Implant Coating Manufactured by Micro-Arc Oxidation and Dip Coating in Resorbable Polylactide for Antimicrobial Applications in Orthopedics
Abstract
1. Introduction
2. Materials and Methods
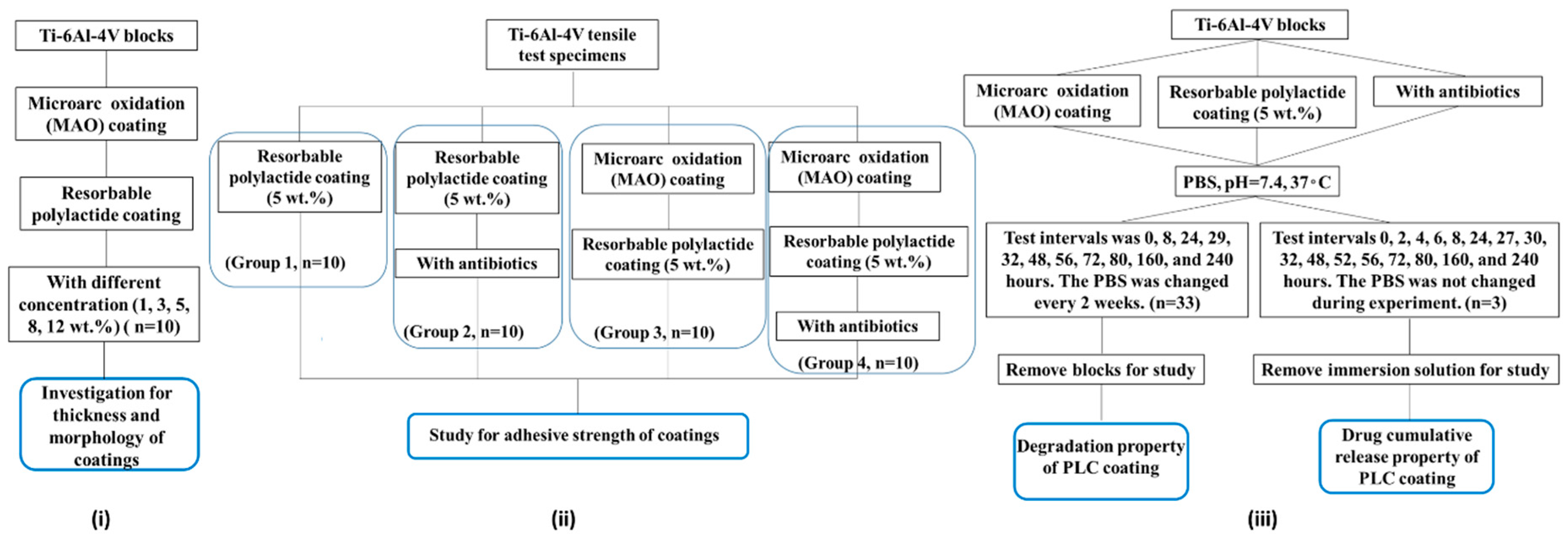
2.1. Preparation of Coatings on Ti-6Al-4V Blocks
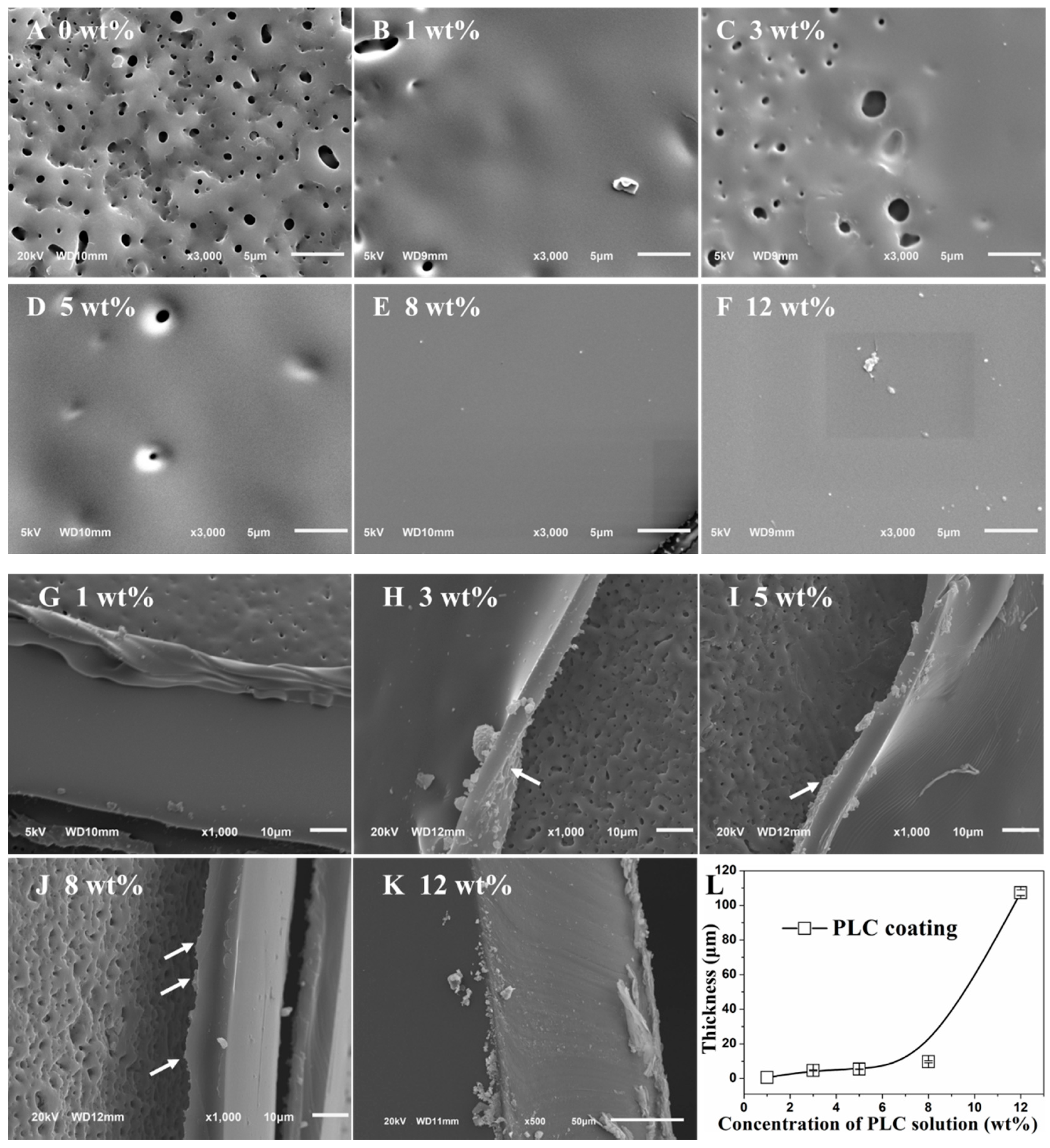
2.2. Morphology and Thickness of Double Coatings
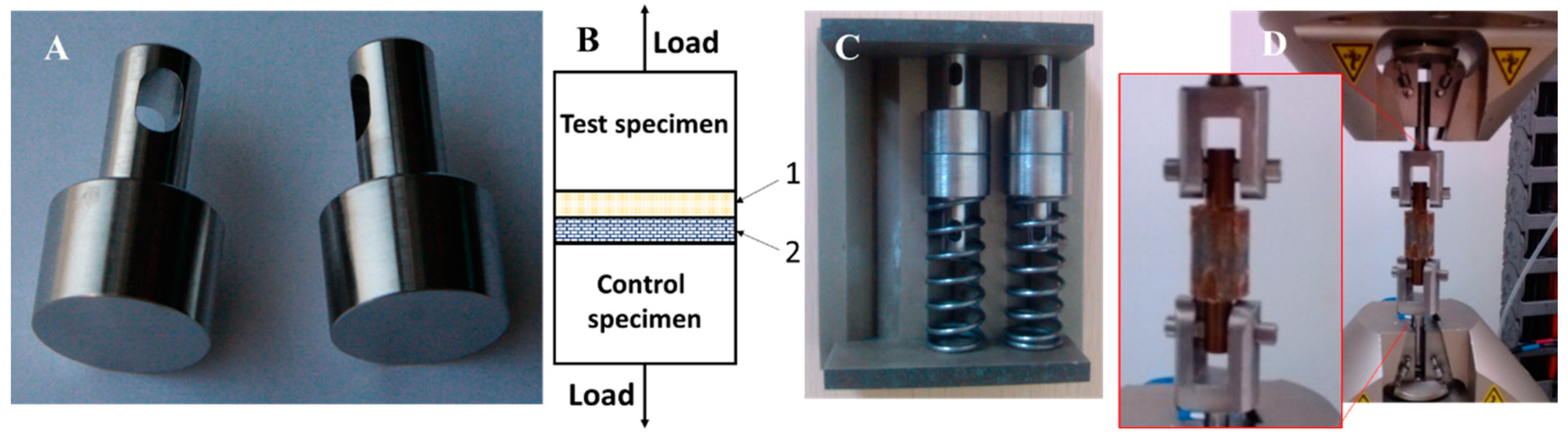
2.3. Tensile Test for Adhesion Strength between MAO Coating and Resorbable PLC Coating
2.4. Degradation Rate and Antibiotic Delivery Rate from Resorbable PLC Coating
3. Results
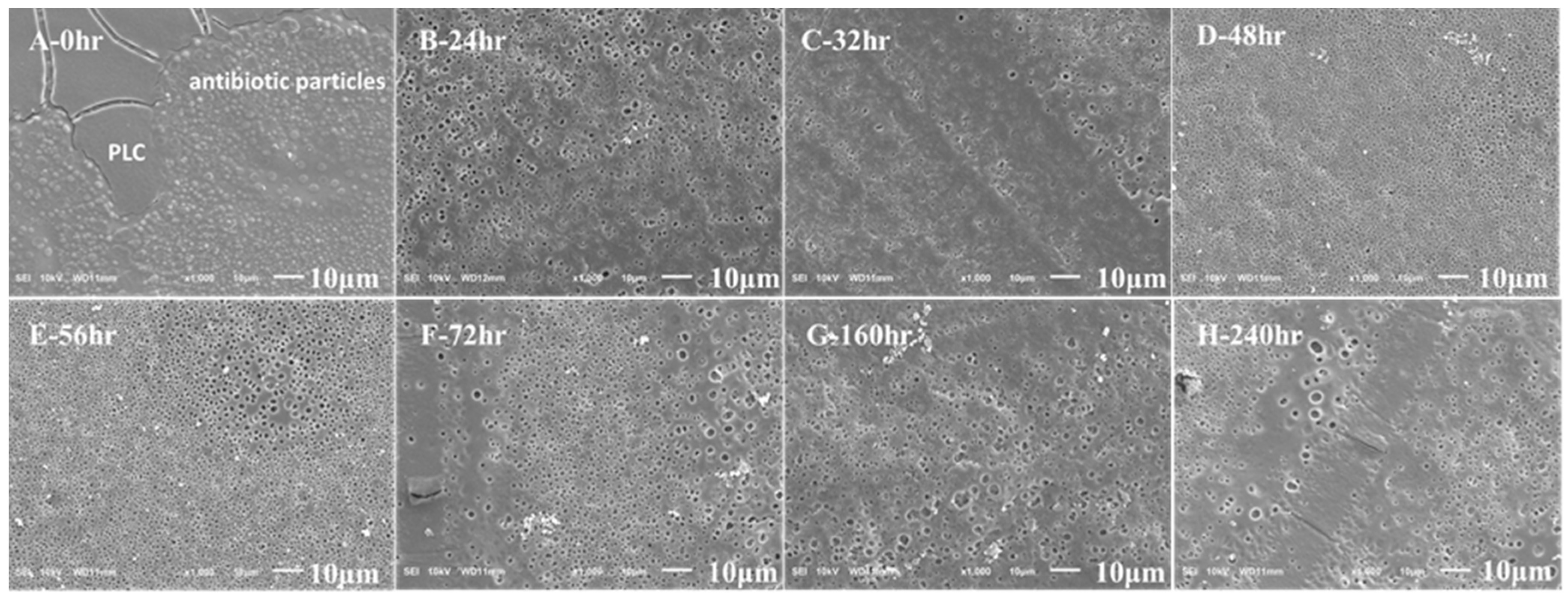
3.1. Morphology and Thickness Characterization of Double Coatings on Ti6-Ai4-V Blocks
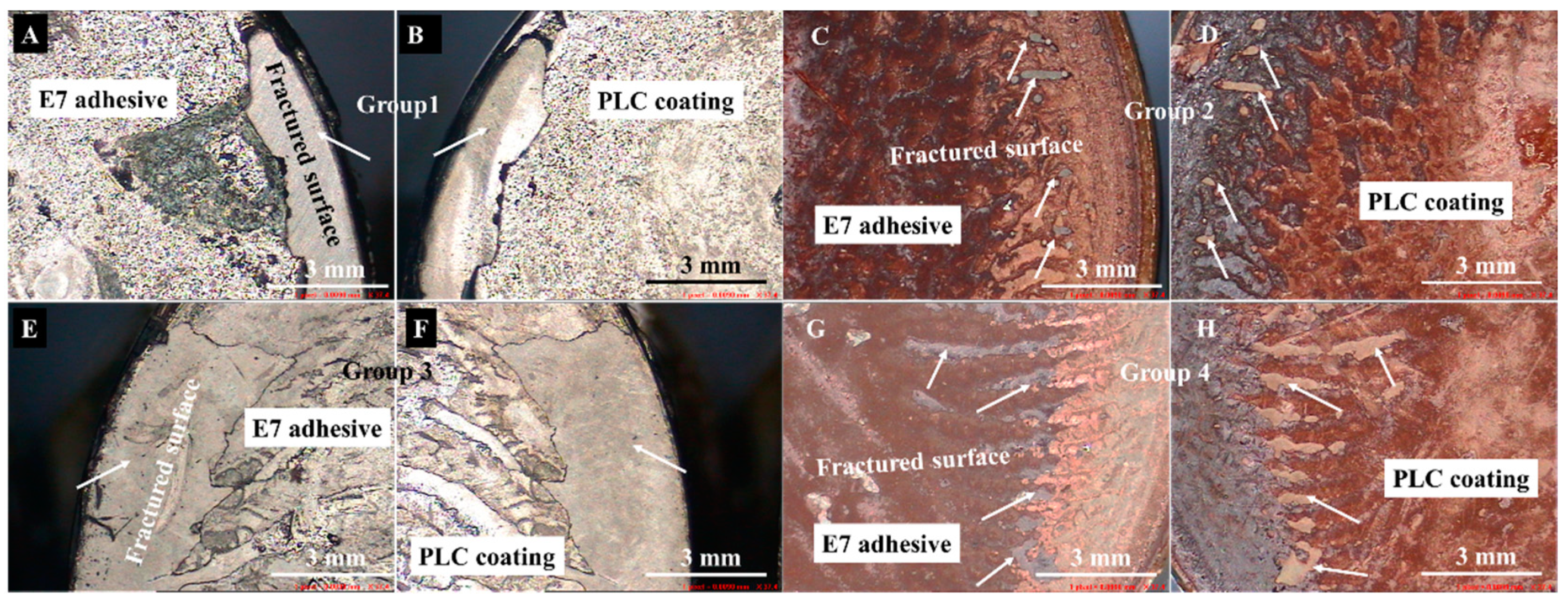
3.2. Adhesion Strength between MAO Coating and Resorbable PLC Coating
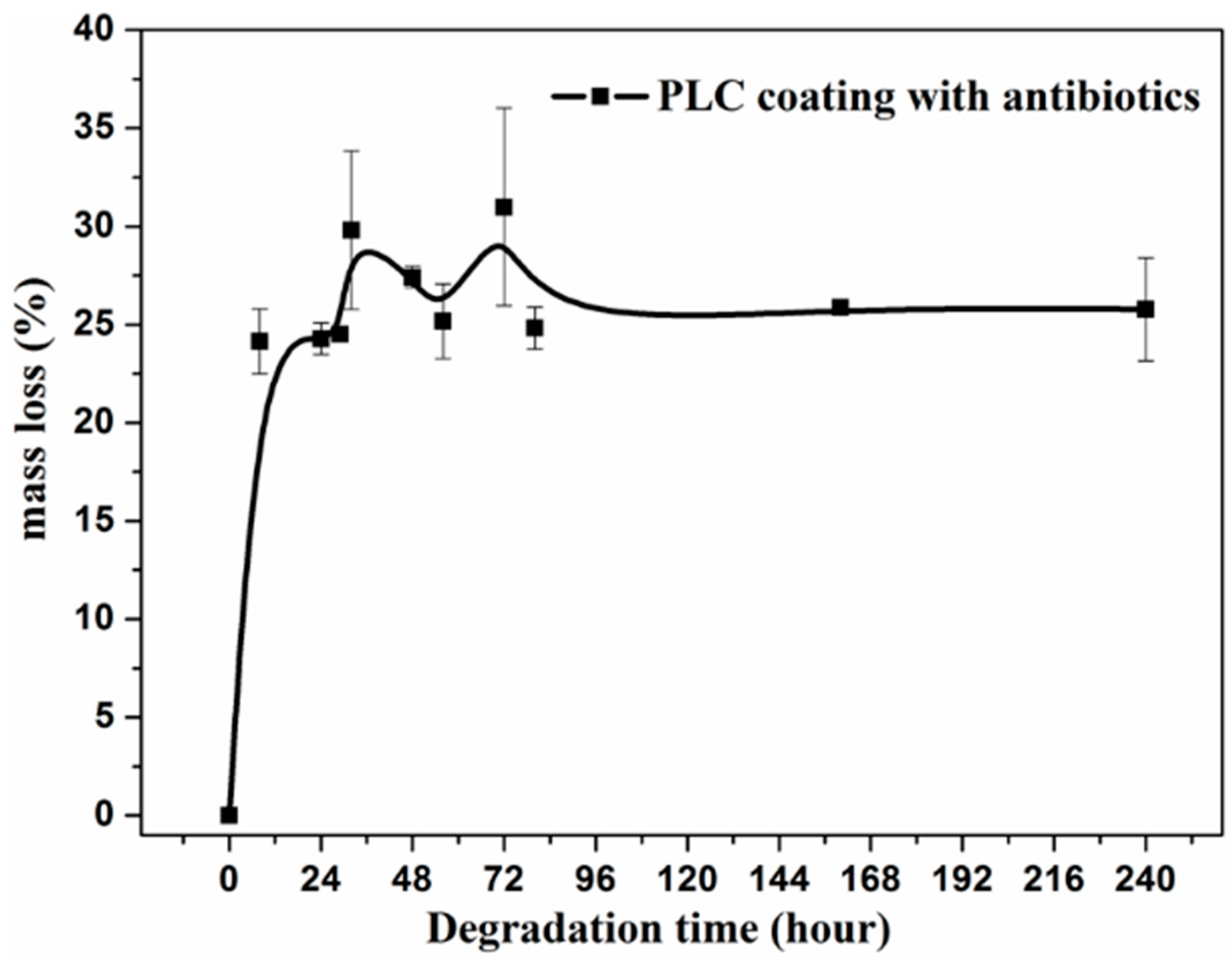
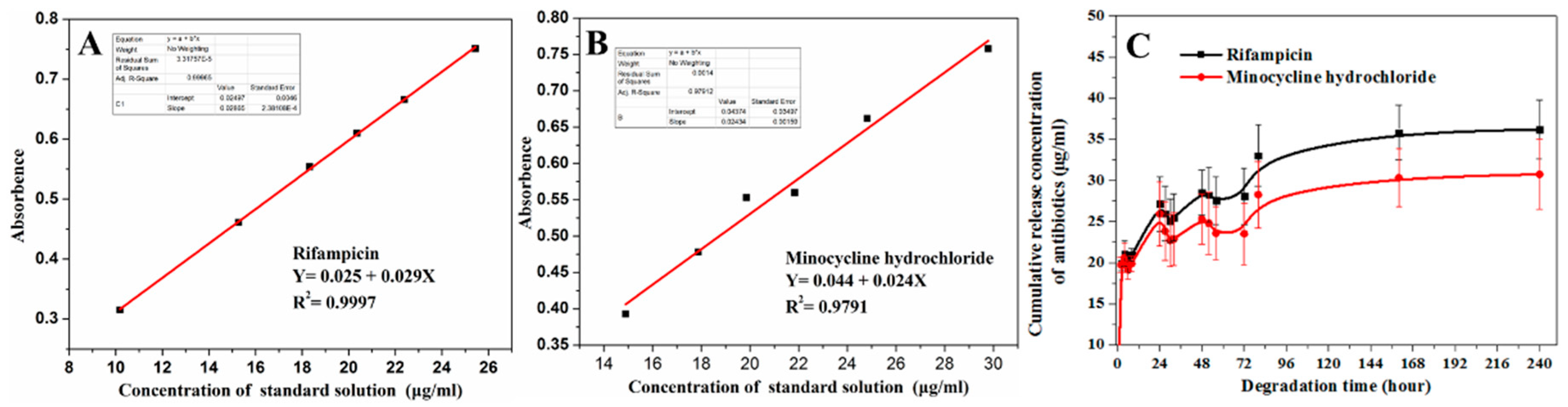
3.3. Degradation and Antibiotic Delivery Rate of Resorbable PLC Coating
4. Discussion
4.1. Interaction between MAO and PLC Coatings
4.2. Degradation and Antibiotic Release Rate of Double Coatings
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, X.; Zhang, X.; Wang, X.; Qin, L. Review of antibacterial activity of titanium-based implants’ surfaces fabricated by micro-arc oxidation. Coatings 2017, 7, 45. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Baruzzi, F.; de Candia, S.; Giangregorio, M.M.; Giannossa, L.C.; Dicarlo, M.; Mattioli-Belmonte, M.; Sabbatini, L.; De Giglio, E. Silver-loaded chitosan coating as an integrated approach to face titanium implant-associated infections: Analytical characterization and biological activity. Anal. Bioanal. Chem. 2017, 409, 7211–7221. [Google Scholar] [CrossRef]
- Young, S.; Lie, S.A.; Hallan, G.; Zirkle, L.G.; Engesaeter, L.B.; Havelin, L.I. Low infection rates after 34,361 intramedullary nail operations in 55 low- and middle-income countries: Validation of the surgical implant generation network (sign) online surgical database. Acta Orthop. 2011, 82, 737–743. [Google Scholar] [CrossRef]
- Gollwitzer, H. Antibacterial poly(d,l-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.A.; Beenken, K.E.; Skinner, R.A.; Meeker, D.G.; Smeltzer, M.S.; Haggard, W.O.; Troxel, K.S. Antibiotic-loaded phosphatidylcholine inhibits staphylococcal bone infection. World J. Orthop. 2016, 7, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Malizos, K.; Blauth, M.; Danita, A.; Capuano, N.; Mezzoprete, R.; Logoluso, N.; Drago, L.; Romano, C.L. Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: A multicenter randomized controlled trial. J. Orthop. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2017, 18, 159–169. [Google Scholar] [CrossRef]
- Jennings, J.A.; Carpenter, D.P.; Troxel, K.S.; Beenken, K.E.; Smeltzer, M.S.; Courtney, H.S.; Haggard, W.O. Novel antibiotic-loaded point-of-care implant coating inhibits biofilm. Clin. Orthop. Relat. Res. 2015, 473, 2270–2282. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.I.; Kinnari, T.J.; Aarnisalo, A.A.; Kuusela, P.; Jero, J. Comparison of bacterial adherence to polylactides, silicone, and titanium. Acta Oto-Laryngol. 2009, 127, 587–593. [Google Scholar] [CrossRef]
- Gimeno, M.; Pinczowski, P.; Mendoza, G.; Asin, J.; Vazquez, F.J.; Vispe, E.; Garcia-Alvarez, F.; Perez, M.; Santamaria, J.; Arruebo, M.; et al. Antibiotic-eluting orthopedic device to prevent early implant associated infections: Efficacy, biocompatibility and biodistribution studies in an ovine model. J. Biomed. Mater. Research. Part Bapplied Biomater. 2018, 106, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, M.; Pinczowski, P.; Vazquez, F.J.; Perez, M.; Santamaria, J.; Arruebo, M.; Lujan, L. Porous orthopedic steel implant as an antibiotic eluting device: Prevention of post-surgical infection on an ovine model. Int. J. Pharm. 2013, 452, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Gnedenkov, S.V.; Sharkeev, Y.P.; Sinebryukhov, S.L.; Khrisanfova, O.A.; Legostaeva, E.V.; Zavidnaya, A.G.; Puz’, A.V.; Khlusov, I.A.; Opra, D.P. Functional coatings formed on the titanium and magnesium alloys as implant materials by plasma electrolytic oxidation technology: Fundamental principles and synthesis conditions. Corros. Rev. 2016, 34, 65–83. [Google Scholar] [CrossRef]
- Cerchier, P.; Pezzato, L.; Brunelli, K.; Dolcet, P.; Bartolozzi, A.; Bertani, R.; Dabala, M. Antibacterial effect of peo coating with silver on AA7075. Mater. Sci. Eng. C 2017, 75, 554–564. [Google Scholar] [CrossRef]
- Mohedano, M.; Luthringer, B.J.C.; Mingo, B.; Feyerabend, F.; Arrabal, R.; Sanchez-Egido, P.J.; Blawert, C.; Willumeit-Römer, R.; Zheludkevich, M.L.; Matykina, E. Bioactive plasma electrolytic oxidation coatings on Mg-Ca alloy to control degradation behaviour. Surf. Coat. Technol. 2017, 315, 454–467. [Google Scholar] [CrossRef]
- Durdu, S. Characterization, bioactivity and antibacterial properties of copper-based TiO2 bioceramic coatings fabricated on titanium. Coatings 2018, 9, 1. [Google Scholar] [CrossRef]
- Pezzato, L.; Cerchier, P.; Brunelli, K.; Bartolozzi, A.; Bertani, R.; Dabalà, M. Plasma electrolytic oxidation coatings with fungicidal properties. Surf. Eng. 2018, 35, 325–333. [Google Scholar] [CrossRef]
- Vester, H.; Wildemann, B.; Schmidmaier, G.; Stockle, U.; Lucke, M. Gentamycin delivered from a pdlla coating of metallic implants: In vivo and in vitro characterisation for local prophylaxis of implant-related osteomyelitis. Injury 2010, 41, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Ginjupalli, K.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Nagaraja Upadhya, P. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535. [Google Scholar] [CrossRef]
- Zilberman, M.; Elsner, J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- ter Boo, G.J.; Grijpma, D.W.; Moriarty, T.F.; Richards, R.G.; Eglin, D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials 2015, 52, 113–125. [Google Scholar] [CrossRef]
- Torabi, A.; Etsell, T.H.; Sarkar, P. Dip coating fabrication process for micro-tubular sofcs. Solid State Ion. 2011, 192, 372–375. [Google Scholar] [CrossRef]
- ISO 4624-Paints Varnishes and Plastics–Pull-off Test for Adhesion; ISO Technical Committees: Geneva, Switzerland, 2003.
- Cheng, S.; Wei, D.; Zhou, Y. Mechanical and corrosion resistance of hydrophilic sphene/titania composite coatings on titanium and deposition and release of cefazolin sodium/chitosan films. Appl. Surf. Sci. 2011, 257, 2657–2664. [Google Scholar] [CrossRef]
- Khanna, R.; Ong, J.; Oral, E.; Narayan, R. Progress in wear resistant materials for total hip arthroplasty. Coatings 2017, 7, 99. [Google Scholar] [CrossRef]
- Zhao, Q.-M.; Cheng, L.; Liu, Z.-T.; Zhao, J.-J. Surface characteristics of Zinc–TiO2 coatings prepared via micro-arc oxidation. Compos. Interfaces 2014, 21, 585–593. [Google Scholar] [CrossRef]
- Li, G.; Cao, H.; Zhang, W.; Ding, X.; Yang, G.; Qiao, Y.; Liu, X.; Jiang, X. Enhanced osseointegration of hierarchical micro/nanotopographic titanium fabricated by microarc oxidation and electrochemical treatment. Acs Appl. Mater. Interfaces 2016, 8, 3840–3852. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, D.; Zhou, Y. Structure of microarc oxidized coatings containing Si, Ca and Na on titanium and deposition of cefazolin sodium/chitosan composite film. Surf. Coat. Technol. 2011, 205, 3798–3804. [Google Scholar] [CrossRef]
- Clmneck, N.; Marcelis, L.; Amiri-Lamraski, M.H.; Gordts, B. Treatment of severe staphylococcal infections with treatment of severe staphylococcal infections with. J. Antimicrob. Chemother. 1984, 13, 17–22. [Google Scholar] [CrossRef]
- Goto, Y.; Hiramatsu, K.; Nasu, M. Improved efficacy with nonsimultaneous administration of netilmicin and minocycline against methicillin–resistant staphylococcus aureus in in vitro and in vivo models. Int. J. Antimicrob. Agents 1999, 39–46. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Sin, L.V.; Yu, J.; Francis, K.P.; Purchio, T.F.; Contag, P.R. Noninvasive optical imaging method to evaluate postantibiotic effects on biofilm infection in vivo. Antimicrob. Agents Chemother. 2004, 48, 2283–2287. [Google Scholar] [CrossRef]
- Gomez, E.O.; Jafary, A.; Dever, L.L. Daptomycin and rifampin for the treatment of methicillin-resistant staphylococcus aureus septic pulmonary emboli in the absence of endocarditis. Microb. Drug Resist. 2010, 16, 241–244. [Google Scholar] [CrossRef]
- Kastoris, A.C.; Rafailidis, P.I.; Vouloumanou, E.K.; Gkegkes, I.D.; Falagas, M.E. Synergy of fosfomycin with other antibiotics for gram-positive and gram-negative bacteria. Eur. J. Clin. Pharmacol. 2010, 66, 359–368. [Google Scholar] [CrossRef]
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef]
- Mendes, G.C.C.; Brandão, T.R.S.; Silva, C.L.M. Ethylene oxide sterilization of medical devices: A review. Am. J. Infect. Control 2007, 35, 574–581. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Stemberger, A.; Haas, N.P.; Raschke, M. Biodegradable poly(d,l-lactide) coating of implants for continuous release of growth factors. J. Biomed. Mater. Res. Appl. Biomater. 2001, 449–455. [Google Scholar] [CrossRef]

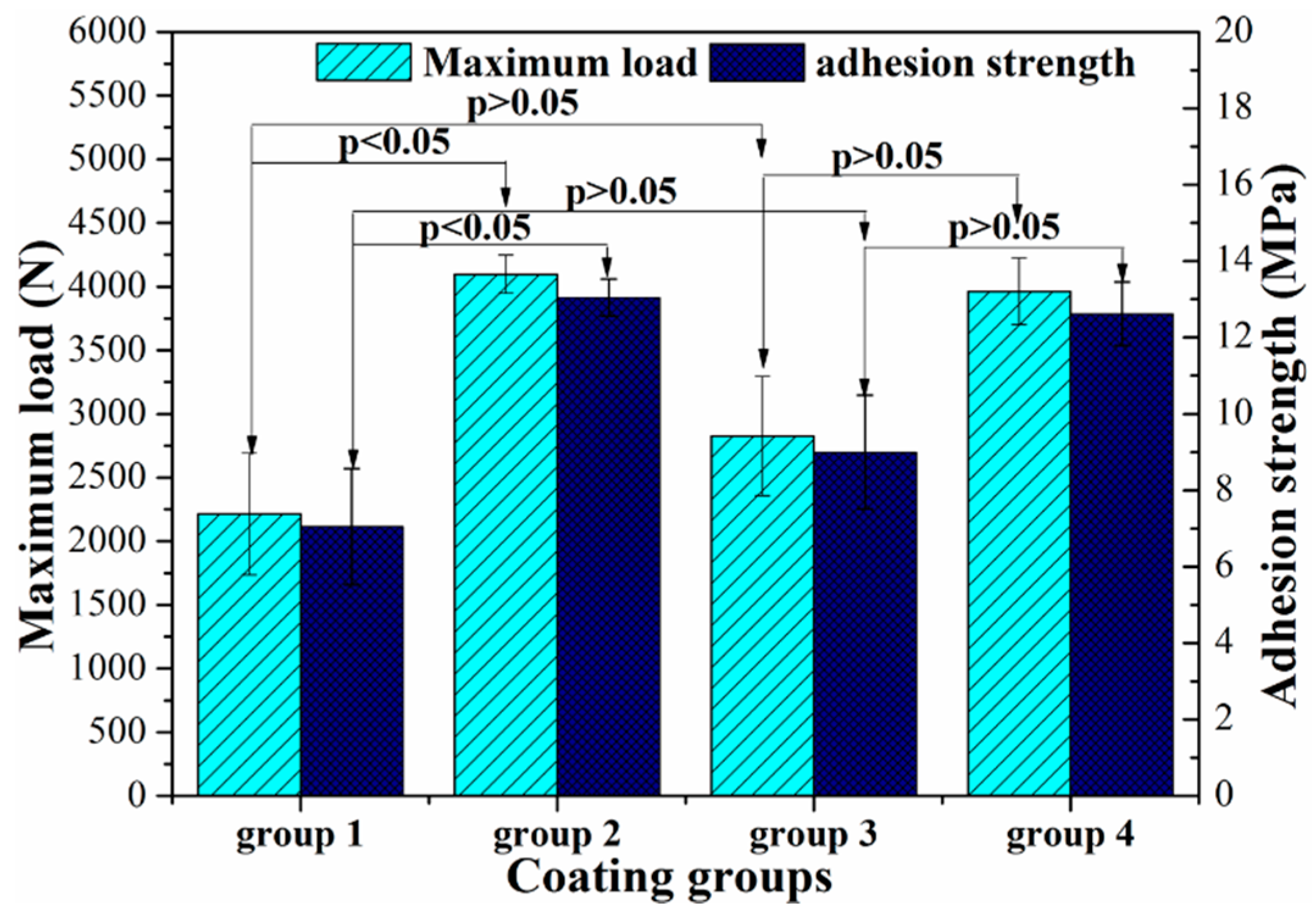
| Group Number | Coatings | Interfaces between the Paired Specimens in Each Group (Figure 3B) | ||
|---|---|---|---|---|
| Test Specimen | Between Specimens | Control Specimen | ||
| 1 | Only PLC coating | Ti-PLC | PLC-E7 | E7-Ti |
| 2 | Only PLC coating with antibiotics | Ti-PLC with antibiotics | PLC-E7 with antibiotics | E7 with antibiotics-Ti |
| 3 | Double coating | Ti-MAO-PLC | PLC-E7 | E7-MAO -Ti |
| 4 | Double coating with antibiotics | Ti-MAO-PLC with antibiotics | PLC-E7 with antibiotics | E7 with antibiotics -MAO-Ti |
| Group Number | Coatings | Strong Adhesion Interface | Failure Mode of Paired Specimens in Each Group | |
|---|---|---|---|---|
| Main Facture Interface | Symmetrical Facture Interface | |||
| Test Specimen | between Specimens | Control Specimen | ||
| 1 | Only PLC coating | Ti-PLC | PLC-E7 | E7-Ti |
| 2 | Only PLC coating with antibiotics | Ti-PLC with antibiotics | PLC-E7 with antibiotics | E7 with antibiotics-Ti |
| 3 | Double coating | Ti-MAO-PLC | PLC-E7 | E7-MAO -Ti |
| 4 | Double coating with antibiotics | Ti-MAO-PLC with antibiotics | PLC-E7 with antibiotics | E7 with antibiotics -MAO-Ti |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.-Y.; Tian, N.; Dong, X.; Cheng, C.-K. Implant Coating Manufactured by Micro-Arc Oxidation and Dip Coating in Resorbable Polylactide for Antimicrobial Applications in Orthopedics. Coatings 2019, 9, 284. https://doi.org/10.3390/coatings9050284
Cao X-Y, Tian N, Dong X, Cheng C-K. Implant Coating Manufactured by Micro-Arc Oxidation and Dip Coating in Resorbable Polylactide for Antimicrobial Applications in Orthopedics. Coatings. 2019; 9(5):284. https://doi.org/10.3390/coatings9050284
Chicago/Turabian StyleCao, Xiao-Yan, Na Tian, Xiang Dong, and Cheng-Kung Cheng. 2019. "Implant Coating Manufactured by Micro-Arc Oxidation and Dip Coating in Resorbable Polylactide for Antimicrobial Applications in Orthopedics" Coatings 9, no. 5: 284. https://doi.org/10.3390/coatings9050284
APA StyleCao, X.-Y., Tian, N., Dong, X., & Cheng, C.-K. (2019). Implant Coating Manufactured by Micro-Arc Oxidation and Dip Coating in Resorbable Polylactide for Antimicrobial Applications in Orthopedics. Coatings, 9(5), 284. https://doi.org/10.3390/coatings9050284





