [Google Scholar] [CrossRef]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant Dent. Relat. Res. 2005, 7, S13–S20. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; van Steenberghe, D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. JOMI 1996, 11, 169–178. [Google Scholar]
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Darius, P.L.; van Steenberghe, D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J. Clin. Periodontol. 1990, 17, 138–144. [Google Scholar] [CrossRef]
- Radford, D.R.; Sweet, S.P.; Challacombe, S.J.; Walter, J.D. Adherence of Candida albicans to denture-base materials with different surface finishes. J. Dent. 1998, 26, 577–583. [Google Scholar] [CrossRef]
- Von Fraunhofer, J.A.; Loewy, Z.G. Factors involved in microbial colonization of oral prostheses. Gen. Dent. 2009, 57, 136–143. [Google Scholar] [PubMed]
- Bürgers, R.; Witecy, C.; Hahnel, S.; Gosau, M. The effect of various topical peri-implantitis antiseptics on Staphylococcus epidermidis, Candida albicans, and Streptococcus sanguinis. Arch. Oral Biol. 2012, 57, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Teutle-Coyotecatl, B.; Contreras-Bulnes, R.; Scougall-Vilchis, R.J.; Almaguer-Flores, A.; Garcia-Perez, V.I.; Rodriguez-Vilchis, L.E.; Arenas Alatorre, J. Adhesion of Streptococcus mutans and Streptococcus sanguinis on Er:YAG laser-irradiated dental enamel: Effect of surface roughness. Photomed. Laser Surg. 2018, 36, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Ota-Tsuzuki, C.; Martins, F.L.; Giorgetti, A.P.; de Freitas, P.M.; Duarte, P.M. In vitro adhesion of Streptococcus sanguinis to dentine root surface after treatment with Er:YAG laser, ultrasonic system, or manual curette. Photomed. Laser Surg. 2009, 27, 735–741. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, A.G.; Juarez, A.; Engel, E.; Gil, F.J. Streptococcus sanguinis adhesion on titanium rough surfaces: Effect of shot-blasting particles. J. Mater. Sci. Mater. Med. 2011, 22, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; van Pelt, A.W.J.; de Boer, P.; de Jong, H.P.; Arends, J. The effect of surface roughening of polymers on measured contact angle of liquids. Coll. Surf. 1984, 9, 319–331. [Google Scholar] [CrossRef]

| Product | Type | Manufacturer |
|---|---|---|
| Ice Zirkon 95H14 | yttria-stabilized zirconia ceramic (YTZP) | Zirkonzahn, Gais, Italy |
| Tecno Med 95H16 | polyetheretherketone (PEEK) | Zirkonzahn |
| PalaXPress | polymethylmathacrylate (PMMA) | Heraeus Kulzer, Hanau, Germany |
| Ti-6Al-4V | titanium alloy (Ti) | Hempel metals and more, Duebendorf-Zurich, Switzerland |
| Roughness Level | Ra (µm) |
|---|---|
| I | <0.1 |
| II | ~0.2 |
| III | 0.7–1 |
| IV | 1.7–2 |
| Parameter | Symbol | |
|---|---|---|
| 1. Maximum peak height of the roughness profile | Rp |  |
| 2. Maximum valley depth of the roughness profile | Rv | |
| 3. Mean roughness depth | Rz | |
| 4. Mean height of profile elements | Rc | |
| 5. Total height of the roughness profile | Rt = Rmax | |
| 6. Arithmetical mean roughness value | Ra | |
| 7. Root-mean-square roughness | Rq = Rms | |
| 8. Skewness of the roughness profile | Rsk | |
| 9. Kurtosis of the roughness profile | Rku | |
| 10. Profile section height between two material ratios | Rdc | |
| 11. Material component of the profile | Rmr | |
| 12. Root mean square deviation of surface topography | Sq |  |
| 13. Skewness of topography height distribution | Ssk | |
| 14. Kurtosis of topography height distribution | Sku | |
| 15. Maximum peak height of the surface topography | Sp | |
| 16. Maximum valley depth of the surface topography | Sv | |
| 17. Maximum height of surface topography | Sz | |
| 18. Arithmetical mean deviation of surface roughness | Sa |
| Roughness Level | Ra (µm) Mean (sd) | ||||
|---|---|---|---|---|---|
| All materials | YTZP | PEEK | PMMA | Ti | |
| I | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | 0.0 * (0.0) |
| II | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.0) | 0.3 (0.1) |
| III | 0.9 (0.2) | 0.9 (0.1) | 0.9 (0.1) | 0.8 (0.2) | 0.8 (0.3) |
| IV | 1.8 (0.3) | 1.9 (0.2) | 2.0 (0.1) | 1.7 (0.3) | 1.9 (0.2) |
| Microorganism | Material | Parameter | R2 | LCL | UCL | Sig. |
|---|---|---|---|---|---|---|
| C. albicans | YTZP | Rdc | 87 | −23 | 197 | no |
| Ra | 80 | −27 | 187 | |||
| PEEK | Rdc | 127 | −1 | 255 | no | |
| Ra | 123 | −3 | 249 | |||
| PMMA | Rdc | 308 | 150 | 466 | no | |
| Ra | 297 | 140 | 454 | |||
| Ti | Sa | 146 | 12 | 280 | no | |
| Ra | 135 | 5 | 265 | |||
| S. sanguinis | YTZP | Sa | 2 | −16 | 20 | no |
| Ra | 2 | −16 | 20 | |||
| PEEK | Sa | 42 | −39 | 123 | no | |
| Ra | 39 | −39 | 117 | |||
| PMMA | Sa | 157 | 20 | 294 | no | |
| Ra | 153 | 17 | 289 | |||
| Ti | Sa | 22 | −38 | 82 | no | |
| Ra | 19 | −36 | 74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, A.; Wassmann, T.; Holtappels, M.; Kurbad, O.; Krohn, S.; Bürgers, R. Predictability of Microbial Adhesion to Dental Materials by Roughness Parameters. Coatings 2019, 9, 456. https://doi.org/10.3390/coatings9070456
Schubert A, Wassmann T, Holtappels M, Kurbad O, Krohn S, Bürgers R. Predictability of Microbial Adhesion to Dental Materials by Roughness Parameters. Coatings. 2019; 9(7):456. https://doi.org/10.3390/coatings9070456
Chicago/Turabian StyleSchubert, Andrea, Torsten Wassmann, Mareike Holtappels, Oliver Kurbad, Sebastian Krohn, and Ralf Bürgers. 2019. "Predictability of Microbial Adhesion to Dental Materials by Roughness Parameters" Coatings 9, no. 7: 456. https://doi.org/10.3390/coatings9070456
APA StyleSchubert, A., Wassmann, T., Holtappels, M., Kurbad, O., Krohn, S., & Bürgers, R. (2019). Predictability of Microbial Adhesion to Dental Materials by Roughness Parameters. Coatings, 9(7), 456. https://doi.org/10.3390/coatings9070456





