Novel Preparation of Noncovalent Modified GO Using RAFT Polymerization to Reinforce the Performance of Waterborne Epoxy Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly[oligo(Ethylene Glycol) Methyl Ether Methacrylate] (POEGMA950-RAFT)
2.3. Synthesis of POEGMA950-Block-PAA (POEGMA950-b-PAA)
2.4. Preparation of GO by Oxidation of Graphite
2.5. Preparation of Modified GO (MGO)
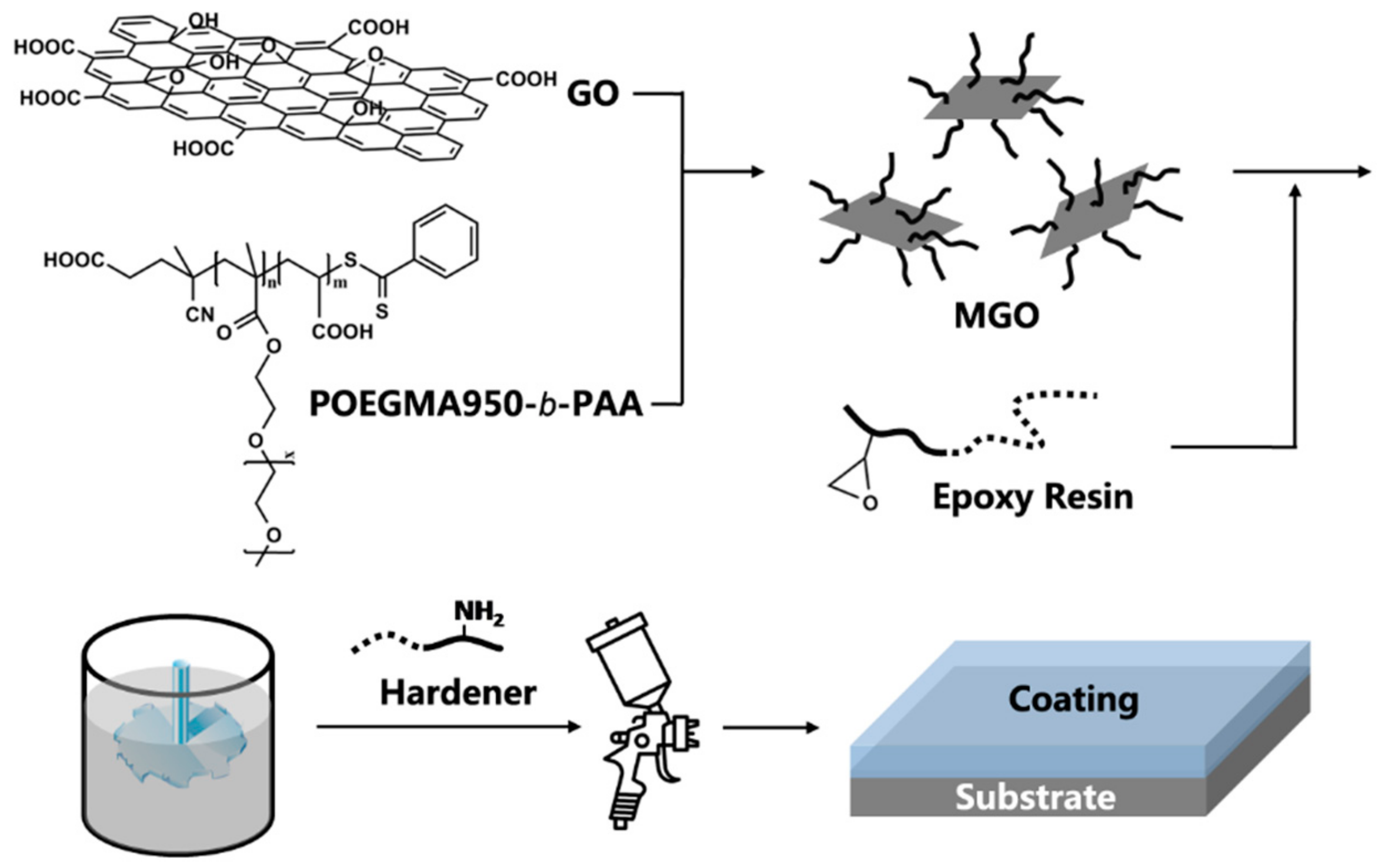
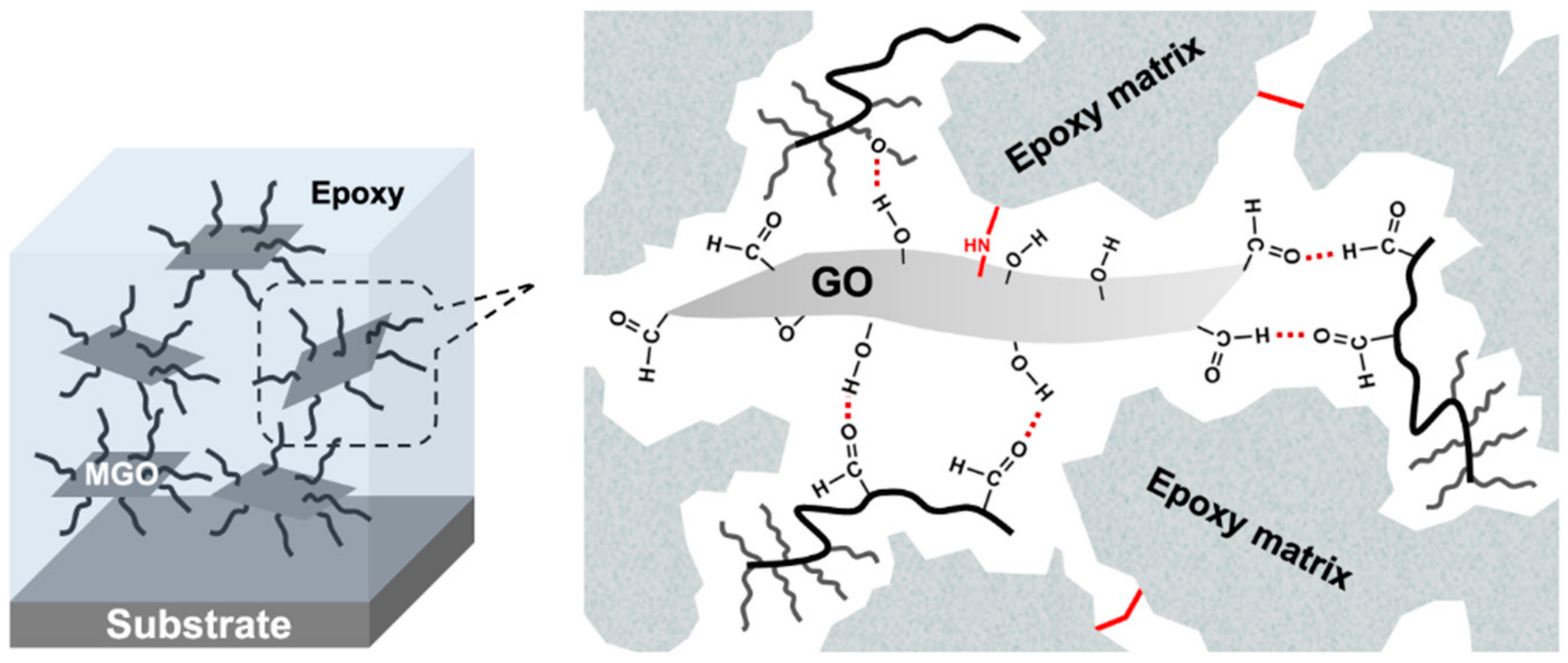
2.6. Preparation of the Waterborne Epoxy-GO Coatings (WEGC)
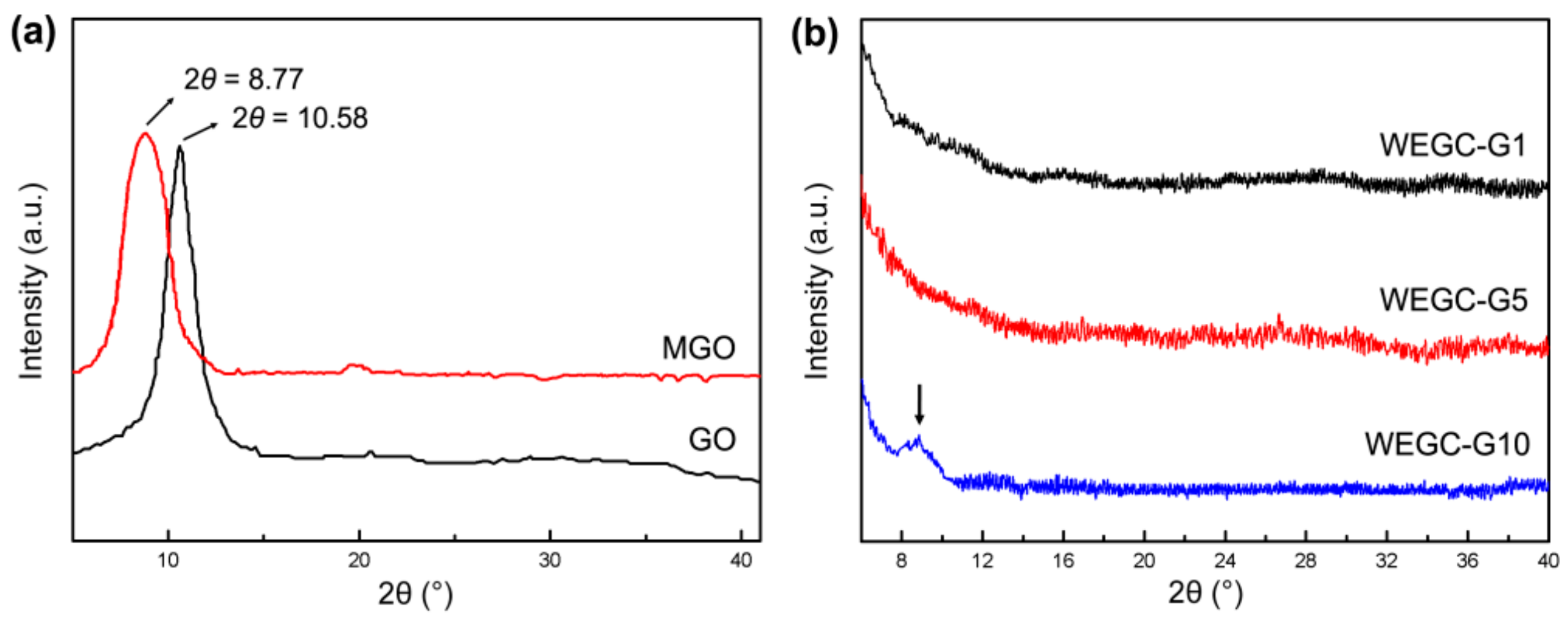
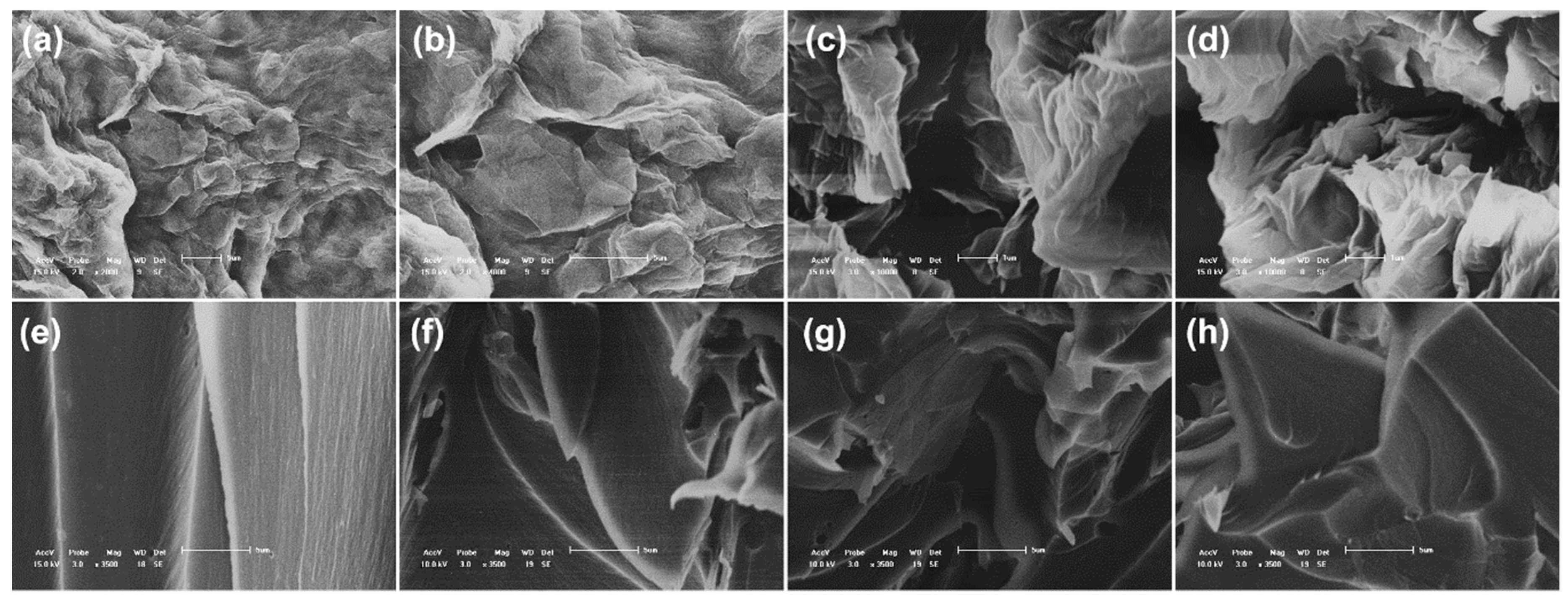
2.7. Characterization
2.8. Salt Spray Tests
2.9. Determination of the Water Absorption Rate
2.10. Pull-Off Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Upadhyay, V.; Battocchi, D. Localized electrochemical characterization of organic coatings: A brief review. Prog. Org. Coat. 2016, 99, 365–377. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q. Review on modern study methods of organic coatings. Mater. Sci. Eng. 2003, 21, 763–768. [Google Scholar]
- Cui, M.; Ren, S.; Chen, J.; Liu, S.; Zhang, G.; Zhao, H.; Wang, L.; Xue, Q. Anticorrosive performance of waterborne epoxy coatings containing water-dispersible hexagonal boron nitride (h-BN) nanosheets. Appl. Surf. Sci. 2017, 397, 77–86. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.N.; Chen, J.S.; Zhang, J.; Fang, Q.H. Dopamine modified metal-organic frameworks on anti-corrosion properties of waterborne epoxy coatings. Prog. Org. Coat. 2017, 109, 126–134. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Di, G. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef]
- Wang, S.T.; Liu, K.S.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2005, 115, 8230–8293. [Google Scholar] [CrossRef]
- Guillaumin, V.; Landolt, D. Effect of dispersion agent on the degradation of a water borne paint on steel studied by scanning acoustic microscopy and impedance. Corros. Sci. 2002, 44, 179–189. [Google Scholar] [CrossRef]
- Wang, Y.G.; Chen, H.H.; Pei, S.F.; Zhang, T. Development of waterborne inorganic zinc silicate anticorrosion coatings. Corros. Sci. Prot. Technol. 2006, 18, 41–45. (In Chinese) [Google Scholar]
- Liu, M.; Mao, X.; Zhu, H.; Lin, A.; Wang, D. Water and corrosion resistance of epoxy-acrylic-amine waterborne coatings: Effects of resin molecular weight, polar group and hydrophobic segment. Corros. Sci. 2013, 75, 106–113. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Li, X.; Qiu, S.; Ye, Y.; Yang, F.; Zhao, H. Effect of self-assembled tetraaniline nanofiber on the anticorrosion performance of waterborne epoxy coating. Prog. Org. Coat. 2019, 128, 137–147. [Google Scholar] [CrossRef]
- He, Y.; Chen, C.; Xiao, G.; Zhong, F.; Wu, Y.; He, Z. Improved corrosion protection of waterborne epoxy/graphene coating by combining non-covalent and covalent bonds. React. Funct. Polym. 2019, 137, 104–115. [Google Scholar] [CrossRef]
- Nie, J.; Liu, D.; Li, S.; Qiu, Z.; Ma, N.; Sui, G. Improved dispersion of the graphene and corrosion resistance of waterborne epoxy—Graphene composites by minor cellulose nanowhiskers. J. Appl. Polym. Sci. 2019, 136, 47631. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Zhong, F.; Li, H.; Wu, Y.; Chen, J. Synergistic effect of grapheme oxide@phosphate-intercalated hydrotalcite for improved anti-corrosion and self-healable protection of waterborne epoxy coating in salt environments. J. Mater. Chem. C 2019, 7, 2318–2326. [Google Scholar] [CrossRef]
- Ding, J.H.; Rahman, O.; Peng, W.J.; Dou, H.M.; Yu, H.B. A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne graphene/epoxy coatings. Appl. Surf. Sci. 2018, 427, 981–991. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, T.; Zhang, X.; Xin, Z.X.; Deng, X.; Prakashan, K. Mechanically stable superhydrophobic polymer films by a simple hot press lamination and peeling process. RSC Adv. 2016, 6, 12530–12536. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzadeh, B.; Moghadam, M.M.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, T.C.; Peng, C.W.; Yeh, T.C.; Lu, H.I.; Hung, W.I.; Weng, C.J.; Yang, T.I.; Yeh, J.M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Posner, R.; Ozcan, O.; Grundmeier, G. Water and ions at polymer/metal interfaces. In Design of Adhesive Joints under Humid Conditions; da Silva, L.F.M., Sato, C., Eds.; Springer: Berlin, Germany, 2013; pp. 21–52. [Google Scholar]
- Monetta, T.; Acquesta, A.; Bellucci, F. Graphene/epoxy coating as multifunctional material for aircraft structures. Aerospace 2015, 2, 423–434. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved thermal conductivity for chemically functionalized exfoliated graphite/epoxy composites. Carbon 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Gu, L.; Liu, S.; Zhao, H.; Yu, H. Facile preparation of water-dispersible graphene sheets stabilized by carboxylatedoligoanilines and their anticorrosion coatings. ACS Appl. Mater. Interfaces 2015, 7, 17641–17648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, X.; Wang, H.; Li, J.; Fei, G. Electrochemical and anti-corrosion behaviors of water dispersible graphene/acrylic modified alkyd resin latex composites coated carbon steel. J. Appl. Polym. Sci. 2017, 134, 44445. [Google Scholar] [CrossRef]
- Jackson, M.; Kaushik, M.; Nazarenko, S.; Ward, S.; Maskell, R.; Wiggins, J. Effect of free volume hole-size on fluid ingress of glassy epoxy networks. Polymer 2011, 52, 4528–4535. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.H.; Wang, X.; Gui, T.J.; Li, B.J.; Han, P.; Tian, H.W.; Liu, A.; Wang, X.; Liu, X.J.; et al. In-situ reduction and deposition of Ag nanoparticles on black phosphorus nanosheets co-loaded with graphene oxide as a broad spectrum photocatalyst for enhanced photocatalytic performance. J. Alloy. Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Verdejo, R.; Bernal, M.M.; Romasanta, L.J.; Lopez-Manchado, M.A. Graphene filled polymer nanocomposites. J. Mater. Chem. 2011, 21, 3301–3310. [Google Scholar] [CrossRef]
- Qiu, S.H.; Liu, G.; Li, W.; Zhao, H.C.; Wang, L.P. Noncovalent exfoliation of graphene and its multifunctional composite coating with enhanced anticorrosion and tribological performance. J. Alloy. Compd. 2018, 747, 60–70. [Google Scholar] [CrossRef]
- Morsch, S.; Lyon, S.; Greensmith, P.; Smith, S.D.; Gibbon, S.R. Water transport in an epoxy-phenolic coating. Prog. Org. Coat. 2015, 78, 293–299. [Google Scholar] [CrossRef]
- Sharma, J.; Tewari, K.; Arya, R.K. Diffusion in polymeric systems—A review on free volume theory. Prog. Org. Coat. 2017, 111, 83–92. [Google Scholar] [CrossRef]
- Hayatgheib, Y.; Ramezanzadeh, B.; Kardar, P.; Mahdavian, M. A comparative study on fabrication of a highly effective corrosion protective system based on graphene oxide-polyanilinenanofibers/epoxy composite. Corros. Sci. 2018, 133, 358–373. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Yu, C.; Chen, Q.; Wang, A.; Zhou, X.; Wu, S.; Ran, Q. Improved dispersibility of multi-wall carbon nanotubes with reversible addition-fragmentation chain transfer polymer modification. Polym. Int. 2015, 64, 1219–1224. [Google Scholar] [CrossRef]
- Qiao, M.; Wu, S.S.; Wang, Y.W.; Ran, Q.P. Brush-like block copolymer synthesized via RAFT polymerization for graphene oxide aqueous suspensions. RSC Adv. 2017, 7, 4776–4782. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano. 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- ASTM B117-18 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2018; Volume 03.02.
- ASTM D714-02 Standard Test Method for Evaluating Degree of Blistering of Paints; ASTM International: West Conshohocken, PA, USA, 2017; Volume 06.01.
- ASTM D4541-17 Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2017; Volume 06.02.
- Namvari, M.; Namazi, H. Sweet graphene I: Toward hydrophilic graphenenanosheets via click grafting alkyne-saccharides onto azide-functionalized graphene oxide. Carbohydr. Res. 2014, 396, 1–8. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Doable resonant Raman scattering in graphite. Phys. Rev. Lett. 2000, 85, 5214–5217. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Nandi, S.; Das, P.; Nandi, A.K. Fluorescent graphene oxide via polymer grafting: An efficient nanocarrier for both hydrophilic and hydrophobic drugs. ACS Appl. Mater. Interfaces 2015, 7, 3512–3523. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.L.; Xiang, S.; Wang, Y.; Wang, C.; Jiang, W.; Zhou, H.; Yang, Y.W.; Tang, J. Covalent modification of graphene oxide with polynorbornene by surface-initiated ring-opening metathesis polymerization. Polymer 2014, 55, 6044–6050. [Google Scholar] [CrossRef]
- Jiang, T.; Kuila, T.; Kim, N.H.; Lee, J.H. Effects of surface-modified silica nanoparticles attached graphene oxide using isocyanate-terminated flexible polymer chains on the mechanical properties of epoxy composites. J. Mater. Chem. A 2014, 2, 10557–10567. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Roghani-Mamaqani, H.; Haddadi-Asl, V.; Khezri, K.; Salami-Kalajahi, M.; Najafi, M. Kinetic study of styrene atom transfer radical polymerization from hydroxyl groups of graphenenanoplatelets: Heterogeneities in chains and graft densities. Polym. Eng. Sci. 2015, 55, 1720–1732. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, P.; Qian, Y.; Liu, J. The synthesis of a novel graphene-based inorganic-organic hybrid flame retardant and its application in epoxy resin. Compos. Part B 2014, 60, 341–349. [Google Scholar] [CrossRef]
- Neetika, G.; Kumar, D.; Tomar, S.K. Thermal behaviour of chemically synthesized polyanilines/polystyrene sulphonic acid composites. Int. J. Mater. Chem. 2012, 2, 79–85. [Google Scholar] [CrossRef]
- Shreepathi, S.; Naik, S.M.; Vattipalli, M.R. Water transportation through organic coatings: Correlation between electrochemical impedance measurements, gravimetry, and water vapor permeability. J. Coat. Technol. Res. 2011, 9, 411–422. [Google Scholar] [CrossRef]

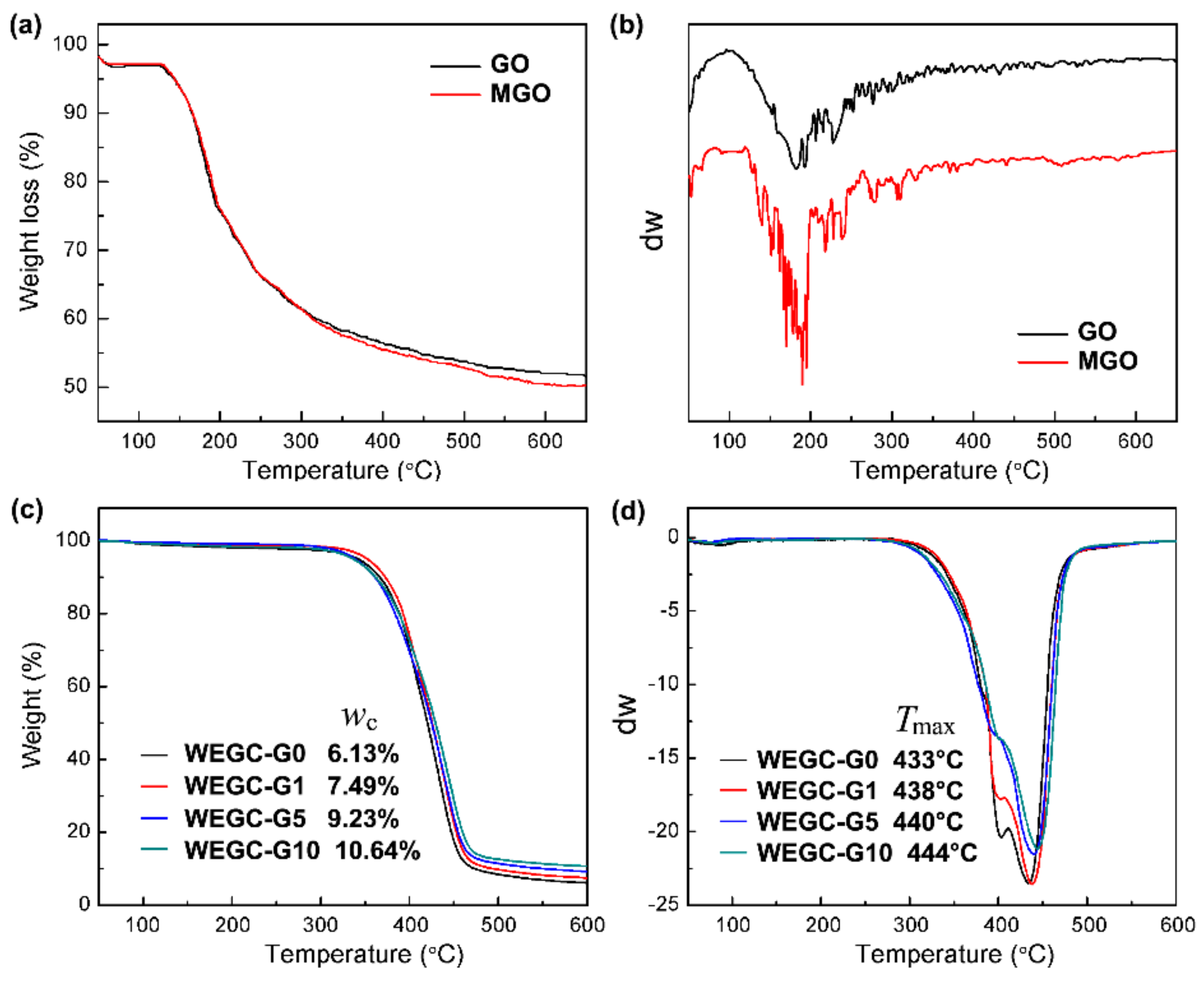
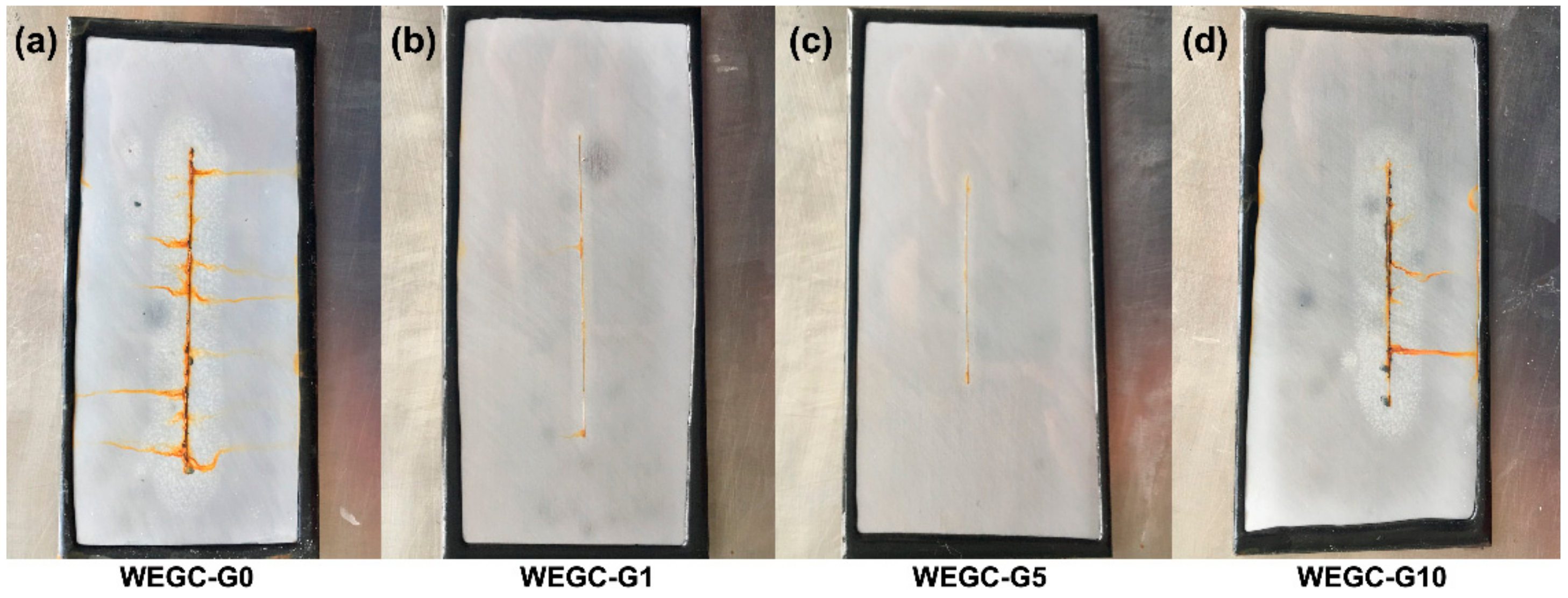
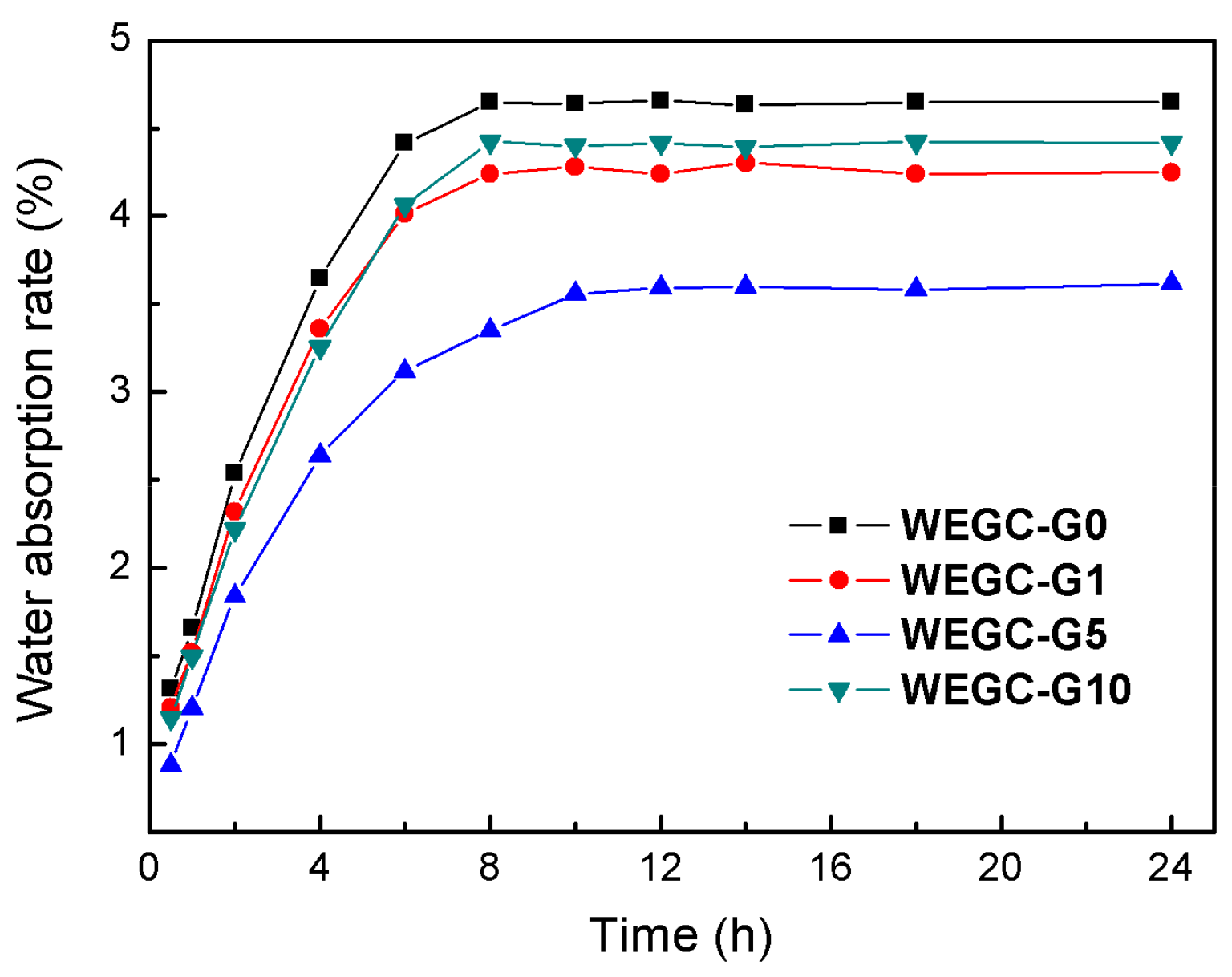
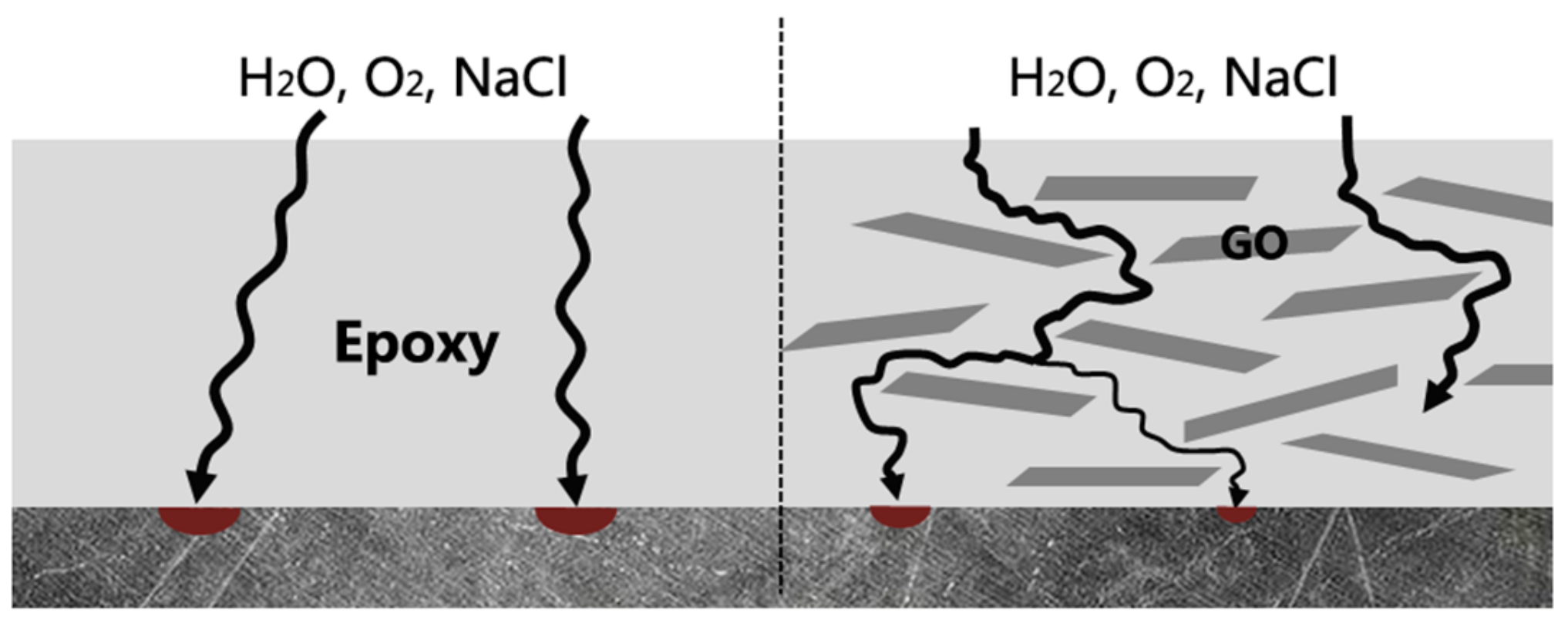
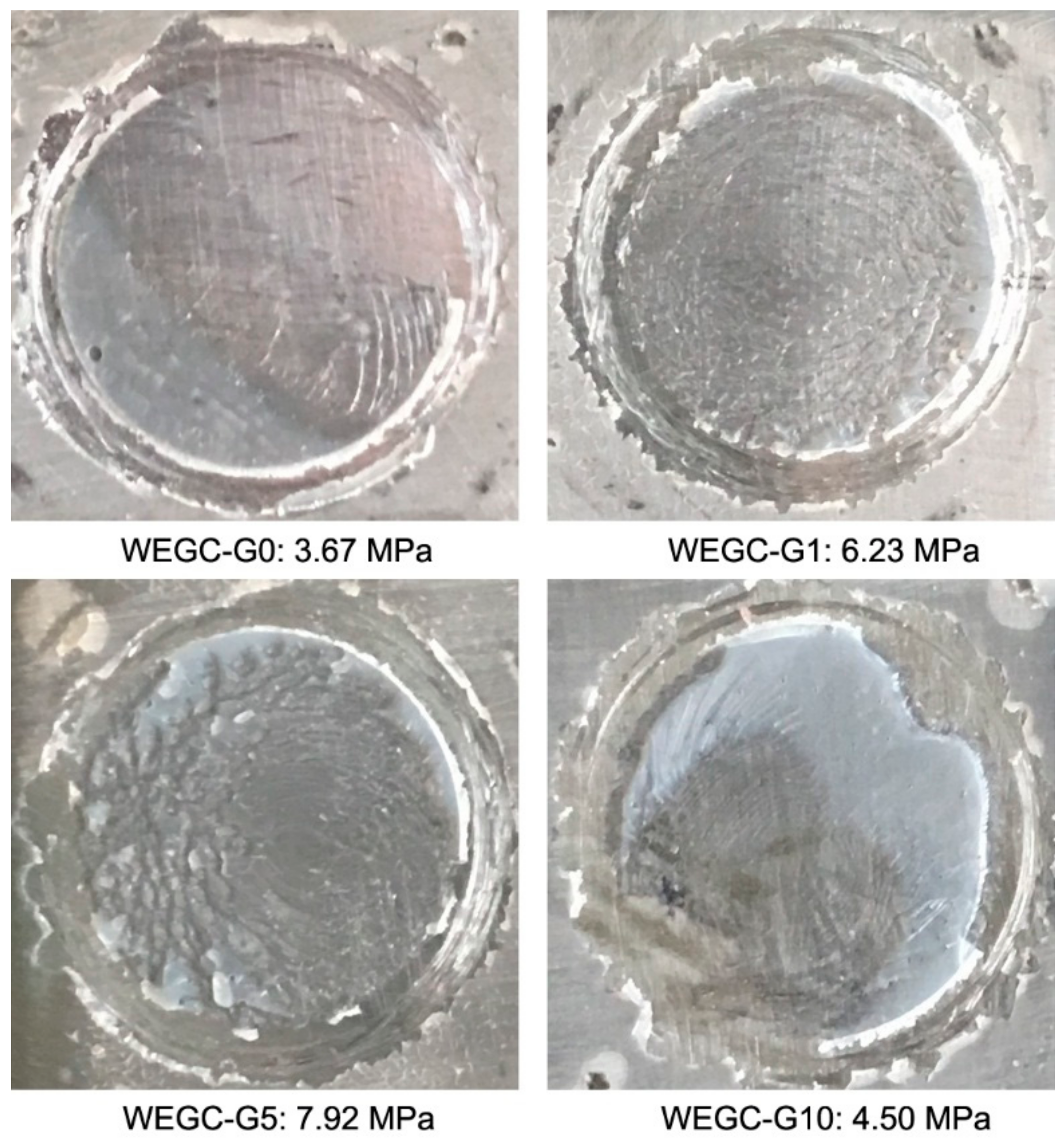
| Sample | WEGC-G0 (MPa) | WEGC-G1 (MPa) | WEGC-G5 (MPa) | WEGC-G10 (MPa) |
|---|---|---|---|---|
| 1 | 3.54 | 6.21 | 8.15 | 4.31 |
| 2 | 3.82 | 6.45 | 7.88 | 4.58 |
| 3 | 3.65 | 6.02 | 7.74 | 4.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Wang, M.; Liang, Y.; Zhang, Z.; Ren, G.; Liu, Y.; Wu, S.; Shen, J. Novel Preparation of Noncovalent Modified GO Using RAFT Polymerization to Reinforce the Performance of Waterborne Epoxy Coatings. Coatings 2019, 9, 348. https://doi.org/10.3390/coatings9060348
Liu B, Wang M, Liang Y, Zhang Z, Ren G, Liu Y, Wu S, Shen J. Novel Preparation of Noncovalent Modified GO Using RAFT Polymerization to Reinforce the Performance of Waterborne Epoxy Coatings. Coatings. 2019; 9(6):348. https://doi.org/10.3390/coatings9060348
Chicago/Turabian StyleLiu, Baolei, Mingqian Wang, Ying Liang, Zhicheng Zhang, Guohong Ren, Yajun Liu, Shishan Wu, and Jian Shen. 2019. "Novel Preparation of Noncovalent Modified GO Using RAFT Polymerization to Reinforce the Performance of Waterborne Epoxy Coatings" Coatings 9, no. 6: 348. https://doi.org/10.3390/coatings9060348
APA StyleLiu, B., Wang, M., Liang, Y., Zhang, Z., Ren, G., Liu, Y., Wu, S., & Shen, J. (2019). Novel Preparation of Noncovalent Modified GO Using RAFT Polymerization to Reinforce the Performance of Waterborne Epoxy Coatings. Coatings, 9(6), 348. https://doi.org/10.3390/coatings9060348






