Modified Electrodeposited Cobalt Foam Coatings as Sensors for Detection of Free Chlorine in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Preparation and Deposition of Cobalt Foams
2.2. Modification of Co Foams and Detection of Free Chlorine
2.3. Instrumentation
3. Results and Discussion
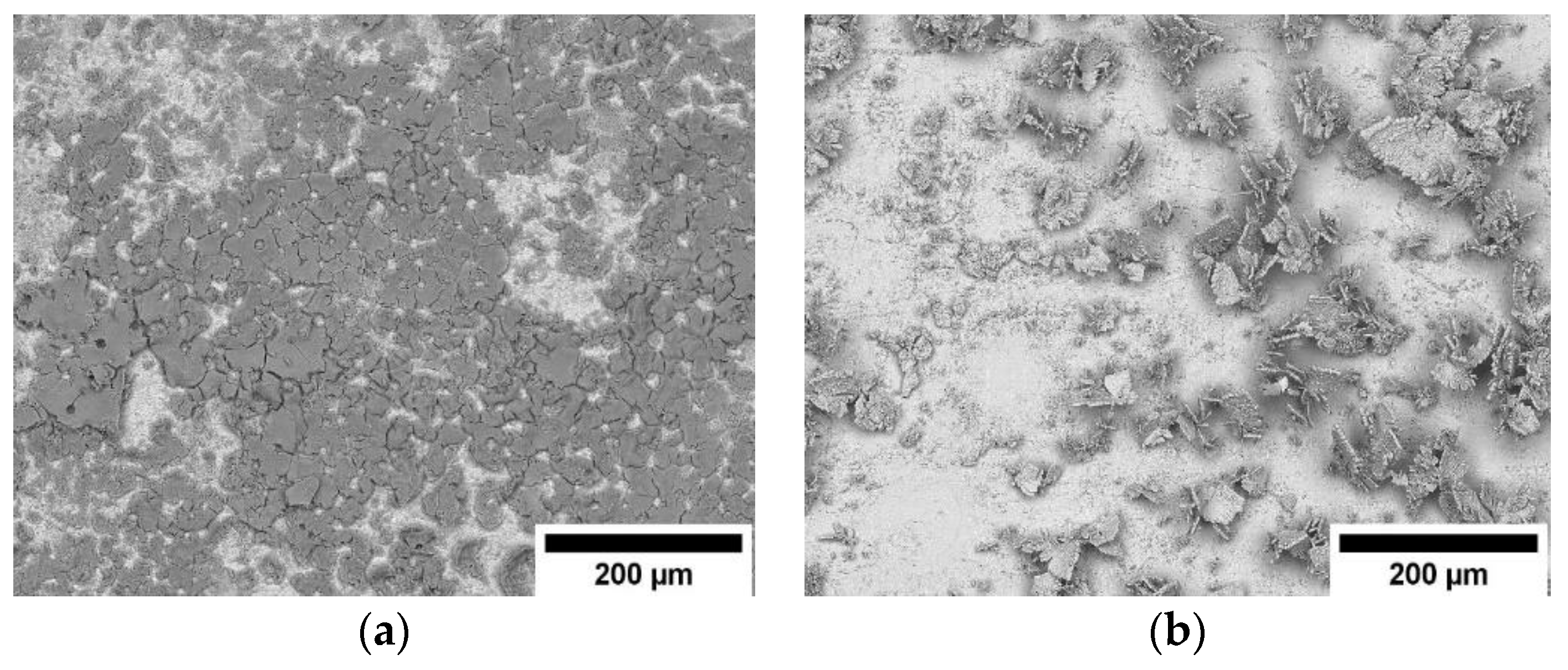
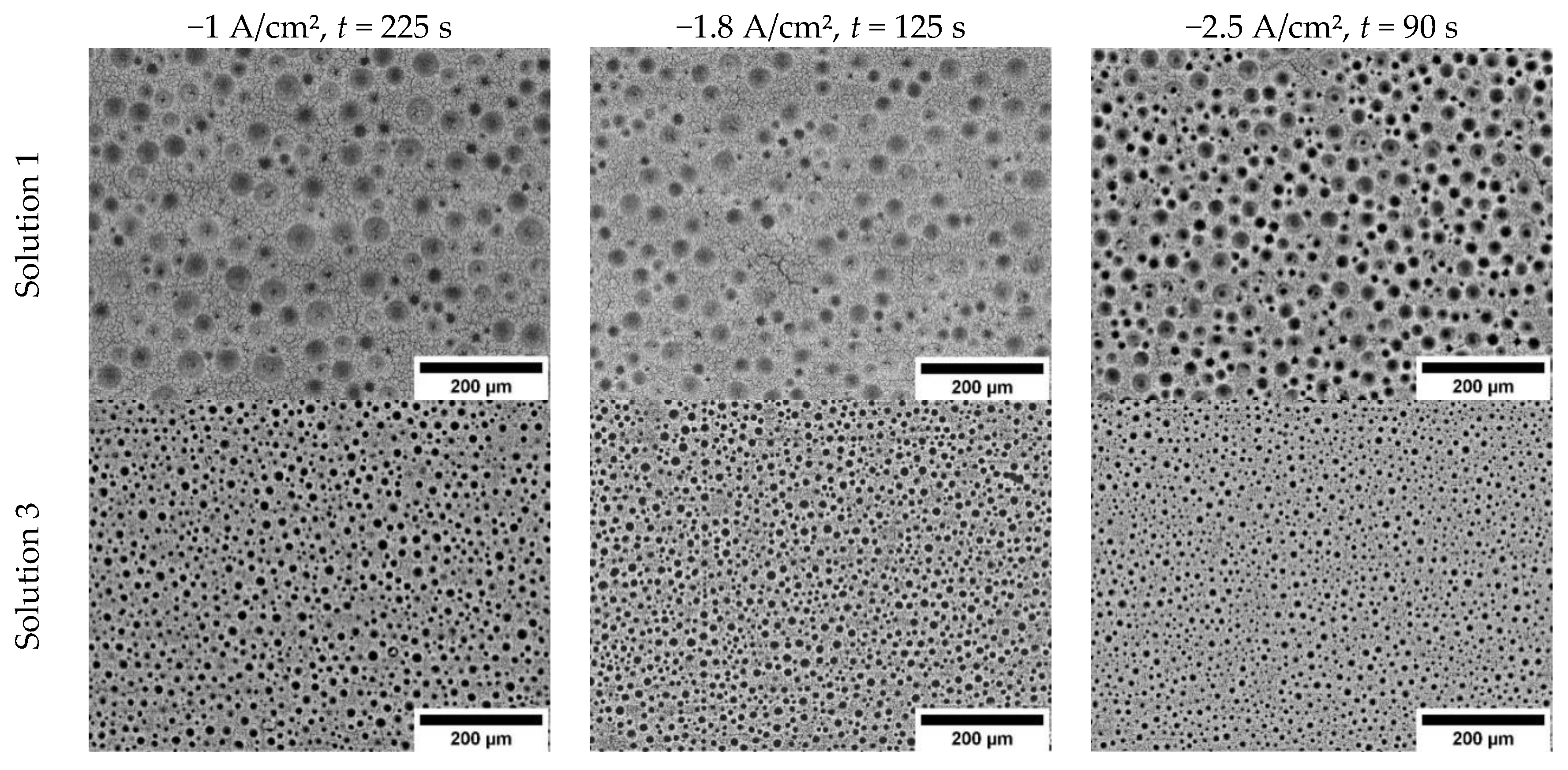
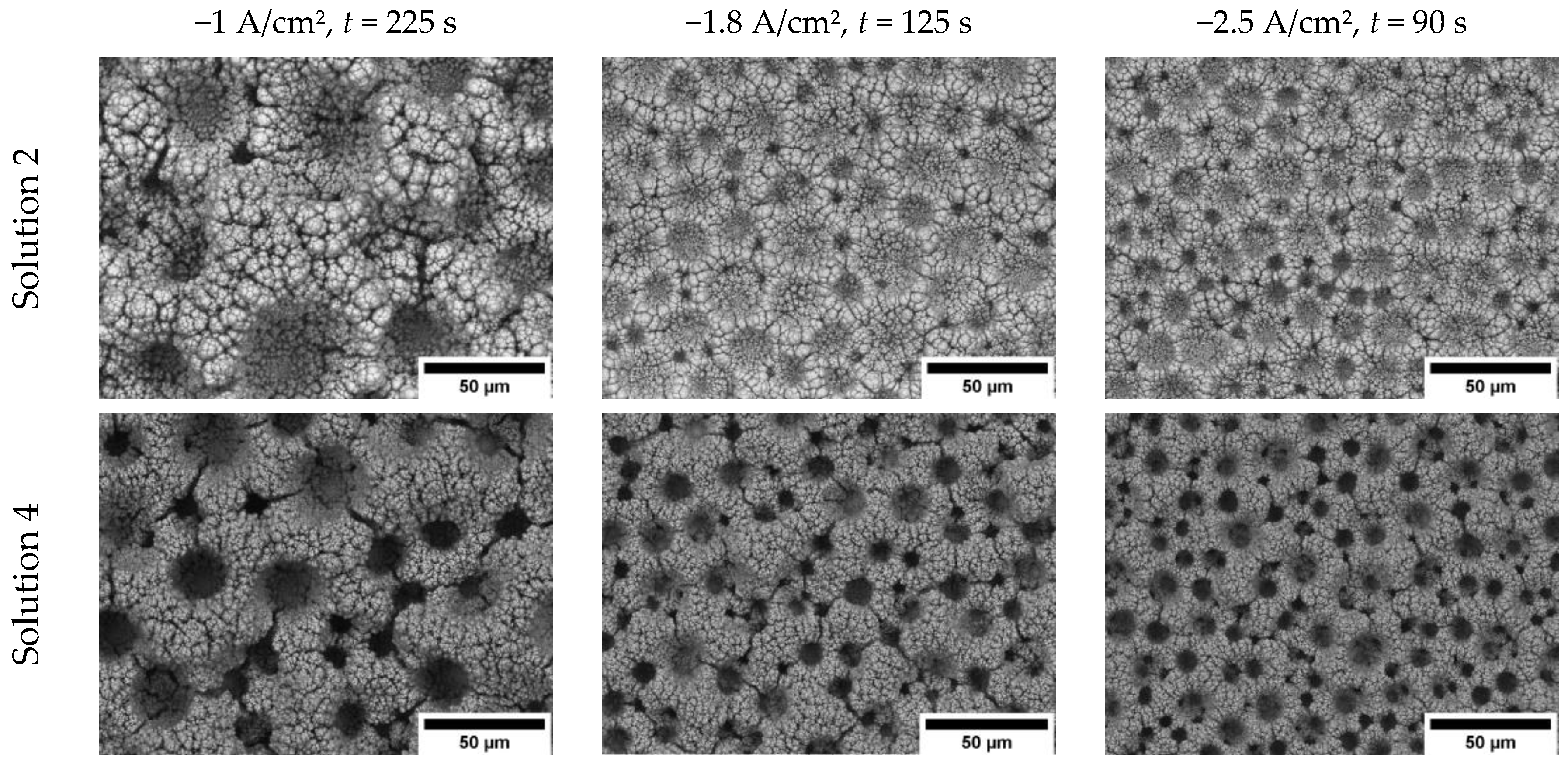
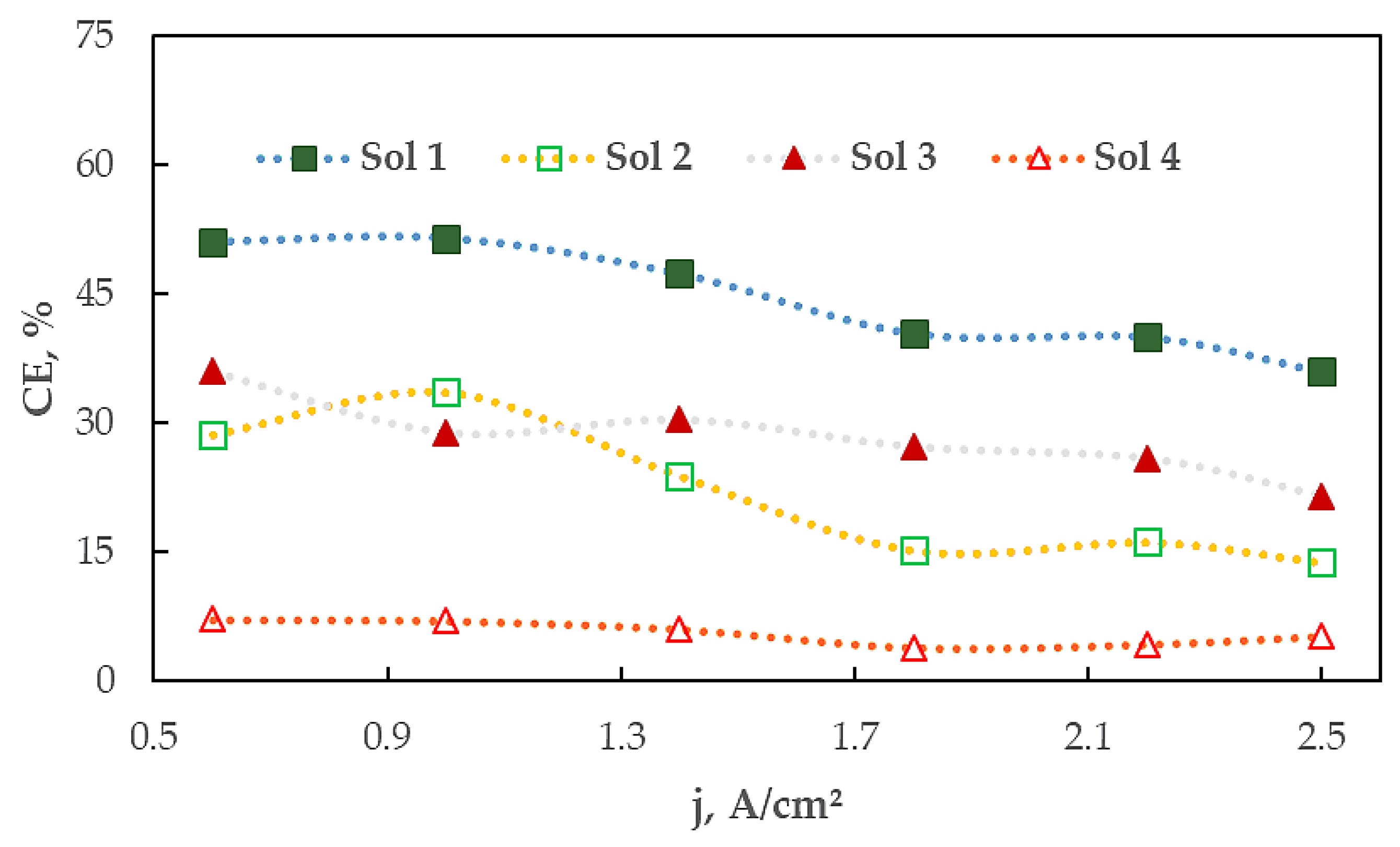
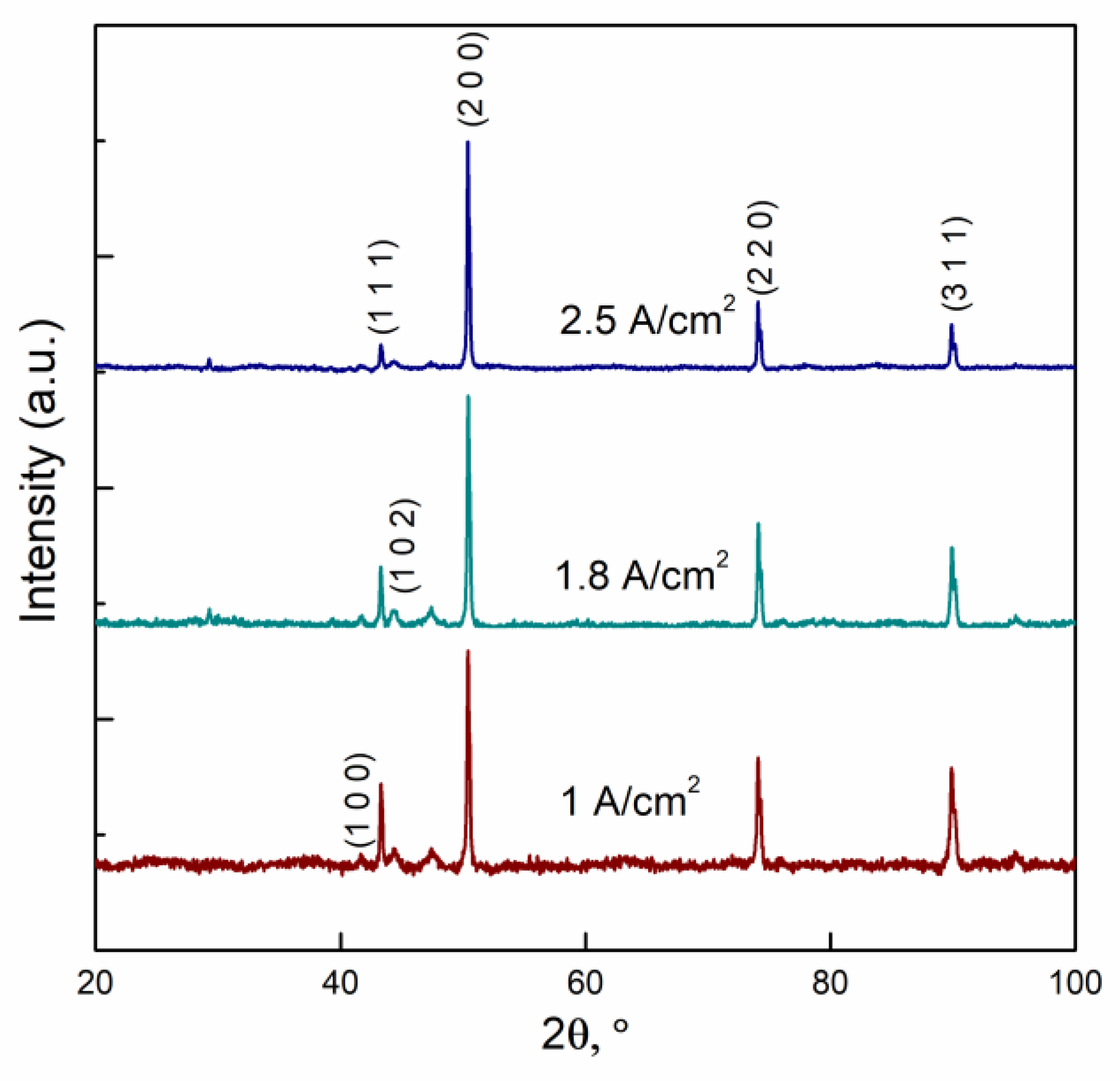
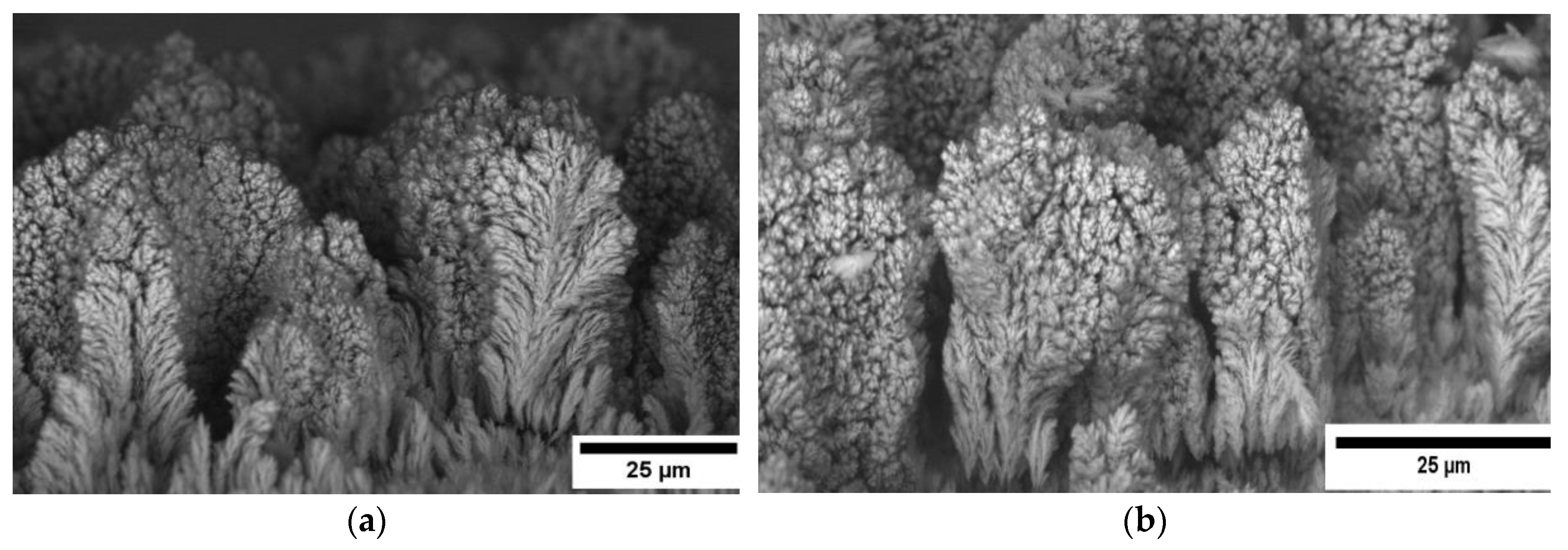
3.1. The Influence of Electrolyte Composition and Operating Conditions on Co Coatings Deposition
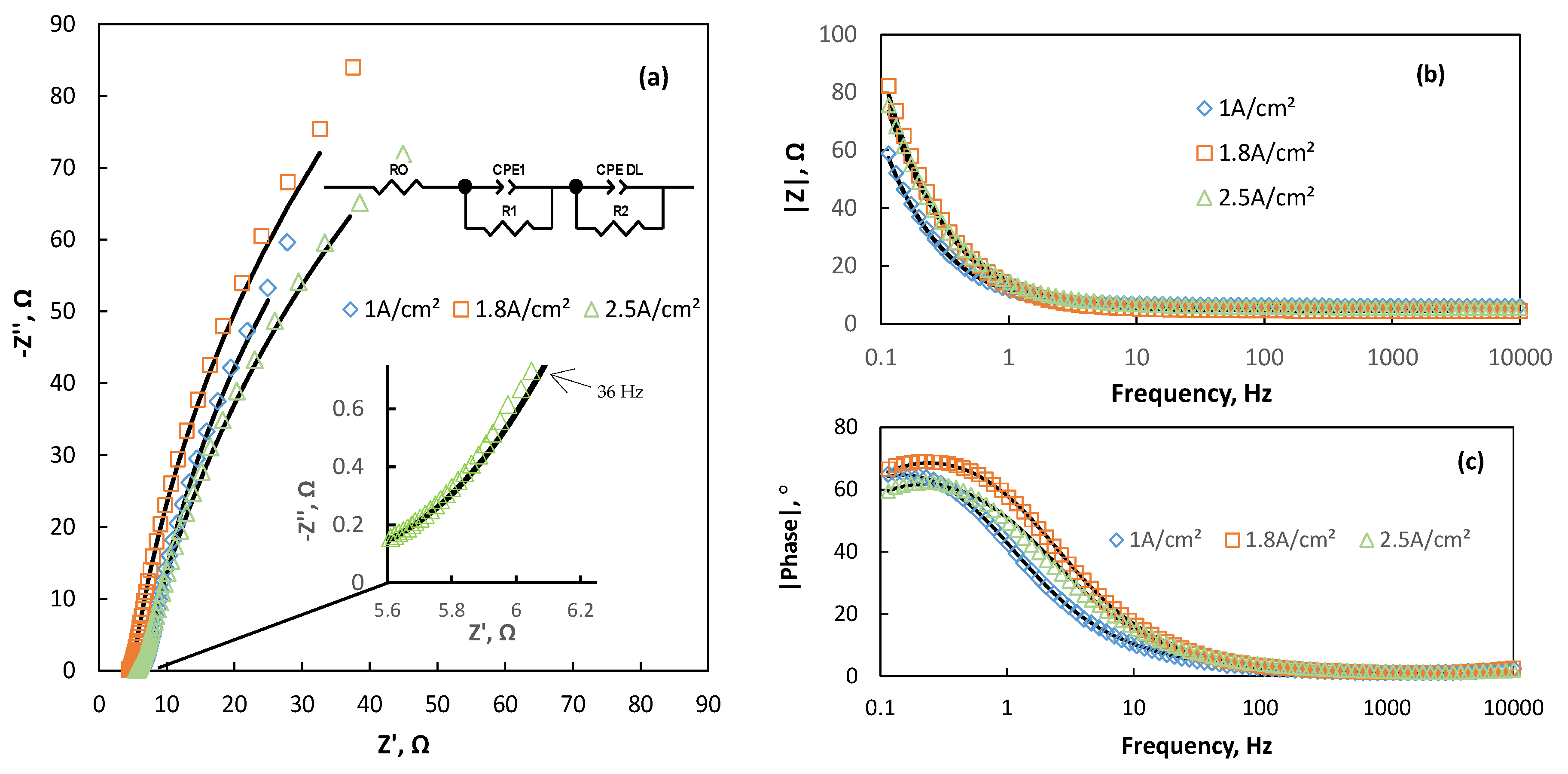
3.2. The Mechanism of Co Foams Depositon and Determination of True Surface Area
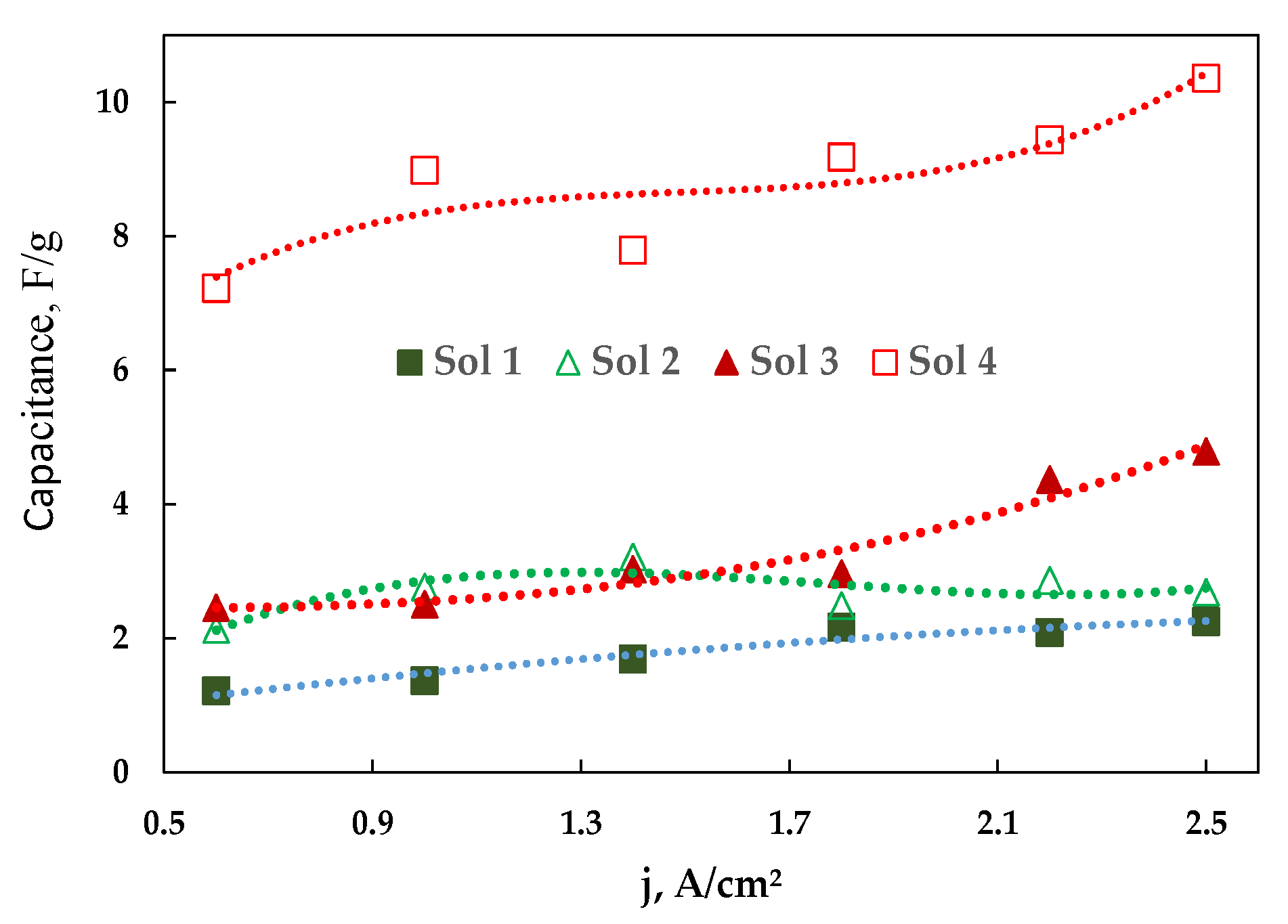
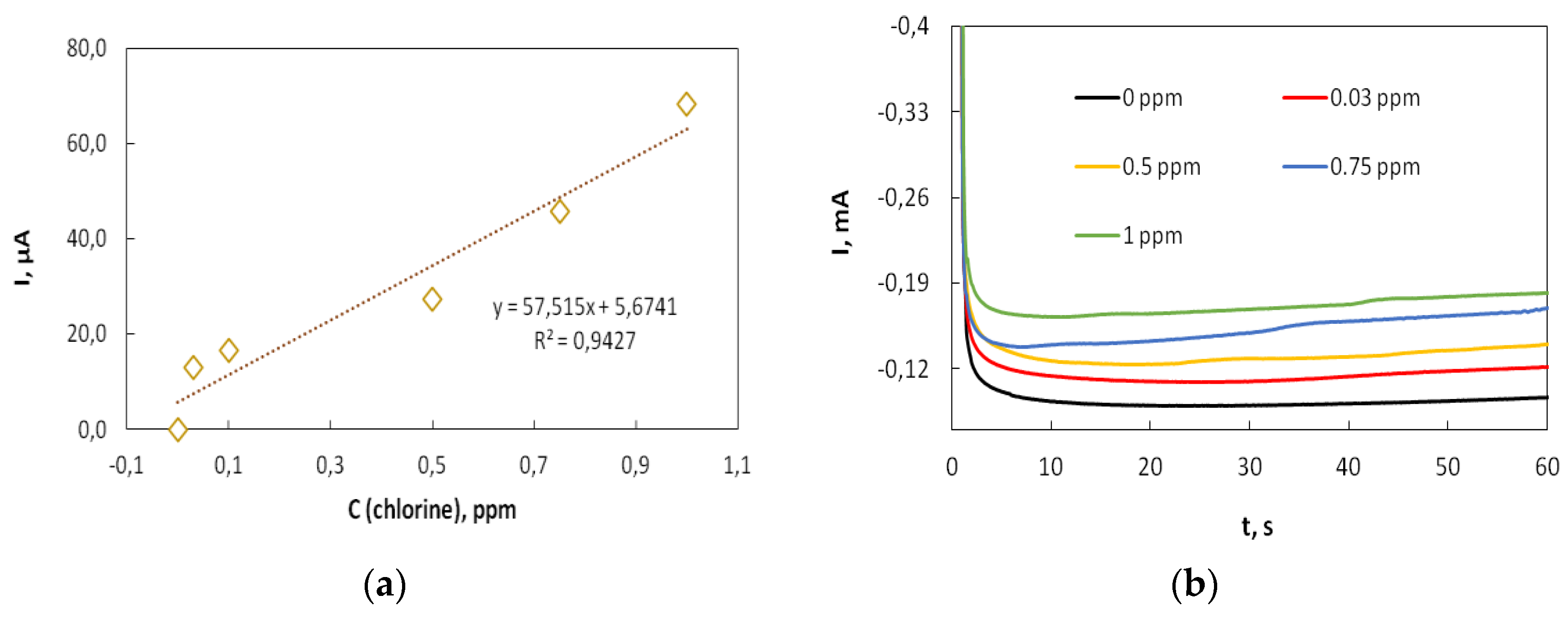
3.3. Modification of Cobalt Foams and Detection of Free Chlorine
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations sustainable development. Available online: https://www.un.org/sustainabledevelopment/blog/2015/12/sustainable-development-goals-kick-off-with-start-of-new-year (accessed on 23 February 2019).
- Prüss, A.; Kay, D.; Fewtrell, L.; Bartram, J. Estimating the burden of disease from water sanitation and hygiene at a global level. Environ. Health Perspect. 2002, 110, 537–542. [Google Scholar] [CrossRef]
- Bridle, H. Waterborne Pathogens: Detection Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444595430. [Google Scholar]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Richardson, S.D.; Postigo, C. Drinking water disinfection by-products. In Emerging Organic Contaminants and Human Health; Barceló, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 93–137. ISBN 978-3-642-28132-7. [Google Scholar]
- Wolfe, T.D. Water monitoring system. U.S. Patent 9,015,003B2, 17 December 1998. [Google Scholar]
- Berry, D.; Xi, C.; Raskin, L. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 2006, 17, 297–302. [Google Scholar] [CrossRef]
- Clark, R.M.; Skov, K.; Goodrich, J.A.; Deininger, R.A.; Grayman, W.M. Measuring and modeling chlorine propagation in water distribution systems. J. Water Resour. Plan. Manag. 2006, 120, 871–887. [Google Scholar] [CrossRef]
- Moberg, L.; Karlberg, B. An improved N,N’-diethyl-p-phenylenediamine (DPD) method for the determination of free chlorine based on multiple wavelength detection. Anal. Chim. Acta 2000, 407, 127–133. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; García-García, F.J.; González-Mora, J.L.; González-Elipe, A.R. A novel and improved surfactant-modified Prussian Blue electrode for amperometric detection of free chlorine in water. Sens. Actuators B Chem. 2015, 213, 116–123. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; González-Mora, J.L.; González-Elipe, A.R. Application of prussian blue electrodes for amperometric detection of free chlorine in water samples using flow injection analysis. Talanta 2016, 146, 410–416. [Google Scholar] [CrossRef]
- Del Campo, F.J.; Ordeig, O.; Muñoz, F.J. Improved free chlorine amperometric sensor chip for drinking water applications. Anal. Chim. Acta 2005, 554, 98–104. [Google Scholar] [CrossRef]
- Kodera, F.; Umeda, M.; Yamada, A. Determination of free chlorine based on anodic voltammetry using platinum, gold, and glassy carbon electrodes. Anal. Chim. Acta 2005, 537, 293–298. [Google Scholar] [CrossRef]
- Okumura, A.; Hirabayashi, A.; Sasaki, Y.; Miyake, R. Simple miniaturized amperometric flow cell for monitoring residual chlorine in tap water. Anal. Sci. 2005, 17, 1113–1115. [Google Scholar] [CrossRef]
- Kishioka, S.Y.; Kosugi, T.; Yamada, A. Electrochemical determination of a free chlorine residual using cathodic potential-step chronocoulometry. Electroanalysis 2005, 17, 724–726. [Google Scholar] [CrossRef]
- Jin, J.; Suzuki, Y.; Ishikawa, N.; Takeuchi, T. A miniaturized FIA system for the determination of residual chlorine in environmental water samples. Anal. Sci. 2004, 20, 205–207. [Google Scholar] [CrossRef][Green Version]
- Yu, H.; Chen, H.; Pan, M.; Tang, Y.; Zeng, K.; Peng, F.; Wang, H. Effect of the metal foam materials on the performance of methanol steam micro-reformer for fuel cells. Appl. Catal. A Gen. 2007, 327, 106–113. [Google Scholar] [CrossRef]
- Shahbazi, P.; Kiani, A. Fabricated Cu2O porous foam using electrodeposition and thermal oxidation as a photocatalyst under visible light toward hydrogen evolution from water. Int. J. Hydrog. Energy 2016, 41, 2–11. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, S.; He, P.; Dong, F.; He, M.; Zhang, Y.; Wang, S.; Li, C.; Liu, M.; Jia, L. Samarium oxide modified Ni-Co nanosheets based three-dimensional honeycomb film on nickel foam: A highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2019, 299, 405–414. [Google Scholar] [CrossRef]
- Siwek, K.I.; Eugénio, S.; Santos, D.M.F.; Silva, M.T.; Montemor, M.F. 3D nickel foams with controlled morphologies for hydrogen evolution reaction in highly alkaline media. Int. J. Hydrog. Energy 2019, 44, 1701–1709. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, F.; Li, X.; Chu, M.; Li, N.; Li, X.; Wang, L.; Qu, D.; Dong, Y.; Xie, Z.; et al. A porous FeCuNi-based electrocatalyst supported by nickel foam for oxygen evolution reaction in alkaline conditions. J. Electrochem. Soc. 2018, 165, F1127–F1132. [Google Scholar] [CrossRef]
- Jin, J.; Xia, J.; Qian, X.; Wu, T.; Ling, H.; Hu, A.; Li, M.; Hang, T. Exceptional electrocatalytic oxygen evolution efficiency and stability from electrodeposited NiFe alloy on Ni foam. Electrochim. Acta 2019, 299, 567–574. [Google Scholar] [CrossRef]
- Pei, Y.; Ge, Y.; Chu, H.; Smith, W.; Dong, P.; Ajayan, P.M.; Ye, M.; Shen, J. Controlled synthesis of 3D porous structured cobalt-iron based nanosheets by electrodeposition as asymmetric electrodes for ultra-efficient water splitting. Appl. Catal. B Environ. 2019, 244, 583–593. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Feng, H.; Ji, S.; Mao, X.; Wang, R. Three-dimensional iron, nitrogen-doped carbon foams as efficient electrocatalysts for oxygen reduction reaction in alkaline solution. Electrochim. Acta 2014, 142, 317–323. [Google Scholar] [CrossRef]
- Sanzó, G.; Taurino, I.; Antiochia, R.; Gorton, L.; Favero, G.; Mazzei, F.; De Micheli, G.; Carrara, S. Bubble electrodeposition of gold porous nanocorals for the enzymatic and non-enzymatic detection of glucose. Bioelectrochemistry 2016, 112, 125–131. [Google Scholar] [CrossRef]
- Nam, D.-H.; Kim, R.-H.; Lee, C.-L.; Kwon, H.-S. Highly reversible Sn-Co alloy anode using porous Cu foam substrate for Li-Ion batteries. J. Electrochem. Soc. 2012, 159, A1822–A1826. [Google Scholar] [CrossRef]
- Trahey, L.; Vaughey, J.T.; Kung, H.H.; Thackeray, M.M. High-capacity, microporous Cu6Sn5–Sn anodes for Li-ion batteries. J. Electrochem. Soc. 2009, 156, A385. [Google Scholar] [CrossRef]
- Xu, J.; Ji, X.; Zhang, W.; Liu, G. Pool boiling heat transfer of ultra-light copper foam with open cells. Int. J. Multiph. Flow 2008, 34, 1008–1022. [Google Scholar] [CrossRef]
- Hong, S.T.; Herling, D.R. Open-cell aluminum foams filled with phase change materials as compact heat sinks. Scr. Mater. 2006, 55, 887–890. [Google Scholar] [CrossRef]
- Shin, H.C.; Dong, J.; Liu, M. Nanoporous structures prepared by an electrochemical deposition process. Adv. Mater. 2003, 15, 1610–1614. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Kiani, A.; Rashid-Nadimi, S. Fabrication of highly porous Pt coated nanostructured Cu-foam modified copper electrode and its enhanced catalytic ability for hydrogen evolution reaction. Int. J. Hydrog. Energy 2010, 35, 452–458. [Google Scholar] [CrossRef]
- Lange, G.A.; Eugénio, S.; Duarte, R.G.; Silva, T.M.; Carmezim, M.J.; Montemor, M.F. Characterisation and electrochemical behaviour of electrodeposited Cu-Fe foams applied as pseudocapacitor electrodes. J. Electroanal. Chem. 2015, 737, 85–92. [Google Scholar] [CrossRef]
- Eugénio, S.; Silva, T.M.; Carmezim, M.J.; Duarte, R.G.; Montemor, M.F. Electrodeposition and characterization of nickel-copper metallic foams for application as electrodes for supercapacitors. J. Appl. Electrochem. 2014, 44, 455–465. [Google Scholar] [CrossRef]
- Niu, J.; Liu, X.; Xia, K.; Xu, L.; Xu, Y.; Fang, X.; Lu, W. Effect of electrodeposition parameters on the morphology of three-dimensional porous copper foams. Int. J. Electrochem. Sci. 2015, 10, 7331–7340. [Google Scholar]
- Marozzi, C.A.; Chialvo, A.C. Development of electrode morphologies of interest in electrocatalysis. Part 1: Electrodeposited porous nickel electrodes. Electrochim. Acta 2000, 45, 2111–2120. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, R.H.; Kwon, H.S. Preparation of copper foam with 3-dimensionally interconnected spherical pore network by electrodeposition. Electrochem. commun. 2008, 10, 1148–1151. [Google Scholar] [CrossRef]
- Nam, D.; Kim, R.; Han, D.; Kim, J.; Kwon, H. Effects of (NH4)2SO4 and BTA on the nanostructure of copper foam prepared by electrodeposition. Electrochim. Acta 2011, 56, 9397–9405. [Google Scholar] [CrossRef]
- De Tacconi, N.R.; Rajeshwar, K.; Lezna, R.O. Metal hexacyanoferrates: electrosynthesis, in situ characterization, and applications. Chem. Mater. 2003, 15, 3046–3062. [Google Scholar] [CrossRef]
- Kumar, A.; Kanagare, A.; Banerjee, S.; Kumar, P.; Kumar, M.; Sudarsan, V. Synthesis of cobalt hexacyanoferrate nanoparticles and its hydrogen storage properties. Int. J. Hydrog. Energy 2018, 43, 7998–8006. [Google Scholar] [CrossRef]
- Cai, C.-X.; Xue, K.-H.; Xu, S.-M. Electrocatalytic activity of a cobalt hexacyanoferrate modified glassy carbon electrode toward ascorbic acid oxidation. J. Electroanal. Chem. 2000, 486, 111–118. [Google Scholar] [CrossRef]
- Hegner, F.S.; Herraiz-Cardona, I.; Cardenas-Morcoso, D.; López, N.; Galán-Mascarós, J.R.; Gimenez, S. Cobalt hexacyanoferrate on BiVO4 photoanodes for robust water splitting. ACS Appl. Mater. Interfaces 2017, 9, 37671–37681. [Google Scholar] [CrossRef]
- Ho, K.C.; Chen, C.Y.; Hsu, H.C.; Chen, L.C.; Shiesh, S.C.; Lin, X.Z. Amperometric detection of morphine at a Prussian blue-modified indium tin oxide electrode. Biosens. Bioelectron. 2004, 20, 3–8. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef]
- Berkh, O.; Shacham-Diamand, Y.; Gileadi, E. Reduction of ammonium ion on Pt electrodes. J. Electrochem. Soc. 2008, 155, F223. [Google Scholar] [CrossRef]
- Ribeiro, C.P.; Mewes, D. The effect of electrolytes on the critical velocity for bubble coalescence. Chem. Eng. J. 1993, 97, 10192–10197. [Google Scholar] [CrossRef]
- Tsyntsaru, N.; Cesiulis, H.; Pellicer, E.; Celis, J.P.; Sort, J. Structural, magnetic, and mechanical properties of electrodeposited cobalt-tungsten alloys: Intrinsic and extrinsic interdependencies. Electrochim. Acta 2013, 104, 94–103. [Google Scholar] [CrossRef]
- Liu, X.; Wu, N.; Zhou, P.; Bi, N.; Or, S.W.; Cui, C.; Sun, Y. Large scale synthesis of superparamagnetic face-centered cubic Co/C nanocapsules by a facile hydrothermal method and their microwave absorbing properties. Mater. Res. 2015, 18, 756–762. [Google Scholar] [CrossRef][Green Version]
- Liu, B.H.; Li, Z.P.; Suda, S. Nickel- and cobalt-based catalysts for hydrogen generation by hydrolysis of borohydride. J. Alloy. Compd. 2006, 415, 288–293. [Google Scholar] [CrossRef]
- Staszak-Jirkovský, J.; Malliakas, C.D.; Lopes, P.P.; Danilovic, N.; Kota, S.S.; Chang, K.-C.; Genorio, B.; Strmcnik, D.; Stamenkovic, V.R.; Kanatzidis, M.G.; et al. Design of active and stable Co–Mo–Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2015, 15, 197–203. [Google Scholar]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Nickel-cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int. J. Hydrog. Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Mulder, W.; Sluyters, J.; Pajkossy, T.; Nyikos, L. Tafel current at fractal electrodes: Connection with admittance spectra. J. Electroanal. Chem. Interfacial Electrochem. 1990, 285, 103–115. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Budniok, A.; Rówiński, E.; Łągiewka, E.; Lasia, A. Effect of heat-treatment on the mechanism and kinetics of the hydrogen evolution reaction on Ni-P + TiO2 + Ti electrodes. J. Appl. Electrochem. 2004, 34, 507–516. [Google Scholar] [CrossRef]
- Damian, A.; Omanovic, S. Ni and nimo hydrogen evolution electrocatalysts electrodeposited in a polyaniline matrix. J. Power Sources 2006, 158, 464–476. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Budniok, A.; Rówiński, E.; Łagiewka, E.; Lasia, A. The structure, morphology and electrochemical impedance study of the hydrogen evolution reaction on the modified nickel electrodes. Int. J. Hydrog. Energy 2004, 29, 145–157. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Kelsall, G.H. Models of hypochlorite production in electrochemical reactors with plate and porous anodes. J. Appl. Electrochem. 2007, 37, 1203–1217. [Google Scholar] [CrossRef]
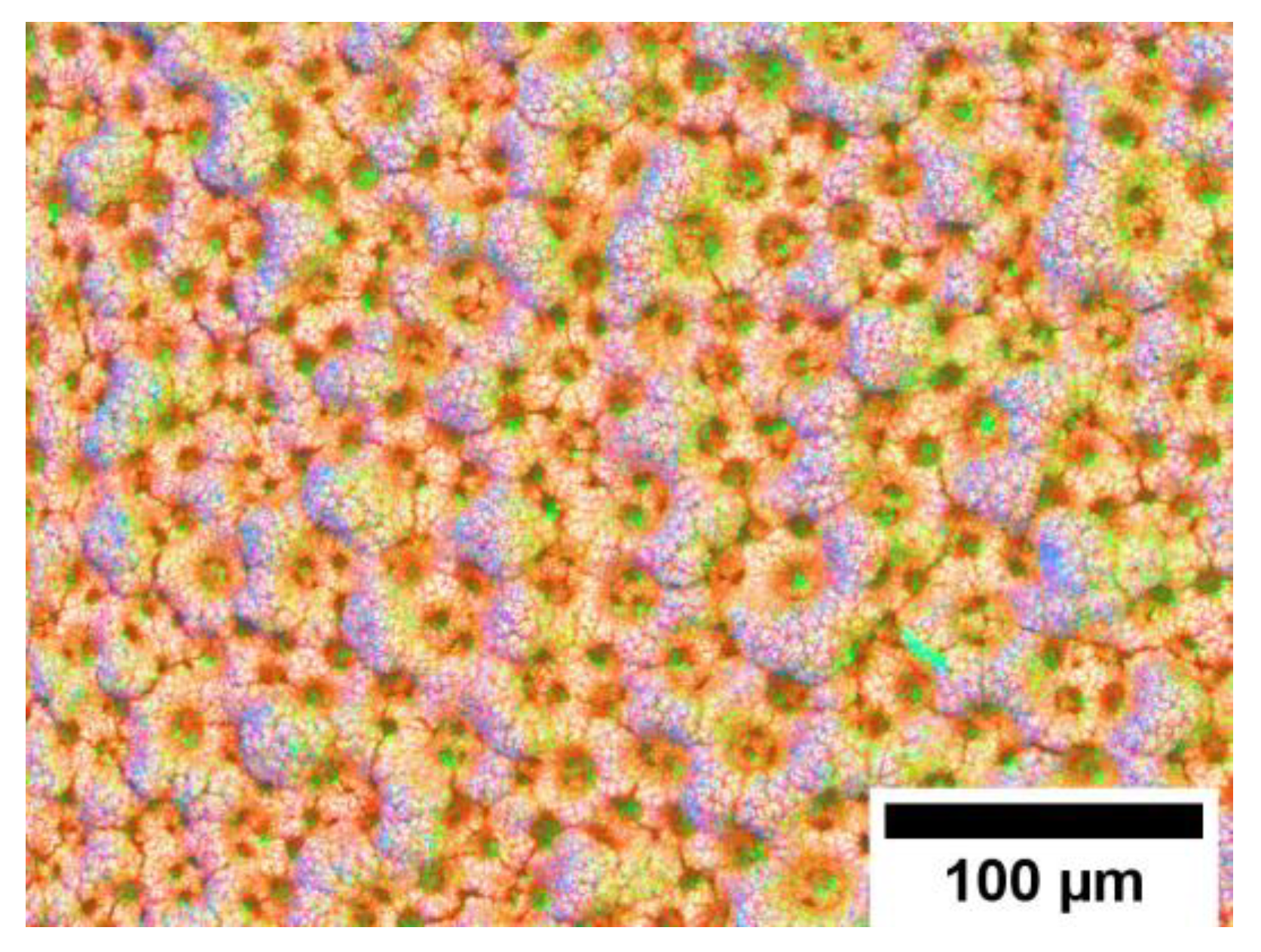
| Solution No | CoCl2 (mol/L) | NH4Cl (mol/L) | CoSO4 (mol/L) | (NH4)2SO4 (mol/L) | Isopropyl Alcohol (mol/L) |
|---|---|---|---|---|---|
| 1 | 0.2 | 2 | − | − | − |
| 2 | 0.2 | 2 | − | − | 2 |
| 3 | − | − | 0.2 | 1 | − |
| 4 | − | − | 0.2 | 1 | 2 |
| Solution Used for Co-Foam Deposition | R0, Ω | CPE1, Fsn−1 | n | R1, Ω | CPEDL, Fsn−1 | n | R2, Ω |
|---|---|---|---|---|---|---|---|
| 1 | 3.668 ± 0.50% | 0.1131 ± 1.7% | 0.5 | 0.7317 ± 3.5% | 0.0342 ± 0.91% | 0.9321 ± 0.54% | 133.7 ± 5.5% |
| 2 | 4.081 ± 0.27% | 0.0499 ± 1.6% | 0.7652 ± 1.4% | 0.0112 ± 0.37% | 0.8844 ± 0.17% | 349.7 ± 1.8% | |
| 3 | 4.872 ± 0.43% | 0.0468 ± 1.9% | 0.878 ± 2.1% | 0.028 ± 0.82% | 0.8313 ± 0.48% | 508.8 ± 10.7% | |
| 4 | 5.427 ± 0.53% | 0.046681 ± 2.9% | 0.80871 ± 2.3% | 0.01736 ± 0.94% | 0.85157 ± 0.45% | 311.9 ± 9.1% | |
| Pure Co | 8.442 ± 0.17% | − | − | − | 0.000181 ± 0.402% | 0.905 ± 0.0868% | 1458 ± 0.79% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vainoris, M.; Tsyntsaru, N.; Cesiulis, H. Modified Electrodeposited Cobalt Foam Coatings as Sensors for Detection of Free Chlorine in Water. Coatings 2019, 9, 306. https://doi.org/10.3390/coatings9050306
Vainoris M, Tsyntsaru N, Cesiulis H. Modified Electrodeposited Cobalt Foam Coatings as Sensors for Detection of Free Chlorine in Water. Coatings. 2019; 9(5):306. https://doi.org/10.3390/coatings9050306
Chicago/Turabian StyleVainoris, Modestas, Natalia Tsyntsaru, and Henrikas Cesiulis. 2019. "Modified Electrodeposited Cobalt Foam Coatings as Sensors for Detection of Free Chlorine in Water" Coatings 9, no. 5: 306. https://doi.org/10.3390/coatings9050306
APA StyleVainoris, M., Tsyntsaru, N., & Cesiulis, H. (2019). Modified Electrodeposited Cobalt Foam Coatings as Sensors for Detection of Free Chlorine in Water. Coatings, 9(5), 306. https://doi.org/10.3390/coatings9050306







