Corrosion Behavior of AZ91D Magnesium Alloy with a Calcium–Phosphate–Vanadium Composite Conversion Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Surface Preparation
2.2. Preparation of the Conversion Coating
2.3. Surface Characterization
2.4. Electrochemical Measurements
2.5. Scratch Immersion Test
3. Results and Discussion
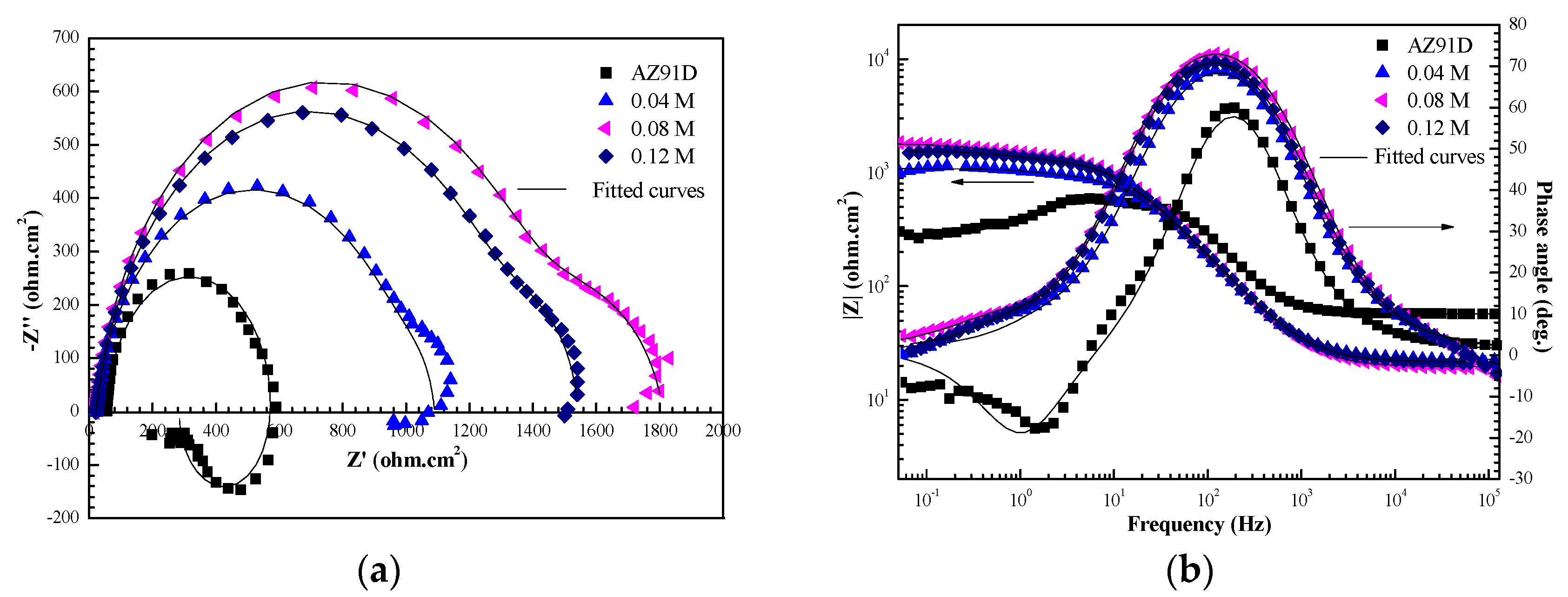
3.1. Effect of NaVO3 Concentration on the Corrosion Resistance of the Ca–P–V Composite Coating
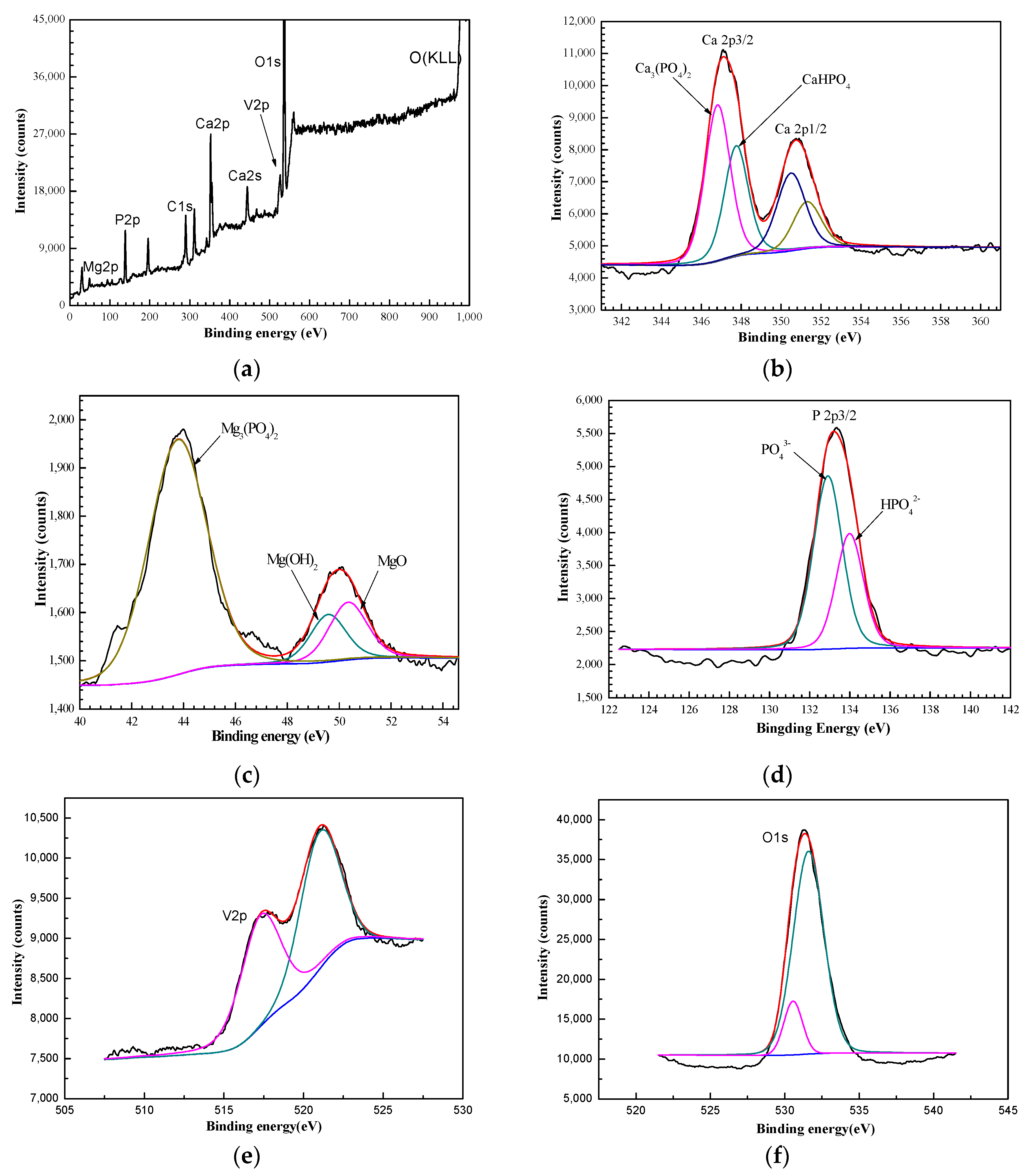
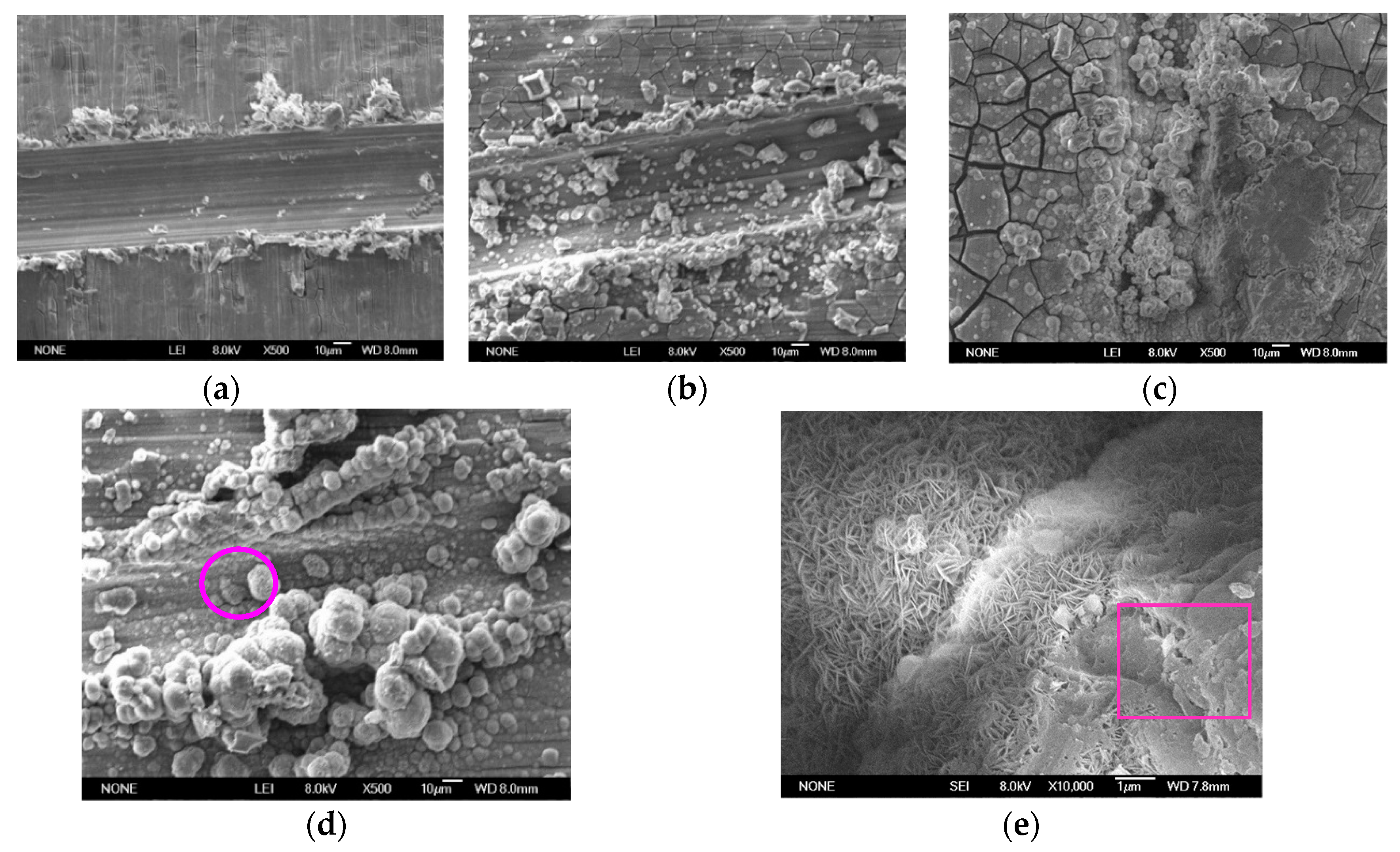
3.2. Morphology and Composition of the Ca–P–V Composite Coating
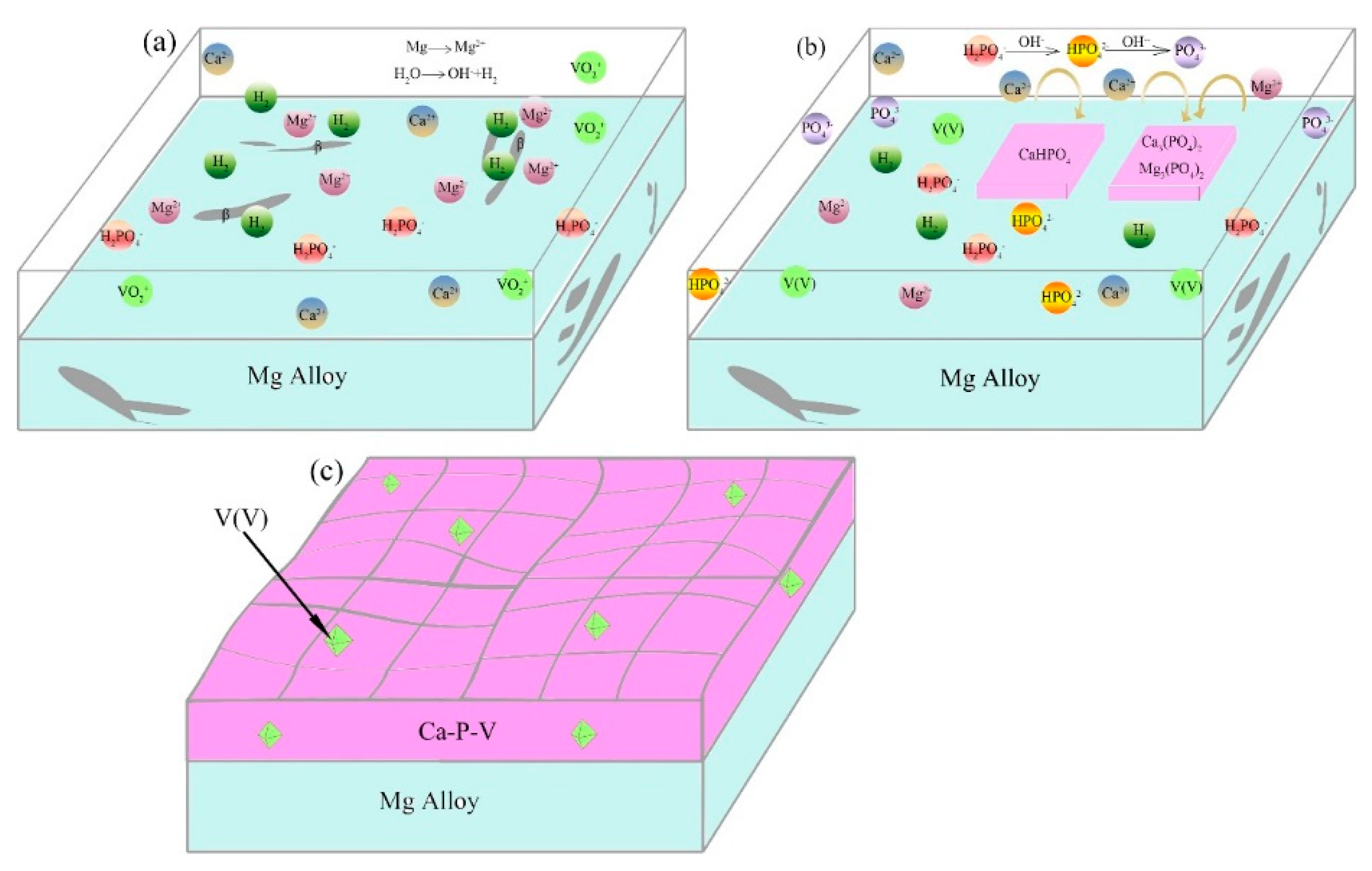
3.3. Formation Mechanism of the Ca–P–V Composite Coating
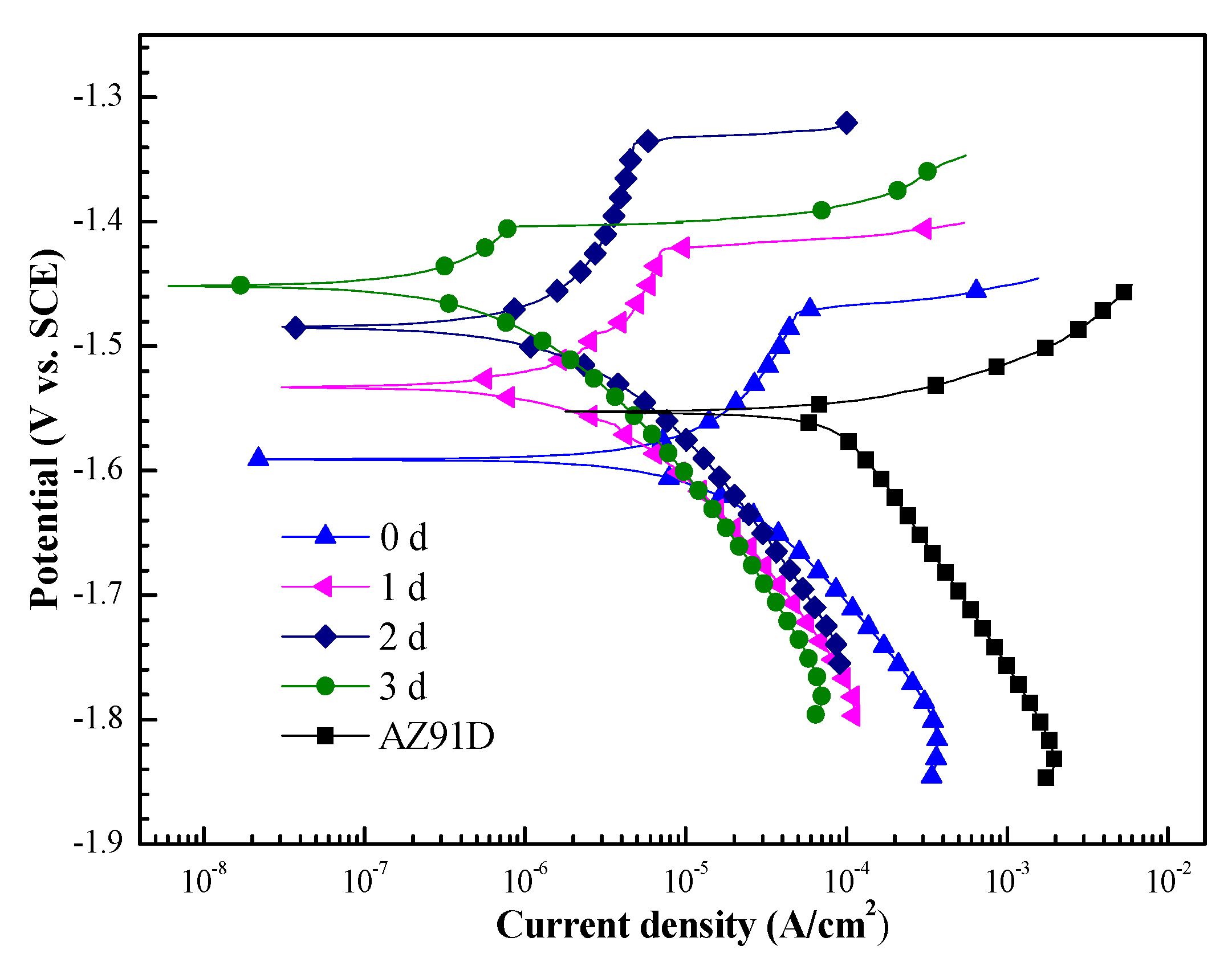
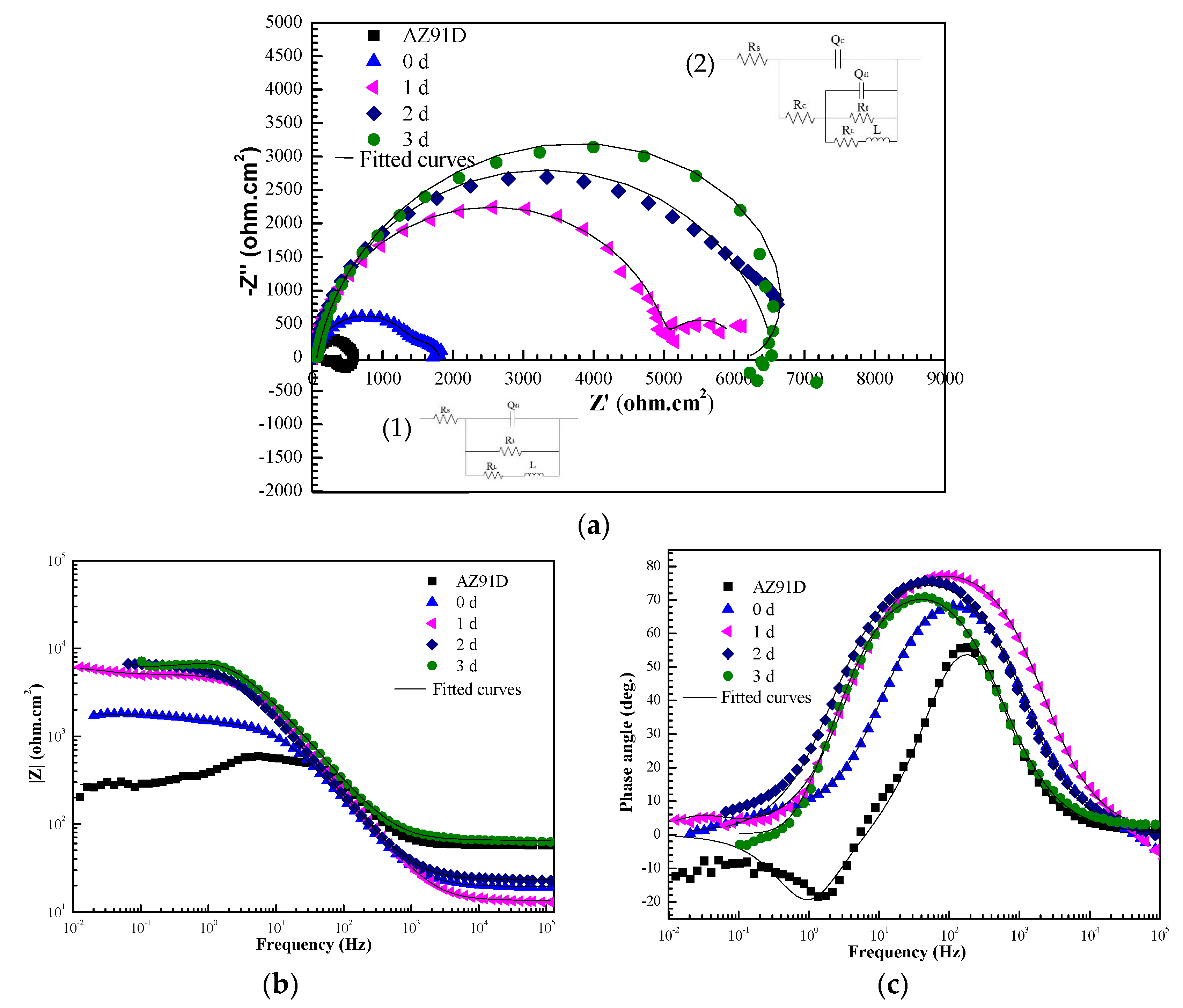
3.4. Anticorrosion Property of the Ca–P–V Coated Samples
3.5. Self-Healing Behaviour of the Scratched Ca–P–V Coated Specimens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayaraj, J.; Raj, S.A.; Srinivasan, A.; Ananthakumar, S.; Pillai, U.T.S.; Dhaipule, N.G.K.; Mudali, U.K. Composite magnesium phosphate coatings for improved corrosion resistance of magnesium AZ31 alloy. Corros. Sci. 2016, 113, 104–115. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. A simple route towards a hydroxyapatite-Mg(OH)2 conversion coating for magnesium. Corros. Sci. 2011, 53, 2263–2268. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Hu, J.; Lin, T. Formation mechanism and corrosion resistance of the hydrophobic coating on anodized magnesium. Corros. Sci. 2016, 111, 334–343. [Google Scholar] [CrossRef]
- Gray, J.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloy Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Wang, J.L.; Ke, C.; Pohl, K.; Birbilis, N.; Chen, X.B. The unexpected role of benzotriazole in mitigating magnesium alloy corrosion: A nucleating agent for crystalline nanostructured magnesium hydroxide film. J. Electrochem. Soc. 2015, 162, C403–C411. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing treatments for magnesium alloys and their effect on corrosion resistance in various environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y. Corrosion fatigue behavior of AM60 magnesium alloy with anodizing layer and chemical-conversion-coating layer. Mater. Corros. 2015, 66, 940–948. [Google Scholar] [CrossRef]
- Wang, S.H.; Guo, X.W.; Sun, C.; Gong, J.; Peng, L.M.; Ding, W.J. Electrodeposition of Cu coating with high corrosion resistance on Mg–3.0Nd–0.2Zn–0.4Zr magnesium alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 3810–3817. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Yu, S.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Kainer, K.U.; Zheludkevich, M.L. Investigation of the formation mechanisms of plasma electrolytic oxidation coatings on Mg alloy AM50 using particles. Electrochim. Acta 2016, 196, 680–691. [Google Scholar] [CrossRef]
- Klein, M.; Lu, X.; Blawert, C.; Kainer, K.U.; Zheludkevich, M.L.; Walther, F. Influence of plasma electrolytic oxidation coatings on fatigue performance of AZ31 Mg alloy. Mater. Corros. 2017, 68, 50–57. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Ivanova, A.A.; Tian, Q.M.; Pittman, R.; Jiang, W.S.; Lin, J.J.; Liu, H.H.; Surmenev, R.A. Bone marrow derived mesenchymal stem cell response to the RF magnetron suppter deposited hydroxyapatite coating on AZ91 magnesium alloy. Mater. Chem. Phys. 2019, 221, 89–98. [Google Scholar] [CrossRef]
- Mukhametkaliyev, T.M.; Surmeneva, M.A.; Vladescu, A.; Cotrut, C.M.; Braic, M.; Dinu, M.; Vranceanu, M.D.; Pana, I.; Mueller, M.; Surmenev, R.A. A biodegradable AZ91 magnesium alloy coated with a thin nanostructured hydroxyapatite for improving the corrosion resistance. Mat. Sci. Eng. C 2017, 75, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Liu, Y.; Liu, Z.Y.; Zhao, X.Y.; Li, W. Improving corrosion resistance of AZ91D magnesium alloy by laser surface melting and micro-arc oxidation. Mater. Corros. 2015, 66, 963–970. [Google Scholar] [CrossRef]
- Yang, N.; Li, Q.; Chen, F.; Cai, P.; Tan, C.; Xi, Z. A solving-reprecipitation theory for self-healing functionality of stannate coating with a high environmental stability. Electrochim. Acta 2015, 174, 1192–1201. [Google Scholar] [CrossRef]
- Pommiers, S.; Frayret, J.; Castetbon, A.; Potin-Gautier, M. Alternative conversion coatings to chromate for the protection of magnesium alloys. Corros. Sci. 2014, 84, 135–146. [Google Scholar] [CrossRef]
- Qi, J.; Gao, L.; Li, Y.; Wang, Z.; Thompson, G.E.; Skeldon, P. An optimized trivalent chromium conversion coating process for AA2024-T351 alloy. J. Electrochem. Soc. 2017, 164, C390–C395. [Google Scholar] [CrossRef]
- Van Phuong, N.; Lee, K.; Chang, D.; Kim, M.; Lee, S.; Moon, S. Zinc phosphate conversion coatings on magnesium alloys: a review. Met. Mater. Int. 2013, 19, 273–281. [Google Scholar] [CrossRef]
- Sun, R.X.; Wang, P.F.; Zhao, D.D.; Sun, Z.Z.; Li, C.Q.; Chen, K.Z. An environment-friendly calcium phosphate conversion coating on AZ91D alloy and its corrosion resistance. Mater. Corros. 2015, 66, 383–386. [Google Scholar] [CrossRef]
- Zeng, R.C.; Zhang, F.; Lan, Z.D.; Cui, H.Z.; Han, E.H. Corrosion resistance of calcium-modified zinc phosphate conversion coatings on magnesium-aluminium alloys. Corros. Sci. 2014, 88, 452–459. [Google Scholar] [CrossRef]
- Chen, X.B.; Zhou, X.; Abbott, T.B.; Easton, M.A.; Birbilis, N. Double-layered manganese phosphate conversion coating on magnesium alloy AZ91D: Insights into coating formation, growth and corrosion resistance. Surf. Coat. Technol. 2013, 217, 147–155. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Doench, I.; Möhwald, H. Assessment of a one-step intelligent self-healing vanadia protective coatings for magnesium alloys in corrosive media. Electrochim. Acta 2011, 56, 2493–2502. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Doench, I.; Möhwald, H. Vanadia-based coatings of self-repairing functionality for advanced magnesium Elektron ZE41 Mg-Zn-rare earth alloy. Surf. Coat. Technol. 2012, 206, 3686–3692. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.; Lei, T.; Xiao, T. Optimization of process factors for self-healing vanadium-based conversion coating on AZ31 magnesium alloy. Appl. Sur. Sci. 2015, 353, 811–819. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, R.; Jiang, S. Microstructure and corrosion resistance of Ce–V conversion coating on AZ31 magnesium alloy. Appl. Sur. Sci. 2015, 341, 166–174. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, X.; Jia, Y.; Liu, Y. Self-repairing vanadium–zirconium composite conversion coating for aluminum alloys. Appl. Surf. Sci. 2013, 280, 489–493. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.H. A novel phosphate conversion film on Mg–8.8Li alloy. Surf. Coat. Technol. 2009, 203, 1107–1113. [Google Scholar] [CrossRef]
- Zeng, R.C.; Lan, Z.D.; Kong, L.H.; Huang, Y.D.; Cui, H.Z. Characterization of calcium-modified zinc phosphate conversion coatings and their influences on corrosion resistance of AZ31 alloy. Surf. Coat. Technol. 2011, 205, 3347–3355. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V 2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Wu, Q.H.; Thissen, A.; Jaegermann, W.; Liu, M. Photoelectron spectroscopy study of oxygen vacancy on vanadium oxides surface. Appl. Sur. Sci. 2004, 236, 473–478. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Zou, Z.L.; Li, N.; Li, D.Y.; Liu, H.P.; Mu, S.L. A vanadium-based conversion coating as chromate replacement for electrogalvanized steel substrates. J. Alloy Compd. 2011, 509, 503–507. [Google Scholar] [CrossRef]
- Chu, Y.R.; Lin, C.S. Citrate gel conversion coating on AZ31 magnesium alloys. Corros. Sci. 2014, 87, 288–296. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Effect of [Ca2+] and [PO43−] levels on the formation of calcium phosphate conversion coatings on die-cast magnesium alloy AZ91D. Corros. Sci. 2012, 55, 226–232. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.H. Formation mechanism of phosphate conversion film on Mg-8.8Li alloy. Corros. Sci. 2009, 51, 62–69. [Google Scholar] [CrossRef]
- Su, Y.; Niu, L.; Lu, Y.; Lian, J.; Li, G. Preparation and corrosion behavior of calcium phosphate and hydroxyapatite conversion coatings on AM60 magnesium alloy. J. Electrochem. Soc. 2013, 160, C536–C541. [Google Scholar] [CrossRef]
- Livage, J. Sol-gel chemistry and electrochemical properties of vanadium oxide gels. Solid State Ionics 1996, 86, 935–942. [Google Scholar] [CrossRef]
- Li, J.R.; Jiang, Q.T.; Sun, H.Y.; Li, Y.T. Effect of heat treatment on corrosion behavior of AZ63 alloy in 3.5 wt. % sodium chloride solution. Corros. Sci. 2016, 111, 288–301. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.Y.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-healing plasma electrolytic oxidation coating doped with benzotrizaole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Nadaraia, K.V.; Gnedenkov, A.S.; Bouznik, V.M. Composite fluoropolymer coatings on the MA8 magnesium alloy surface. Corros. Sci. 2016, 111, 175–185. [Google Scholar] [CrossRef]
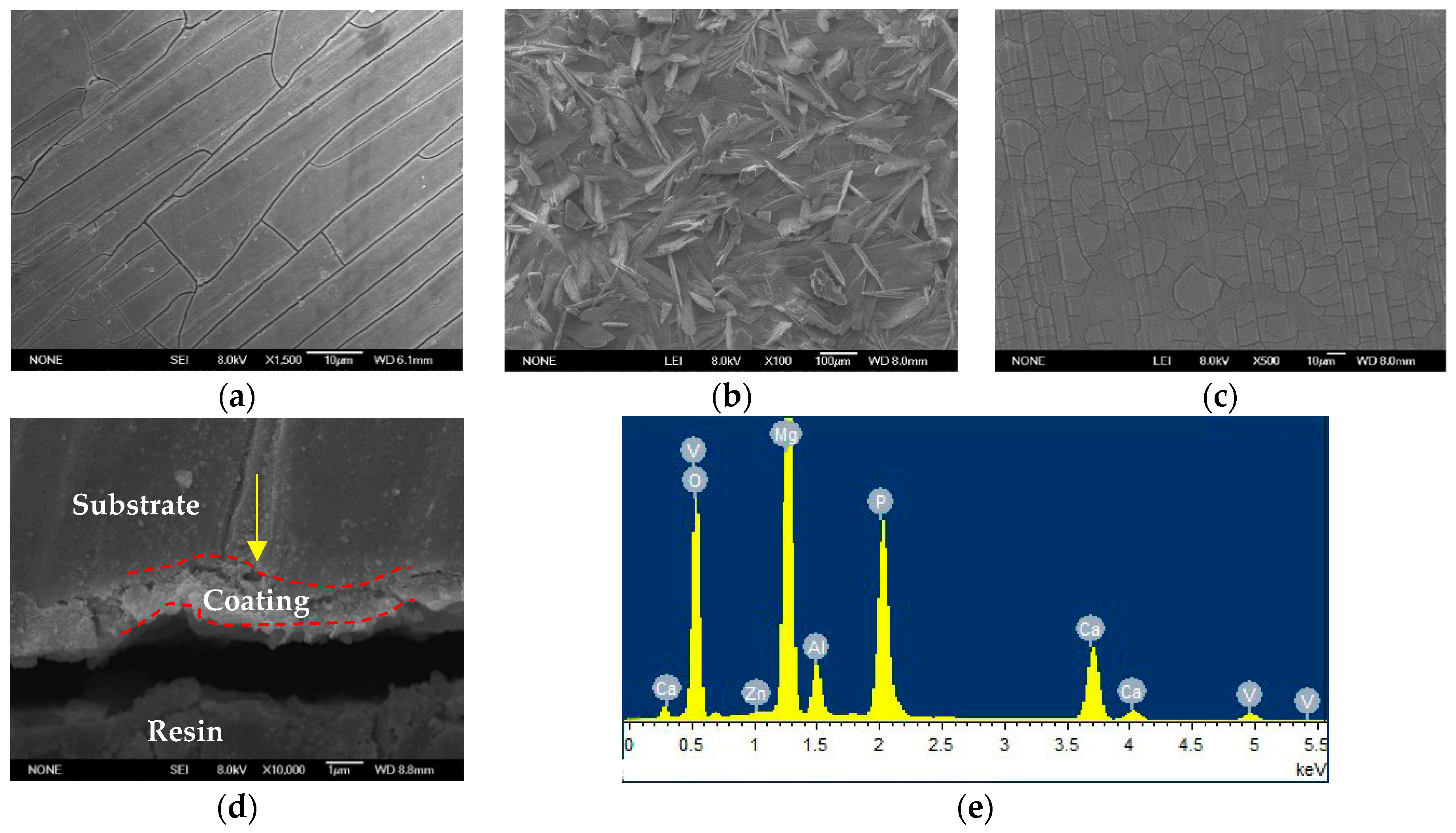
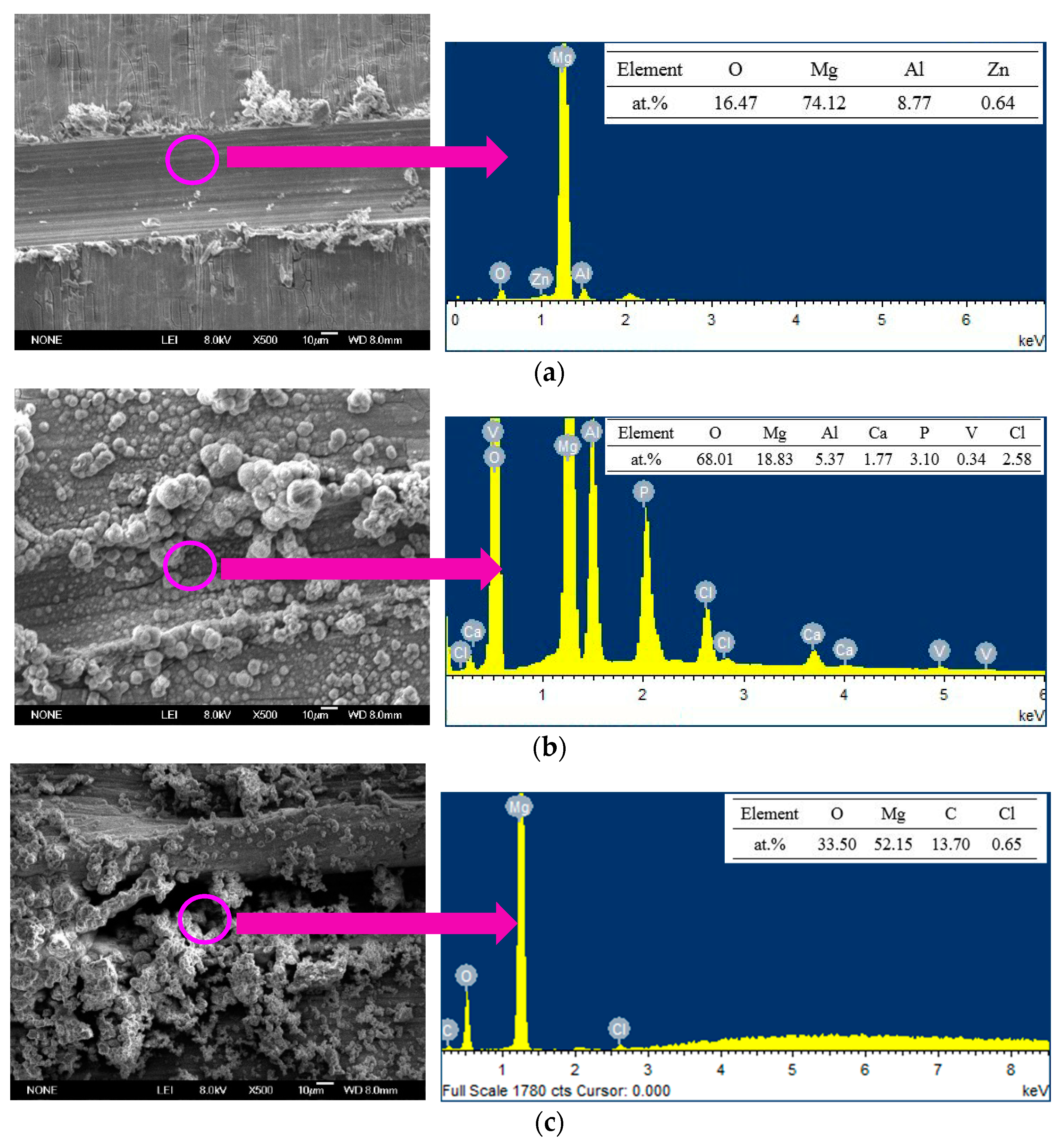
| Elements | Mg | Al | Zn | O | Ca | P | V |
|---|---|---|---|---|---|---|---|
| at. % | 19.2 | 2.7 | 0.1 | 63.0 | 4.3 | 10.1 | 0.6 |
| wt % | 22.6 | 3.5 | 0.2 | 48.7 | 8.4 | 15.1 | 1.6 |
| Samples | Ecorr (V/SCE) | βa(mV/dec) | βc (mV/dec) | Icorr (A/cm2) |
|---|---|---|---|---|
| 0 d | −1.59 ± 0.02 | 84.3 | −71.7 | 9.1 × 10−6 |
| 1 d | −1.53 ± 0.03 | 88.1 | −69.1 | 1.39 × 10−6 |
| 2 d | −1.48 ± 0.01 | 98.1 | −64.0 | 1.04 × 10−6 |
| 3 d | −1.45 ± 0.02 | 95.7 | −65.1 | 3.84 × 10−7 |
| AZ91D | −1.55 ± 0.01 | 42.3 | −462.8 | 1.56 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Yang, S.; Lv, T. Corrosion Behavior of AZ91D Magnesium Alloy with a Calcium–Phosphate–Vanadium Composite Conversion Coating. Coatings 2019, 9, 379. https://doi.org/10.3390/coatings9060379
Sun R, Yang S, Lv T. Corrosion Behavior of AZ91D Magnesium Alloy with a Calcium–Phosphate–Vanadium Composite Conversion Coating. Coatings. 2019; 9(6):379. https://doi.org/10.3390/coatings9060379
Chicago/Turabian StyleSun, Ruixue, Shuaikang Yang, and Tao Lv. 2019. "Corrosion Behavior of AZ91D Magnesium Alloy with a Calcium–Phosphate–Vanadium Composite Conversion Coating" Coatings 9, no. 6: 379. https://doi.org/10.3390/coatings9060379
APA StyleSun, R., Yang, S., & Lv, T. (2019). Corrosion Behavior of AZ91D Magnesium Alloy with a Calcium–Phosphate–Vanadium Composite Conversion Coating. Coatings, 9(6), 379. https://doi.org/10.3390/coatings9060379




