Antifungal Starch–Gellan Edible Coatings with Thyme Essential Oil for the Postharvest Preservation of Apple and Persimmon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of CFS
2.3. Rheological Behaviour and Contact Angle of the CFS
2.4. Quality of Coated Fruit
2.4.1. Surface Density of Solids (SDS)
2.4.2. Weight Loss
2.4.3. Respiration Rates
2.4.4. Fruit Firmness
2.5. In Vivo Antifungal Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. CFS Properties
3.2. Effect of the Incorporation of Tween 85 into CFS on Apple Quality
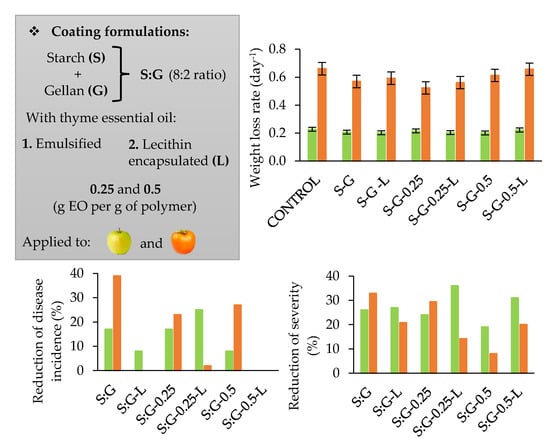
3.3. Effect of CFS on Postharvest Behaviour and Quality of Apples and Persimmons
3.4. Fungal Decay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, D.; Sharma, R.R. Postharvest diseases of fruits and vegetables and their management. In Postharvest Disinfection of Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: London, UK, 2018; pp. 1–52. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Troncoso-Rojas, R.; Tiznado-Hernández, M.E. Alternaria alternata (black rot, black spot). In Postharvest Decay: Control Strategies; Bautista-Baños, S., Ed.; Academic Press: London, UK, 2014; pp. 147–187. [Google Scholar] [CrossRef]
- Batta, Y.A. Postharvest biological control of apple gray mold by Trichoderma harzianum Rifai formulated in an invert emulsion. Crop Prot. 2004, 23, 19–26. [Google Scholar] [CrossRef]
- Palou, L.; Montesinos-Herrero, C.; Tarazona, I.; Besada, C.; Taberner, V. Incidence and etiology of postharvest fungal diseases of persimmon (Diospyros Kaki Thunb. Cv. Rojo Brillante) in Spain. Plant Dis. 2015, 99, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; He, J.; Liu, H.; Zhou, H. The phenylpropanoid pathway affects apple fruit resistance to Botrytis cinerea. J. Phytopathol. 2018, 166, 206–215. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E. Botrytis cinerea (gray mold). In Postharvest Decay: Control Strategies; Bautista-Baños, S., Ed.; Academic Press: London, UK, 2014; pp. 131–146. [Google Scholar] [CrossRef]
- Prusky, D.; Eshel, D.; Kobiler, I.; Yakoby, N.; Beno-Moualem, D.; Ackerman, M.; Zuthji, Y.; Ben Arie, R. Postharvest chlorine treatments for the control of the persimmon black spot disease caused by Alternaria alternata. Postharvest Biol. Technol. 2001, 22, 271–277. [Google Scholar] [CrossRef]
- Biton, E.; Kobiler, I.; Feygenberg, O.; Yaari, M.; Kaplunov, T.; Ackerman, M.; Friedman, H.; Prusky, D. The mechanism of differential susceptibility to alternaria black spot, caused by Alternaria alternata, of stem-and bottom-end tissues of persimmon fruit. Postharvest Biol. Technol. 2014, 94, 74–81. [Google Scholar] [CrossRef]
- Olivas, G.; Barbosa-Cánovas, G. Edible films and coatings for fruits and vegetables. In Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer: New York, NY, USA, 2009; pp. 211–244. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008, 43, 951–957. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Morice, I.M.; Shorland, F.B. Composition of the surface waxes of apple fruits and changes during storage. J. Sci. Food Agric. 1973, 24, 1331–1339. [Google Scholar] [CrossRef]
- Prusky, D.; Kobiler, I.; Akerman, M.; Miyara, I. Effect of acidic solutions and acidic prochloraz on the control of postharvest decay caused by Alternaria alternata in mango and persimmon fruit. Postharvest Biol. Technol. 2006, 42, 134–141. [Google Scholar] [CrossRef]
- Da Rocha Neto, A.C.; Navarro, B.B.; Canton, L.; Maraschin, M.; Di Piero, R.M. Antifungal activity of palmarosa (Cymbopogon martinii), tea tree (Melaleuca alternifolia) and star anise (Illicium verum) essential oils against Penicillium expansum and their mechanisms of action. LWT-Food Sci. Technol. 2019, 105, 385–392. [Google Scholar] [CrossRef]
- El Ouadi, Y.; Manssouri, M.; Bouyanzer, A.; Majidi, L.; Bendaif, H.; Elmsellem, H.; Shariati, M.A.; Melhaoui, A.; Hammouti, B. Essential oil composition and antifungal activity of Melissa officinalis originating from North-Est Morocco, against postharvest phytopathogenic fungi in apples. Microb. Pathog. 2017, 107, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Desjobert, J.M.; Cristofari, G.; Paolini, J.; Costa, J.; Majidi, L.; Znini, M. Essential oil composition and antifungal activity of Pulicaria mauritanica coss., against postharvest phytopathogenic fungi in apples. LWT-Food Sci. Technol. 2013, 54, 564–569. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Daniel, C.K.; Lennox, C.L.; Vries, F.A. In vivo application of garlic extracts in combination with clove oil to prevent postharvest decay caused by Botrytis cinerea, Penicillium expansum and Neofabraea alba on apples. Postharvest Biol. Technol. 2014, 99, 88–92. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; Lennox, C.L.; van Reenen, A.J.; Opara, U.L. In vitro and in vivo antifungal activity of chitosan-essential oils against pomegranate fruit pathogens. Postharvest Biol. Technol. 2017, 129, 9–22. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant-and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control. 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Plotto, A.; Roberts, D.D.; Roberts, R.G. Evaluation of plant essential oils as natural postharvest disease control of tomato (Lycopersicon esculentum). Acta Hortic. 2003, 628, 737–745. [Google Scholar] [CrossRef]
- Cano, A.; Fortunati, E.; Cháfer, M.; Kenny, J.M.; Chiralt, A.; González-Martínez, C. Properties and ageing behaviour of pea starch films as affected by blend with poly(vinyl alcohol). Food Hydrocoll. 2015, 48, 84–93. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Jiménez, M.; Jiménez, A.; Atarés, L.; Vargas, M.; Chiralt, A. Influence of liposome encapsulated essential oils on properties of chitosan films. Polym. Int. 2016, 65, 979–987. [Google Scholar] [CrossRef]
- Sapper, M.; Bonet, M.; Chiralt, A. Wettability of starch-gellan coatings on fruits, as affected by the incorporation of essential oil and/or surfactants. LWT-Food Sci. Technol. under review.
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; Hagenmaier, R.H. Alternatives to shellac coatings provide comparable gloss, internal gas modification, and quality for “delicious” apple fruit. HortScience 2002, 37, 559–563. [Google Scholar] [CrossRef]
- Marín, A.; Atarés, L.; Cháfer, M.; Chiralt, A. Properties of biopolymer dispersions and films used as carriers of the biocontrol agent Candida sake CPA-1. LWT-Food Sci. Technol. 2017, 79, 60–69. [Google Scholar] [CrossRef]
- Castelló, M.L.; Fito, P.J.; Chiralt, A. Changes in respiration rate and physical properties of strawberries due to osmotic dehydration and storage. J. Food Eng. 2010, 97, 64–71. [Google Scholar] [CrossRef]
- Saei, A.; Tustin, D.S.; Zamani, Z.; Talaie, A.; Hall, A.J. Cropping effects on the loss of apple fruit firmness during storage: the relationship between texture retention and fruit dry matter concentration. Sci. Hortic. 2011, 130, 256–265. [Google Scholar] [CrossRef]
- Belding, R.; Blankenship, S.; Young, E.; Leidy, R. Composition and variability of epicuticular waxes in apple cultivars. J. Am. Soc. Hortic. Sci. 1998, 123, 348–356. [Google Scholar] [CrossRef]
- Ju, Z.; Bramlage, W.J. Developmental changes of cuticular constituents and their association with ethylene during fruit ripening in “Delicious” apples. Postharvest Biol. Technol. 2001, 21, 257–263. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.B.; Marques, J.R.; Pristijono, P.; Chockchaisawasdee, S.; Scarlett, C.J.; Stathopoulos, C.E. Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of ‘Valencia’ oranges. Postharvest Biol. Technol. 2018, 137, 9–20. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Sothornvit, R.; Pérez-Gago, M.B. Effect of plasticizer type and amount on hydroxypropyl methylcellulose-beeswax edible film properties and postharvest quality of coated plums (cv. Angeleno). J. Agric. Food Chem. 2008, 56, 9502–9509. [Google Scholar] [CrossRef] [PubMed]
- Pastor, C.; Sanchez-Gonzalez, L.; Marcilla, A.; Chiralt, A.; Cháfer, M.; Gonzalez-Martinez, C. Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible coatings containing propolis extract. Postharvest Biol. Technol. 2011, 60, 64–70. [Google Scholar] [CrossRef]
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Pérez-Gago, M.B. Hydroxypropyl methylcellulose-beeswax edible coatings formulated with antifungal food additives to reduce Alternaria black spot and maintain postharvest quality of cold-stored cherry tomatoes. Sci. Hortic. (Amsterdam) 2015, 193, 249–257. [Google Scholar] [CrossRef]
- Fagundes, C.; Carciofi, B.A.M.; Monteiro, A.R. Estimate of respiration rate and physicochemical changes of fresh-cut apples stored under different temperatures. Food Sci. Technol. 2013, 33, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.; Vicente, A.A.; Teixeira, J.A.; Miranda, C. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol. Technol. 2007, 44, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Harker, F.R.; Feng, J.; Johnston, J.W.; Gamble, J.; Alavi, M.; Hall, M.; Chheang, S.L. Influence of postharvest water loss on apple quality: The use of a sensory panel to verify destructive and non-destructive instrumental measurements of texture. Postharvest Biol. Technol. 2019, 148, 32–37. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K.; Banerji, R.; Chansouria, J.P.N. Evaluation of some essential oils as botanical fungitoxicants in management of post-harvest rotting of citrus fruits. World J. Microbiol. Biotechnol. 2004, 20, 317–321. [Google Scholar] [CrossRef]
- Basiak, E.; Linke, M.; Debeaufort, F.; Lenart, A.; Geyer, M. Dynamic behaviour of starch-based coatings on fruit surfaces. Postharvest Biol. Technol. 2019, 147, 166–173. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; del Río, M.A.; Pérez-Gago, M.B. Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 872–900. [Google Scholar] [CrossRef] [PubMed]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involve in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Pérez, M.A.; Sanfuentes, E.A.; Figueroa, N.E.; Figueroa, C.R.; Rivas, B.L. Thermoplastic starch/clay nanocomposites loaded with essential oil constituents as packaging for strawberries—In vivo antimicrobial synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 2015, 99, 37–43. [Google Scholar] [CrossRef]
| CFS | Rheological Behavior | Contact Angle (θ) | |||
|---|---|---|---|---|---|
| n | K (mPa·s) n | η at 100 s−1 (mPa·s) | Apple | Persimmon | |
| S:G | 0.854 ± 0.001 d | 65.0 ± 0.2 a | 33.1 ± 0.1 c | 96 ± 2 e | 67 ± 3 cd |
| S:G-L | 0.74 ± 0.01 a | 114 ± 3 b | 35.0 ± 0.1 d | 85 ± 3 d | 72 ± 2 e |
| S:G-0.25 | 0.86 ± 0.01 d | 59 ± 9 a | 31 ± 3 b | 69 ± 3 a | 65 ± 3 c |
| S:G-0.25-L | 0.815 ± 0.001 c | 59.7 ± 0.5 a | 25.5 ± 0.3 a | 73 ± 2 b | 50 ± 6 a |
| S:G-0.5 | 0.766 ± 0.004 b | 124 ± 3 c | 42.2 ± 0.2 e | 77 ± 2 c | 68 ± 2 d |
| S:G-0.5-L | 0.809 ± 0.002 c | 60 ± 1 a | 25.05 ± 0.03 a | 74 ± 2 b | 55 ± 4 b |
| Control | Control | S:G | S:G-Tween 85 | |
|---|---|---|---|---|
| Initial Time | 7 days | |||
| Weight loss rate | – | 0.36 ± 0.02 a | 0.36 ± 0.01 a | 0.66 ± 0.06 b |
| Fmax | 43 ± 7 | 46 ± 6 a | 49 ± 8 a | 46 ± 4 a |
| dmax | 3.0 ± 0.3 | 3.6 ± 0.4 a | 3.9 ± 0.5 a | 4.6 ± 0.7 b |
| R O2 | 12.94 ± 0.05 | 12.9 ± 1.3 b | 11.4 ± 0.8 b | 7.77 ± 0.02 a |
| R CO2 | 13.9 ± 0.6 | 15.2 ± 0.7 b | 18 ± 1 c | 11.0 ± 0.2 a |
| RQ | 1.07 ± 0.05 | 1.18 ± 0.07 a | 1.58 ± 0.03 c | 1.41 ± 0.03 b |
| R O2 | R CO2 | RQ | Fmax | dmax | |
|---|---|---|---|---|---|
| Apple | 13.7 ± 1.5 | 12.2 ± 0.2 | 0.9 ± 0.1 | 29 ± 2 | 2.2 ± 0.1 |
| Persimmon | 5.7 ± 1.1 | 5.4 ± 0.9 | 0.94 ± 0.02 | 21.1 ± 0.5 | 2.5 ± 0.3 |
| Control | S:G | S:G-L | S:G-0.25 | S:G-0.25-L | S:G-0.5 | S:G-0.5-L | |
|---|---|---|---|---|---|---|---|
| Apple | |||||||
| SSD | – | 1.3 ± 0.3 c | 0.8 ± 0.1 a | 1.4 ± 0.3 c | 1.1 ± 0.1 b | 1.5 ± 0.2 c | 1.0 ± 0.1 b |
| Weight loss rate (14 d) | 0.23 ± 0.03 a | 0.21 ± 0.03 a | 0.20 ± 0.05 a | 0.21 ± 0.03 a | 0.20 ± 0.03 a | 0.20 ± 0.03 a | 0.22 ± 0.03 a |
| R O2 (7 d) | 6.0 ± 1.4 ab | 6.0 ± 1.5 ab | 6.5 ± 1.6 ab | 6.6 ± 0.8 ab | 6.5 ± 1.0 ab | 7.6 ± 0.6 b | 5.1 ± 0.9 a |
| R CO2 (7 d) | 6.5 ± 1.2 a | 7.8 ± 0.8 ab | 8.4 ± 2.0 ab | 8.8 ± 0.1 ab | 8.3 ± 0.7 ab | 9.7 ± 1.0 b | 6.8 ± 0.9 a |
| RQ (7 d) | 1.1 ± 0.1 a | 1.3 ± 0.1 b | 1.29 ± 0.04 b | 1.4 ± 0.1 b | 1.3 ± 0.1 b | 1.3 ± 0.1 b | 1.3 ± 0.1 b |
| R O2 (14 d) | 5.3 ± 0.8 a | 8.4 ± 1.1 d | 7.3 ± 0.5 cd | 7.0 ± 0.1 bcd | 5.9 ± 0.8 ab | 6.5 ± 0.9 abc | 5.6 ± 0.9 ab |
| R CO2 (14 d) | 6.2 ± 1.3 a | 10.4 ± 1.1 d | 9.0 ± 0.9 cd | 8.1 ± 0.2 bc | 7.2 ± 0.6 ab | 7.9 ± 0.6 bc | 7.2 ± 0.6 ab |
| RQ (14 d) | 1.2 ± 0.1 a | 1.2 ± 0.1 a | 1.23 ± 0.05 a | 1.2 ± 0.1 a | 1.2 ± 0.1 a | 1.2 ± 0.1 a | 1.3 ± 0.1 a |
| Fmax (14 d) | 27 ± 2 ab | 31 ± 3 c | 30 ± 4 bc | 29 ± 3 abc | 30.4 ± 1.5 bc | 32 ± 2 c | 26.1 ± 1.2 a |
| dmax (14 d) | 3 ± 1 a | 3 ± 1 a | 3 ± 1 a | 3 ± 0 a | 3 ± 1 a | 3 ± 1 a | 3.4 ± 0.5 a |
| Persimmon | |||||||
| SSD | – | 0.5 ± 0.1 a | 0.7 ± 0.1 ab | 0.7 ± 0.2 b | 0.8 ± 0.1 bc | 0.9 ± 0.1 c | 0.7 ± 0.2 b |
| Weight loss rate (14 d) | 0.7 ± 0.1 b | 0.6 ± 0.1 ab | 0.6 ± 0.1 ab | 0.52 ± 0.03 a | 0.56 ± 0.06 ab | 0.6 ± 0.1 ab | 0.7 ± 0.1 b |
| R O2 (7 d) | 5.2 ± 0.3 a | 3.3 ± 1.6 a | 3.8 ± 0.6 a | 2.9 ± 0.2 a | 3.9 ± 2.1 a | 3.6 ± 0.3 a | 4.1 ± 1.7 a |
| R CO2 (7 d) | 5.6 ± 0.5 a | 3.8 ± 1.0 a | 4.2 ± 0.9 a | 3.4 ± 0.4 a | 4.5 ± 2.0 a | 4.6 ± 0.7 a | 4.8 ± 2.1 a |
| RQ (7 d) | 1.08 ± 0.04 a | 1.2 ± 0.3 a | 1.11 ± 0.05 a | 1.19 ± 0.03 a | 1.3 ± 0.3 a | 1.3 ± 0.1 a | 1.2 ± 0.1 a |
| R O2 (14 d) | 3.7 ± 0.6 a | 4.2 ± 1.8 a | 3.0 ± 1.1 a | 3.3 ± 0.4 a | 2.2 ± 0.2 a | 2.6 ± 0.6 a | 3.5 ± 1.9 a |
| R CO2 (14 d) | 3.9 ± 1.1 a | 5.3 ± 2.6 a | 4.1 ± 1.0 a | 3.9 ± 1.8 a | 2.2 ± 0.2 a | 2.9 ± 0.4 a | 4.2 ± 1.3 a |
| RQ (14 d) | 1.0 ± 0.1 a | 1.3 ± 0.1 a | 1.4 ± 0.2 a | 1.2 ± 0.4 a | 1.0 ± 0.0 a | 1.1 ± 0.1 a | 1.3 ± 0.3 a |
| Fmax (14 d) | 21 ± 4 a | 27 ± 3 bc | 23 ± 4 ab | 27 ± 5 bc | 28 ± 6 c | 29 ± 6 c | 28 ± 6 c |
| dmax (14 d) | 5 ± 1 ab | 6 ± 1 bc | 5 ± 1a | 5 ± 1 a | 5.2 ± 0.4 abc | 6 ± 1 c | 6 ± 1 bc |
| Disease Incidence (%) | Reduction of Incidence (%) | Disease Severity (mm) | Reduction of Severity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 7 Days | 12 Days | 7 Days | 12 Days | 7 Days | 12 Days | 7 Days | 12 Days | |
| Apple gray mold | ||||||||
| Control | 100 ± 0 a | 100 ± 0 a | – | – | 70 ± 5 b | 100 ± 5 b | – | – |
| S:G | 75 ± 25 a | 83 ± 14 a | 25 | 17 | 44 ± 5 a | 74 ± 13 a | 32 | 26 |
| S:G-L | 92 ± 14 a | 92 ± 14 a | 8 | 8 | 47 ± 11 a | 73 ± 21 a | 27 | 27 |
| S:G-0.25 | 75 ± 25 a | 83 ± 14 a | 25 | 17 | 53 ± 8 ab | 76 ± 15 a | 19 | 24 |
| S:G-0.25-L | 75 ± 25 a | 75 ± 25 a | 25 | 25 | 44 ± 11 a | 64 ± 17 a | 33 | 36 |
| S:G-0.5 | 92 ± 14 a | 92 ± 14 a | 8 | 8 | 45 ± 10 a | 81 ± 7 ab | 32 | 19 |
| S:G-0.5-L | 100 ± 0 a | 100 ± 0 a | 0 | 0 | 47 ± 1 a | 69 ± 10 a | 29 | 31 |
| Persimmon black spot | ||||||||
| Control | 68 ± 3 b | 73 ± 5 bc | – | – | 10.6 ± 0.8 a | 21.9 ± 1.9 b | – | – |
| S:G | 38 ± 9 a | 45 ± 12 a | 44 | 39 | 9.3 ± 1.5 a | 15.0 ± 3.0 a | 12 | 32.9 |
| S:G-L | 70 ± 10 b | 78 ± 7 bc | 0 | 0 | 9.7 ± 1.2 a | 17.0 ± 3.0 a | 9 | 20.8 |
| S:G-0.25 | 42 ± 7 a | 57 ± 10 ab | 39 | 23 | 10.8 ± 1.1 a | 15.5 ± 1.4 a | 0 | 29.4 |
| S:G-0.25-L | 58 ± 8 ab | 72 ± 6 bc | 14 | 2 | 12.7 ± 0.6 a | 18.8 ± 1.9 ab | 0 | 14.2 |
| S:G-0.5 | 42 ± 7 a | 53 ± 7 ab | 39 | 27 | 11.4 ± 0.3 a | 20.2 ± 0.5 ab | 0 | 8 |
| S:G-0.5-L | 72 ± 7 b | 82 ± 8 c | 0 | 0 | 10.4 ± 0.8 a | 17.6 ± 0.6 a | 2 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapper, M.; Palou, L.; Pérez-Gago, M.B.; Chiralt, A. Antifungal Starch–Gellan Edible Coatings with Thyme Essential Oil for the Postharvest Preservation of Apple and Persimmon. Coatings 2019, 9, 333. https://doi.org/10.3390/coatings9050333
Sapper M, Palou L, Pérez-Gago MB, Chiralt A. Antifungal Starch–Gellan Edible Coatings with Thyme Essential Oil for the Postharvest Preservation of Apple and Persimmon. Coatings. 2019; 9(5):333. https://doi.org/10.3390/coatings9050333
Chicago/Turabian StyleSapper, Mayra, Lluís Palou, María B. Pérez-Gago, and Amparo Chiralt. 2019. "Antifungal Starch–Gellan Edible Coatings with Thyme Essential Oil for the Postharvest Preservation of Apple and Persimmon" Coatings 9, no. 5: 333. https://doi.org/10.3390/coatings9050333
APA StyleSapper, M., Palou, L., Pérez-Gago, M. B., & Chiralt, A. (2019). Antifungal Starch–Gellan Edible Coatings with Thyme Essential Oil for the Postharvest Preservation of Apple and Persimmon. Coatings, 9(5), 333. https://doi.org/10.3390/coatings9050333