Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources
Abstract
1. Introduction
2. Review of Literature
2.1. Inclusion Criteria
2.2. Exclusion Criteria
3. BioHA vs. Synthetic HA
4. Preparation of Materials
Powder Preparation
4.1.1. Extraction of HA from Mammalian Bones
4.1.2. Extraction of HA from Fish Sources
4.1.3. Extraction of HA from Biogenic Sources
5. Pulsed Laser Deposition Method
5.1. Method Overview
- The irradiation source is situated outside the deposition chamber, which offers a high degree of flexibility in using the material, set-up, and adjustment of deposition parameters;
- most solid materials can be laser ablated and deposited as films;
- due to the laser operating in a pulsed regime, the film growth rate can be controlled with a highly precise degree (10−2 –10−1 nm/pulse);
- in optimal conditions, the stoichiometry of the deposited layer coincides with the one of the targets, even for very complex materials with a high degree of instability;
- the high energy of ablated species determines the synthesis of extremely adherent layers;
- one can obtain species with electronic states different from the equilibrium ones and new, metastable phases of the material;
- even though their thickness might have very low values, the films uniformly cover the substrate and prevent the release of ions from the implant into the body;
- when reducing the thickness, the risk of delamination decreases.
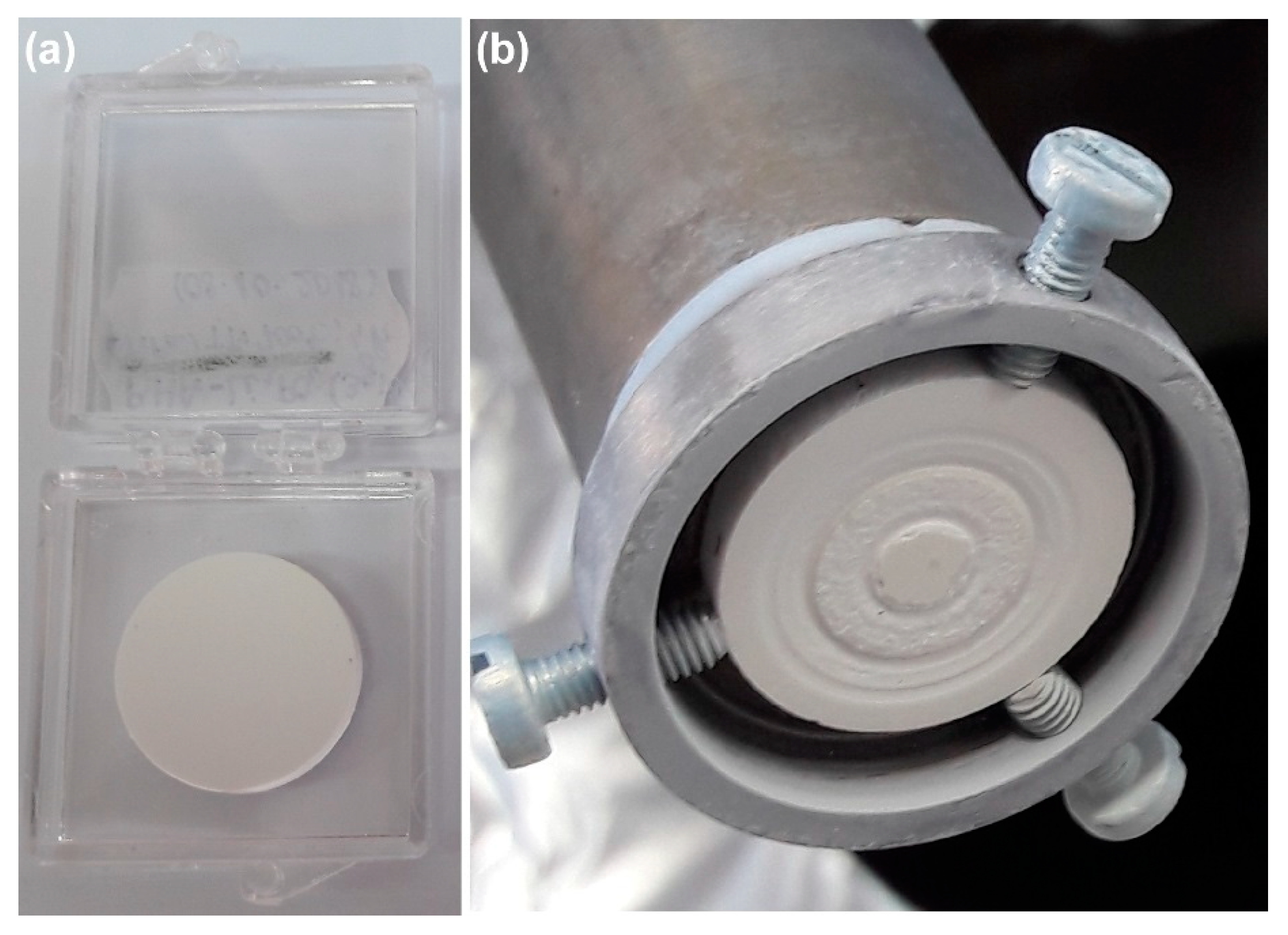
5.2. BioHA Targets Preparation
The Importance of Thermal Treatments in the Case of Targets
5.3. Substrates Used as Pulsed Laser Deposition Collectors
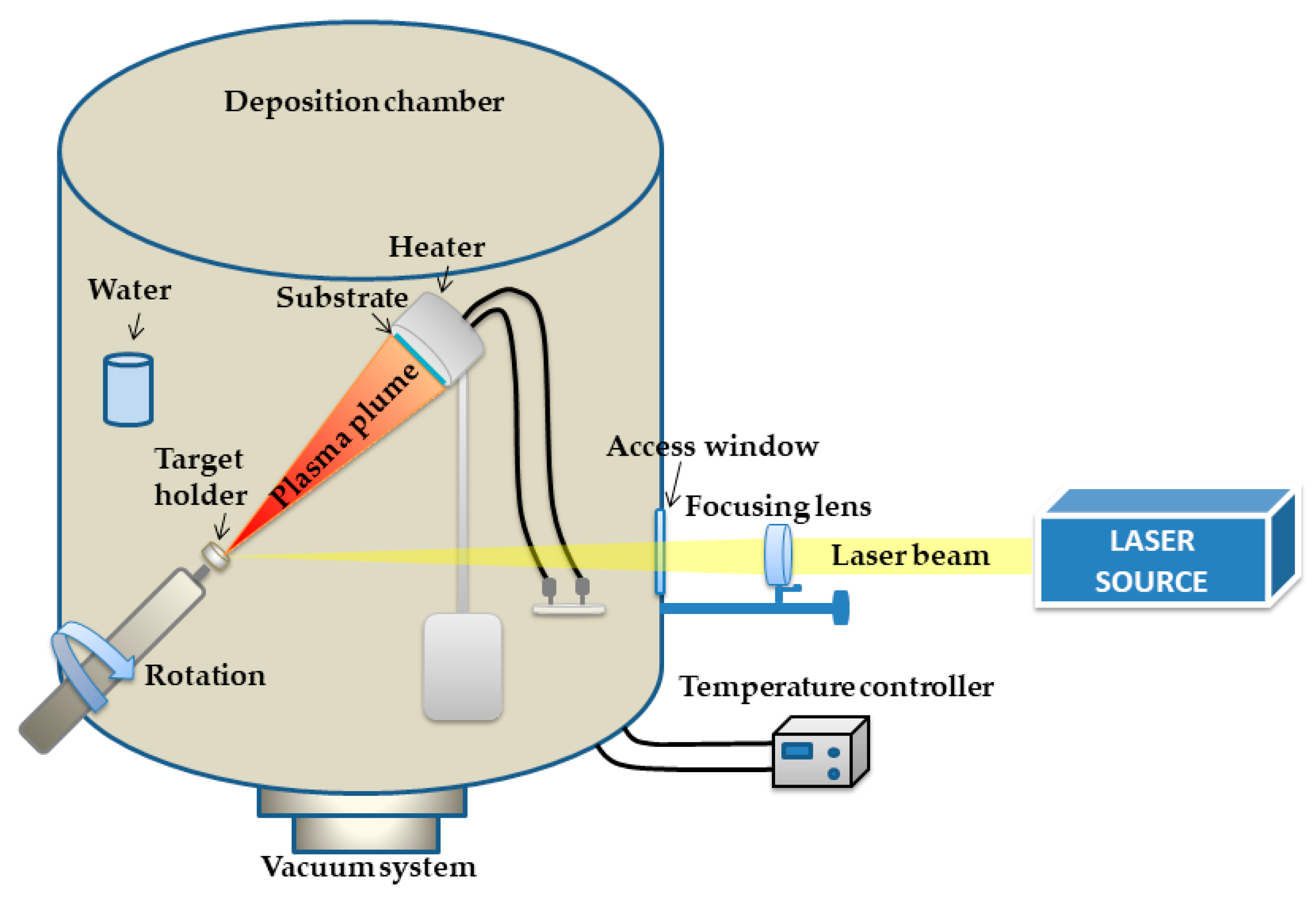
5.4. Pulsed Laser Deposition Experimental Set-Up
5.5. Thermal Treatments Applied to Pulsed Laser Deposited Coatings
6. Characterization of Pulsed Laser Deposited Coatings of Undoped and Doped Animal-Origin Hydroxyapatite
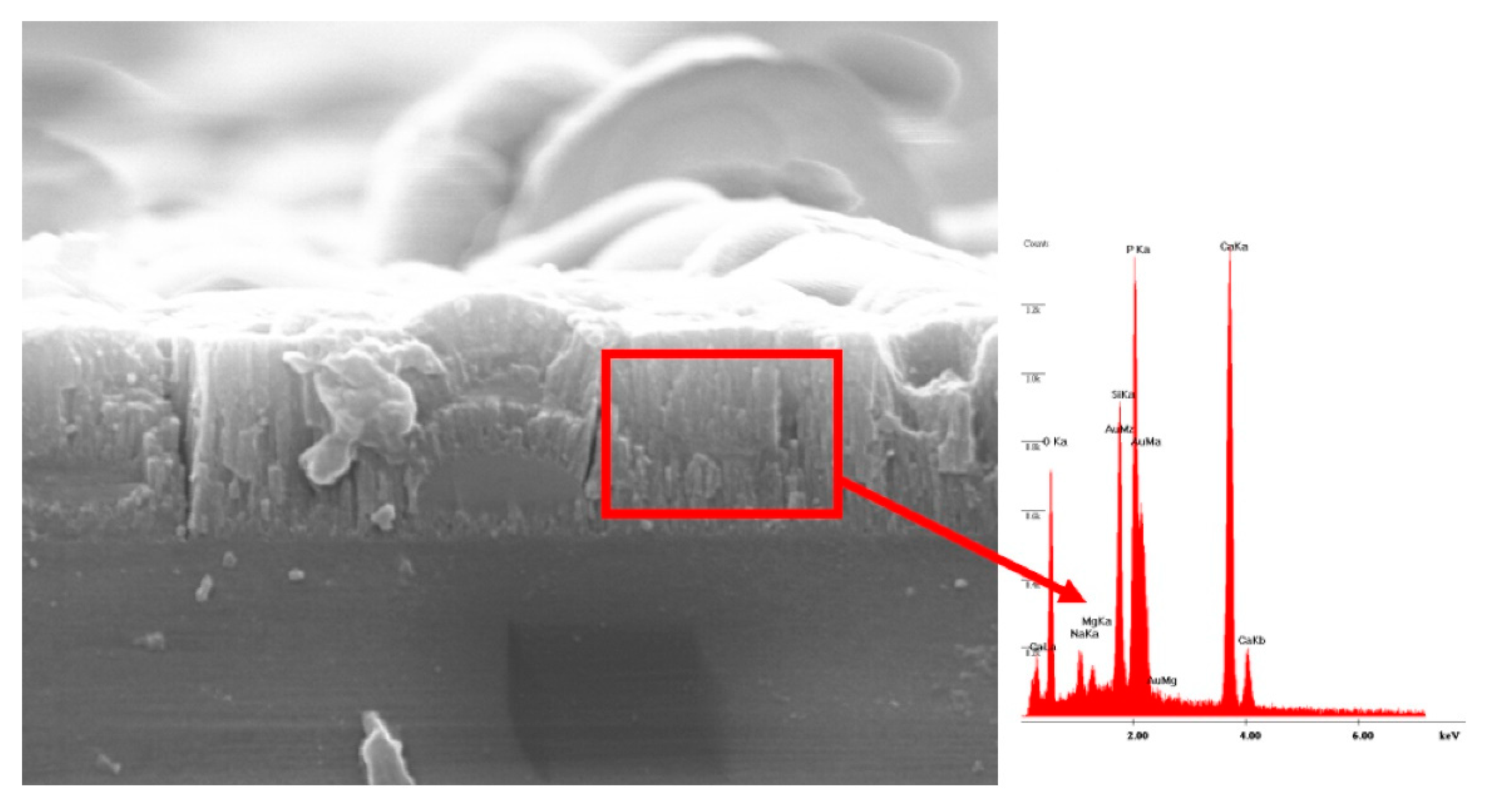
6.1. Morphological and Compositional Analyses
6.2. Structural Investigations
6.3. Bonding Strength Tests
6.4. In vitro Biological Observations
6.4.1. Bioactivity Effect
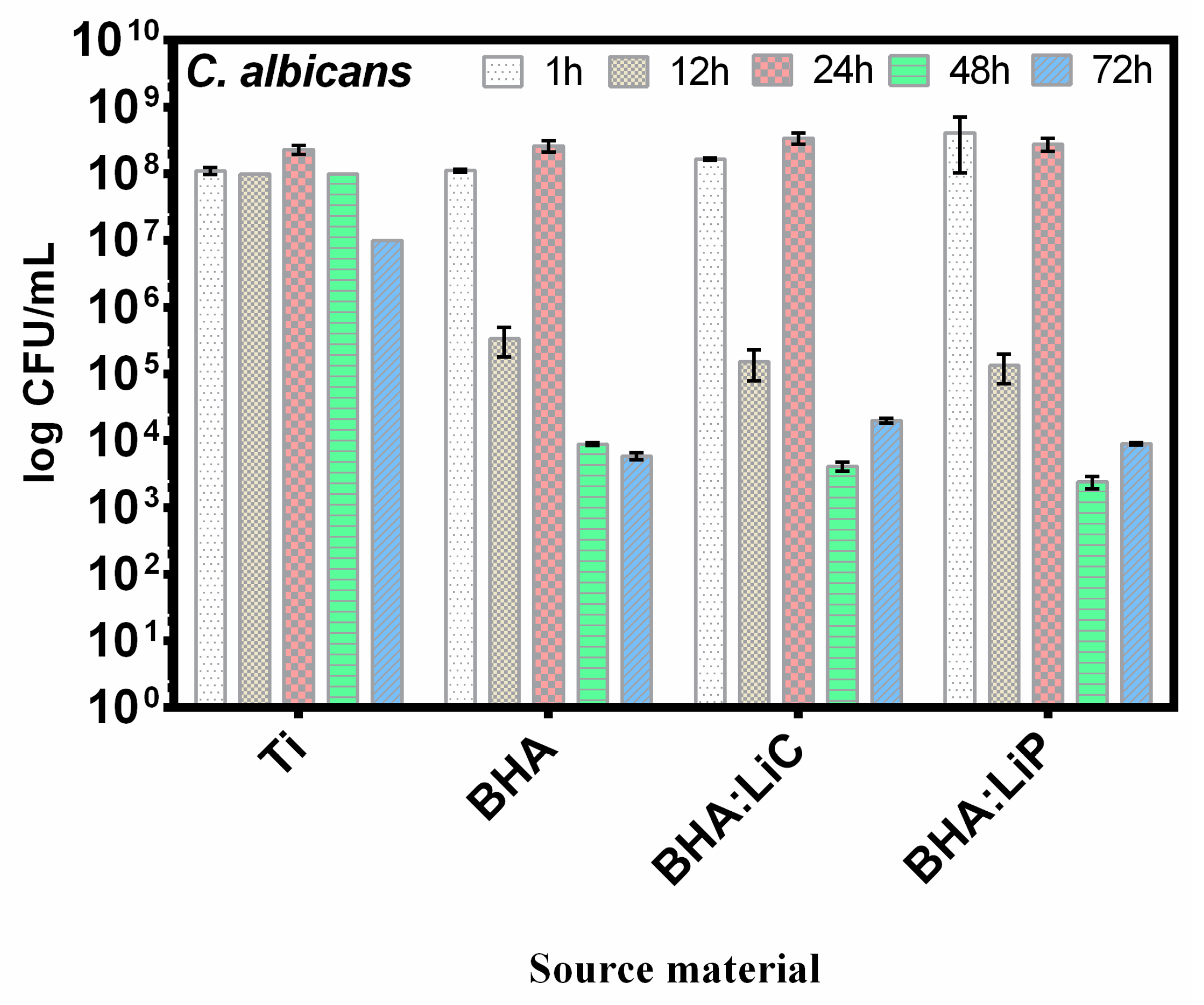
6.4.2. Antibacterial Effect
6.5. In Vivo Tests
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oladele, I.; Agbabiaka, O.; Olasunkanmi, O. Non-synthetic sources for the development of hydroxyapatite. J. Appl. Biotechnol. Bioeng. 2018, 5, 92–99. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Bigi, A.; Bracci, B.; Cuisinier, F.; Elkaim, R.; Fini, M.; Mayer, I.; Mihailescu, I.N.; Socol, G.; Sturba, L.; Torricelli, P. Human osteoblast response to pulsed laser deposited calcium phosphate coatings. Biomaterials 2005, 26, 2381–2385. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Torricelli, P.; Bigi, A.; Mayer, I.; Iliescu, M.; Werckmann, J.; Socol, G.; Miroiu, F.; Cuisinier, F.; Elkaim, R.; et al. Calcium phosphate thin films synthesized by pulsed laser deposition: Physico-chemical characterization and in vitro cell response. Appl. Surf. Sci. 2005, 248, 344–348. [Google Scholar] [CrossRef]
- Dorozhkin, S. History of Calcium Phosphates in Regenerative Medicine. In Advances in Calcium Phosphate Biomaterials; Ben-Nissan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2, pp. 435–483. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Ristoscu, C.; Bigi, A.; Mayer, I. Advanced biomimetic implants based on nanostructured coatings synthesized by pulsed laser technologies. In Laser-Surface Interactions for New Materials Production, Tailoring Structure and Properties; Miotello, A., Ossi, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 235–268. [Google Scholar] [CrossRef]
- O’Hare, P.; Meenan, B.J.; Burke, G.A.; Byrne, G.; Dowling, D.; Hunt, J.A. Biological responses to hydroxyapatite surfaces deposited via a co-incident microblasting technique. Biomaterials 2010, 31, 515–522. [Google Scholar] [CrossRef]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; van Blitterswijk, C.A. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar] [CrossRef]
- Marini, E.; Ballanti, P.; Silvestrini, G.; Valdinucci, F.; Bonucci, E. The presence of different growth factors does not influence bone response to hydroxyapatite: preliminary results. J. Orthopaed. Traumatol. 2004, 5, 34–43. [Google Scholar] [CrossRef]
- Rabiee, S.; Moztarzadeh, F.; Solati-Hashjin, M. Synthesis and characterization of hydroxyapatite cement. J. Mol. Struct. 2010, 969, 172–175. [Google Scholar] [CrossRef]
- Duta, L.; Chifiriuc, M.C.; Popescu-Pelin, G.; Bleotu, C.; (Pircalabioru) Gradisteanu, G.; Anastasescu, M.; Achim, A.; Popescu, A. Pulsed Laser Deposited Biocompatible Lithium-Doped Hydroxyapatite Coatings with Antimicrobial Activity. Coatings 2019, 9, 54. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Bioceramic Layers with Antifungal Properties. Coatings 2018, 8, 276. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Belyavskaya, O.A.; Linders, J.; Loza, K.; Prymak, O.; Mayer, C.; Rau, J.V.; Epple, M.; Sharkeev, Y.P. Glancing Angle Deposition of Zn-Doped Calcium Phosphate Coatings by RF Magnetron Sputtering. Coatings 2019, 9, 220. [Google Scholar] [CrossRef]
- León, B.; Jansen, J.A. Thin calcium phosphate coatings for medical implants; Springer: New York, NY, USA, 2009; p. 328. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.M.; Vallet-Regí, M.; Ferreira, J.M.F.; Ginebra, M.P.; Aparicio, C.; Planell, J.A. Hydroxyapatite ceramic bodies with tailored mechanical properties for different applications. J. Biomed. Mater. Res. 2002, 60, 159–166. [Google Scholar] [CrossRef]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. 2010, 95, 1203–1214. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Wang, G.; Wang, Z.; Wu, X.; Tan, Z. Characteristics of (002) Oriented Hydroxyapatite Coatings Deposited by Atmospheric Plasma Spraying. Coatings 2018, 8, 258. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Hubler, G.K. Pulsed Laser Deposition of Thin Films, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 1–649. [Google Scholar]
- Eason, R. Pulsed Laser Deposition of Thin Films‒Applications-Led Growth of Functional Materials; Wiley-Interscience: Hoboken, NJ, USA, 2006; pp. 1–682. [Google Scholar]
- Yang, Y.; Wu, Q.; Wang, M.; Long, J.; Mao, Z.; Chen, X. Hydrothermal Synthesis of Hydroxyapatite with Different Morphologies: Influence of Supersaturation of the Reaction System. Cryst. Growth Des. 2014, 14, 4864–4871. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, L.; Liu, C.; Zhang, C.; Wu, N. Nucleation and growth of hydroxyapatite nanocrystals by hydrothermal method. AIP Adv. 2018, 8, 085221. [Google Scholar] [CrossRef]
- Pham, V.H.; Van, H.N.; Tam, P.D.; Ha, H.N.T. A novel 1540 nm light emission from erbium doped hydroxyapatite/beta-tricalcium phosphate through co-precipitation method. Mater. Lett. 2016, 167, 145–147. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Cerium-doped hydroxyapatite nanoparticles synthesized by the co-precipitation method. J. Serb. Chem. Soc. 2016, 81, 433–446. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalina, O.O.; Modin, E.B.; Yu Mayorov, V.; Portnyagin, A.S.; Kobylyakov, S.P.; Golub, A.V.; Medkov, M.A.; Tananaev, I.G.; Avramenk, V.A. Wollastonite ceramics with bimodal porous structure prepared by Sol-Gel and SPS techniques. RSC Adv. 2016, 6, 34066–34073. [Google Scholar] [CrossRef]
- Duta, L.; Mihailescu, N.; Popescu, A.C.; Luculescu, C.R.; Mihailescu, I.N.; Cetin, G.; Gunduz, O.; Oktar, F.N.; Popa, A.C.; Kuncser, A.; et al. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017, 413, 129–139. [Google Scholar] [CrossRef]
- Oktar, F. Microstructure and mechanical properties of sintered enamel hydroxyapatite. Ceram. Int. 2007, 33, 1309–1314. [Google Scholar] [CrossRef]
- Goller, G.; Oktar, F.N.; Agathopoulos, S.; Tulyaganov, D.U.; Ferreira, J.M.F.; Kayali, E.S.; Peker, I. Effect of sintering temperature on mechanical and microstructural properties of bovine hydroxyapatite (BHA). J. Sol-Gel Sci. Techn. 2006, 37, 111–115. [Google Scholar] [CrossRef]
- Eisenhart, S. EU Regulation 722. New EU Animal Tissue Regulations in Effect for Some Medical Devices; Emergo: Hong Kong, China, 2013; Available online: https://www.emergobyul.com/blog/2013/09/new-eu-animaltissue-regulations-effect-some-medical-devices (accessed on 29 April 2019).
- ISO 22442-1. Medical Devices Utilizing Animal Tissues and Their Derivatives‒Part 1: Application of Risk Management; International Organization for Standardization: Berlin, Germany, 2015. [Google Scholar]
- Barakat, N.A.M.; Khil, M.S.; Omran, A.M.; Sheikh, F.A.; Kim, H.Y. Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods. J. Mater. Process. Technol. 2009, 209, 3408–3415. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.C.; Wilson, R.M.; Dowker, S.E.P. Apatite structures. Adv. X-ray Anal. 2002, 45, 172–181. [Google Scholar]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Ben-Nissan, B. Advances in calcium phosphate biomaterials; Part of the Springer Series in Biomaterials Science and Engineering book series; Springer: Berlin, Germany, 2014; Volume 2, p. 547. [Google Scholar]
- Mucalo, M. Hydroxyapatite (HAp) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; p. 404. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Coupled substitution of type A and B carbonate in sodium-bearing apatite. Biomaterials 2007, 28, 916–926. [Google Scholar] [CrossRef]
- Astala, R.; Stott, M.J. First principles investigation of mineral component of bone: CO3 substitutions in hydroxyapatite. Chem. Mater. 2005, 17, 4125–4133. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Mattioli-Belmonte, M.; Celotti, G.; Sandri, M.; Gigante, A.; Fava, P.; Biagini, G. Biomimetic Mg- and Mg, CO3-substituted hydroxyapatites: Synthesis characterization and in vitro behaviour. J. Eur. Ceram. Soc. 2006, 26, 2593–2601. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Verdelis, K.; Lukashova, L.; Wright, J.T.; Mendelsohn, R.; Peterson, M.G.E.; Doty, S.; Boskey, A.L. Maturational changes in dentin mineral properties. Bone 2007, 40, 1399–1407. [Google Scholar] [CrossRef]
- Sun, R.-X.; Lv, Y.; Niu, Y.-R.; Zhao, X.-H.; Cao, D.-S.; Tang, J.; Sun, X.-C.; Chen, K.-Z. Physicochemical and biological properties of bovine-derived porous hydroxyapatite/collagen composite and its hydroxyapatite powders. Ceram. Int. 2017, 43, 16792–16798. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.A.; Akhtar, N.; Haq, I. Serum hormonal, electrolytes and trace element profiles in the rutting and non-rutting one-humped male camel (Camelus dromedarius). Anim. Reprod. Sci. 2007, 101, 172–178. [Google Scholar]
- Rahavi, S.S.; Ghaderi, O.; Monshi, A.; Fathi, M.H. A comparative study on physicochemical properties of hydroxyapatite powders derived from natural and synthetic sources. Russ. J. Non-Ferrous Metals 2017, 58, 276–286. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Rajendran, A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O.; Pattanayak, D.K. Synthesis of organic derived hydroxyapatite scaffold from pig bone waste for tissue engineering applications. Adv. Powder Technol. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Labarthe, J.C.; Bonel, G.; Montel, G. Structure and properties of B-type phosphocalcium carbonate apatites. Ann. Chim. 1973, 8, 289–301. [Google Scholar]
- Wu, Y.; Glimcher, M.J.; Rey, C.; Ackerman, J.L. A unique protonated phosphate group in bone mineral not present in synthetic calcium phosphates. Identification by phosphorus-31 solid state NMR spectroscopy. J. Mol. Biol. 1994, 244, 423–435. [Google Scholar] [CrossRef]
- Winand, L. Etude physico-chimique du phosphate tricalcique hydraté et de l’hydroxyapatite. Ann. Chim. 1961, 6, 951–967. [Google Scholar]
- Riggs, B.L.; Hodgson, S.F.; O’Fallon, W.M.; Chao, E.Y.; Wahner, H.W.; Muhs, J.M.; Cedel, S.L.; Melton III, J. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N. Engl. J. Med. 1990, 322, 802–809. [Google Scholar] [CrossRef]
- Kay, H.M.; Wilson, M. The in vitro effects of amine fluorides on plaque bacteria. J. Periodontol. 1988, 59, 266–269. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.N.; Stan, G.E.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I.N. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.E.; Duta, L.; Chifiriuc, C.M.; Coralia, B.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF2 or MgO for implants functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J Food Sci Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef]
- Kim, S.-H.; Shin, J.-W.; Park, S.-A.; Kim, Y.K.; Park, M.S.; Mok, J.M.; Yang, W.I.; Lee, J.W. Chemical, structural properties, and osteoconductive effectiveness of bone block derived from porcine cancellous bone. J. Biomed. Mater. Res. Part B 2004, 68, 69–74. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Khalil, K.A.; Sheikh, F.A.; Omran, A.M.; Gaihre, B.; Khil, S.M.; Kim, H.Y. Physiochemical characterizations of hydroxyapatite extracted from bovine bones by three different methods: extraction of biologically desirable Hap. Mater. Sci. Eng. C-Mater. 2008, 28, 1381–1387. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into Biological Apatite: Physiochemical Properties and Preparation Approaches. Biomed Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef]
- Seo, D.S.; Kim, Y.G.; Lee, J.K. Sintering and dissolution of bone ash-derived hydroxyapatite. Met. Mater.-Int. 2010, 16, 687–692. [Google Scholar] [CrossRef]
- Popescu, A.C.; Florian, P.E.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.N.; Trusca, R.; Sima, L.E.; Roseanu, A.; et al. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Figueiredo, M.; Fernando, A.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram. Int. 2010, 36, 2383–2393. [Google Scholar] [CrossRef]
- Ramesh, S.; Loo, Z.Z.; Tan, C.Y.; Chew, W.J.K.; Ching, Y.C.; Tarlochan, F.; Chandran, H.; Krishnasamy, S.; Bang, L.T.; Sarhan, A.A.D.M. Characterization of biogenic hydroxyapatite derived from animal bones for biomedical applications. Ceram. Int. 2018, 44, 10525–10530. [Google Scholar] [CrossRef]
- Akyurt, N.; Yetmez, M.; Karacayli, U.; Gunduz, O.; Agathopoulos, S.; Gökçe, H.; Öveçoğlu, M.L.; Oktar, F.N. A New Natural Biomaterial: Sheep Dentine Derived Hydroxyapatite. Key. Eng. Mater 2012, 493–494, 281–286. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite – hardstonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Khandan, A.; Abdellahi, M.; Barenji, R.V.; Ozada, N.; Karamian, E. Introducing natural hydroxyapatite-diopside(NHA-Di) nano-bioceramic coating. Ceram. Int. 2015, 41, 12355–12363. [Google Scholar] [CrossRef]
- Giraldo-Betancur, A.L.; Espinosa-Arbelaez, D.G.; del Real-López, A.; Millan-Malo, B.M.; Rivera-Muñoz, E.M.; Gutierrez-Cortez, E.; Pineda-Gomez, P.; Jimenez-Sandoval, S.; Rodriguez-García, M.E. Comparison of physicochemical properties of bio and commercial hydroxyapatite. Curr. Appl. Phys. 2013, 13, 1383–1390. [Google Scholar] [CrossRef]
- Rincón-López, J.A.; Hermann-Muñoz, J.A.; Giraldo-Betancur, A.L.; De Vizcaya-Ruiz, A.; Alvarado-Orozco, J.M.; Muñoz-Saldaña, J. Synthesis, Characterization and In Vitro Study of Synthetic and Bovine-Derived Hydroxyapatite Ceramics: A Comparison. Materials 2018, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Gambardella, A.; Graziani, G.; Liscio, F.; Maltarello, M.C.; Boi, M.; Berni, M.; Bellucci, D.; Marchiori, G.; Valle, F.; et al. Plasma-assisted deposition of bone apatite-like thin films from natural apatite. Mater. Lett. 2017, 199, 32–36. [Google Scholar] [CrossRef]
- Pramanik, S.; Hanif, A.S.M.; Pingguan-Murphy, B.; Osman, N.A.A. Morphological change of heat treated bovine bone: a comparative study. Mater. 2013, 6, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Kusrini, E.; Sontang, M. Characterization of x-ray diffraction and electron spin resonance: Effects of sintering time and temperature on bovine hydroxyapatite. Radiat. Phys. Chem. 2012, 81, 118–125. [Google Scholar] [CrossRef]
- Bahrololoom, M.E.; Javidi, M.; Javadpour, S.; Ma, J. Characterisation of natural hydroxyapatite extracted from bovine cortical bone ash. J. Ceram. Process. Res. 2009, 10, 129–138. [Google Scholar]
- Bano, N.; Jikan, S.S.; Basri, H.; Bakar, S.A.S.A.; Nuhu, A.H. Natural hydroxyapatite extracted from bovine bone. J. Sci. Technol. 2017, 9, 22–28. [Google Scholar]
- Ruksudjarit, A.; Pengpat, K.; Rujijanagul, G.; Tunkasiri, T. Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr. Appl. Phys. 2008, 8, 270–272. [Google Scholar] [CrossRef]
- Nasiri-Tabrizi, B.; Fahami, A.; Ebrahimi-Kahrizsangi, R. A comparative study of hydroxyapatite nanostructures produced under different milling conditions and thermal treatment of bovine bone. J. Ind. Eng. Chem. 2014, 20, 245–258. [Google Scholar] [CrossRef]
- Rakmae, S.; Lorprayoon, C.; Ekgasit, S.; Suppakarn, N. Influence of heat-treated bovine bone-derived hydroxyapatite on physical properties and in vitro degradation behavior of poly (lactic acid) composites. Polym. Plast. Technol. 2013, 52, 1043–1053. [Google Scholar] [CrossRef]
- Nirmala, R.; Sheikh, F.A.; Kanjwal, M.A.; Lee, J.H.; Park, S.-J.; Navamathavan, R.; Kim, H.Y. Synthesis and characterization of bovine femur bone hydroxyapatite containing silver nanoparticles for the biomedical applications. J. Nanopart. Res. 2011, 13, 1917–1927. [Google Scholar] [CrossRef]
- Herliansyah, M.K.; Hamdi, M.; Ide-Ektessabi, A.; Wildan, M.W.; Toque, J.A. The influence of sintering temperature on the properties of compacted bovine hydroxyapatite. Mater. Sci. Eng. C 2009, 29, 1674–1680. [Google Scholar] [CrossRef]
- Ayatollahi, M.R.; Yahya, M.Y.; Asgharzadeh Shirazi, H.; Hassan, S.A. Mechanical and tribological properties of hydroxyapatite nanoparticles extracted from natural bovine bone and the bone cement developed by nano-sized bovine hydroxyapatite filler. Ceram. Int. 2015, 41, 10818–10827. [Google Scholar] [CrossRef]
- Jaber, H.L.; Hammood, A.S.; Parvin, N. Synthesis and characterization of hydroxyapatite powder from natural Camelus bone. J. Aust. Ceram. Soc. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Duta, L.; Serban, N.; Oktar, F.N.; Mihailescu, I.N. Biological hydroxyapatite thin films synthesized by pulsed laser deposition. Optoelectron. Adv. Mater.-Rapid Commun. 2013, 7, 1040–1044. [Google Scholar]
- Maidaniuc, A.; Miculescu, F.; Voicu, S.I.; Andronescu, C.; Miculescu, M.; Matei, E.; Mocanu, A.C.; Pencea, I.; Csaki, I.; Machedon-Pisu, T.; et al. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 158–166. [Google Scholar] [CrossRef]
- Ferraro, V.; Carvalho, A.P.; Piccirillo, C.; Santos, M.M.; Castro, P.M.L.; Pintado, M.E. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues-A review. Mat. Sci. Eng. C-Mater. 2013, 33, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Qian, Z.J.; Ryu, M.; Thomas, N.V.; Kim, S.K. A comparative study of thermal calcination and an alkaline hydrolysis method in the isolation of hydroxyapatite from Thunnus obesus bone. Biomed. Mater. 2011, 6, 12. [Google Scholar] [CrossRef]
- Kannan, S.; Rocha, J.H.G.; Agathopoulos, S.; Ferreira, J.M.F. Fluorine-substituted hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. Acta Biomater. 2007, 3, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C-Mater. Biol. Appl. 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Huang, Y.C.; Hsiao, P.C.; Chai, H.J. Hydroxyapatite extracted from fish scale: Effects on MG63 osteoblast-like cells. Ceram. Int. 2011, 37, 1825–1831. [Google Scholar] [CrossRef]
- Piccirillo, C.; Silva, M.F.; Pullar, R.C.; Braga da Cruz, I.; Jorge, R.; Pintado, M.M.; Castro, P.M. Extraction and characterisation of apatite- and tricalcium phosphate-based materials from cod fish bones. Mat. Sci. Eng. C-Mater. 2013, 33, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Senthil, R.; Vedakumari, S.W.; Sastry, T.P. Hydroxyapatite and Demineralized Bone Matrix from Marine Food Waste – A Possible Bone Implant. Am. J. Mat. Synth. Proc. 2018, 3, 1–6. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; Aguilera-Correa, J.J.; López-Álvarez, M.; Romera, D.; Esteban, J.; Gonzalez, P.; Serra, J. Fluor-carbonated hydroxyapatite coatings by pulsed laser deposition to promote cell viability and antibacterial properties. Surf. Coat. Technol. 2018, 349, 736–744. [Google Scholar] [CrossRef]
- Aguiar, H.; Chiussi, S.; López-Álvarez, M.; González, P.; Serra, J. Structural characterization of bioceramics and mineralized tissues based on Raman and XRD techniques. Ceram. Int. 2018, 44, 495–504. [Google Scholar] [CrossRef]
- Lopez-Alvarez, M.; Vigo, E.; Rodriguez-Valencia, C.; Outeirino-Iglesias, V.; Gonzalez, P.; Serra, J. In vivo evaluation of shark teeth-derived bioapatites. Clin. Oral Impl. Res. 2016, 28, e91–e100. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alvarez, M.; Pérez-Davila, S.; Rodríguez-Valencia, C.; González, P.; Serra, J. The improved biological response of shark tooth bioapatites in a comparative in vitro study with synthetic and bovine bone grafts. Biomed. Mater. 2016, 11, 035011. [Google Scholar] [CrossRef]
- Rivera, E.M.; Araiza, M.; Brostow, W.; Castano, V.M.; Estrada, J.R.D.; Hernandez, R.; Rodrigues, J.R. Synthesis of hydroxyapatite from eggshells. Mater. Lett. 1999, 41, 128–134. [Google Scholar] [CrossRef]
- Elizondo-Villarreal, N.; Martínez-de-la-Cruz, A.; Obregón Guerra, R.; Gómez-Ortega, J.L.; Torres-Martínez, L.M.; Castaño, V.M. Biomaterials from Agricultural Waste: Eggshell-based Hydroxyapatite. Water Air Soil Pollut. 2012, 223, 3643–3646. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Mondal, B.; Modak, D.K.; Pramanik, K.; Chaudhuri, B.K. Preparation and characterization of nanocrystalline hydroxyapatite from eggshell and K2HPO4 solution. Mater. Lett. 2013, 97, 148–150. [Google Scholar] [CrossRef]
- Tamasan, M.; Ozyegin, L.S.; Oktar, F.N.; Simon, V. Characterization of calcium phosphate powders originating from Phyllacanthus imperialis and Trochidae Infundibulum concavus marine shells. Mat. Sci. Eng. C-Mater 2013, 33, 2569–2577. [Google Scholar] [CrossRef]
- Gunduz, O.; Sahin, Y.M.; Agathopoulos, S.; Ben-Nissan, B.; Oktar, F.N. A New Method for Fabrication of Nanohydroxyapatite and TCP from the Sea Snail Cerithium vulgatum. J. Nanomater. 2014, 2014, 382861. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Jing, X.; Liu, Q.; Saba, J.; Mann, T.; Zhang, M.; Wei, H.; Chen, R.; Liu, L. Conversion of calcined eggshells into flower-like hydroxyapatite agglomerates by solvothermal method using Hydrogen peroxide/N,N-dimethylformamide mixed solvents. J. Am. Ceram. Soc. 2012, 95, 3377–3379. [Google Scholar] [CrossRef]
- Ho, W.-F.; Hsu, H.-C.; Hsu, S.-K.; Hung, C.-W.; Wu, S.-C. Calcium phosphate bioceramics synthesized from eggshell powders through a solid state reaction. Ceram. Int. 2013, 39, 6467–6473. [Google Scholar] [CrossRef]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on Ionic Substitutions in Hydroxyapatite Thin Films: Towards Complete Biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Schneider, C.W.; Lippert, T. Laser Ablation and Thin Film Deposition. In Laser Processing of Materials; Schaaf, P., Ed.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 139, pp. 89–112. [Google Scholar]
- Caricato, A.P.; Martino, M.; Romano, F.; Mirchin, N.; Peled, A. Pulsed laser photodeposition of a-Se nanofilms by ArF laser. Appl. Surf. Sci. 2007, 253, 6517–6521. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ueda, M.; Kohiga, Y.; Imura, K.; Hontsu, S. Application of fluoridated hydroxyapatite thin film coatings using KrF pulsed laser deposition. Dent Mater J. 2018, 37, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; Blythe, H.J.; Heald, S.M.; Fox, A.M.; Gehring, G.A. Growth of high quality yttrium iron garnet films using standard pulsed laser deposition technique. J. Magn. Magn. Mater. 2018, 453, 254–257. [Google Scholar] [CrossRef]
- Novotný, M.; Vondráček, M.; Marešová, E.; Fitl, P.; Bulíř, J.; Pokornýa, P.; Havlová, Š.; Abdellaoui, N.; Pereira, A.; Hubík, P.; et al. Optical and structural properties of ZnO:Eu thin films grown by pulsed laser deposition. Appl. Surf. Sci. 2019, 476, 271–275. [Google Scholar] [CrossRef]
- Gyorgy, E.; Ristoscu, C.; Mihailescu, I.N. Role of laser pulse duration and gas pressure in deposition of AlN thin films. J. Appl. Phys. 2001, 90, 456. [Google Scholar] [CrossRef]
- Boyd, I.W. Thin film growth by pulsed laser deposition. Ceram. Int. 1996, 22, 429–434. [Google Scholar] [CrossRef]
- Popescu, A.C.; Duta, L.; Dorcioman, G.; Mihailescu, I.N.; Stan, G.E.; Pasuk, I.; Zgura, I.; Beica, T.; Enculescu, I.; Ianculescu, A.; et al. Radical modification of the wetting behavior of textiles coated with ZnO thin films and nanoparticles when changing the ambient pressure in the pulsed laser deposition process. J. Appl. Phys. 2011, 110, 064321. [Google Scholar] [CrossRef]
- Murray, M.; Jose, G.; Richards, B.; Jha, A. Femtosecond pulsed laser deposition of silicon thin films. Nanoscale Res. Lett. 2013, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Fehse, M.; Trócoli, R.; Hernández, E.; Ventosa, E.; Sepúlveda, A.; Morata, A.; Tarancón, A. An innovative multi-layer pulsed laser deposition approach for LiMn2O4 thin film cathodes. Thin Solid Films 2018, 648, 108–112. [Google Scholar] [CrossRef]
- Stock, F.; Diebold, L.; Antoni, F.; Chowde Gowda, C.; Muller, D.; Haffner, T.; Pfeiffer, P.; Roques, S.; Mathiot, D. Silicon and silicon-germanium nanoparticles obtained by Pulsed Laser Deposition. Appl. Surf. Sci. 2019, 466, 375–380. [Google Scholar] [CrossRef]
- Craciun, D.; Socol, G.; Stefan, N.; Miroiu, M.; Mihailescu, I.N.; Galca, A.C.; Craciun, V. Structural investigations of ITO-ZnO films grown by the combinatorial pulsed laser deposition technique. Appl. Surf. Sci. 2009, 255, 5288–5291. [Google Scholar] [CrossRef]
- Rasoga, O.; Sima, L.; Chiritoiu, M.; Popescu-Pelin, G.; Fufa, O.; Grumezescu, V.; Socol, M.; Stanculescu, A.; Zgura, I.; Socol, G. Biocomposite coatings based on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/calcium phosphates obtained by MAPLE for bone tissue engineering. Appl. Surf. Sci. 2017, 417, 204–212. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Gyorgy, E. Pulsed Laser Deposition: An overview. In International Trends in Optics and Photonics; Asakura, T., Ed.; Part of the Springer Series in Optical Sciences book series; Springer: Heidelberg, Germany, 1999; Volume 74, pp. 201–214. [Google Scholar] [CrossRef]
- Ozyegin, L.S.; Oktar, F.N.; Goller, G.; Kayali, S.; Yazici, T. Plasma-sprayed bovine hydroxyapatite coatings. Mater. Lett. 2004, 58, 2605–2609. [Google Scholar] [CrossRef]
- Aguzzi, A. Prion diseases of humans and farm animals: epidemiology, genetics, and pathogenesis. J. Neurochem. 2006, 97, 1726–1739. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R-Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Park, J.-W.; Park, K.-B.; Suh, J.-Y. Effects of calcium ion incorporation on bone healing of Ti6Al4V alloy implants in rabbit tibiae. Biomaterials 2007, 28, 3306–3313. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Duncan, W.; Swain, M.; Lee, M.H. One-step approach for hydroxyapatite-incorporated TiO2 coating on titanium via a combined technique of micro-arc oxidation and electrophoretic deposition. Appl. Surf. Sci. 2011, 257, 7010–7018. [Google Scholar] [CrossRef]
- Liu, L.-S.; Thompson, A.Y.; Heidaran, M.A.; Poser, J.W.; Spiro, R.C. An osteoconductive collagen/hyaluronate matrix for bone regeneration. Biomaterials 1999, 20, 1097–1108. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science7—An Introduction to Materials in Medicine, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2004; p. 864. [Google Scholar]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Traini, T.; Murmura, G.; Sinjari, B.; Perfetti, G.; Scarano, A.; D’Arcangelo, C.; Caputi, S. The Surface Anodization of Titanium Dental Implants Improves Blood Clot Formation Followed by Osseointegration. Coatings 2018, 8, 252. [Google Scholar] [CrossRef]
- Han, A.; Tsoi, J.K.-H.; Matinlinna, J.P.; Chen, Z. Influence of Grit-Blasting and Hydrofluoric Acid Etching Treatment on Surface Characteristics and Biofilm Formation on Zirconia. Coatings 2017, 7, 130. [Google Scholar] [CrossRef]
- Zhou, G.; Bi, Y.; Ma, Y.; Wang, L.; Wang, X.; Yu, Y.; Mutzk, A. Large current ion beam polishing and characterization of mechanically finished titanium alloy (Ti6Al4V) surface. Appl. Surf. Sci. 2019, 476, 905–913. [Google Scholar] [CrossRef]
- Chen, R.; Li, S.; Wang, Z.; Lu, X. Mechanical model of single abrasive during chemical mechanical polishing: molecular dynamics simulation. Tribol. Int. 2019, 133, 40–46. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, V.S.; Singh, S.; Dogra, M. Nanofluids assisted environmental friendly lubricating strategies for the surface grinding of titanium alloy: Ti6Al4V-ELI. J. Manuf. Process. 2019, 39, 241–249. [Google Scholar] [CrossRef]
- Eliaz, N.; Ritman-Hertz, O.; Aronov, D.; Weinberg, E.; Shenhar, Y.; Rosenman, G.; Weinreb, M.; Ron, E. The effect of surface treatments on the adhesion of electrochemically deposited hydroxyapatite coating to titanium and on its interaction with cells and bacteria. J. Mater. Sci. Mater. Med. 2011, 22, 1741–1752. [Google Scholar] [CrossRef]
- Liu, C.-F.; Li, S.-J.; Hou, W.-T.; Hao, Y.-L.; Huang, H.-H. Enhancing Corrosion Resistance and Biocompatibility of Interconnected Porous β-type Ti-24Nb-4Zr-8Sn Alloy Scaffold through Alkaline Treatment and Type I Collagen Immobilization. Appl. Surf. Sci. 2019, 476, 325–334. [Google Scholar] [CrossRef]
- Peng, F.; Shaw, M.T.; Olson, J.R.; Wei, M. Influence of surface treatment and biomimetic hydroxyapatite coating on the mechanical properties of hydroxyapatite/poly(L-lactic acid) fibers. J. Biomater. Appl. 2013, 27, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef]
- Koh, A.T.T.; Foong, Y.M.; Chua, D.H.C. Cooling rate and energy dependence of pulsed laser fabricated graphene on nickel at reduced temperature. Appl. Phys. Lett. 2010, 97, 114102. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, J. Progress in pulsed laser deposited two-dimensional layered materials for device applications. J. Mater. Chem. C 2016, 4, 8859–8878. [Google Scholar] [CrossRef]
- Christen, H.M.; Eres, G. Recent advances in pulsed-laser deposition of complex oxides. J. Phys. Condens. Matter. 2008, 20, 264005. [Google Scholar] [CrossRef]
- Saju, K.K.; Reshmi, R.; Jayadas, N.H.; James, J.; Jayaraj, M.K. Polycrystalline coating of hydroxyapatite on TiAl6V4 implant material grown at lower substrate temperatures by hydrothermal annealing after pulsed laser deposition. Proc. Inst. Mech. Eng. Part H-J. Eng. Med. 2009, 223, 1049–1057. [Google Scholar] [CrossRef]
- Lynn, A.K.; DuQuesnay, D.L. Hydroxyapatite-coated Ti–6Al–4V Part 2: the effects of post-deposition heat treatment at low temperatures. Biomaterials 2002, 23, 1947–1953. [Google Scholar] [CrossRef]
- Lu, Y.P.; Chen, Y.M.; Li, S.T.; Wang, J.H. Surface nanocrystallization of hydroxyapatite coating. Acta Biomater. 2008, 4, 1865–1872. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Thermal treatment optimization of electrodeposited hydroxyapatite coatings on Ti6Al4V substrate. Adv. Eng. Mater. 2012, 14, 377–382. [Google Scholar] [CrossRef]
- Azis, S.A.A.; Kennedy, J.; Cao, P. Effect of annealing on microstructure of hydroxyapatite coatings and their behaviours in simulated body fluid. Adv. Mater. Res. 2014, 922, 657–662. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Agrawal, C.M.; Ong, J.L. Influence of post-deposition heating time and the presence of water vapor on sputter-coated calcium phosphate crystallinity. J. Dent. Res. 2003, 82, 833–837. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Kim, K.H.; Agrawal, C.M.; Ong, J.L. Effect of post-deposition heating temperature and the presence of water vapor during heat treatment on crystallinity of calcium phosphate coatings. Biomaterials 2003, 24, 5131–5137. [Google Scholar] [CrossRef]
- Dinda, G.P.; Shin, J.; Mazumder, J. Pulsed laser deposition of hydroxyapatite thin films on Ti-6Al-4V: Effect of heat treatment on structure and properties. Acta Biomaterialia 2009, 5, 1821–1830. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Chen, J.; Zeng, S.; De Groot, K. High temperature characteristics of synthetic hydroxyapatite. J. Mater. Sci.: Mater. In Med. 1993, 4, 83–85. [Google Scholar] [CrossRef]
- Stan, G.E.; Pasuk, I.; Husanu, M.A.; Enculescu, I.; Pina, S.; Lemos, A.F.; Tulyaganov, D.U.; Mabrouk, K.E.L.; Ferreira, J.M.F. Highly adherent bioactive glass thin films synthesized by magnetron sputtering at low temperature. J. Mater. Sci.-Mater. Med. 2011, 22, 2693–2710. [Google Scholar] [CrossRef]
- DFD® INSTRUMENTS. Available online: www.dfdinstruments.com (accessed on 29 April 2019).
- Bao, Q.; Chen, C.; Wang, D.; Ji, Q.; Lei, T. Pulsed laser deposition and its current research status in preparing hydroxyapatite thin films. Appl. Surf. Sci. 2005, 252, 1538–1544. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: a review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Stan, G.E. Adherent functional graded hydroxylapatite coatings produced by sputtering deposition techniques. J. Optolelectron. Adv. Mater. 2009, 11, 1132–1138. [Google Scholar]
- Stan, G.E.; Morosanu, C.O.; Marcov, D.A.; Pasuk, I.; Miculescu, F.; Reumont, G. Effect of annealing upon the structure and adhesion properties of sputtered bio-glass/titanium coatings. Appl. Surf. Sci. 2009, 255, 9132–9138. [Google Scholar] [CrossRef]
- Stan, G.E.; Popa, A.C.; Galca, A.C.; Aldica, G.; Ferreira, J.M.F. Strong bonding between sputtered bioglass–ceramic films and Ti-substrate implants induced by atomic inter-diffusion post-deposition heat-treatments. Appl. Surf. Sci. 2013, 280, 530–538. [Google Scholar] [CrossRef]
- ISO 13779-2. Implants for Surgery—Hydroxyapatite—Part 2: Coatings of Hydroxyapatite; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- ISO 10993-5: 2009. Biological Evaluation of Medical Devices—Part 5: Tests for in vitro cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Cleare, A.; Pariante, C.M.; Young, A.H.; Anderson, I.M.; Christmas, D.; Cowen, P.J.; Dickens, C.; Ferrier, I.N.; Geddes, J.; Gilbody, S.; et al. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. J. Psychopharmacol. 2015, 29, 459–525. [Google Scholar] [CrossRef]
- Cohen, O.; Rais, T.; Lepkifker, E.; Vered, I. Lithium carbonate therapy is not a risk factor for osteoporosis. Horm. Metab. Res. 1998, 30, 594–597. [Google Scholar] [CrossRef]
- Nordenstrom, J.; Elvius, M.; Bagedahl-Strindlund, M.; Zhao, B.; Torring, O. Biochemical hyperparathyroidism and bone-mineral status in patients treated long-term with lithium. Metabolism 1994, 43, 1563–1567. [Google Scholar] [CrossRef]
- Satija, N.K.; Sharma, D.; Afrin, F.; Tripathi, R.P.; Gangenahalli, G. High throughput transcriptome profiling of lithium stimulated human mesenchymal stem cells reveals priming towards osteoblastic lineage. PLoS ONE 2013, 8, e55769. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Li, J.J.; So, K.F.; Chen, J.Y.H.; Cheng, W.S.; Wu, J.; Wang, Z.M.; Gao, F.; Young, W. Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: A double-blind, randomized, placebo-controlled clinical trial. Spinal Cord 2012, 50, 141–146. [Google Scholar] [CrossRef]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Stegmayr, B.; Salander, R.E.; Werneke, U. Lithium intoxication: Incidence, clinical course and renal function—A population-based retrospective cohort study. J. Psychopharmacol. 2016, 30, 1008–1019. [Google Scholar] [CrossRef]
- Rybakowski, J.K. Challenging the negative perception of lithium and optimizing its long-term administration. Front. Mol. Neurosci. 2018, 11, 349. [Google Scholar] [CrossRef]
- Post, R.M. The new news about lithium: An underutilized treatment in the United States. Neuropsychopharmacology 2018, 43, 1174–1179. [Google Scholar] [CrossRef]
- Kandori, K.; Kuroda, T.; Wakamura, M. Protein adsorption behaviors onto photocatalytic Ti(IV)-doped calcium hydroxyapatite particles. Colloid Surf. B Biointerfaces 2011, 87, 472–479. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Cui, J.; Wei, Z. Interaction between low molecular weight organic acids and hydroxyapatite with different degrees of crystallinity. Colloid Surf. A Physicochem. Eng. Aspect 2011, 392, 67–75. [Google Scholar] [CrossRef]
- Pesakova, V.; Kubies, D.; Hulejova, H.; Himmlova, L. The influence of implant surface properties on cell adhesion and proliferation. J. Mater. Sci.-Mater. Med. 2007, 18, 465–473. [Google Scholar] [CrossRef]
- Ng, C.H.; Rao, N.; Law, W.C.; Xu, G.; Cheung, T.L.; Cheng, F.T.; Wang, X.; Man, H.C. Enhancing the cell proliferation performance of NiTi substrate by laser diffusion nitriding. Surf. Coat. Technol. 2017, 309, 59–66. [Google Scholar] [CrossRef]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef]
- Dos Santos, E.A.; Farina, M.; Soares, G.A.; Anselme, K. Surface energy of hydroxyapatite and beta-tricalcium phosphate ceramics driving serum protein adsorption and osteoblast adhesion. J. Mater. Sci.: Mater. Med. 2008, 19, 2307–2316. [Google Scholar] [CrossRef]
- Redey, S.A.; Razzouk, S.; Rey, C.; Bernache-Assollant, D.; Leroy, G.; Nardin, M.; Cournot, G. Osteoclast adhesion and activity on synthetic hydroxyapatite, carbonated hydroxyapatite, and natural calcium carbonate: Relationship to surface energies. J. Biomed. Mater. Res. 1999, 45, 140–147. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Vidic, J.; Stankic, S.; Haque, F.; Ciric, D.; Goffic, R.L.; Vidy, A.; Jupille, J.; Delmas, B. Selective antibacterial effects of mixed ZnMgO nanoparticles. J. Nanoparticle Res. 2013, 15, 1595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.; Zhai, Z.; Liu, L.; Li, H.; Yang, K.; Tan, L.; Wan, P.; Liu, X.; Ouyang, Z.; et al. Antibacterial Properties of Magnesium In Vitro and in an In Vivo Model of Implant-Associated Methicillin-Resistant Staphylococcus aureus Infection. Antimicrob Agents Chemother. 2014, 58, 7586–7591. [Google Scholar] [CrossRef]
- Sawai, J.; Kojima, H.; Igarashi, H.; Hashimoto, A.; Shoji, S.; Sawaki, T.; Hakoda, A.; Kawada, E.; Kokugan, T.; Shimizu, M. Antibacterial characteristics of magnesium oxide powder. World J. Microbiol. Biotechnol. 2000, 16, 187. [Google Scholar] [CrossRef]
- Gayathri, B.; Muthukumarasamy, N.; Velauthapillai, D.; Santhosh, S.B.; asokan, V. Magnesium incorporated hydroxyapatite nanoparticles: Preparation, characterization, antibacterial and larvicidal activity. Arab. J. Chem. 2018, 11, 645–654. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Matter. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica (Cairo) 2017, 2017, 4179326. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.; Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A. A critical review of decades of research on calcium phosphate–based coatings: How far are we from their widespread clinical application? Curr. Opin. Biomed. Eng. 2019, 10, 35–44. [Google Scholar] [CrossRef]
- Chen, L.; Komasa, S.; Hashimoto, Y.; Hontsu, S.; Okazaki, J. In Vitro and In Vivo Osteogenic Activity of Titanium Implants Coated by Pulsed Laser Deposition with a Thin Film of Fluoridated Hydroxyapatite. Int. J. Mol. Sci. 2018, 19, 1127. [Google Scholar] [CrossRef]
- Mróz, W.; Budner, B.; Syroka, R.; Niedzielski, K.; Golański, G.; Slósarczyk, A.; Schwarze, D.; Douglas, T.E. In vivo implantation of porous titanium alloy implants coated with magnesium-doped octacalcium phosphate and hydroxyapatite thin films using pulsed laser deposition. J. Biomed. Mater. Res. Part B 2015, 103, 151–158. [Google Scholar] [CrossRef]
- Peraire, C.; Arias, J.L.; Bernal, D.; Pou, J.; Leon, B.; Arano, A.; Roth, W. Biological stability and osteoconductivity in rabbit tibia of pulsed laser deposited hydroxylapatite coatings. J. Biomed. Mater. Res. Part A 2006, 77A, 370–379. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Lamolle, S.; Socol, G.; Miroiu, F.; Roenold, H.J.; Bigi, A.; Mayer, I.; Cuisinier, F.; Lyngstadaas, S.P. In vivo tensile tests of biomimetic titanium implants pulsed laser coated with nanostructured Calcium Phosphate thin films. Optoelectron. Adv. Mater.-Rapid Commun. 2008, 2, 337–341. [Google Scholar]
- Dostalova, T.; Himmlova, L.; Jelinek, M.; Grivas, C. Osseointegration of loaded dental implant with KrF laser hydroxylapatite films on Ti6Al4V alloy by minipigs. J. Biomed. Opt. 2001, 6, 239–243. [Google Scholar] [CrossRef]
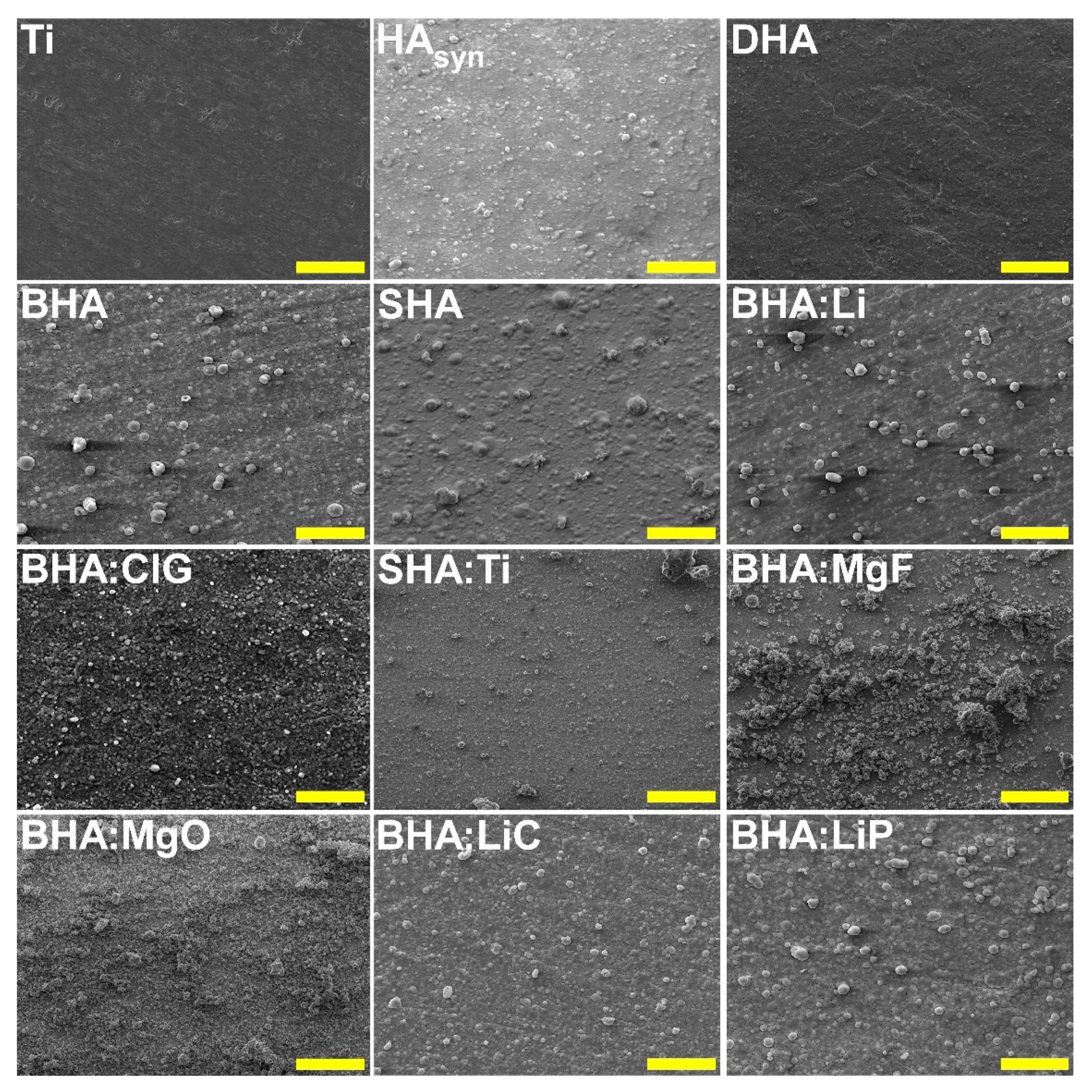
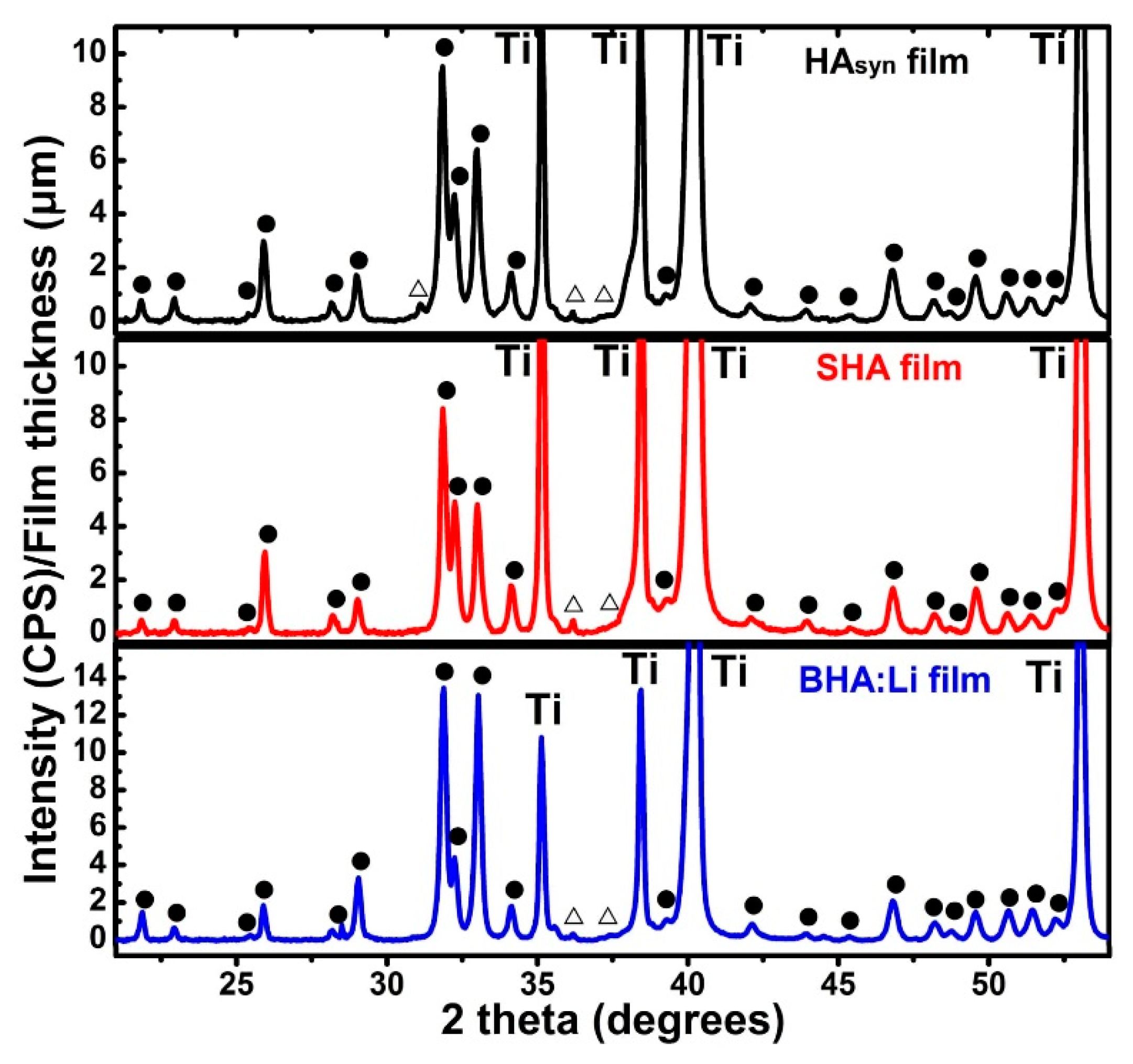

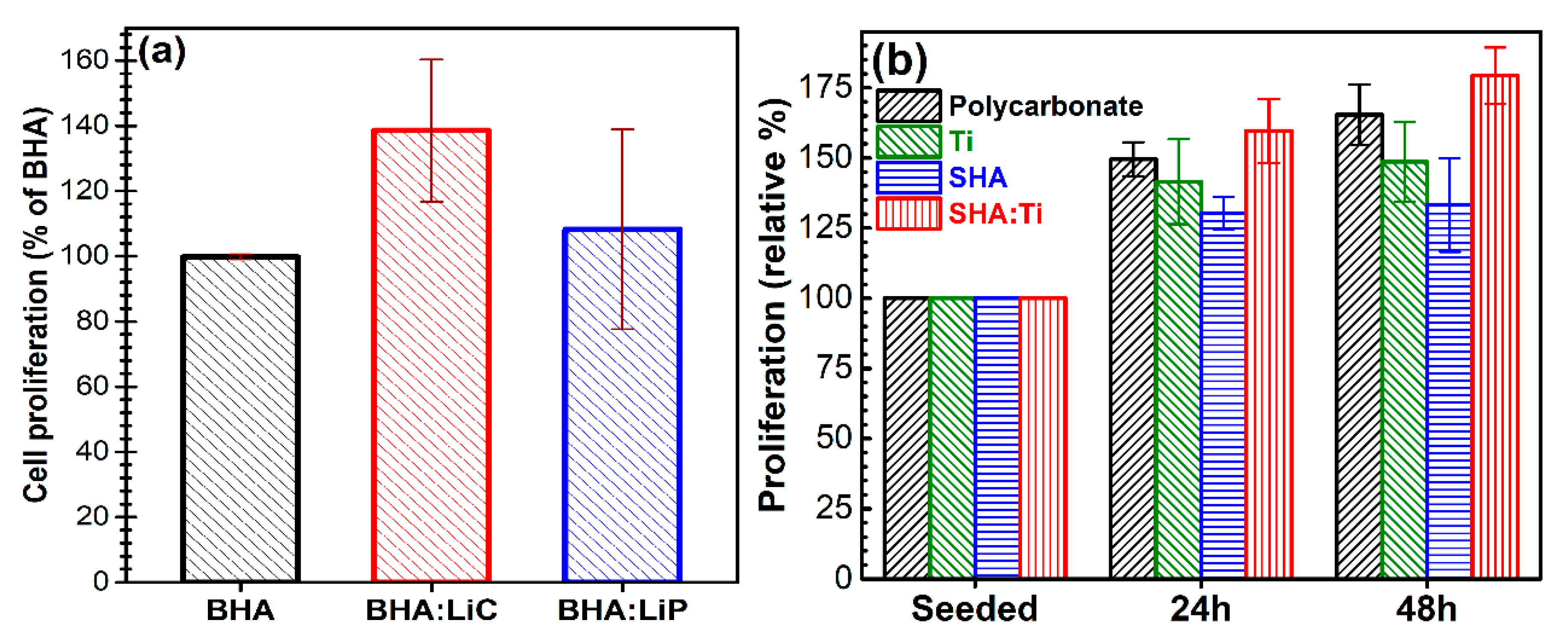
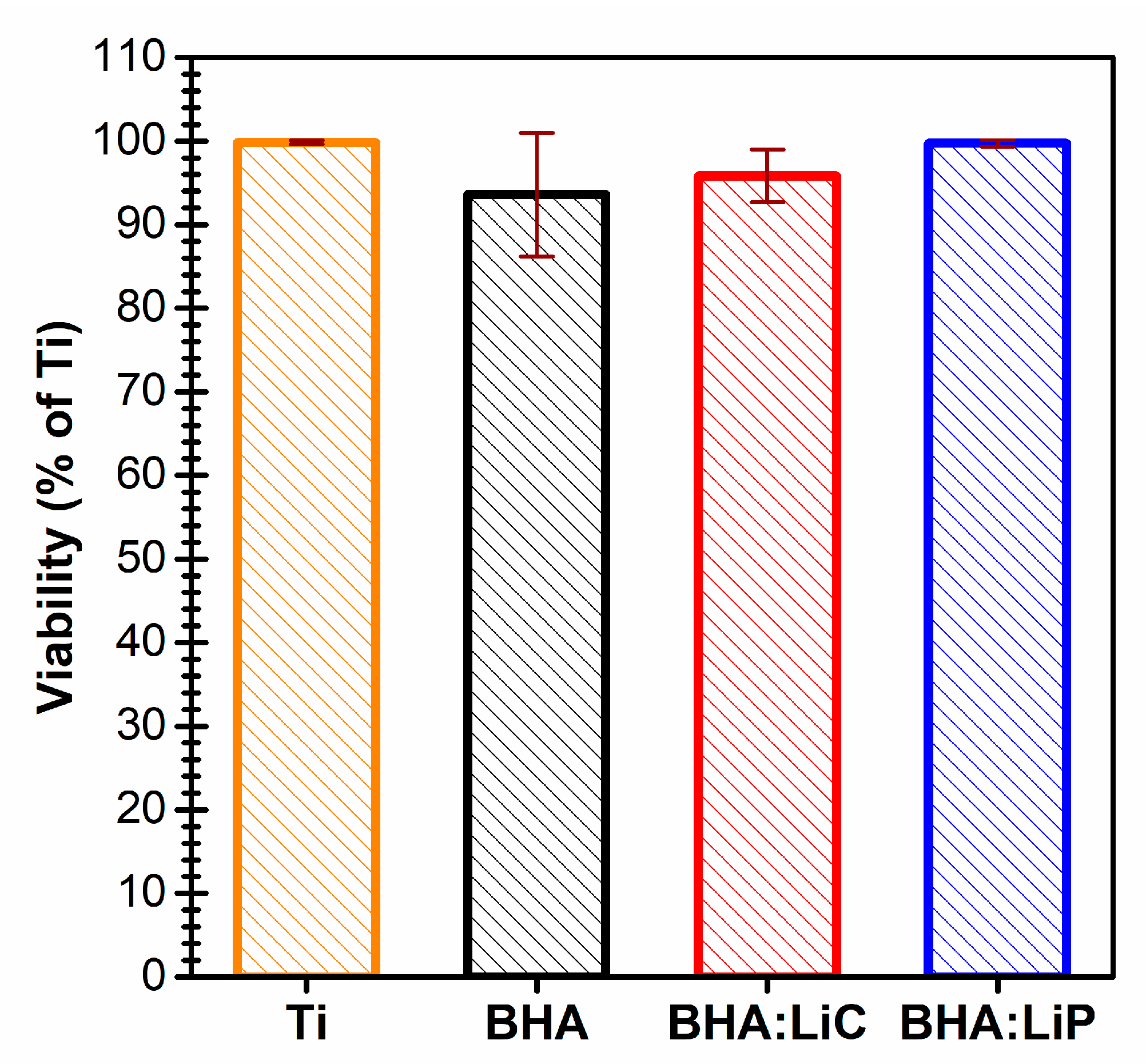
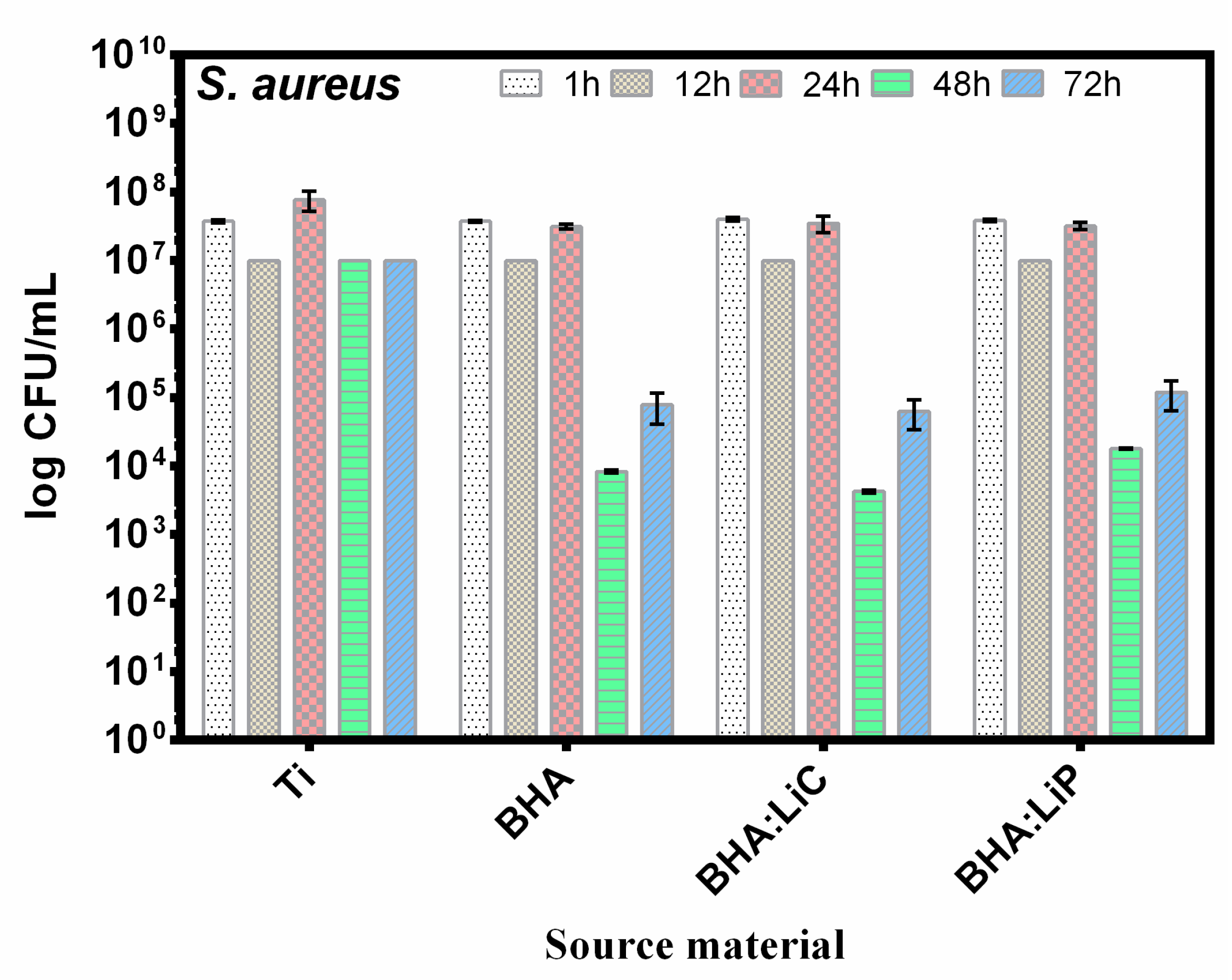


| Sample Code | Sample Description |
|---|---|
| Ti | Titanium (control specimen or deposition substrate) |
| HAsyn | Synthetic hydroxyapatite |
| DHA | Dentine hydroxyapatite |
| BHA | Bovine hydroxyapatite |
| SHA | Ovine (sheep) hydroxyapatite |
| BHA:Li | Bovine hydroxyapatite doped with Li2O |
| BHA:CIG | Bovine hydroxyapatite doped with commercial inert glass |
| SHA:Ti | Ovine (sheep) hydroxyapatite doped with titanium |
| BHA:MgF | Bovine hydroxyapatite doped with MgF2 |
| BHA:MgO | Bovine hydroxyapatite doped with MgO |
| BHA:LiC | Bovine hydroxyapatite doped with Li2CO3 |
| BHA:LiP | Bovine hydroxyapatite doped with Li3PO4 |
| Sample Material | D002 (nm) | D300 (nm) | D002/D300 | Reference |
|---|---|---|---|---|
| HAsyn | 59.2 | 47.4 | 1.25 | [53] |
| SHA | 50.1 | 48.5 | 1.03 | [25] |
| BHA | 152.8 | 103.8 | ~1.47 | [54] |
| SHA:Ti | 41.6 | 32.1 | 1.30 | [25] |
| BHA:Li | 83.5 | 56 | 1.49 | [53] |
| BHA:CIG | 48.9 | 23.4 | 2.1 | |
| BHA:MgF | 100.0 | 89.6 | ~1.11 | [54] |
| BHA:MgO | 169.7 | 141.2 | ~1.20 |
| Source Material | Pre-Treatment | Calcination | Dopants | Reference | |
|---|---|---|---|---|---|
| [°C] | Time [min] | ||||
| Bovine bones (femur) | Immersion in 2.6 wt.% sodium hypochlorite solution for 14 days | − | – | – | [68] |
| Bovine bones (femur) | Boiling in distilled water for 2.5 h, ultrasonication with acetone for 5 min, dried at 120 °C for 12 h in an oven | 350–900 | 180 | – | [69] |
| Bovine bones | Boiling in deionized water for 30 min, petroleum ether with constant agitation at 30 °C and sodium hydroxide solution, drying in a vacuum oven at 1.33 Pa and 70 °C for 5 h | 400–900 | 180 | – | [66] |
| Bovine bones (femur) | Boiling water followed by sun drying | 500–1400 | 120–240 | – | [70] |
| Bovine, caprine, and galline bones | Autoclave at 100 °C for 1 h, rinsing with water and drying for 3 h at 70 °C in a box oven | 600–1000 | 120 | – | [62] |
| Bovine bones | Applying direct flame from a gas torch to the cleaned bones | 600–1100 | 180 | – | [71] |
| Bovine bones | Boiling with water for 2 to 3 h, drying in an oven at 80 °C for 72 h | 600–1100 | 180 | – | [72] |
| Human, bovine, porcine bones | Boiling in distilled water for 30 min, immersion in ethanol, hydrogen peroxide, formaldehyde solution, drying in a vacuum oven at 50 °C for three days | 600–1200 | 1080 | – | [61] |
| Bovine bones | Immersion for 14 days in an alkali solution of 1% sodium hypochlorite | 700 | 240 | 1 wt.% Li2CO3, 1 wt.% Li3PO4 | [60] |
| Bovine bones | Washing by water and acetone, drying at 160 °C for 48 h | 750 | 360 | – | [30] |
| Bovine bones | Boiling in distilled water for 8 h, drying overnight at 200 °C | 800 | 180 | – | [73] |
| Bovine bones (femur) | Keeping in boiling distilled water for 2 h, heated at 60 °C for 24 h | 800 | 120 | – | [74] |
| Bovine bones | Boiling for 15 min, filtrating, washing with distilled water for several times and drying at 100 °C in a vacuum oven for 48 h | 800–1100 | 180 | – | [75] |
| Ovine and bovine bones | Immersion for 14 days in an alkali solution of 1% sodium hypochlorite | 850 | 240 | 1.5 wt.% Ti | [53] |
| Veal bones | Sodium hydroxide solution in a beaker, neutralization with distilled water, drying in an oven | 850 | 180 | – | [64] |
| Calf bones | Sodium hydroxide solution in a beaker, neutralization with distilled water, drying in an oven | 850 | 180 | – | [65] |
| Bovine bones (femur) | Cleaning and washing with distilled water, immersion for 14 days in an alkali solution of 1% sodium hypochlorite | 850 | 240 | 1 wt.% of Li2CO3 and Li3PO4 | [11] |
| Bovine bones | Washing by water and acetone, drying at 160 °C for 48 h | 850 | 60 | – | [76] |
| Bovine bones | Boiling and sun drying | 900 | 120 | – | [77] |
| Bovine bones | Boiling in distilled water for 3 h, drying in an oven at 100 °C for 24 h | 900 | 120 | – | [78] |
| Bovine bones (femur) | Immersion for 14 days in an alkali solution of 1% sodium hypochlorite | 1000 | 240 | 5 wt.% MgO, 2 wt.% MgF2 | [54] |
| Ovine dentine bones | Cleaning and washing, drying at 750 °C for 5–6 h | 1000 | 240 | 1.5 wt.% Ti | [25] |
| Camelus dromedarius bones (femur) | Boiling in distilled water for 1 h, washing with a strong water jet, drying at 100 °C, for 60 min, drying at RT for 7 days, immersion in acetone for 1 h, washing with distilled water | 1000 | 180 | – | [79] |
| Sheep teeth | Cleaning and washing, drying at 750 °C for 5 to 6 h | 1000–1300 | 240 | – | [63] |
| Human dentine, ovine, and bovine bones | Immersion for 14 days in an alkali solution of 1% sodium hypochlorite | 1100 | 240 | – | [80] |
| Bovine bones | Boiling in deionized water for 30 min, petroleum ether with constant agitation at 30 °C and sodium hydroxide solution, drying in a vacuum oven at 1.33 Pa and 70 °C for 5 h | 1200 | 120–240 | – | [67] |
| Bovine bones | Boiling in distilled water for 2 h, heating in an electrical furnace at 500 °C for 6 h | 1200 | 240 | – | [81] |
| Source Material | Pre-Treatment | Calcination | Reference | |
|---|---|---|---|---|
| [°C] | Time [min] | |||
| Cuttlefish bones | Heating at 200 °C for 6, 12, and 24 h and drying | 100–1200 | 60 | [84] |
| Sword fish and tuna bones | Boiling in water for 1 h and washing by a strong water jet, drying at RT in air for 24 h | 600–950 | 720 | [85] |
| Fish bone wastes | Incineration at 300 °C for 3 h | 750 | 300 | [88] |
| Fish scale | Hydrolyzation under 1% protease N for 2.5 h, and 0.5% flavourzyme for 0.5 h, stirring and heating in boiling water for 10 min, drying by hot air, and storing at –20 °C | 800 | 240 | [86] |
| Cod fish bones | Immersion in CaCl2·2H2O, Ca(C2H3O2)2 and NaF solutions, for different time intervals, stirring at 75 °C | 900–1200 | 60 | [87] |
| Tuna bones | Washing with hot water for two days, mixing with 1.0% sodium hydroxide and acetone, drying at 60 °C for 24 h | 900 | 300 | [83] |
| Shark tooth enameloid | Keeping in boiling water for 3 h, drying in a laboratory oven at 60 °C for 24 h | 950 | 720 | [89] |
| Dentine and enameloid of shark teeth and deer antlers | Boiling water for 3 h and drying in a laboratory oven at 60 °C for 24 h | 950 | 720 | [90,91,92] |
| Source Material | Pre-Treatment | Calcination | Reference | |
|---|---|---|---|---|
| [°C] | Time [min] | |||
| Shell of sea snail | Cleaning thoroughly from sand particles and other foreign materials, drying, solution of H3PO4, hot-plate stirring at 80 °C for 8 h, filtration, and drying at 100 °C overnight in an incubator. | 400–800 | 240 | [97] |
| Sputnik Sea urchin and sea snail shells | Heating on a hotplate at 80 °C for 15 min | 450–850 | 240 | [96] |
| Chicken eggshells | Cleaning with distilled water and keeping into 1 M H2O2 solution for a week, drying at 90 °C | 700 | 300 | [94] |
| Egg shell | Cleaning and washing with flowing distilled water, drying at 300 °C for 1 h | 850–900 | 180 | [95] |
| Eggshells | Cleaning in boiling water | 900 | 120 | [98] |
| Hen eggshell | Stripping the membrane off the eggshell, rinsing with water, drying | 1000 | 180 | [99] |
| HA Source Material/Used Substrate | Type of Laser Used | Target-to-Substrate Separation Distance [cm] | Energy [mJ] | Temperature during Deposition [°C] | Water Vapor Atmosphere [mbar] | Pulse Frequency [Hz] | Number of Applied Laser Pulses | Reference |
|---|---|---|---|---|---|---|---|---|
| Enameloid of shark teeth/Ti6Al4V disc | ArF* (193 nm) | – | 320 | 460 | 0.15–0.45 | 10 | – | [89] |
| Ovine and bovine bone/Ti disc and Si wafer | KrF* (248 nm) | 5 | 330 | 500 | 0.50 | 10 | 15,000 | [53] |
| Human dentine, ovine, and bovine bones/Ti disc and Si wafer | 350 | [80] | ||||||
| Bovine bone/Ti disc and Si wafer | [54] | |||||||
| Sheep dentine/Ti disc and Si wafer | 330 | [25] | ||||||
| Bovine bone/Ti disc and Si wafer | 360 | [60] | ||||||
| Bovine bone/Ti disc | [11] |
| Sample Material | Density (Particles/cm2) |
|---|---|
| SHA | (5.5 ± 0.8) × 107 |
| BHA:Li | (11.2 ± 0.7) × 107 |
| BHA:CIG | (13.3 ± 1.1) × 107 |
| HAsyn | (1.4 ± 0.5) × 107 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta, L.; Popescu, A.C. Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources. Coatings 2019, 9, 335. https://doi.org/10.3390/coatings9050335
Duta L, Popescu AC. Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources. Coatings. 2019; 9(5):335. https://doi.org/10.3390/coatings9050335
Chicago/Turabian StyleDuta, Liviu, and Andrei C. Popescu. 2019. "Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources" Coatings 9, no. 5: 335. https://doi.org/10.3390/coatings9050335
APA StyleDuta, L., & Popescu, A. C. (2019). Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources. Coatings, 9(5), 335. https://doi.org/10.3390/coatings9050335






