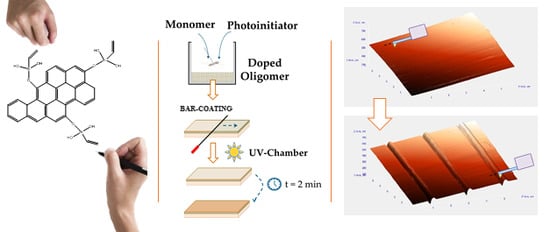
Custom-Made Chemically Modified Graphene Oxide to Improve the Anti-Scratch Resistance of Urethane-Acrylate Transparent Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Coatings
2.2. Graphene Derivatives Synthesis
2.2.1. Graphene Oxide + Chemical Reduction Process (rGO) Synthesis
2.2.2. Graphene Oxide + VTES (GOvtes) Synthesis
2.2.3. Graphene Oxide + VTES + Chemical Reduction Process (rGOvtes) Synthesis
2.3. Fabrication of Coatings with Graphene Derivatives as Nanofiller
2.4. Graphene Derivatives and Coatings Characterization
2.4.1. Graphene Derivatives Characterization
2.4.2. Optical Analysis of Coatings
2.4.3. Wolf–Wilburn Method
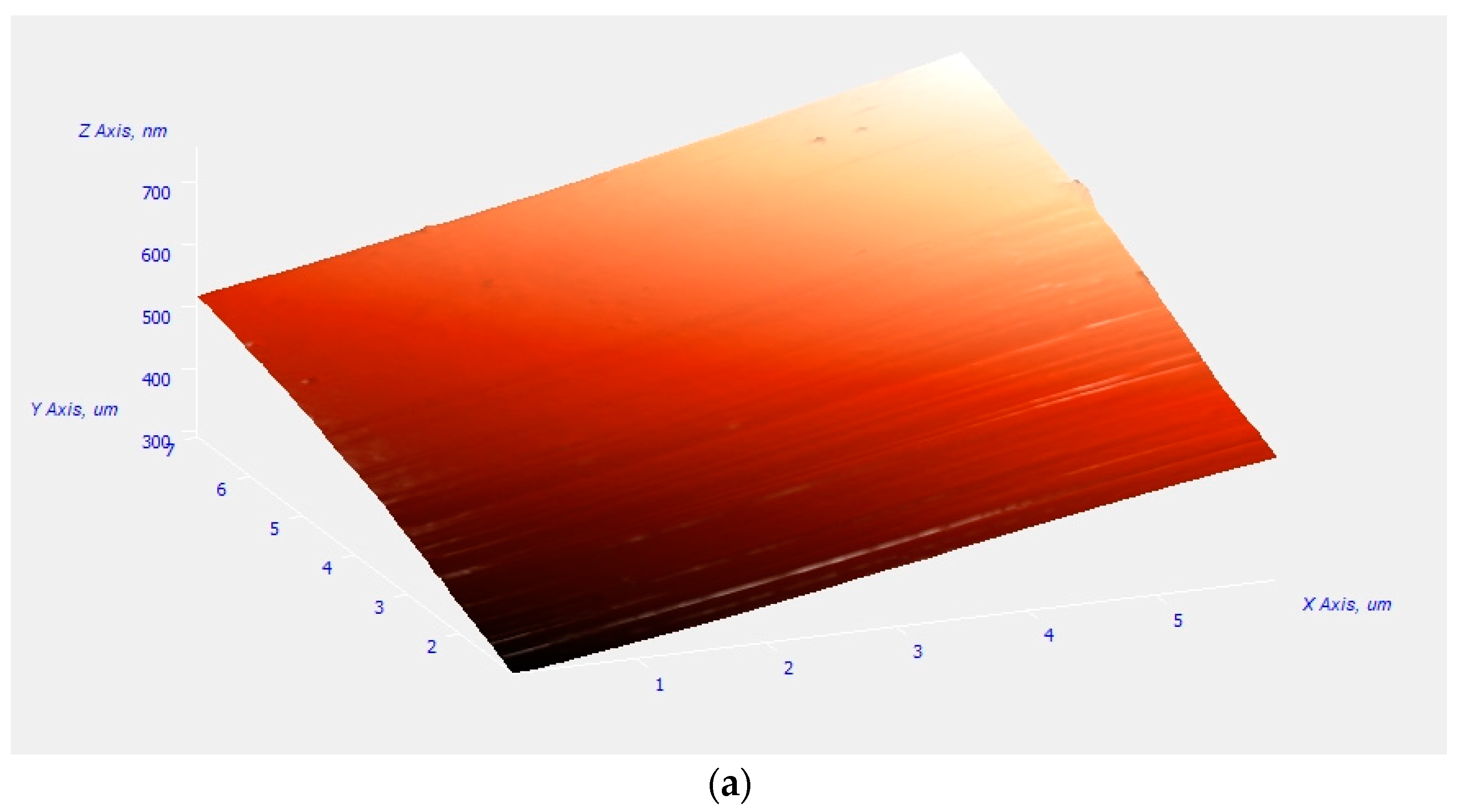
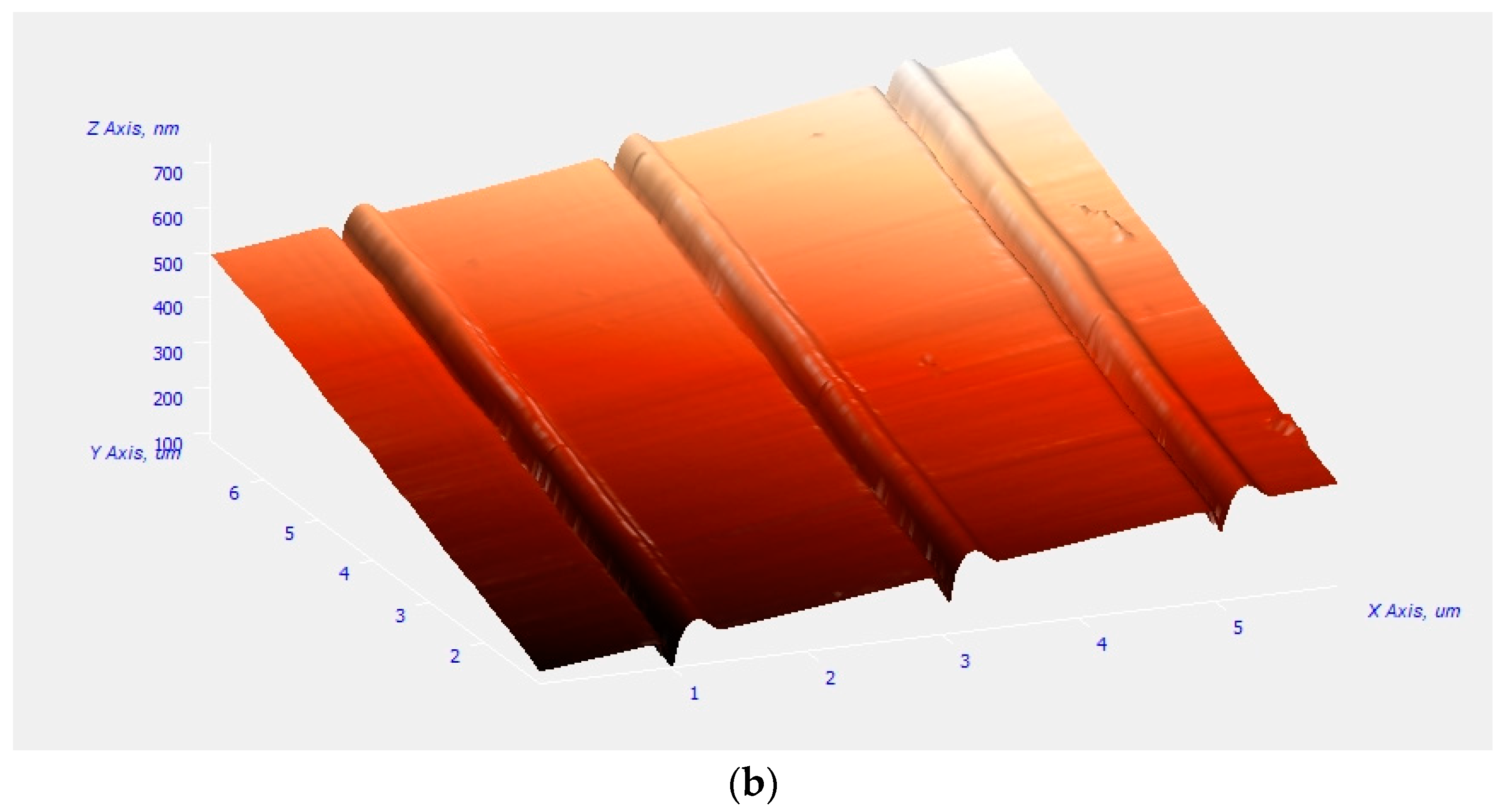
2.4.4. Atomic Force Microscopy (AFM)
3. Results and Discussion
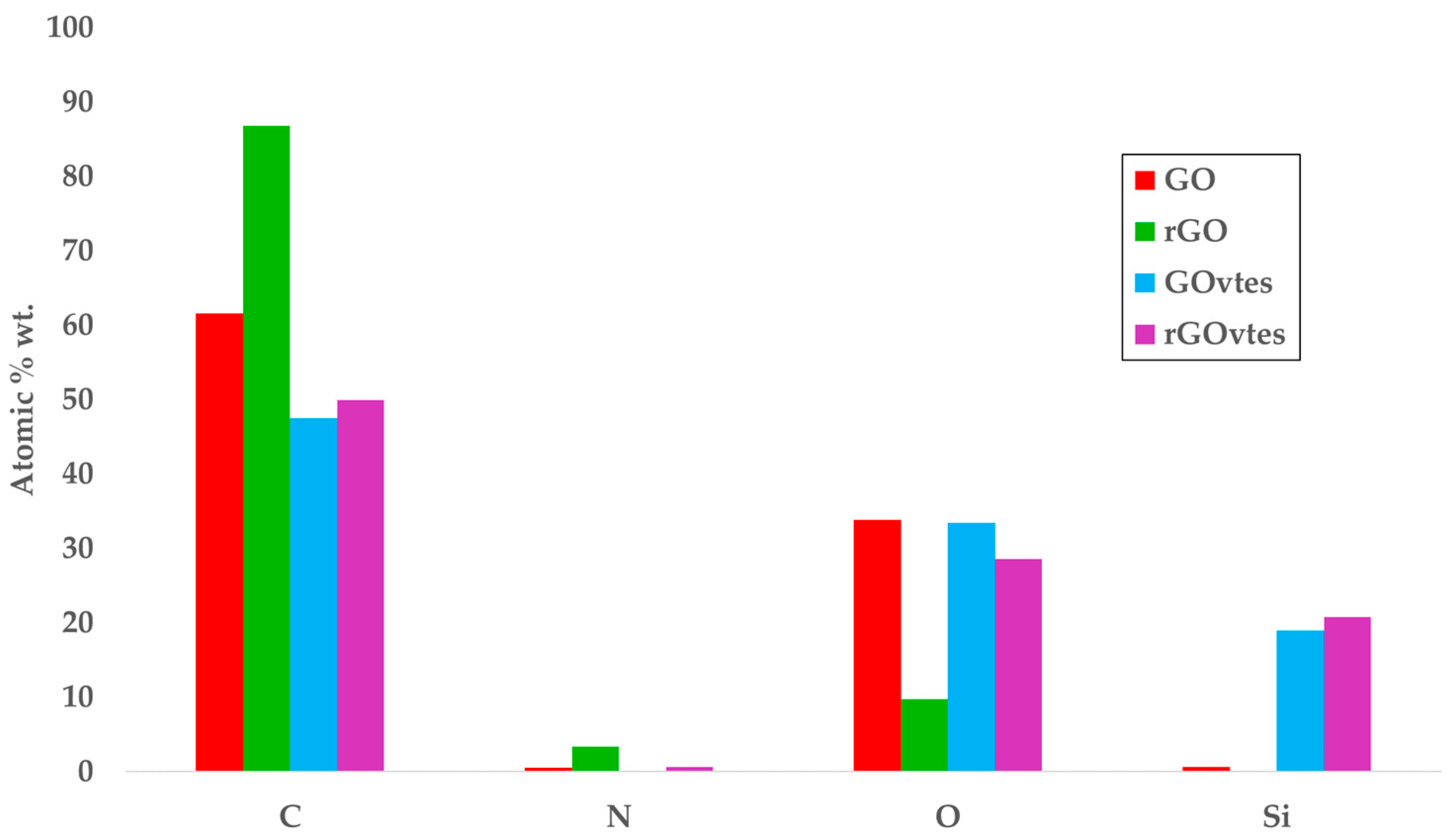
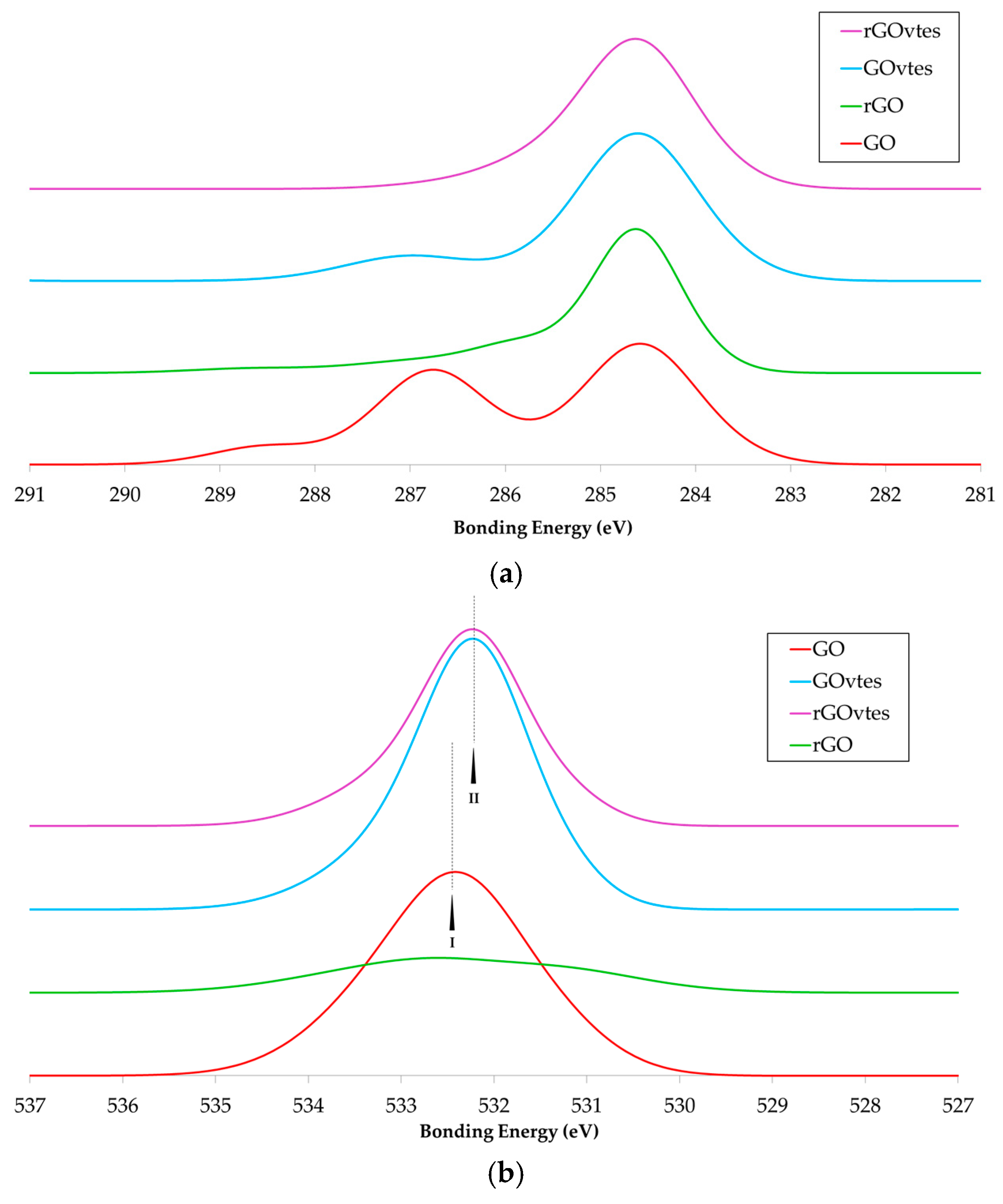
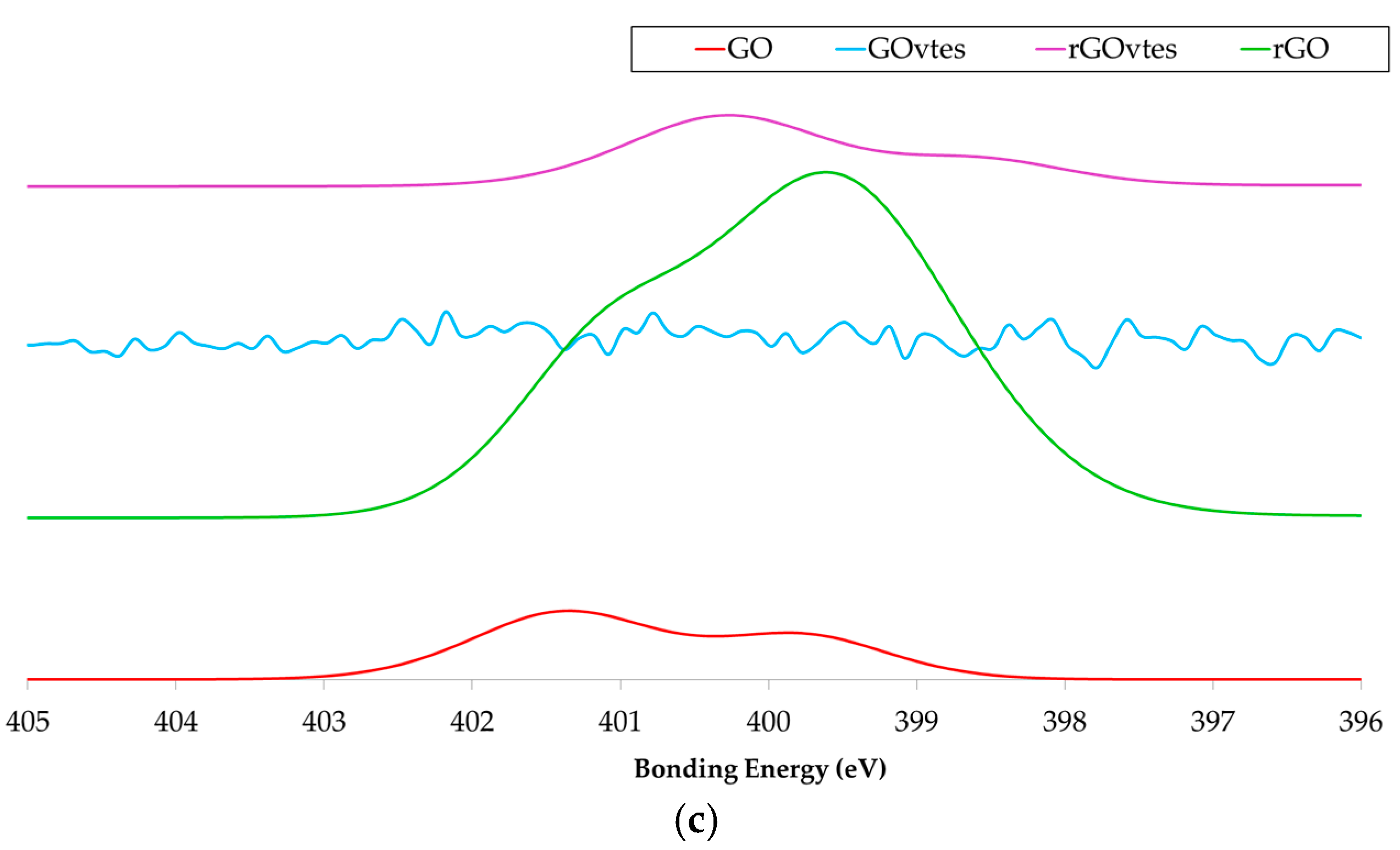
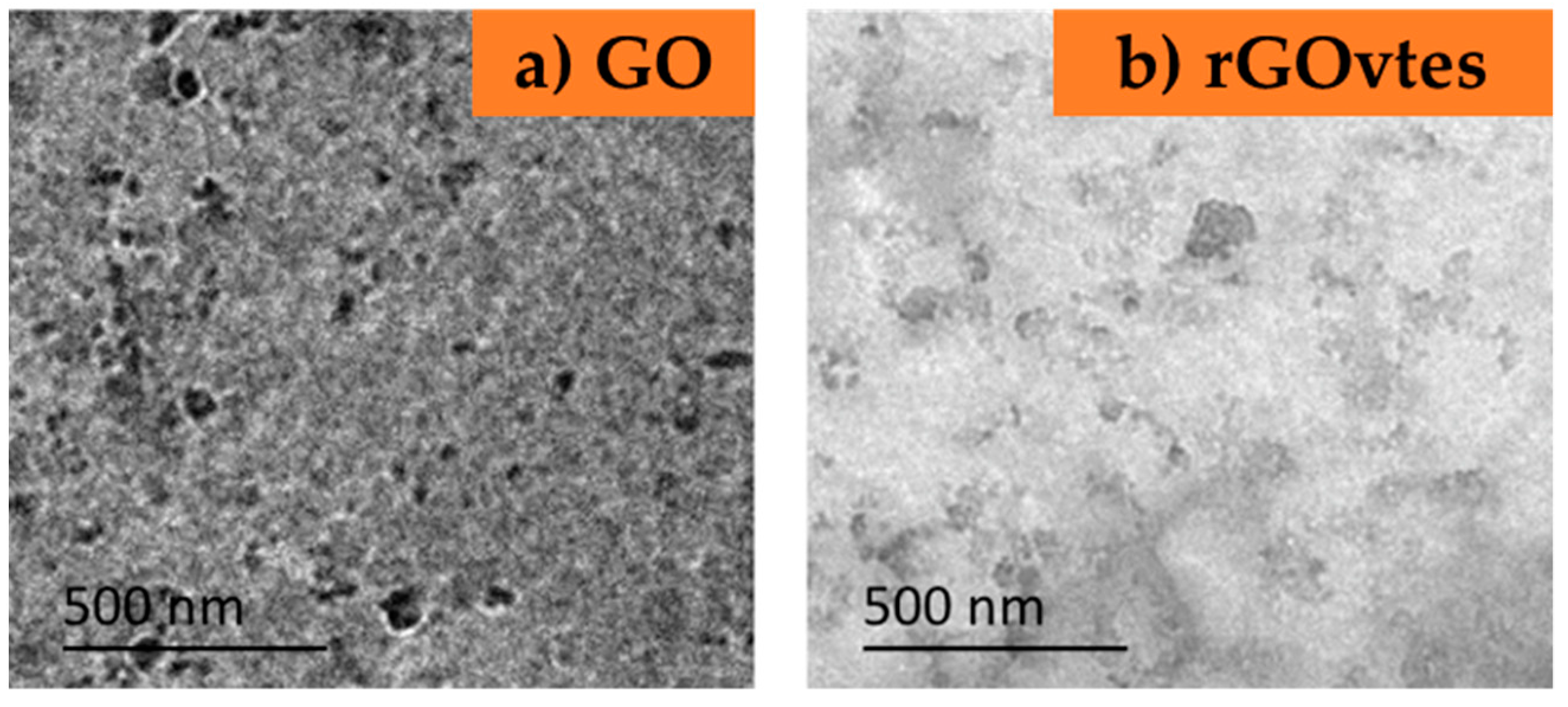
3.1. Graphene Derivatives Characterization
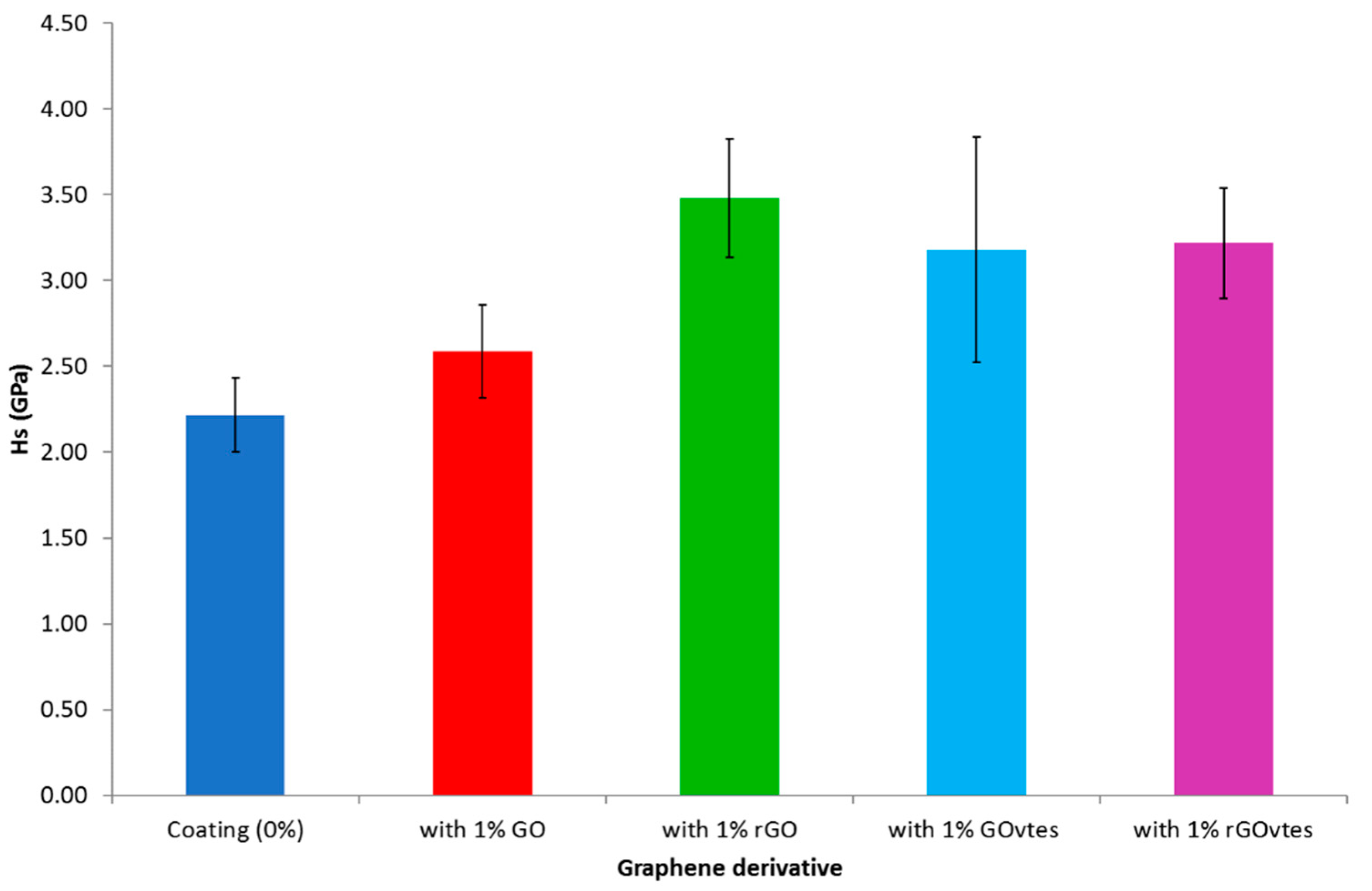
3.2. Cured Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaewpirom, S.; Kunwong, D. Curing behavior and cured film performance of easy-to-clean UV-curable coatings based on hybrid urethane acrylate oligomers. J. Polym. Res. 2012, 19, 9995. [Google Scholar] [CrossRef]
- Llorente, O.; Fernández-Berridi, M.J.; González, A.; Irusta, L. Study of the crosslinking process of waterborne UV curable polyurethane acrylates. Prog. Org. Coat. 2016, 99, 437–442. [Google Scholar] [CrossRef]
- Lee, B.-H.; Kim, H.-J. Influence of isocyanate type of acrylated urethane oligomer and of additives on weathering of UV-cured films. Polym. Degrad. Stab. 2006, 91, 1025–1035. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Song, L.; Yu, B.; Hu, Y.; Yeoh, G.H. Preparation of UV-curable functionalized graphene/polyurethane acrylate nanocomposite with enhanced thermal and mechanical behaviors. React. Funct. Polym. 2013, 73, 854–858. [Google Scholar] [CrossRef]
- Avila-Herrera, C.A.; Gómez-Guzmán, O.; Almaral-Sánchez, J.L.; Yáñez-Limón, J.M.; Muñoz-Saldaña, J.; Ramírez-Bon, R. Mechanical and thermal properties of SiO2–PMMA monoliths. J. Non. Cryst. Solids. 2006, 352, 3561–3566. [Google Scholar] [CrossRef]
- Spadaro, G.; Dispenza, C.; Alessi, S.; Tartaglione, G.; Camino, G. Radiation curing of diacrylate glycerolate of bisphenol-A in the presence of an organically modified montmorillonite for the production of flame-resistant polymer—Clay composites. Adv. Polym. Technol. 2006, 25, 109–120. [Google Scholar] [CrossRef]
- Dashtizadeh, A.; Abdouss, M.; Mahdavi, H.; Khorassani, M. Acrylic coatings exhibiting improved hardness, solvent resistance and glossiness by using silica nano-composites. Appl. Surf. Sci. 2011, 257, 2118–2125. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Hsieh, C.-C.; Lin, Y.-M.; Li, A.-K. Preparation of transparent silica—PMMA nanocomposite hard coatings. Prog. Org. Coat. 2008, 62, 436–439. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, L.; Zhang, Z.; Gu, L.; Xu, Y.; Eger, C. Wear-resistant and transparent acrylate-based coating with highly filled nanosilica particles. Tribol. Int. 2010, 43, 83–91. [Google Scholar] [CrossRef]
- Suriano, R.; Ciapponi, R.; Griffini, G.; Levi, M.; Turri, S. Fluorinated zirconia-based sol-gel hybrid coatings on polycarbonate with high durability and improved scratch resistance. Surf. Coat. Technol. 2017, 311, 80–89. [Google Scholar] [CrossRef]
- Wu, L.Y.L.; Chwa, E.; Chen, Z.; Zeng, X.T. A study towards improving mechanical properties of sol-gel coatings for polycarbonate. Thin Solid Films 2008, 516, 1056–1062. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Ramos-Fernandez, G.; Muñoz, M.; García-Quesada, J.C.; Rodriguez-Pastor, I.; Martin-Gullon, I. Role of graphene oxide surface chemistry on the improvement of the interlaminar mechanical properties of resin infusion processed epoxy-carbon fiber composites. Polym. Compos. 2017, 39, E2116–E2124. [Google Scholar] [CrossRef]
- Bortz, D.R.; Heras, E.G.; Martin-Gullon, I. Impressive fatigue life and fracture toughness improvements in graphene oxide/epoxy composites. Macromolecules 2012, 45, 238–245. [Google Scholar] [CrossRef]
- Lahiri, D.; Hec, F.; Thiesse, M.; Durygin, A.; Zhang, C.; Agarwal, A. Nanotribological behavior of graphene nanoplatelet reinforced ultra high molecular weight polyethylene composites. Tribol. Int. 2014, 70, 165–169. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Todorov, P.; Ivanov, E.; Kaplas, T.; Svirko, Y.; Paddubskaya, A.; Kuzhir, P. Mechanical properties investigation of bilayer graphene/poly(methyl methacrylate) thin films at macro, micro and nanoscale. Carbon 2016, 100, 355–366. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Jiu, J.; Suganuma, K. Efficient assembly of high-performance reduced graphene oxide/silver nanowire transparent conductive film based on in situ light-induced reduction technology. Appl. Surf. Sci. 2018, 459, 732–740. [Google Scholar] [CrossRef]
- Harun, M.H.; Talib, Z.A.; Ibrahim, N.A.; Chyi, J.L.Y.; Salleh, N.G.N.; Alias, M.S.; Mohamed, M.; Othman, N. Characterization of transparent hydrophobic coating with silica and graphene oxide fillers by sol-gel method. Int. J. Nanoelectron. Mater. 2018, 11, 283–292. [Google Scholar]
- Da, S.-X.; Wang, J.; Geng, H.-Z.; Jia, S.-L.; Xu, C.-X.; Li, L.-G.; Shi, P.-P.; Li, G. High adhesion transparent conducting films using graphene oxide hybrid carbon nanotubes. Appl. Surf. Sci. 2017, 392, 1117–1125. [Google Scholar] [CrossRef]
- Valentini, L.; Bon, S.B.; Kenny, J.M. Emerging methods for producing graphene oxide composites in coatings with multifunctional properties. J. Mater. Chem. 2012, 22, 21355–21361. [Google Scholar] [CrossRef]
- Bittolo Bon, S.; Valentini, L.; Kenny, J.M. Preparation of extended alkylated graphene oxide conducting layers and effect study on the electrical properties of PEDOT: PSS polymer composites. Chem. Phys. Lett. 2010, 494, 264–268. [Google Scholar] [CrossRef]
- Akhtar, M.W.; Lee, Y.S.; Yoo, D.J.; Kim, J.S. Alumina-graphene hybrid filled epoxy composite: Quantitative validation and enhanced thermal conductivity. Compos. B Eng. 2017, 131, 184–195. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martín-Gullón, I. A correlation between the Wolf-Wilburn scale and atomic force microscopy for anti-scratch resistance determination. Prog. Org. Coat. 2018, 125, 325–330. [Google Scholar] [CrossRef]
- Lee, S.; Eom, S.H.; Chung, J.S.; Hur, S.H. Large-scale production of high-quality reduced graphene oxide. Chem. Eng. J. 2013, 233, 297–304. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Hu, H.; Wan, L.; Chen, R.; Zheng, H.; Liu, F.; Zhang, M.; Shang, X.; Wang, X. Synthesis, mechanical, and barrier properties of LDPE/graphene nanocomposites using vinyl triethoxysilane as a coupling agent. J. Nanoparticle Res. 2011, 13, 869–878. [Google Scholar] [CrossRef]
- ASTM D3363-05(2011)e2: Standard Test Method for Film Hardness by Pencil Test; ASTM International: West Conshohocken, PA, USA, 2011; Available online: https://www.astm.org/Standards/D3363.htm (accessed on 23 March 2018).
- ASTM D7027-05: Standard Test Method for Evaluation of Scratch Resistance of Polymeric Coatings and Plastics Using an Instrumented Scratch Machine; ASTM International: West Conshohocken, PA, USA, 2005; Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D7027-05.htm (accessed on 23 March 2018).
- Karimzadeh, A.; Ayatollahi, M.R. Investigation of mechanical and tribological properties of bone cement by nano-indentation and nano-scratch experiments. Polym. Test. 2012, 31, 828–833. [Google Scholar] [CrossRef]
- Yan, Y.D.; Sun, T.; Liang, Y.C.; Dong, S. Effects of scratching directions on AFM-based abrasive abrasion process. Tribol. Int. 2009, 42, 66–70. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Vassallo, A.M.; Cole-Clarke, P.A.; Pang, L.S.K.; Palmisano, A.J. Infrared emission spectroscopy of coal minerals and their thermal transformations. Appl. Spectrosc. 1992, 46, 73–78. [Google Scholar] [CrossRef]
- Holmes, J.D.; Ziegler, K.J.; Doty, R.C.; Pell, L.E.; Johnston, K.P.; Korgel, B.A. Highly luminescent silicon nanocrystals with discrete optical transitions. J. Am. Chem. Soc. 2001, 123, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, H.; Yang, W.; Xie, Z.; Miao, H.; An, L. Optical properties of single-crystalline α-Si3N4 nanobelts. Appl. Phys. Lett. 2005, 86, 061908. [Google Scholar] [CrossRef]
- Nagai, N.; Hashimoto, H. FT-IR-ATR study of depth profile of SiO2 ultra-thin films. Appl. Surf. Sci. 2001, 172, 307–311. [Google Scholar] [CrossRef]
- Sachithanadam, M.; Joshi, S.C. Silica Aerogel Composites: Novel Fabrication Methods; Springer: Singapore, 2016. [Google Scholar]
- Rodríguez Pastor, I. Tratamientos De Purificación Y Acondicionamiento De Grafenos Para El Desarrollo De Aplicaciones. Ph.D. Thesis, Universidad de Alicante, Alicante, Spain, March 2014. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=67597 (accessed on 12 March 2018).
- Beamson, G.; Bunn, A.; Briggs, D. High-resolution monochromated XPS of poly (methyl methacrylate) thin films on a conducting substrate. Surf. Interface Anal. 1991, 17, 105–115. [Google Scholar] [CrossRef]
- Long, D.; Li, W.; Ling, L.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Preparation of nitrogen-doped graphene sheets by a combined chemical and hydrothermal reduction of graphene oxide. Langmuir 2010, 26, 16096–16102. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Ren, W.; Cheng, H.; Tang, N.; Wu, W.; Zhong, W.; Du, Y. The doping of reduced graphene oxide with nitrogen and its effect on the quenching of the material’s photoluminescence. Carbon 2012, 50, 5286–5291. [Google Scholar] [CrossRef]
- Hu, T.; Sun, X.; Sun, H.; Xin, G.; Shao, D.; Liu, C.; Lian, J. Rapid synthesis of nitrogen-doped graphene for a lithium ion battery anode with excellent rate performance and super-long cyclic stability. Phys. Chem. Chem. Phys. 2014, 16, 1060–1066. [Google Scholar] [CrossRef]
- Shin, S.; Hwang, B.; Zhao, Z.-J.; Jeon, S.H.; Jung, J.; Lee, J.-H.; Ju, B.-K.; Jeong, J.-H. Transparent displays utilizing nanopatterned quantum dot films. Sci. Rep. 2018, 8, 2463. [Google Scholar] [CrossRef]
- Grepinet, B.; Pla, F.; Hobbes, P.; Swaels, P.; Monge, T. Modeling and simulation of urethane acrylates synthesis. I. Kinetics of uncatalyzed reaction of toluene diisocyanate with a monoalcohol. J. Appl. Polym. Sci. 2000, 75, 705–712. [Google Scholar] [CrossRef]
- Wang, H.; Qin, S.; Yang, X.; Fei, G.; Tian, M.; Shao, Y.; Zhu, K. A waterborne uniform graphene-poly (urethane-acrylate) complex with enhanced anticorrosive properties enabled by ionic interaction. Chem. Eng. J. 2018, 351, 939–951. [Google Scholar] [CrossRef]
- Jimbo, Y.; Tamatsukuri, Y.; Ito, M.; Yokoyama, K.; Hirakata, Y.; Yamazaki, S. Reliability and mechanical durability tests of flexible OLED with ALD coating. J. Soc. Inf. Disp. 2015, 23, 313–318. [Google Scholar] [CrossRef]
- Lu, S.; Shao, J.; Martin, D.C.; Li, Z.; Schwendeman, I.G. Commercialization of sol-gel based transparent functional coatings. J. Sol-Gel Sci. Technol. 2018, 87, 105–112. [Google Scholar] [CrossRef]
- Choi, G.-M.; Jin, J.; Shin, D.; Kim, Y.H.; Ko, J.-H.; Im, H.-G.; Jang, J.; Jang, D.; Bae, B.-S. Flexible hard coating: glass-like wear resistant, yet plastic-like compliant, transparent protective coating for foldable displays. Adv. Mater. 2017, 29, 1700205. [Google Scholar] [CrossRef] [PubMed]


| Coating (0%) | GO | rGO | GOvtes | rGOvtes | |
|---|---|---|---|---|---|
| 40,080 | 37,170 | 34,080 | 36,670 | 29,170 | |
| (%) | 100.00 | 92.72 | 85.02 | 91.50 | 72.78 |
| Resin | Sol-gel Relation | Molar Ratio | Wolf–Wilburn Hardness (Hw) | Ref. |
| Polycarbonate | – | – | 6B–5B | [10,11] |
| Cross-linked siloxane hybrid | – | – | 9H | [49] |
| Fluorinated zirconia-based sol-gel hybrid | Zirconia/Silica | 0.09 | 2H–3H | [10] |
| 0.23 | 2H–3H | |||
| 0.48 | 3H–4H | |||
| Resin | Nanofiller | Concentration | Wolf–Wilburn hardness | Ref. |
| Poly (methyl methacrylate) | – | – | 3H | [8] |
| Modified silica | 10 wt.% | 5H | ||
| Urethane-Acrylate + TMPTA1 | – | – | 1H | [9] |
| Nanosilica | 10 wt.% | 2H | ||
| 40 wt.% | 5H | |||
| GLYMO2 + TEOS3 | – | – | H | [11] |
| Colloidal silica | 10 vol.% | 2H | ||
| 20 vol.% | 3H | |||
| 30 vol.% | 4H | |||
| 40 vol.% | 4H | |||
| Urethane-Acrylate | – | – | 8H | This work |
| GO | 1 wt.% | 8H–10H | ||
| rGO | 11H–13H | |||
| GOvtes | 10H–13H | |||
| rGOvtes | 10H–12H |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domene-López, D.; Sarabia-Riquelme, R.; García-Quesada, J.C.; Martin-Gullon, I. Custom-Made Chemically Modified Graphene Oxide to Improve the Anti-Scratch Resistance of Urethane-Acrylate Transparent Coatings. Coatings 2019, 9, 408. https://doi.org/10.3390/coatings9060408
Domene-López D, Sarabia-Riquelme R, García-Quesada JC, Martin-Gullon I. Custom-Made Chemically Modified Graphene Oxide to Improve the Anti-Scratch Resistance of Urethane-Acrylate Transparent Coatings. Coatings. 2019; 9(6):408. https://doi.org/10.3390/coatings9060408
Chicago/Turabian StyleDomene-López, Daniel, Rubén Sarabia-Riquelme, Juan C. García-Quesada, and Ignacio Martin-Gullon. 2019. "Custom-Made Chemically Modified Graphene Oxide to Improve the Anti-Scratch Resistance of Urethane-Acrylate Transparent Coatings" Coatings 9, no. 6: 408. https://doi.org/10.3390/coatings9060408
APA StyleDomene-López, D., Sarabia-Riquelme, R., García-Quesada, J. C., & Martin-Gullon, I. (2019). Custom-Made Chemically Modified Graphene Oxide to Improve the Anti-Scratch Resistance of Urethane-Acrylate Transparent Coatings. Coatings, 9(6), 408. https://doi.org/10.3390/coatings9060408