A Self-Healing Coating with UV-Shielding Property
Abstract
:1. Introduction
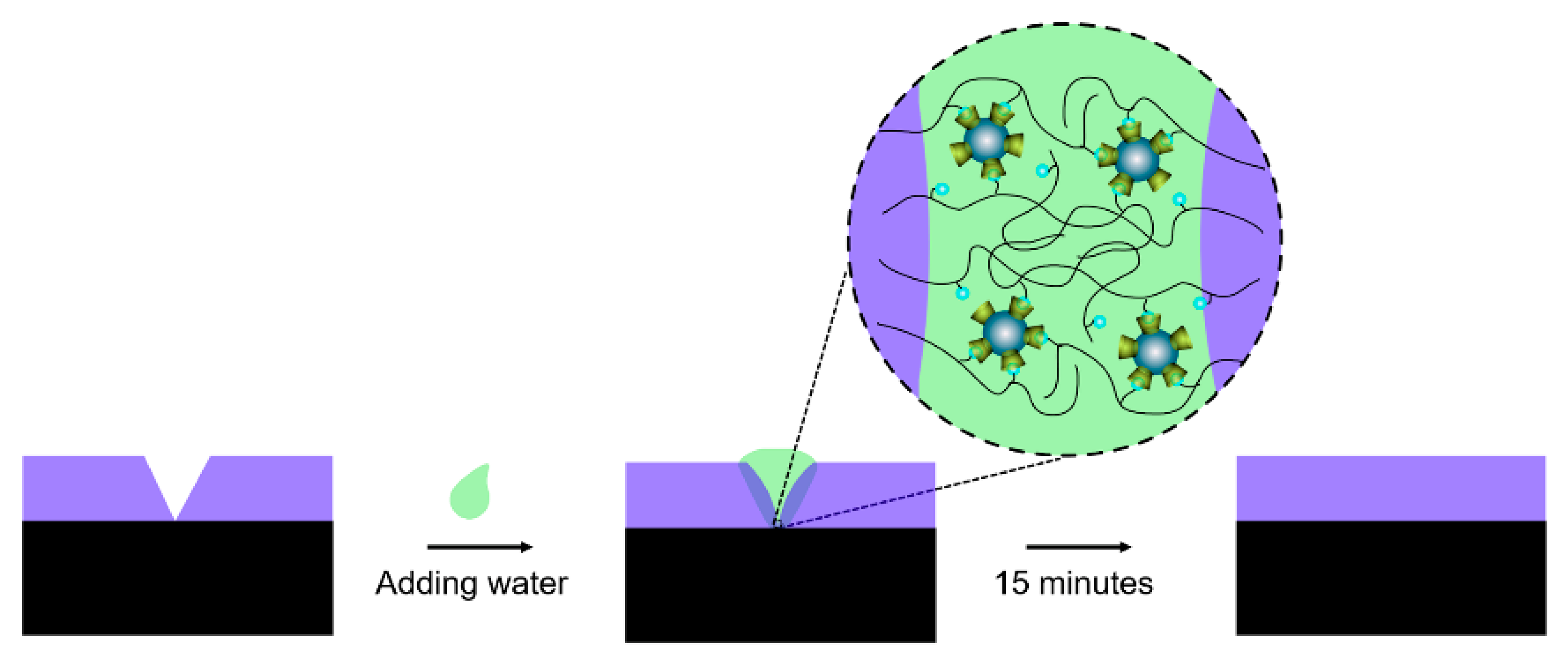
2. Principle
3. Materials and Methods
3.1. Materials
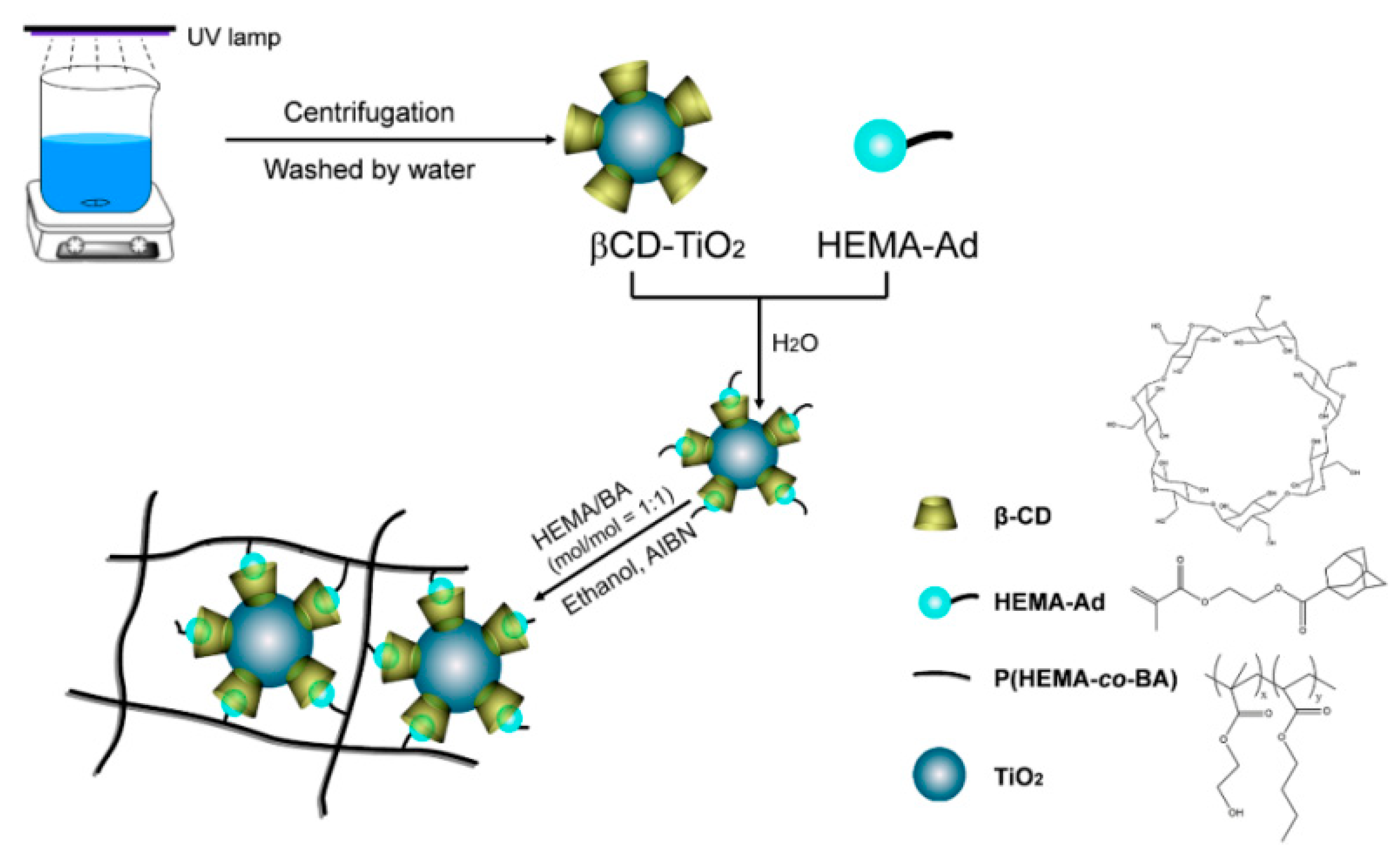
3.2. Sample Fabrication
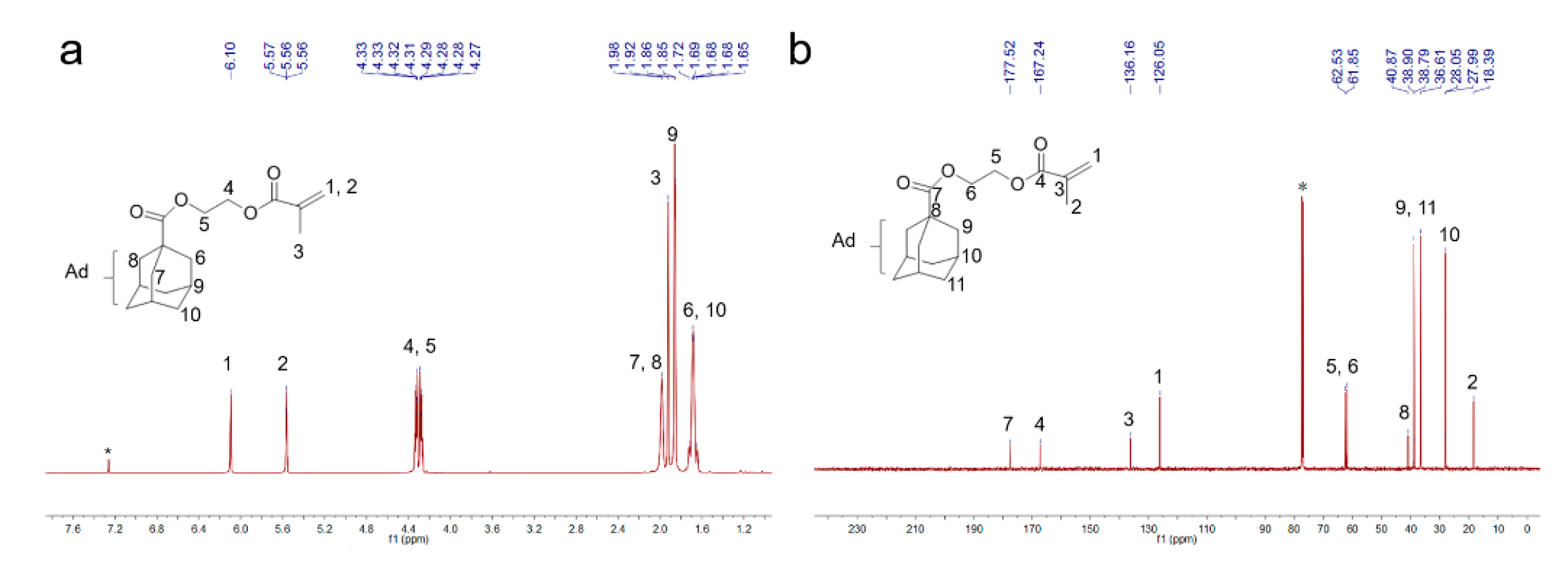
3.2.1. Preparation of the Guest Molecule 2-Hydroxyethyl-methacrylate-adamantane (HEMA-Ad)
3.2.2. Preparation of the Host Molecule β-CD-TiO2
3.2.3. Preparation of the β-CD-TiO2/P(HEMA-co-BA) Coating
3.3. Sample Characterization and Application
4. Results and Discussion
4.1. Characterization of β-CD-TiO2/P(HEMA-co-BA)
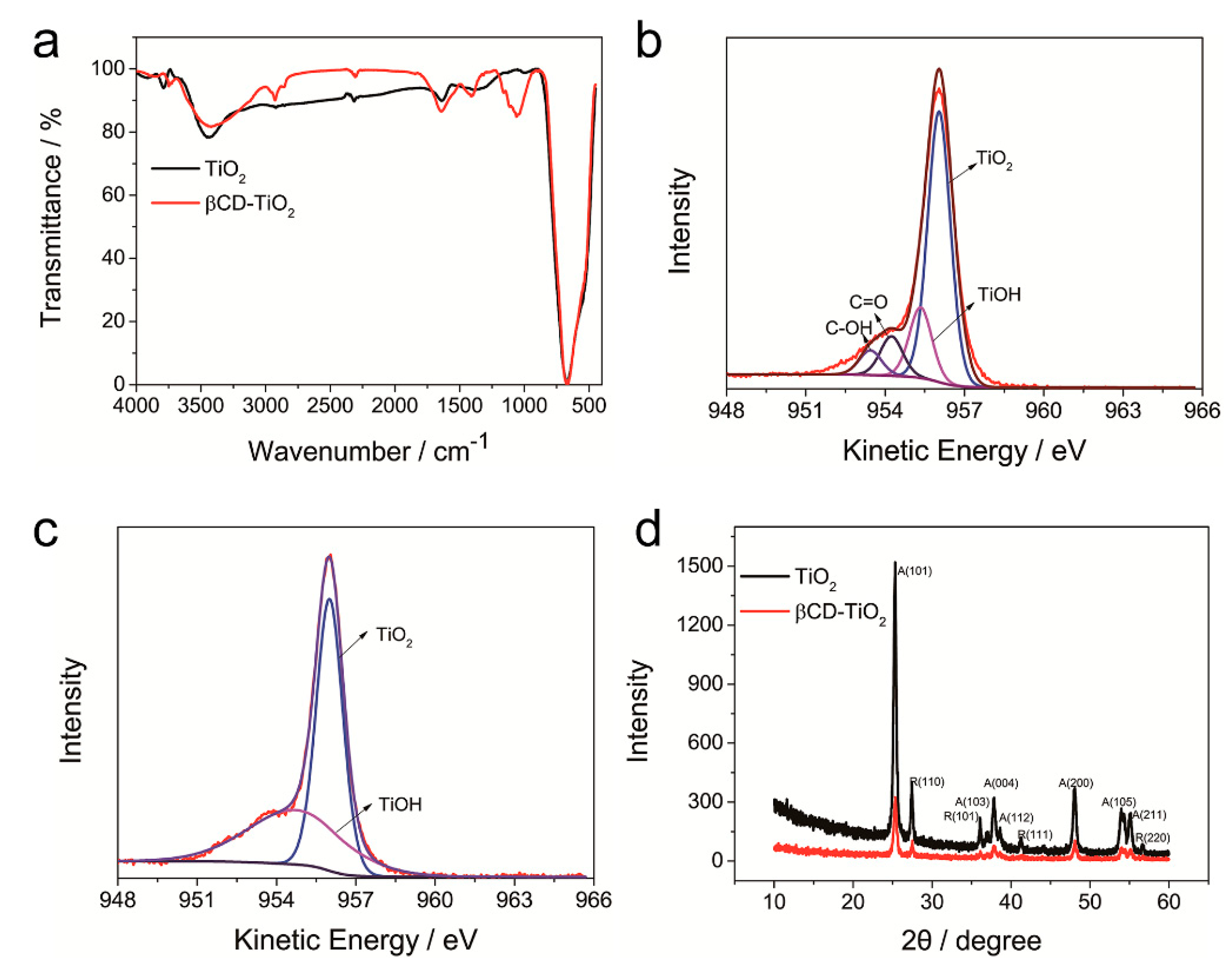
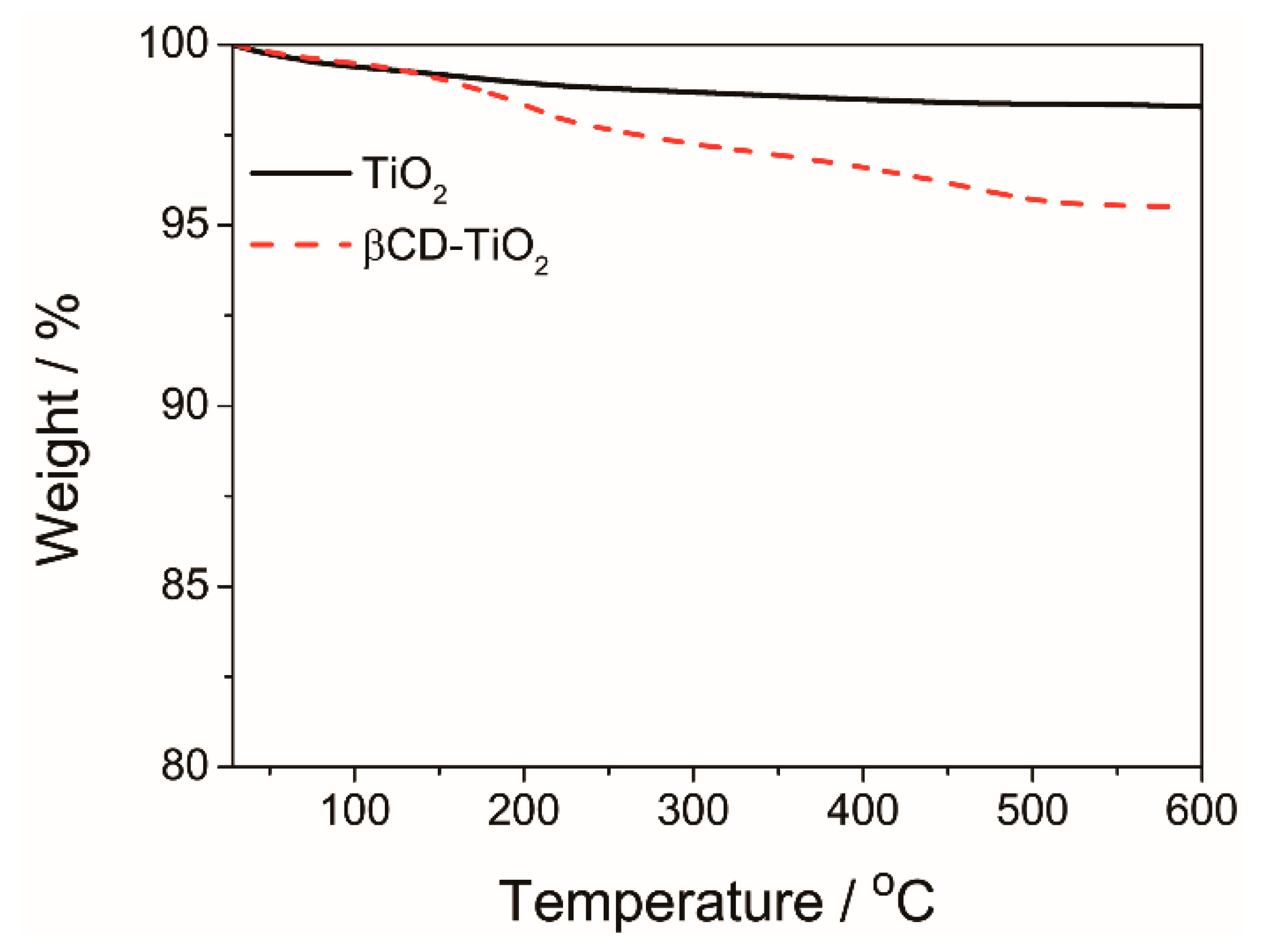
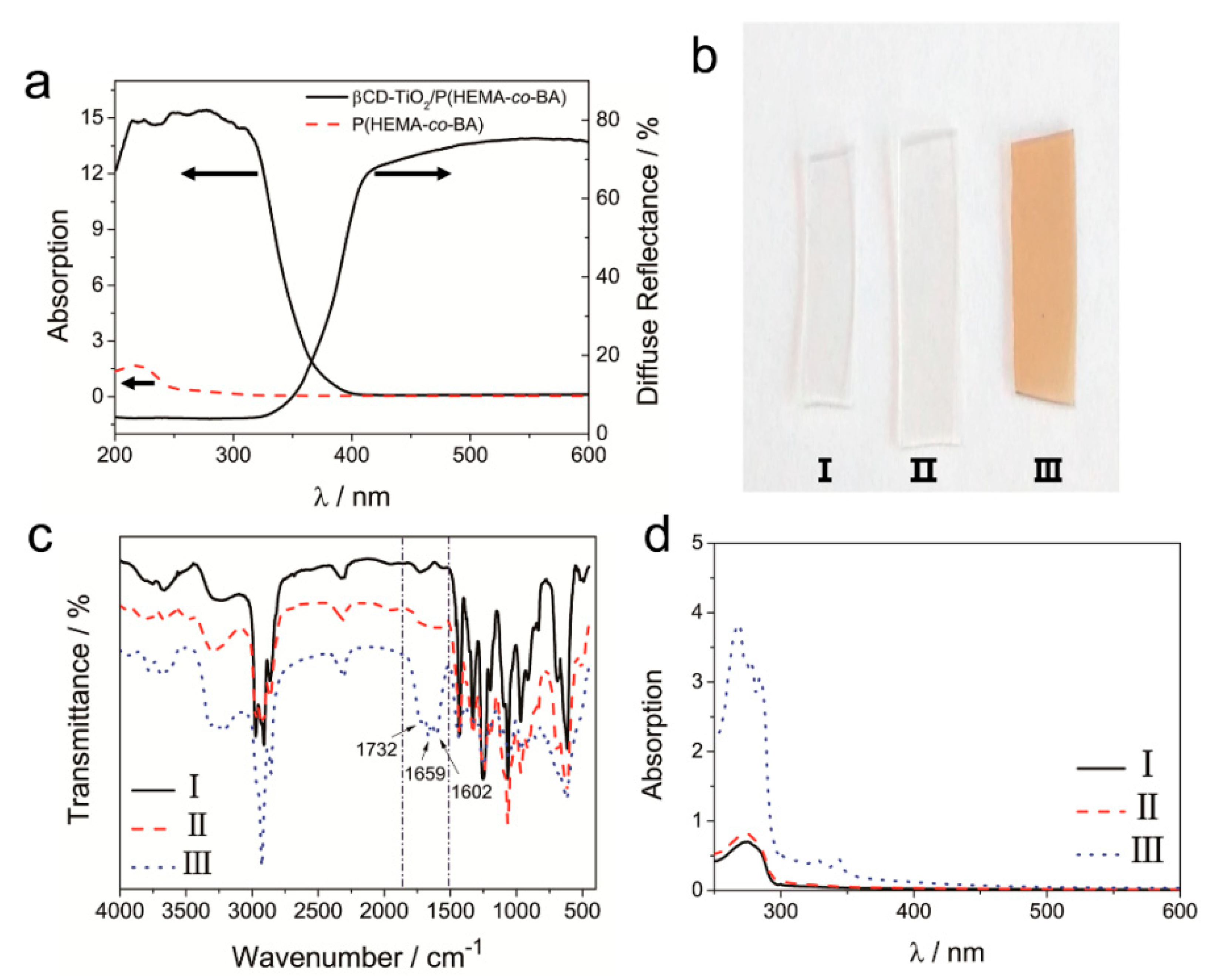
4.1.1. Characterization of β-CD-TiO2 and HEMA-Ad
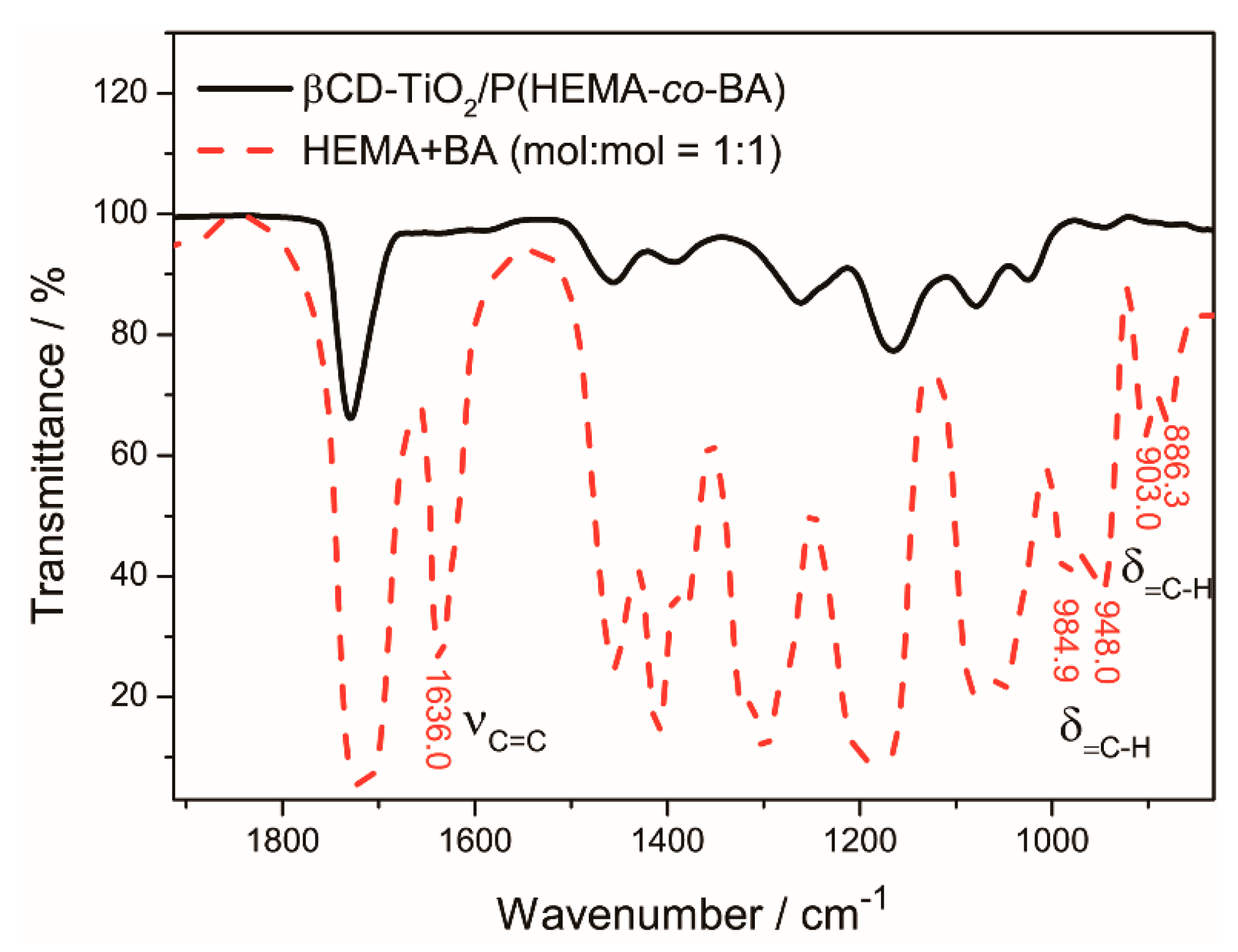
4.1.2. Polymerization of β-CD-TiO2/P(HEMA-co-BA)
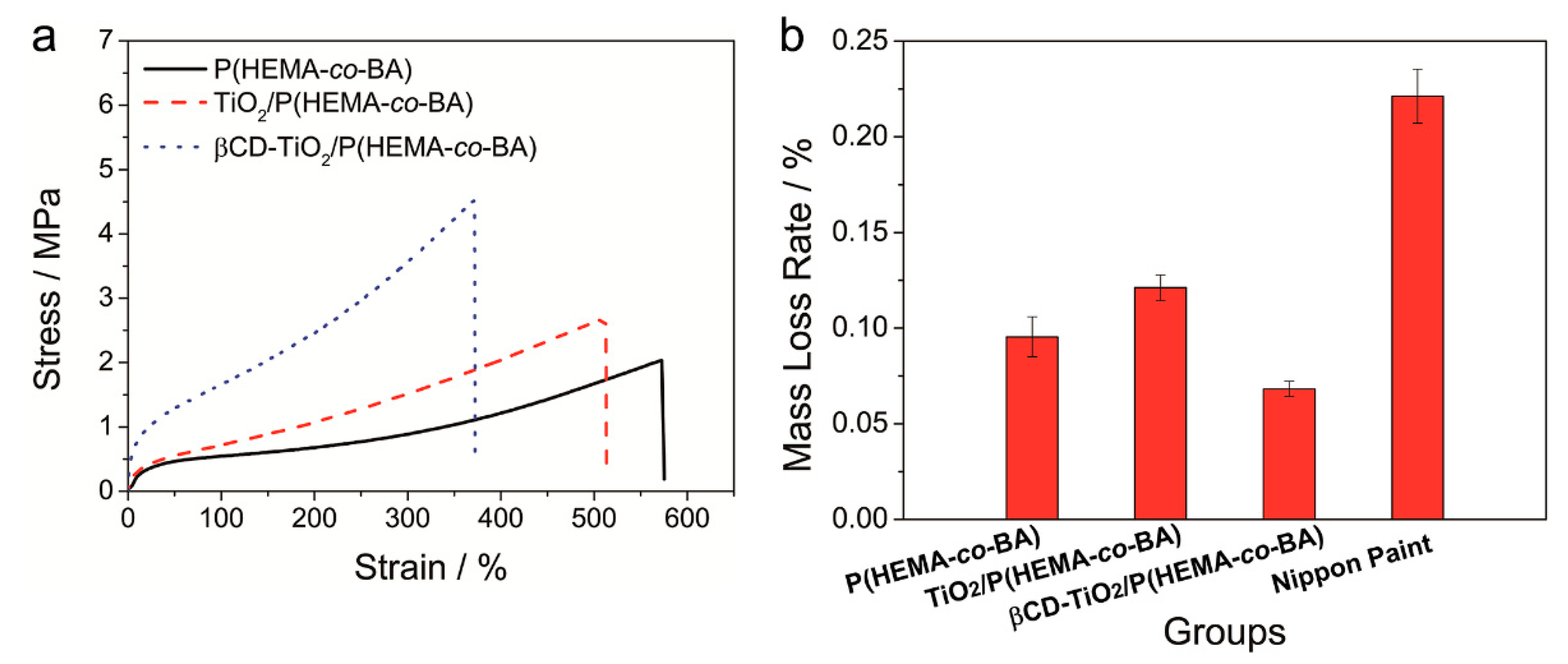
4.2. Mechanical Properties and Wear Resistance of Coatings
4.3. Adhesion of Coating
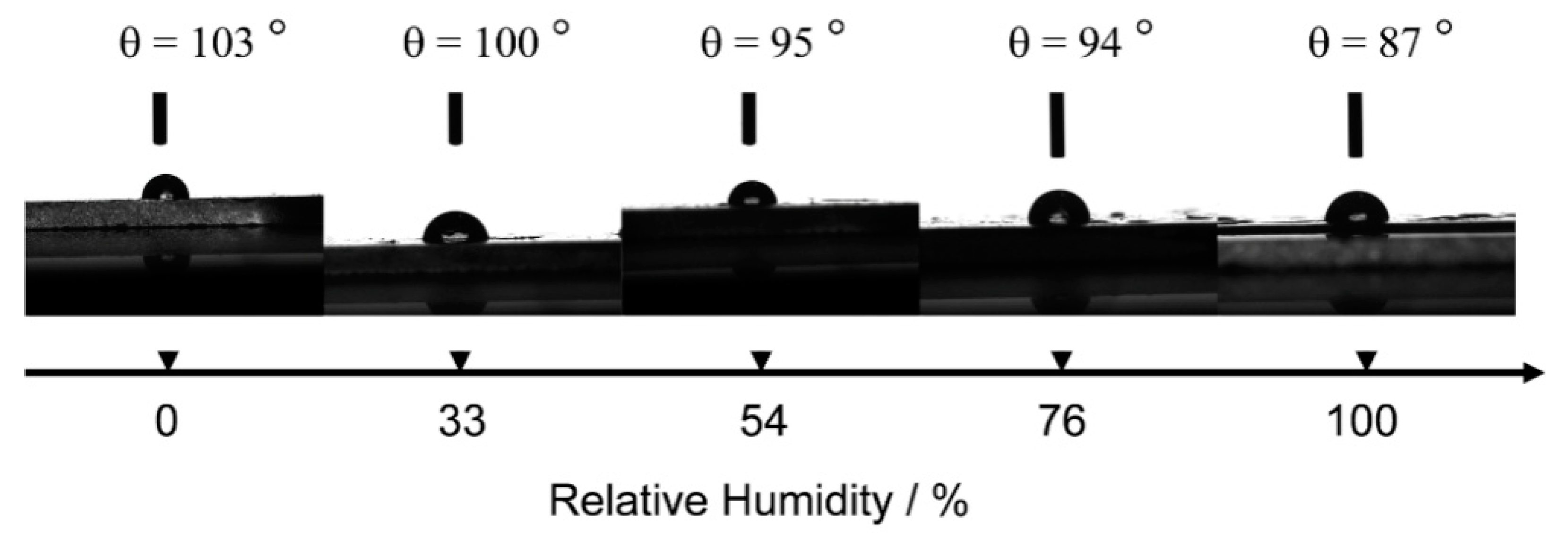
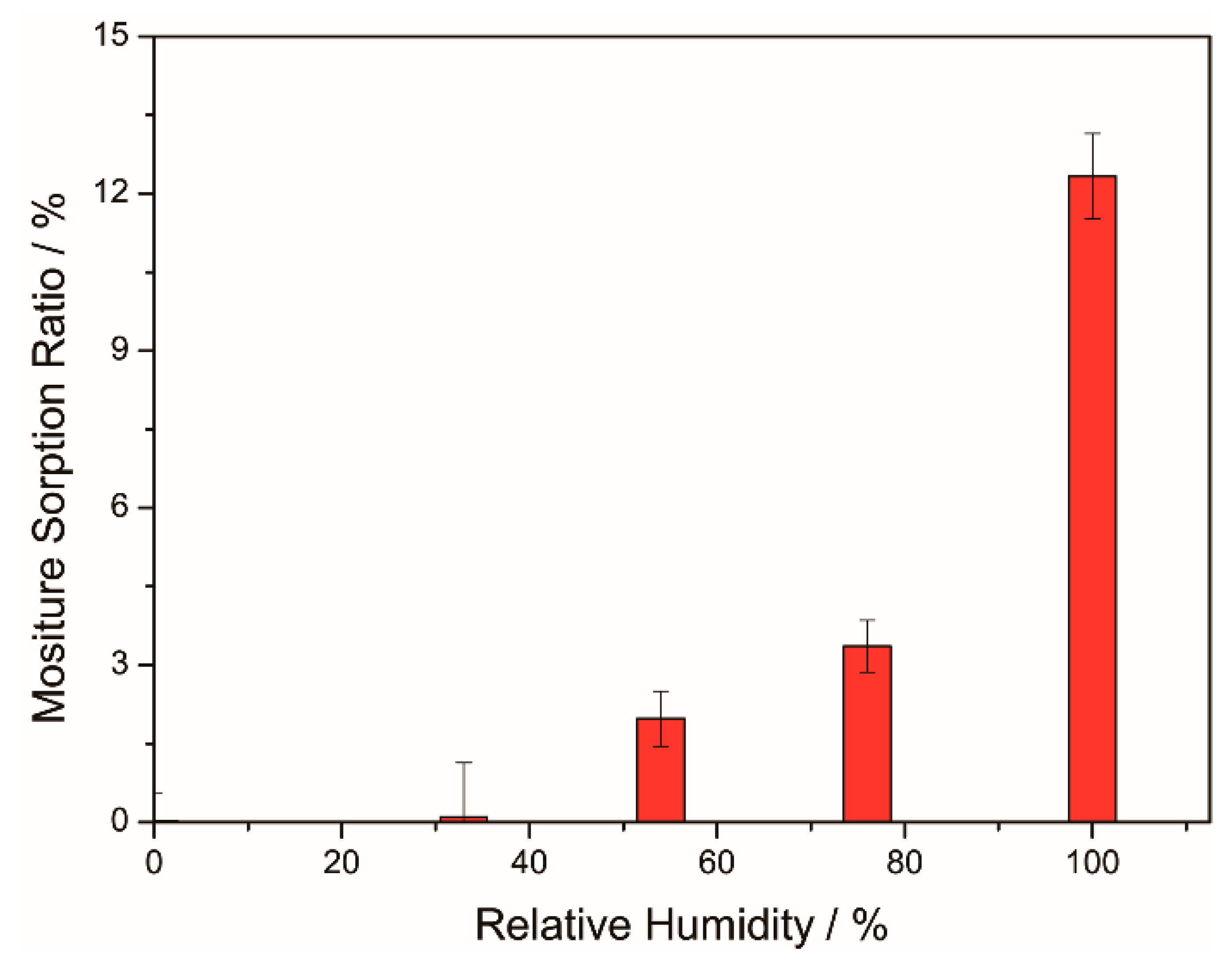
4.4. Hydrophobicity and Hygroscopicity of Coatings
4.5. Coating UV-Shielding Performance
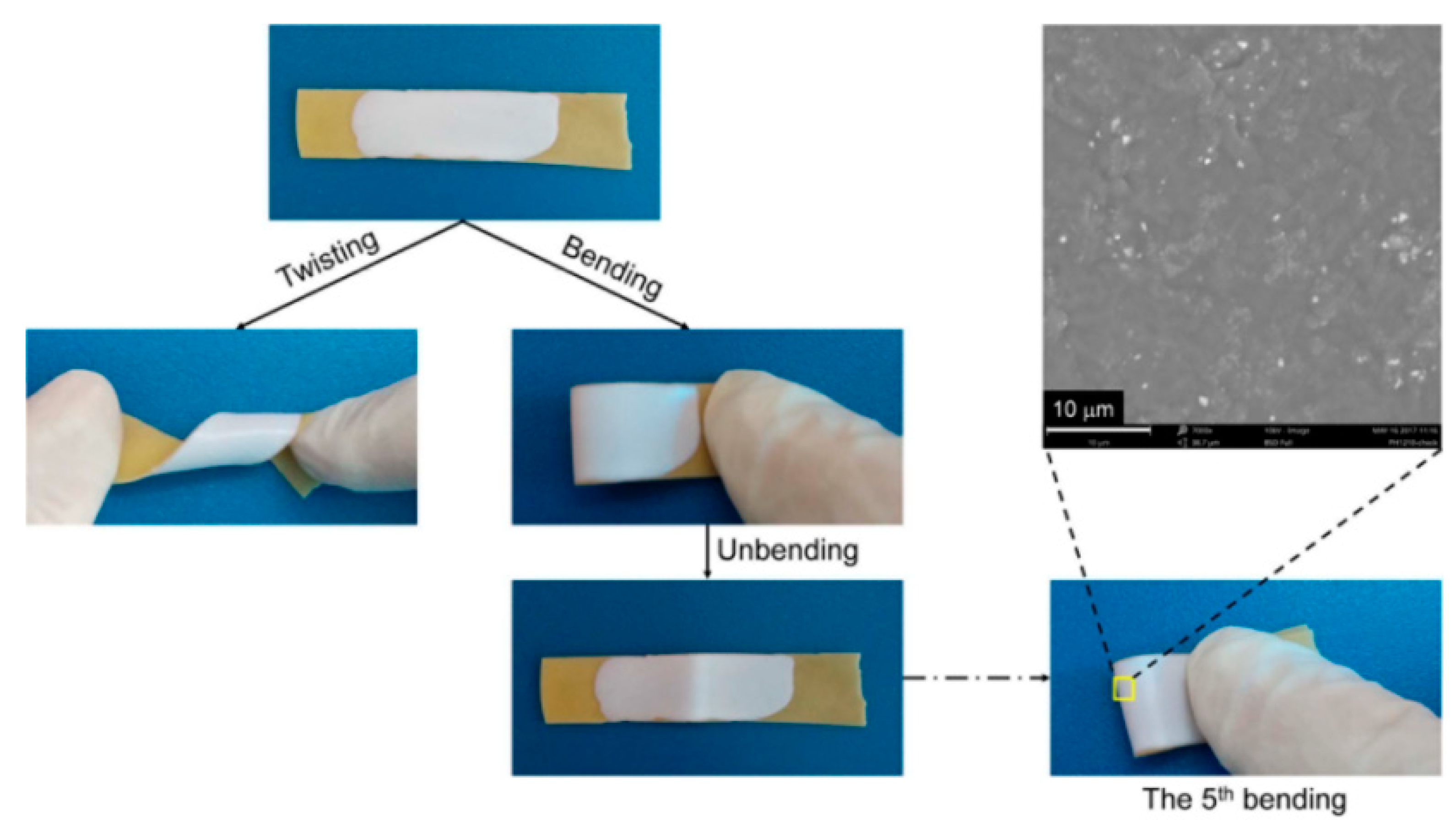
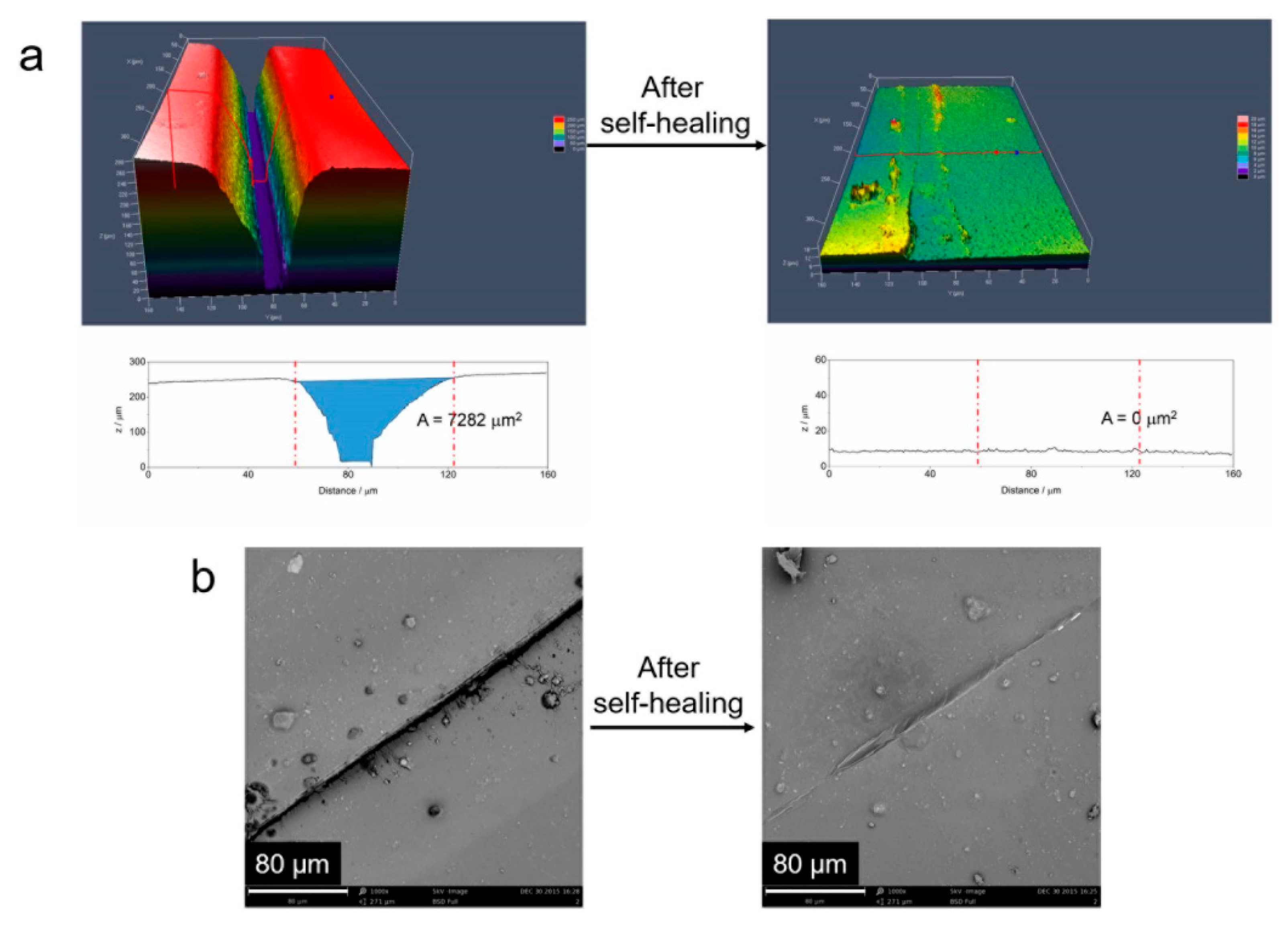
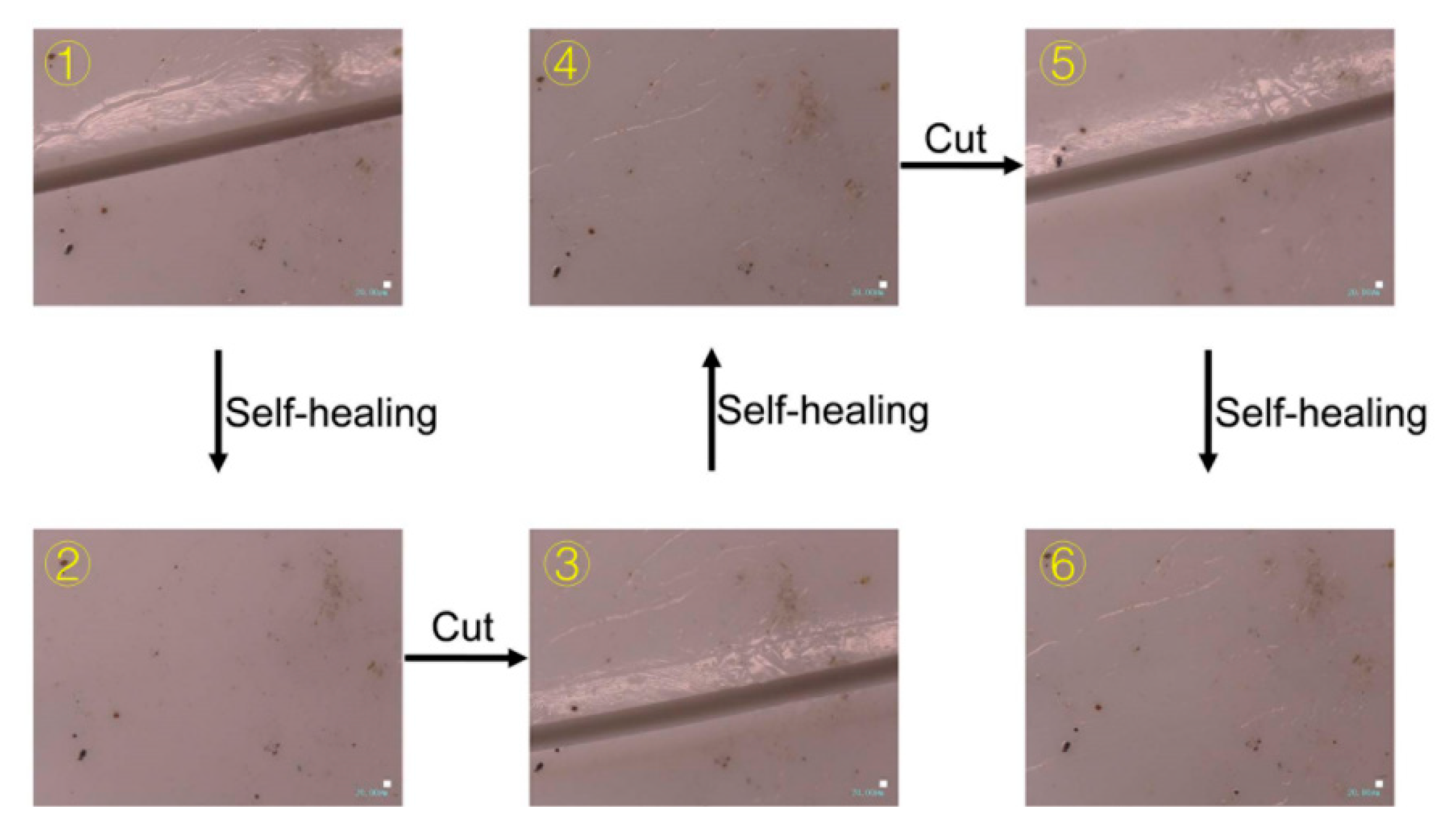
4.6. Self-Healing Properties
4.6.1. Self-Healing of Mechanical Property
4.6.2. Self-Healing of UV-Shielding Property
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mynar, J.L.; Aida, T. Materials science: The gift of healing. Nature 2008, 451, 895. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.W.; White, S.R.; Sottos, N.R. A self-healing poly (dimethyl siloxane) elastomer. Adv. Funct. Mater. 2010, 17, 2399–2404. [Google Scholar] [CrossRef]
- Rule, J.D.; Brown, E.N.; Sottos, N.R.; White, S.R.; Moore, J.S. Wax-protected catalyst microspheres for efficient self-healing materials. Adv. Mater. 2005, 17, 205–208. [Google Scholar] [CrossRef]
- Bleay, S.M.; Loader, C.B.; Hawyes, V.J.; Humberstone, L.; Curtis, P.T. A smart repair system for polymer matrix composites. Compos. Part A Appl. Sci. Manuf. 2001, 32, 1767–1776. [Google Scholar] [CrossRef]
- Tras, R.S.; Williams, G.J.; Bond, I.P. Bioinspired selfhealing of advanced composite structures. JR Soc. Interface 2007, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581. [Google Scholar] [CrossRef] [PubMed]
- Toohey, K.S.; Hansen, C.J.; Lewis, J.A.; White, S.R.; Sottos, N.R. Delivery of two-part self-healing chemistry via microvascular networks. Adv. Funct. Mater. 2009, 19, 1399–1405. [Google Scholar] [CrossRef]
- Chen, X.; Wudl, F.; Mal, A.K.; Shen, H.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Coumarin imparts repeated photochemical remendability to polyurethane. J. Mater. Chem. 2011, 21, 18373–18380. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A healable supramolecular polymer blend based on aromatic π−π stacking and hydrogen-bonding interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.; Zedler, L.; Schacher, F.H.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M.D.; Schubert, U.S. Self-healing polymer coatings based on crosslinked metallosupramolecular copolymers. Adv. Mater. 2013, 25, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-healing supramolecular gels formed by crown ether based host–guest interactions. Angew. Chem. Int. Ed. 2012, 51, 7011–7015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Ju, X.; Li, L.H.; Kang, Y.; Gong, X.L.; Li, B.J.; Zhang, S. An efficient multiple healing conductive composite via host–guest inclusion. Chem. Commun. 2015, 51, 6377–6380. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Meng, D.; Sun, J.; Memon, J.; Huang, Y.; Geng, J. Graphene wrapped TiO2 BASED CATALYSTS with enhanced photocatalytic activity. Adv. Mater. Interfaces 2014, 1, 1300150. [Google Scholar] [CrossRef]
- Wu, Y.; Zuo, F.; Zheng, Z.; Ding, X.; Peng, Y. A novel approach to molecular recognition surface of magnetic nanoparticles based on host–guest effect. Nanoscale Res. Lett. 2009, 4, 738. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Lin, M.; Zhang, S.; Li, L.; Fu, Q.; Hou, J. A Self-Healing Coating with UV-Shielding Property. Coatings 2019, 9, 421. https://doi.org/10.3390/coatings9070421
Peng L, Lin M, Zhang S, Li L, Fu Q, Hou J. A Self-Healing Coating with UV-Shielding Property. Coatings. 2019; 9(7):421. https://doi.org/10.3390/coatings9070421
Chicago/Turabian StylePeng, Lei, Musong Lin, Sheng Zhang, Li Li, Qiang Fu, and Junbo Hou. 2019. "A Self-Healing Coating with UV-Shielding Property" Coatings 9, no. 7: 421. https://doi.org/10.3390/coatings9070421
APA StylePeng, L., Lin, M., Zhang, S., Li, L., Fu, Q., & Hou, J. (2019). A Self-Healing Coating with UV-Shielding Property. Coatings, 9(7), 421. https://doi.org/10.3390/coatings9070421




