1. Introduction
Transparent conductive oxides (TCO) are materials that have attracted a significant amount of interests due to their vast application areas. They are an essential part of various optoelectronic devices, solar cell modules, light emitting diodes, and flexible electronics that involve optical elements and the physical vapor deposition (PVD) viz. sputtering under vacuum is one of the methods of depositing a range of materials as a thin film coating, mobile phone touch screens, perovskite solar cells, and OLEDs are examples of technologies that rely on TCOs [
1]. When sputter deposition of complex materials, such as metal oxides belonging to the TCO family of materials, is carried out, one can imagine how vital the sputtering conditions are to the way that the atoms are deposited and laid on the substrate surface, which will ultimately define the work function, electron affinity, band-gap, and all of the relevant functional parameters of the as-deposited material. However, plasma sputter deposition technique does not behave like a standard chemical reaction or a process with defined steps. For example, researchers who apply this technique for TCO preparation usually report their findings by stating the condition of the sputtering process, such as chamber pressure, plasma power and the gas composition of the chamber during deposition. However, from one sputter machine to another, depending on the dimensions of the chamber, the size of the targets, and a few other design related issues, TCO coatings with different properties are obtained, even if the sputtering conditions are maintained according to an earlier report. The authors have constantly experienced this ambiguity. We believe there is another way of defining the sputtering conditions. Surely, the plasma in the sputtering procedure is the core of the reaction and is fundamental to the deposition of the thin film coatings. By evaluating the plasma, we can define a set standard for deposition procedures, regardless of the machine being used. We explore this concept by considering the sputtering target surface and the plasma as a light source. However, characterising plasma through optical spectroscopy is nothing new. Currently, plasma physicists study and investigate plasma by means of Emission spectroscopic techniques [
2,
3,
4,
5,
6,
7,
8]. The diagnosis of laboratory plasma is usually carried out by optical emission spectroscopy (OES), through which numerous analytical techniques are established to determine the plasma properties, such as electron density, plasma temperature, elements recognition, and quantification of elements that are present in the plasma.
The radiative behavior of the atomic constituents of any plasma can only be predicted if the expected populations of possible states are known and the atoms obey the Boltzmann distribution for every possible state and radiation energy density. Subsequently, it can be assumed that a thermal equilibrium condition is present [
4,
5,
6,
7,
8,
9]. However, creating such a condition in the laboratory would be almost impossible, and hence the concept of local thermal equilibrium (LTE) condition is considered. LTE can be described as a state where the Boltzmann and Saha equation, which governs the distribution of energy level excitation and ionization temperature, are equal to the Maxwell–Boltzmann distribution of free electron velocities [
8,
9,
10]. Achieving the LTE condition depends on defining the plasma by a common temperature
T and the existence of sufficient large electron density. McWhirter proposed a criterion where there is a need for a critical amount of electron density for LTE conditions to exist. This criterion is demonstrated by Equation (1), where
Ne is the electron density,
T is the plasma temperature, and
∆E is the energy gap [
11,
12,
13,
14].
The temperatures of ions and electrons are directly proportional to their random average kinetic energy, and the distribution of velocities for each particle is governed by the Maxwell distribution when thermal equilibrium conditions apply. Under the LTE conditions, the same temperature is assumed for electrons, ions, and atoms in the plasma and the plasma temperature is referred to as the temperature of the electron [
4]. Various methods of plasma temperature determination exist, however, the simplest method by far is the Ratio method, which utilises the intensity of two spectral lines for calculating the temperature of the plasma. The intensity of a spectral line associated with a transition is expressed by Equation (2). In this equation,
Iij is the intensity of transition from state
i to state
j,
Aij is the transition probability between the two states,
c is the speed of light,
N is the number density of electrons,
h is the plank constant,
gj is the statistical weight of the upper level (
j) and
λij is the wavelength corresponding to the transition,
Ej is the energy of level (
j),
T is the excitation temperature and k is the Boltzmann constant, and the
U(T) is referred to as the partition coefficient, which accounts for the probability and degeneracy of the two states (
i and j).
From the spectral data obtained, two spectral lines can be chosen from the same species and their ionization stages, e.g., Ar
I, where there is a large difference in the upper energy level. By taking a ratio (
I1)/(
I2) of the intensities of the two selected lines, the constants cancel each other out and we will be left with Equation (3), where
I1 and
I2 are the intensity of comparative peaks due to their respective
ij transition [
15].
Solving Equation (3) will give the value of T in electron volts. This method is the simplest method of calculating the temperature of the plasma, and its accuracy is conditional upon using two lines with a maximum difference in their upper energy states.
As an alternative to the ratio method discussed, a method that is referred to as the Boltzmann method can be applied to calculate the temperature of the electron. This method also utilises Equation (2); however, with a rearrangement. The equation is rearranged to give Equation (4). Taking the natural logarithm of both sides of Equation (4) would give us Equation (5) [
4,
12,
13,
14,
15].
By plotting the first term of Equation (5) against Ej, the slope of the plot can give us the value of 1/kT, from which value of T can be extracted.
The electron density can be calculated by using various methods, for example, by applying the Stark broadening relationships. The Stark broadening is caused by the electric field of electrons and ions interfering, which results in the broadening of the spectral line. The interference of the mentioned electric fields causes fluctuations in the field of plasma as the radiating atoms are surrounded by the interfering electrons and ions. The electric field of the electrons or ions causes a perturbation of energy levels that are close to the continuum, while simultaneously affecting the externally applied electric field, which ultimately causes the observed spectral broadening. The magnitude of this broadening in terms of its full width half maximum (FWHM) ∆λ
1/2 is given by Equation (6).
In this equation, ω is the electron impact parameter,
A is ionic impact broadening parameter,
Ne is the electron density, and
ND is the Debye shielding parameter. The first term in this equation is associated with broadening that is caused by electrons and the second term is associated with the broadening that is caused by ions [
16]. As the emissions from argon species is considered for the measurements, we can hence eliminate the second term of the equation that relates to the other ions to obtain Equation (7) [
15,
16,
17]. Hence, rearranging the equation and obtaining the ∆λ
1/2 value can calculate the
Ne.
All of the methods that we have mentioned so far are some of the OES methods of measuring the electron density and electron temperature in a plasma. These methods are familiar among plasma physicists; however, when it comes to the application of sputtering plasma and thin film analysis, using the above-mentioned techniques will require the necessary expertise and precision spectroscopy and data interpretation. Many scientists or operators who use plasma deposition for the preparation of thin film coatings would ultimately require a machine that would be easy to use and produce consistent thin film coatings. Our objective is to simplify the operation of the sputtering technique for the users and possibly propose a simpler monitoring diagnostic methodology for the plasma deposition process.
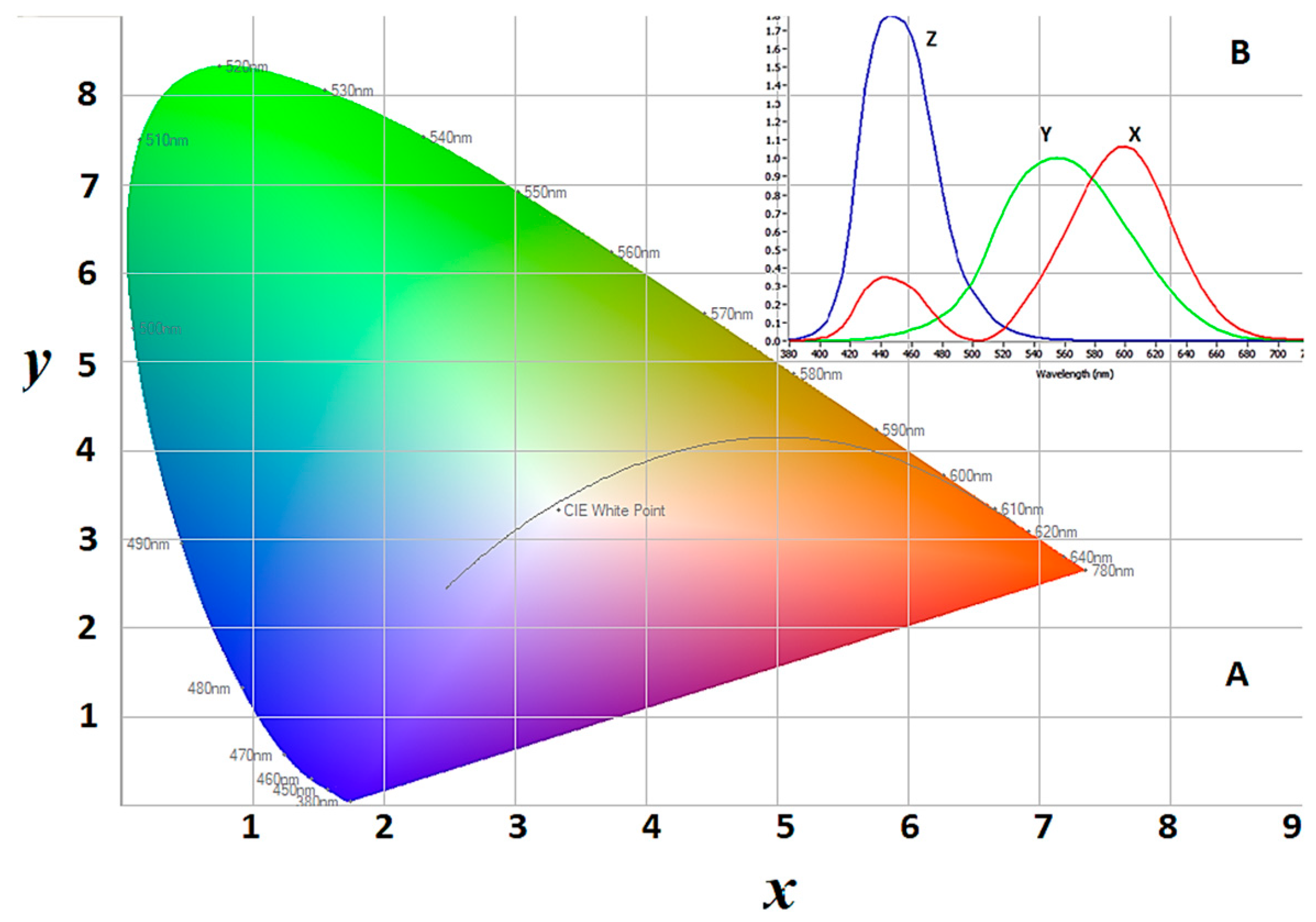
We are reporting a novel strategy of the in-situ characterization of plasma in a sputtering process by translating the emission spectra into a colour indexing (chromaticity) pattern that is able to quickly monitor any changes in the plasma character with a change in the operating parameter conditions during the course of the process, which in turn provides a better handle to reflect the instabilities of the tool and its optimizing parameters. This is a radical conceptual proposition that can potentially simplify the evaluation of the sputtering plasma for applied scientists by theorising a unique characterisation parameter for particular plasma deposition conditions, just like a fingerprint. As an analogy, to clarify our objective, we can divert our attention to the human genome that defines each individual person based on the composition and arrangement of billions of complex genetic coding embedded within each person’s DNA, complex laboratory procedures can be used to sequence and encode the genetic makeup of individuals; however, a simpler approach to distinguish people from one another is to check their fingerprint or iris patterns, rather than conducting the complex genetic complexity that defines the individual. Indeed, that is the objective of this communication. By considering the plasma as a light source, we will focus on its most apparent character; its colour. We will be focusing on the colour of the sputtering plasma light and identifying patterns that may relate its colour to certain fundamental parameters that are involved in generating the plasma, such as the driving power, the working gas, and its pressure. Plans are underway to further investigate the relationship between the thin film coatings that are deposited via this technique and the plasma colour with reports in due time. The colour of any light source can be mathematically described through what is known as colour spaces. A colour space is a completely-specified scheme for describing the colour of light, ordinarily using three numerical values (called coordinates). An important colour space, defined by the International Commission on Illumination (CIE, the initials of its French name), is the CIE XYZ colour space [
18]. However, it is wholly defined in terms of human’s perception of light through their eyes. If two instances of light appear to a viewer to be the same colour, they are the same colour. Colour is usually recognized by the viewer as having two aspects:
Luminance, as an indication of the “brightness” of the light.
Chromaticity, the property that distinguishes colours.
Colour is not a primary physical property, like the temperature or pressure of a gas. Colour is related to the energy of photons that make up the light. Plotting the intensity of the photon energies (power) that are associated with the relevant wavelength will produce a spectral plot. The intensity and distribution of the emitted wavelengths that ultimately shape the plot determine the chromaticity of the light; its overall “vertical scale” determines its luminance [
18]. In fact, there can be several instances of light with different spectrums, which nevertheless have the same colour. This situation is called metamerism. Through mathematics, the
XYZ colour spaces are used to construct the
x,
y colour function, which is presented in
Figure 1A, where the specified area under the defined emission spectrums are used to calculate the coordinates in
Figure 1B.
A luminance-chromaticity space (
Figure 1B) is then constructed by defining two values;
x and
y, as follows:
In these equations, as described in
Figure 1B, the
X,
Y, and
Z represent the area under emission peaks in the regions. By plotting the
x against
y, the CIE
x, y chromaticity diagram is constructed and is used to give a specific sense to a particular colour (
Figure 1B).
Another photometric parameter is the radiance and spectral radiance of the plasma light. The radiance indicates how much radiant flux is emitted by a surface when it is received by an optical system looking at that surface. The radiance value is calculated by measuring the Radiant flux of an emission source, which is the radiant energy emitted per unit time. Spectral radiance expresses radiance as a function of frequency or wavelength and the spectral plot of the emission is based on the radiance value as a magnitude of intensity [
18].
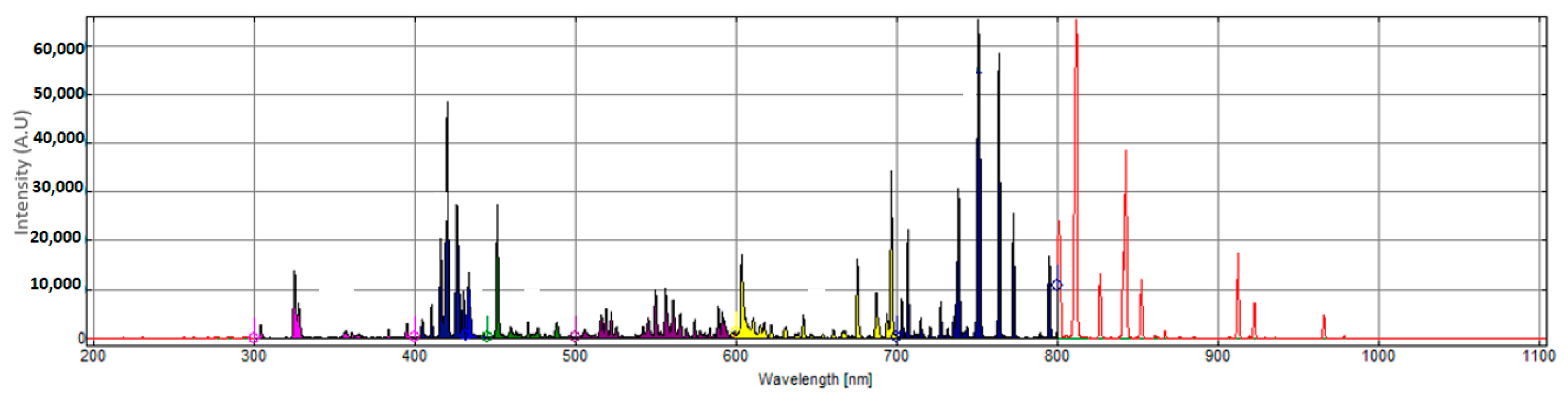
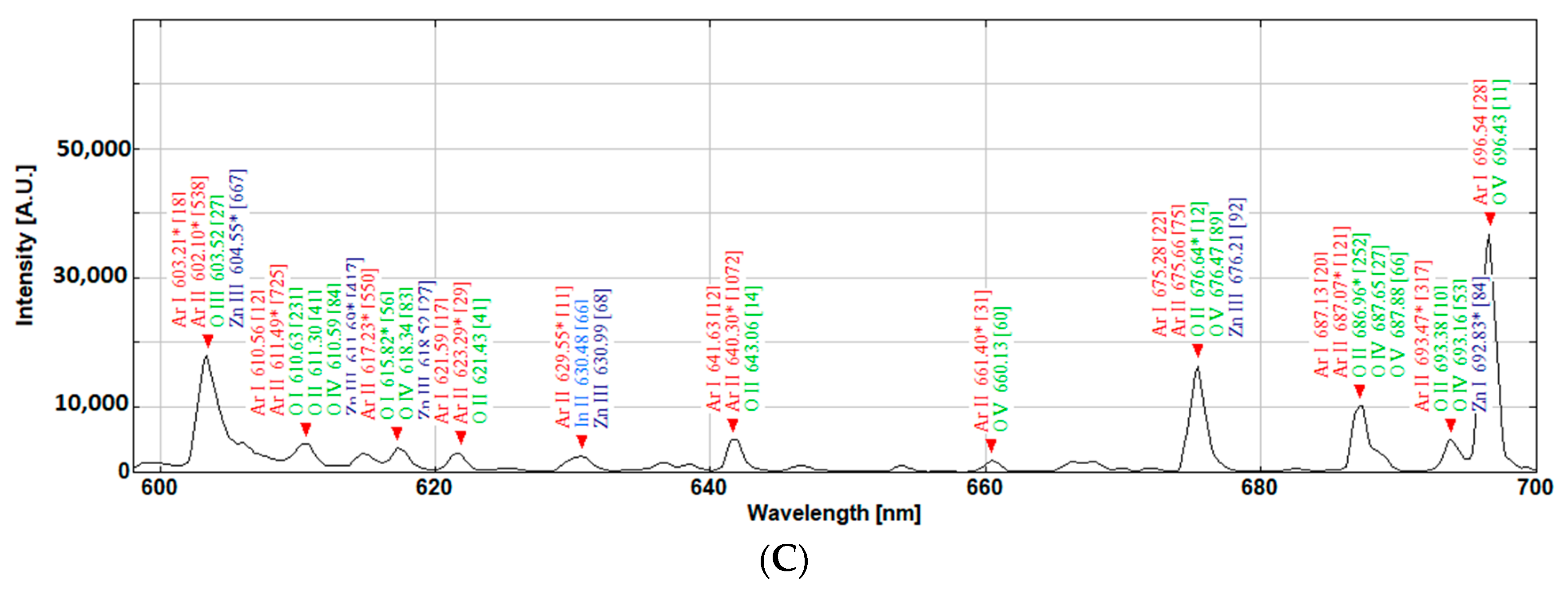
Within a sputtering plasma, a significant number of energy transitions are occurring, leading to a complex emission spectrum. These emissions ultimately define and construct the spectral plot of the plasma as a light source that is observed in plasma. The efficiency and characteristics of the sputtering process depend on the characteristics of the plasma, which in turn depend on the density of the particles and energies within the plasma [
19]. Through complex procedures, some of which we already discussed, the electron temperature and electron density, meta-stable atoms, and ions can be deduced and estimated from the spectral plot [
20,
21,
22,
23]. If the colour parameters can be identified and linked to the existing plasma analysis techniques, it can benefit applied scientists to perform and conduct thin film depositions and can have an easier method of assessing the specifics of the plasma being used.
Due to the extensive optoelectronic applications, high interest in transparent conductive oxides, such as ITO, AZO, and IZO, and their deposition characteristics via plasma sputtering exists and there is plenty of literature available from various research groups [
24,
25]. These materials are of high interest to many photovoltaic researchers, and hence we have focused on evaluating the light from a sputtering magnetron plasma source that was fitted with an IZO target and the objective here is to take a radical diversion from the complexity of the conventional plasma characterisation techniques and observe the colour of the plasma and explore the feasibility of exploiting the colour characteristics of the plasma as an indicator of the coating process. The authors realise that, still, a significant number of energy transitions occur in the UV and IR region of the spectrum that are not accounted for when constructing a conventional chromaticity diagram; however, this work is an initial focus on clarifying the feasibility of such a radical approach, after which further efforts can be invested on to modelling a plasma colour analysis system that can provide a shortcut into the characterisation of a sputtering plasma.
4. Discussion
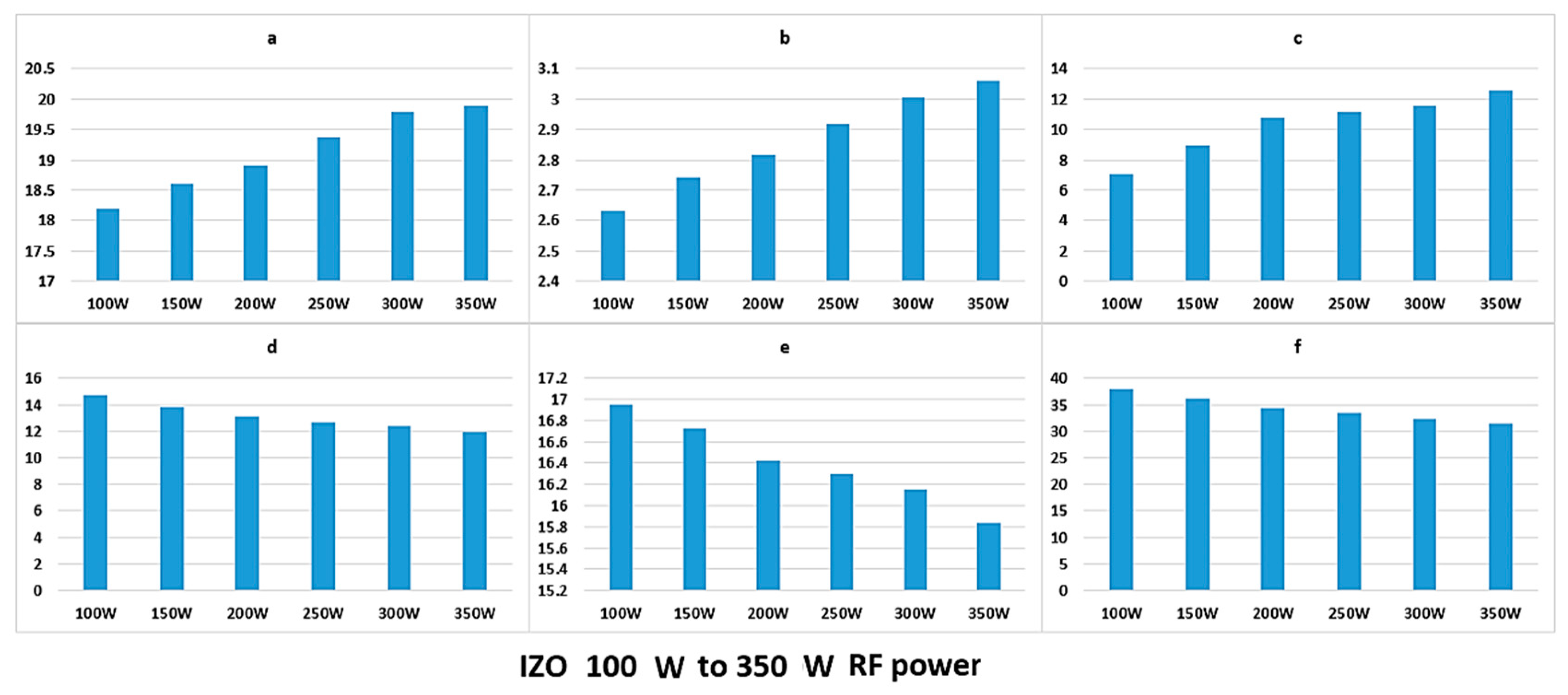
Here, we have demonstrated how the complex analysis of the emission spectrum can be summarised by applying the colour coordinates of the plasma light. The calculation of the colour functions is extracted through the area that is covered under the emission peaks of the plasma light that are affected by various parameters. Higher plasma power simply provides more energy toward the ionization of the working gas atoms. A higher density of ionized atoms, in turn, give a higher flux of emission and, as such, increase the intensity of the spectra. By increasing the power of the plasma, the number and momentum of the argon ions and sputtering particles are both increased, which results in higher bombardment rate of the target surface and higher mobility of the atoms at the deposited thin film surface. The higher mobility of the atoms will reduce the stress at the thin film surface, and hence facilitates further crystallisation [
27].
The discharge current linearly increases with increasing the plasma power, irrespective of the chamber pressure. The energy of the sputtered atoms arriving at the surface of the substrate increases with increasing the plasma power. Parallel to this, the effect of high energy bombardment of the substrate surface is also increased, providing thermal energy at the surface atoms to become more mobile. Overall, the higher plasma power should lead to more crystalline deposition of thin films. However, excessive power can have an adverse effect by causing degradation of the thin film surface, resulting in high defect density [
28]. We have demonstrated that the colour functions demonstrate a linear relationship with the plasma power, hence giving this technique the potential of defining a relationship between the film properties and the
x and
y colour coordinates, we will be exploring this further in future work.
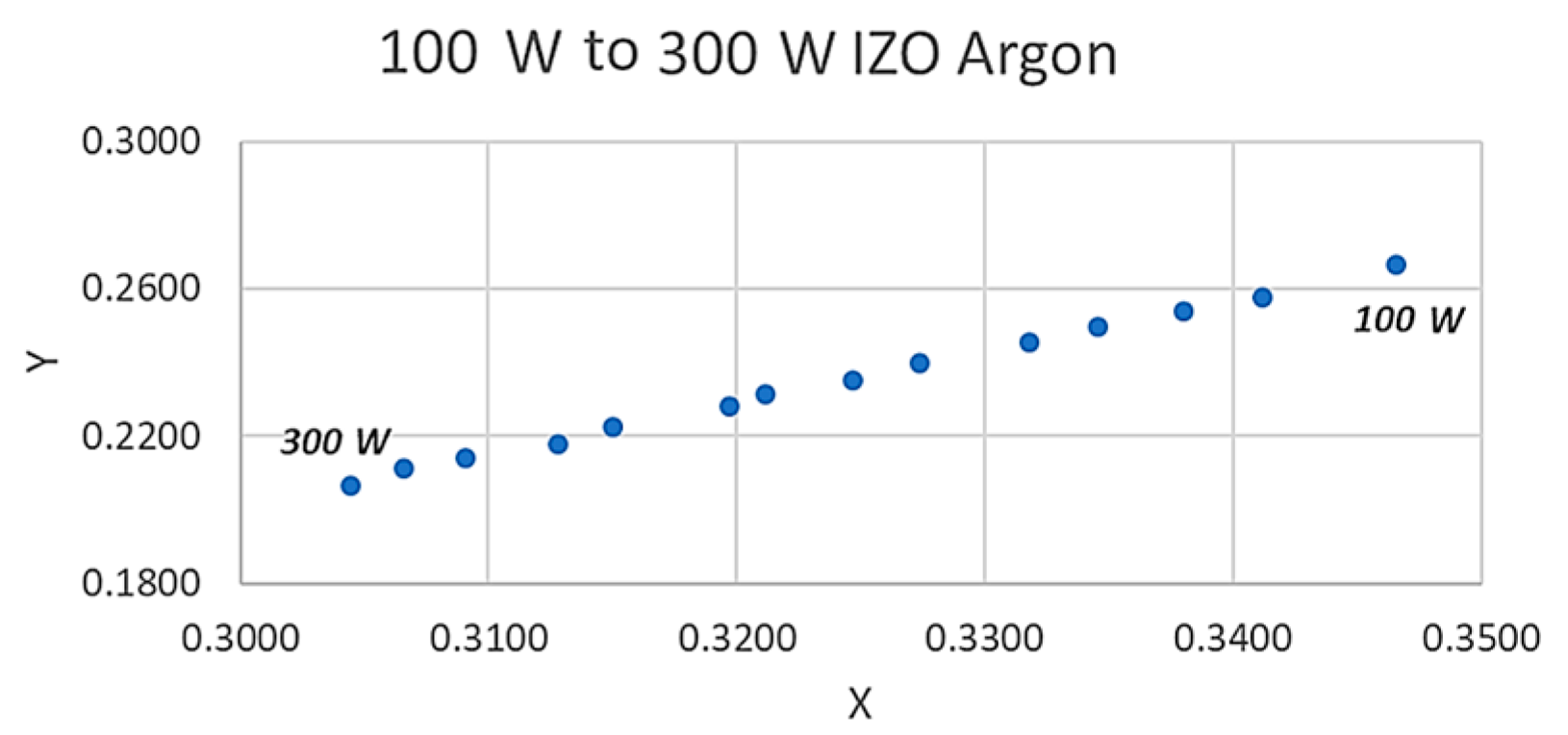
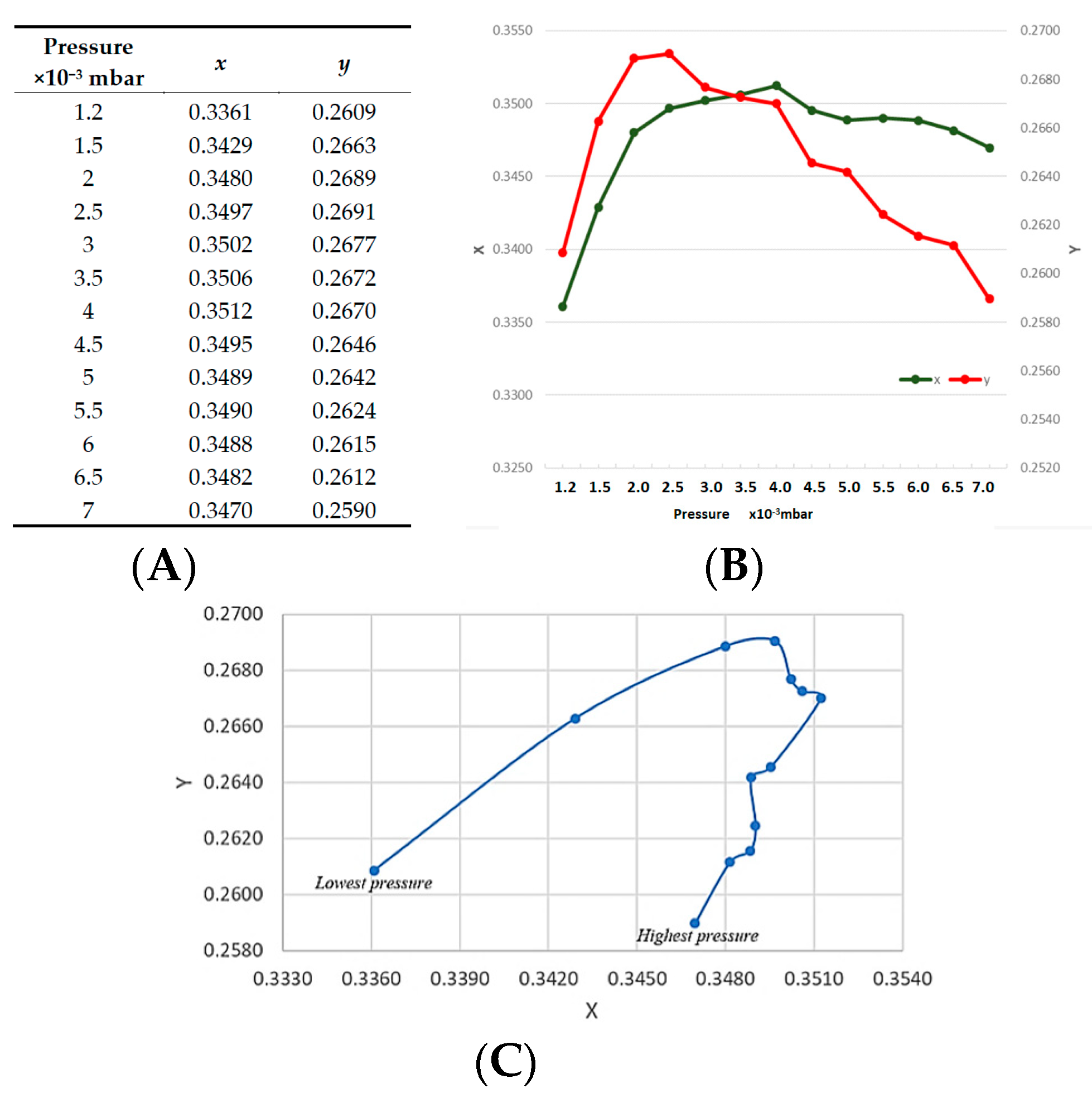
If all of the peaks would increase at the same rate with increasing the power, the chromaticity parameters should not change, but in
Figure 6 we observed a linear trend in how the
x and
y values are plotted against each other. This indicates that as the plasma power is increased and different energy bands tend to respond to the higher magnitude of the power. This is currently under our investigation and the reason that we have the section 400 to 500 nm section of the spectrum divided into three sections is part of our efforts on understanding this observation; however, as yet, our findings are not conclusive and hence no further explanation or claims can be made at present. However, from an applied scientist or an operator of a plasma deposition system, the relationship observed in figure six is a simple observational method if in depth an operator does not require theoretical knowledge. The broadening of the spectral lines, as discussed in the introduction, are ultimately related to the electron density via the stark broadening process. The colour functions are calculated from the area under the emission peaks, hence this broadening is taken into consideration during the colour coordinate measurements and present a simplified form of data for the operator, who may be only interested in basic monitoring protocols and not necessarily wish to indulge in to the complex statistical mechanics of the plasma. A similar argument can be applied for the emission line shapes which are strongly influenced by the interaction of the radiating atoms or ions with surrounding particles. All of the spectral data that were obtained during these experiments were carried out using the same distance between the collimator head and the plasma. It is important to acknowledge that this distance is highly important and it can influence the spectra of the plasma and it is part of our ongoing research objectives to investigate various distances of the collimator and the plasma. Through the data presented in
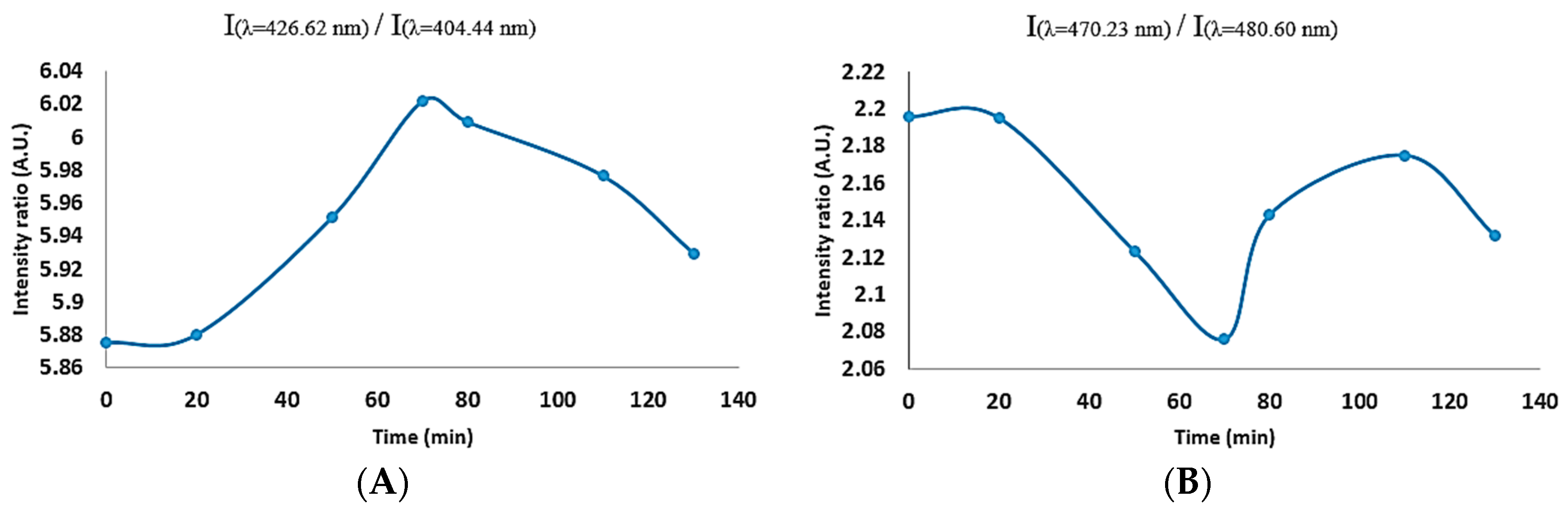
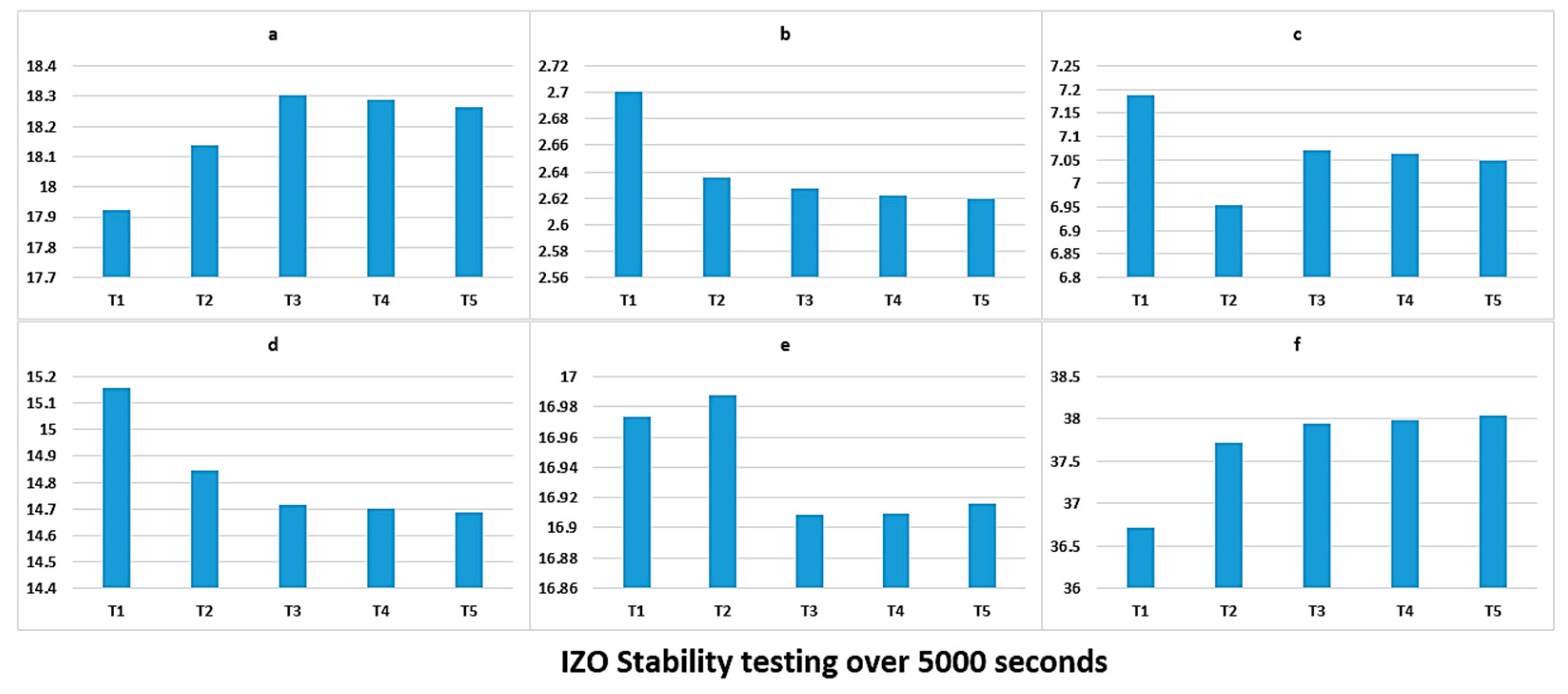
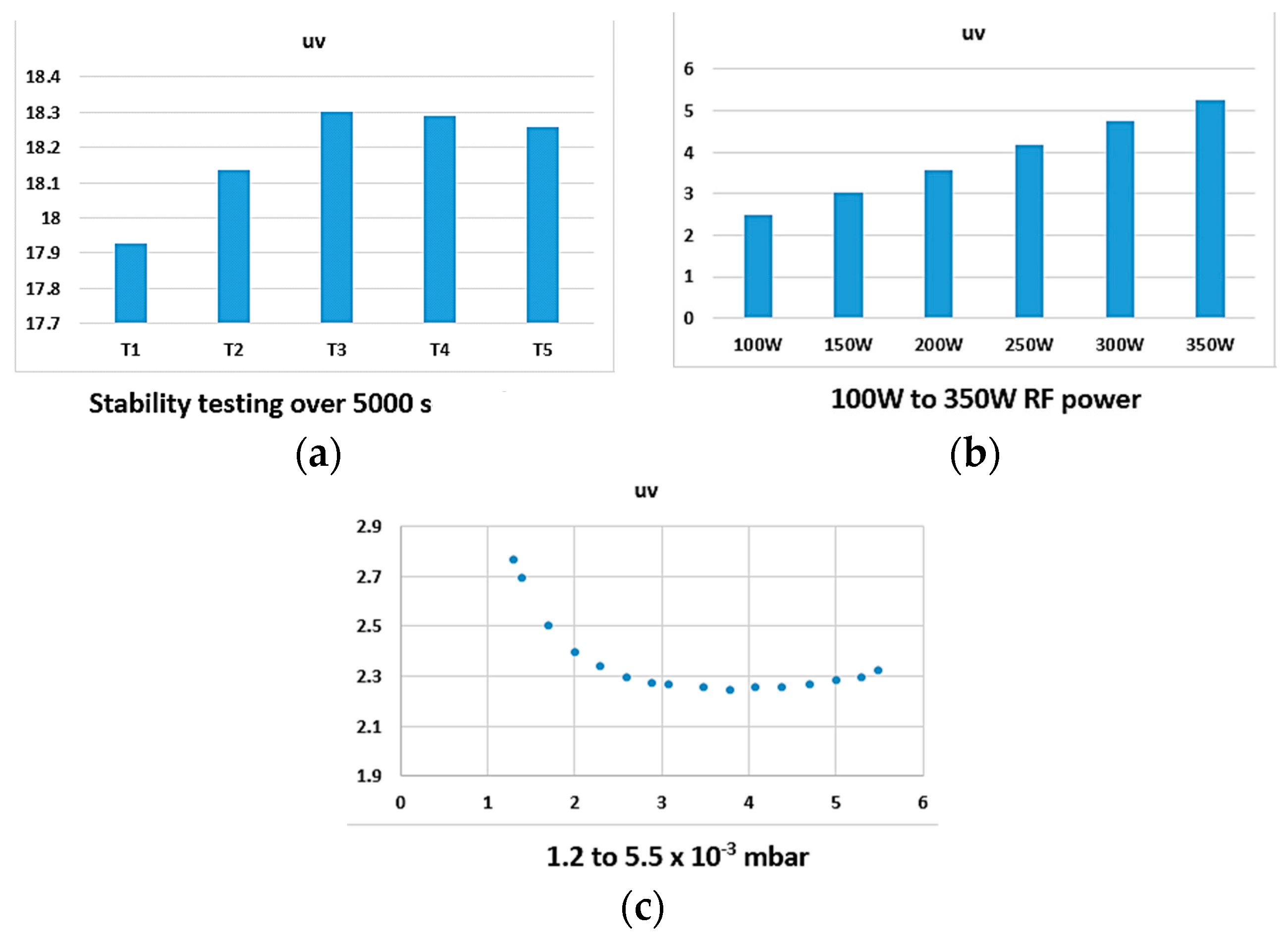
Figure 5, we also demonstrated that, when trying to identify the stability of the plasma through the line ratio method, there may be confusion as certain peaks may increase or decrease, irrespective of their initial magnitude and ratio, while our proposed colour index values can provide a simple root to follow.
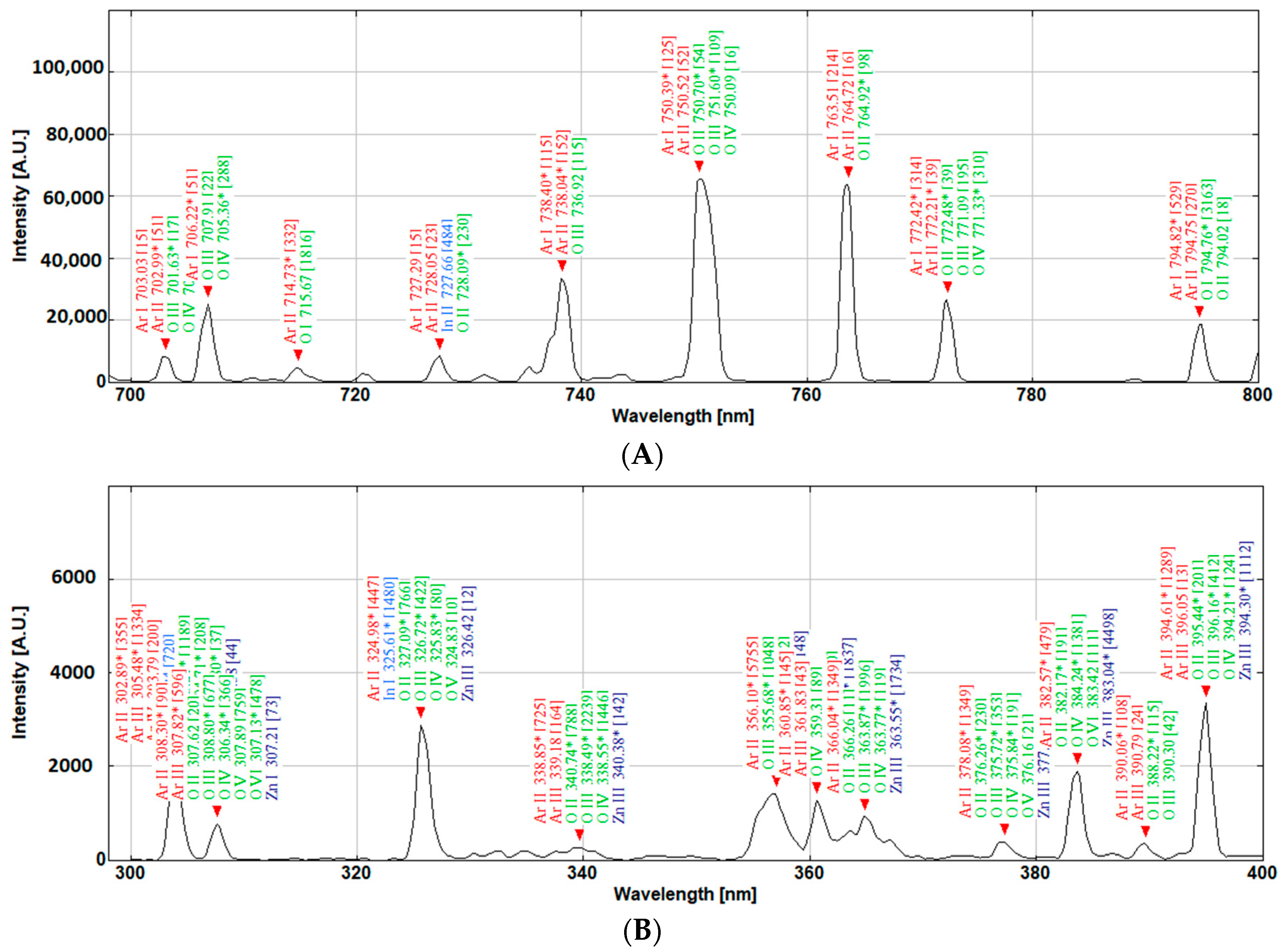
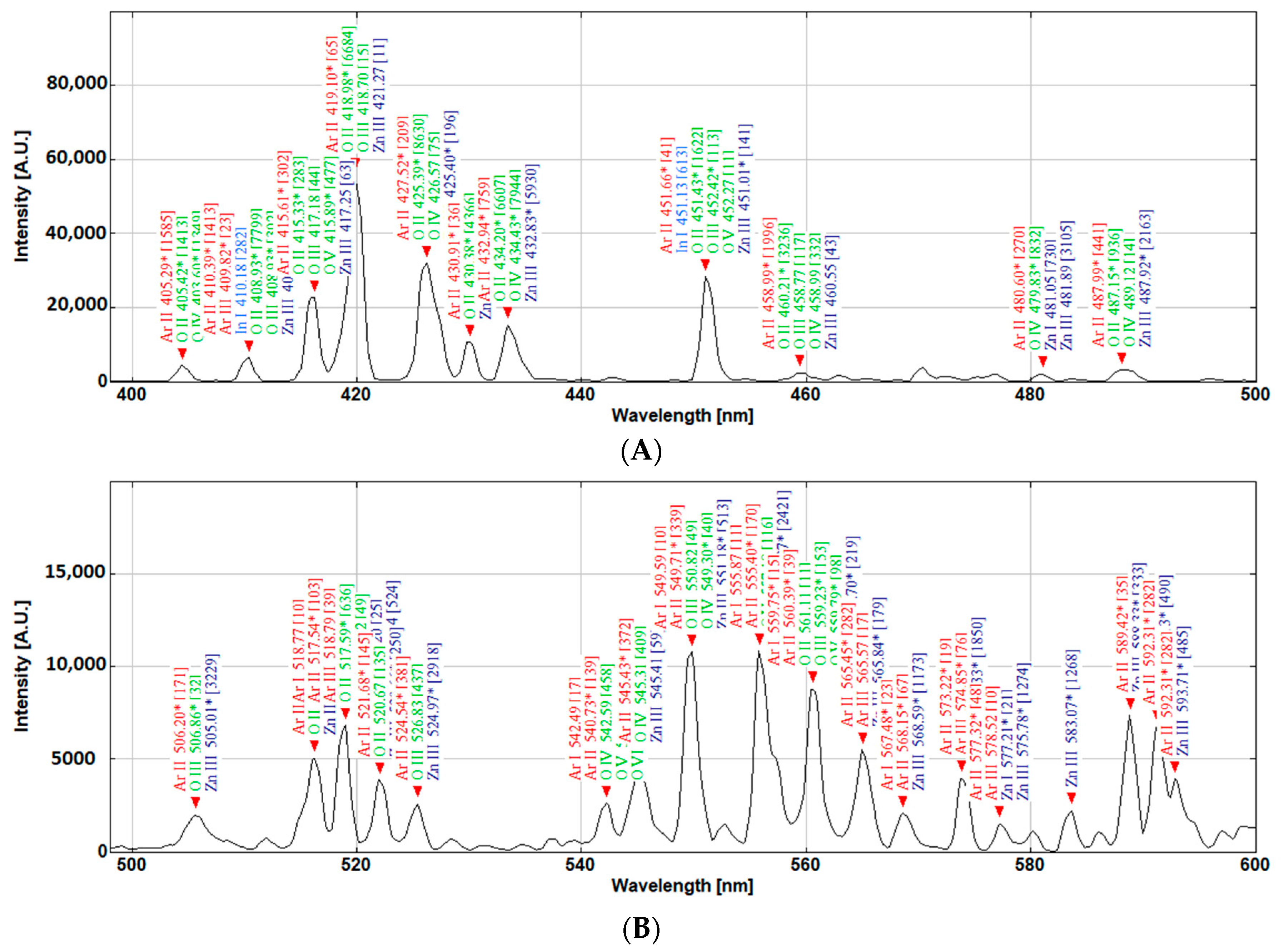
Figure 13 presents some of the possible transitions that need assessment when using the conventional line intensity techniques, while, through colour coordinate calculations, the overall area of the peaks is considered, hence simplifying the monitoring process for a user with limited need of extensive plasma physic knowledge. However, the colour coordinates do not take into consideration the emissions in the UV and far-red part of the spectrum. Hence, an alternative colour function for this application can be envisaged. If this method is matured in the future, then it can be beneficial for applied scientists and those who utilise plasma sputtering for thin film coatings and depositions. The colour of the plasma is derived by the quantity and state of various meta-stable atoms and ions that constitute it. In an analogical overview, a simplified shortcut can be established to characterise the plasma by being able to identify the relationship between the colour of the plasma and physical events within the plasma, just as the fingerprint pattern is used to characterise people rather than their individual genome sequence.
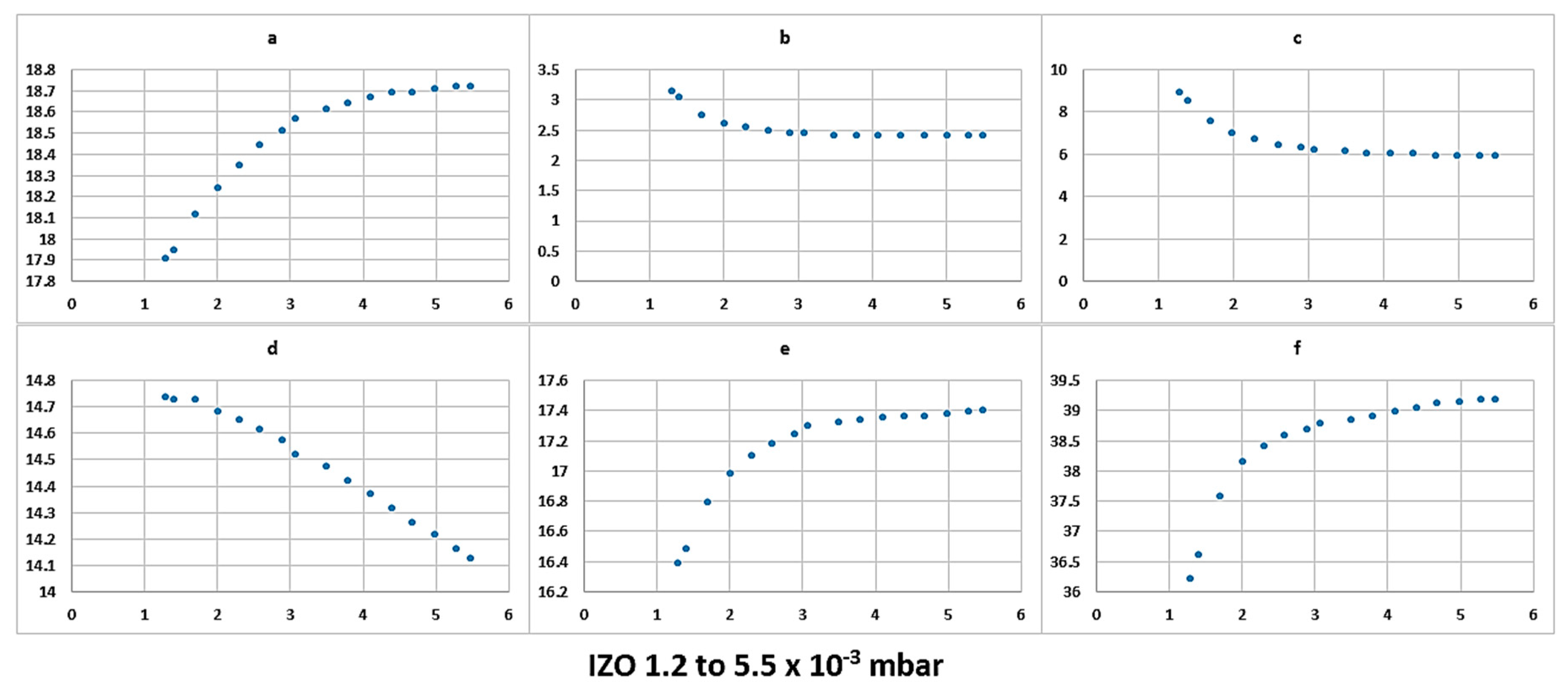
Higher chamber pressure lowers the voltage at which the plasma can be ignited and maintained. This is because higher chamber pressure increases the probability of the ionisation process of argon. Higher chamber pressure on the other hand means that more argon atoms are available to be energised to bombard the target surface; however, a higher concentration of argon atoms means that there will more collision between the sputtered atoms or ions and argon atoms. These collisions can lead to lesser energetic atoms arriving at the surface of the substrate [
29]. A correlation can clearly be seen between the
x and
y coordinates of the chromaticity index of the plasma light and certain parameters, such as plasma stability, the working gas and the associated chamber pressure and the plasma power that affect the
x and
y values. This highlights a clear potential of pursuing and investigating the proposed concept and, if successful for a given target material to be sputtered, regardless of the manufacturer of the sputtering deposition machine, matching the colour parameters can indicate exact similar plasma operating conditions. Meanwhile, theoretically, there is a possibility of operating a plasma under two different conditions and getting similar colour values, we have partially investigated this, where, by altering the chamber pressure and plasma power, similar
x or
y values can be mimicked, but having an identical
x and
y values at the same time so far has not been observed, this itself will be a future experimental protocol.
The x and y coordinates can give an excellent, easy indication of the point at which the plasma is stable when compared to more complex calculation methods. The stabilisation process itself is an interesting concept to consider, slight variation in parameters that are required for driving plasma can create unstable conditions, and the colour factor can be an excellent indicator of this.
We have demonstrated that, under unique chamber pressure and plasma power, we can have a distinct x and y value that can be utilised by the operators of the machine to create a library of the thin film property relevant to particular x and y coordinate values, making the process of monitoring the thin film quality significantly easy. However, what has been reported here is just the beginning of a concept that bears potential. The colour coordinates that are driven from the plasma here do not take into consideration the UV and IR emissions. Additionally, in future efforts, the relationship between the coating properties and the plasma photometric need to be established. Future efforts on further investigation of this concept require establishing the relationship between the chromaticity parameters and the plasma’s inner parameters, such as electron temperature and charge density, to enable us to generate a reliable relationship between the mentioned factors. Thereby, the first step in any future development of this concept is to prepare and create an alternative colour coding that will take the UV and IR emissions into account and will create a colour index beyond our vision.