Effect of Tea Polyphenols on Curdlan/Chitosan Blending Film Properties and Its Application to Chilled Meat Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Film Properties
2.3.1. Mechanical Properties
2.3.2. Water Vapor Permeability (WVP) and Moisture Content (MC)
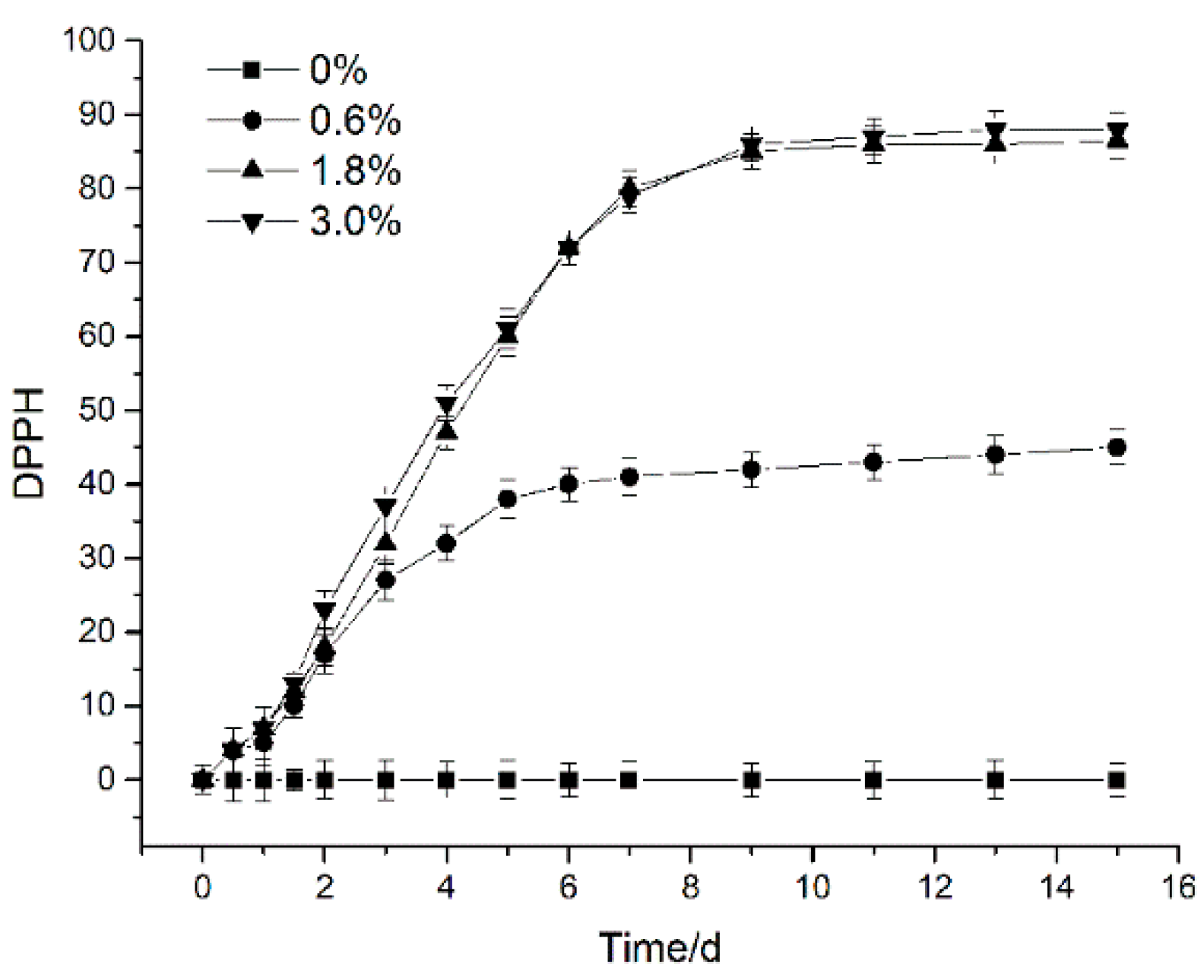
2.3.3. Antioxidant Activity
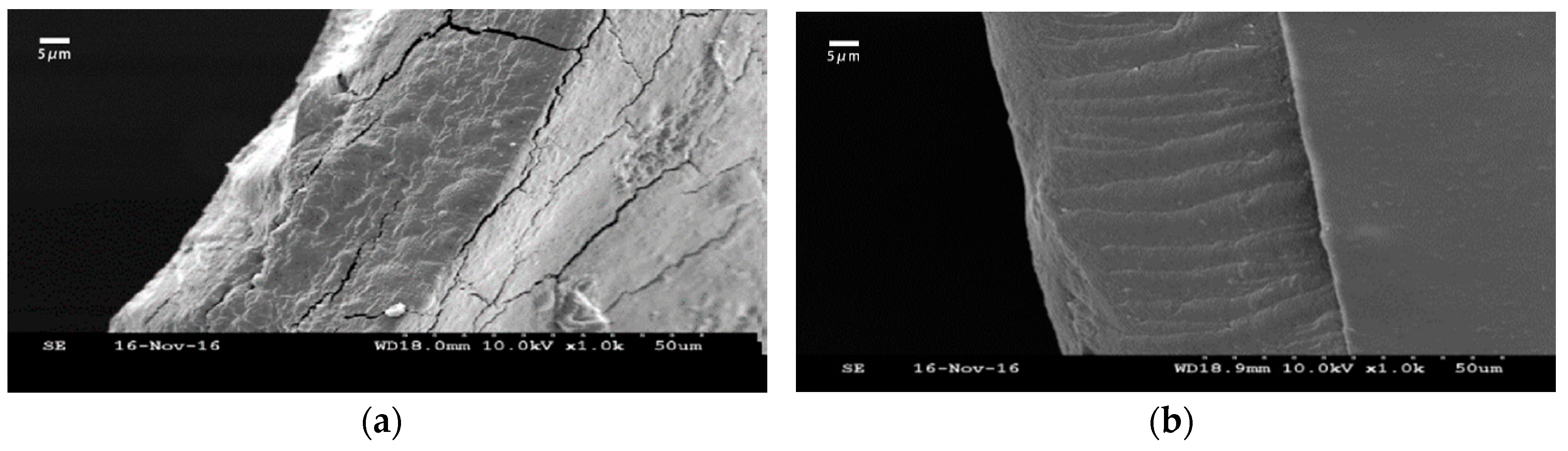
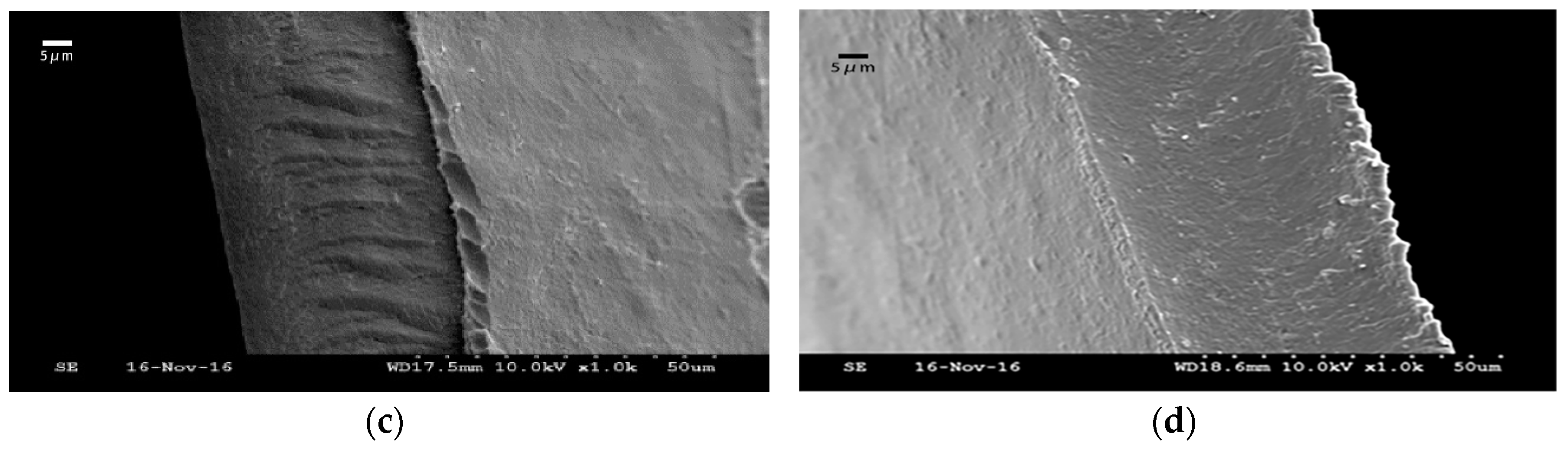
2.3.4. Scanning Electron Microscopy (SEM) Analysis
2.4. Application to Chilled Meat Preservation
2.4.1. Packing Chilled Meat with the Blending Film
2.4.2. Antibacterial Activity Analysis
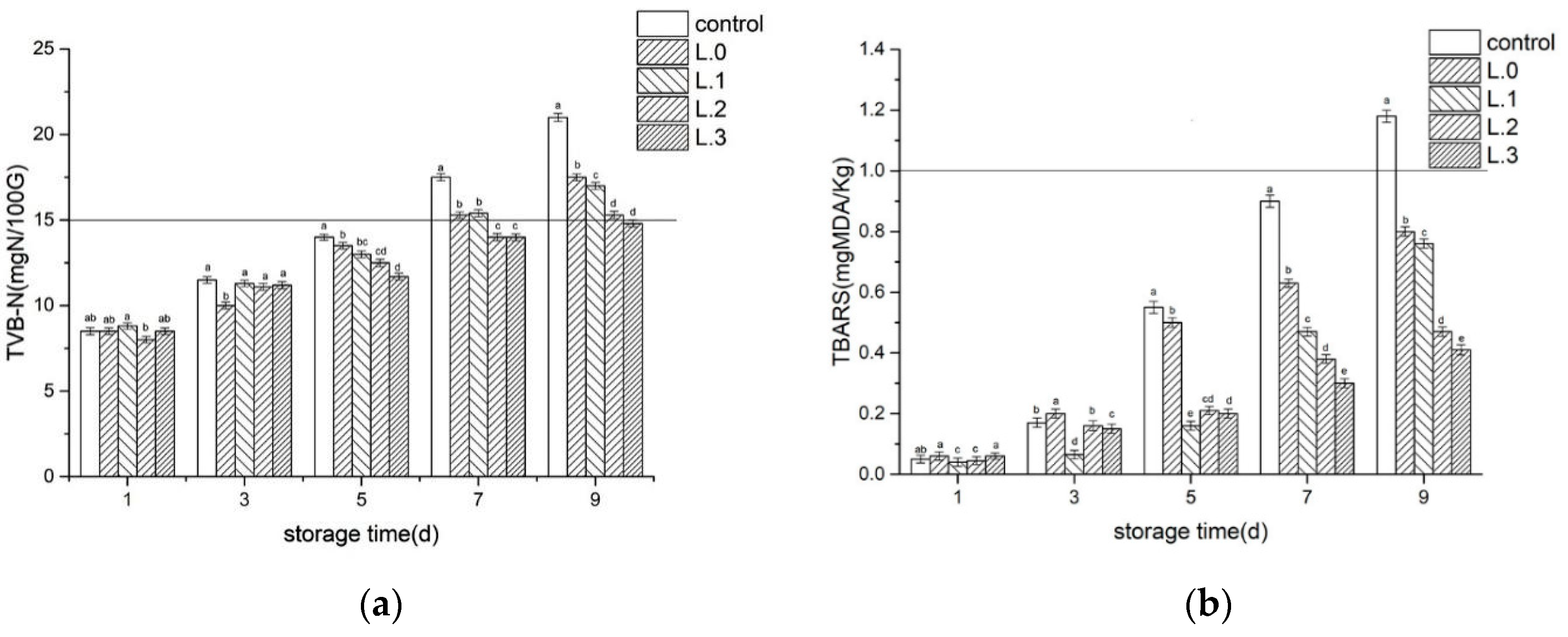
2.4.3. Determination of the total volatile basic nitrogen (TVB-N)
2.4.4. Evaluation of Thiobarbituric Acid Reactive Substances (TBARS)
2.4.5. Color
2.4.6. pH
2.5. Statistical Analysis
3. Results and Discussion
3.1. Film Properties
3.1.1. Mechanical Properties
3.1.2. Water Vapor Permeability and Moisture Content
3.1.3. Antioxidant Properties
3.1.4. Scanning Electron Microscopy (SEM) Analysis
3.2. Application to Chilled Meat Preservation
3.2.1. Microbial Assay
3.2.2. TVB-N
3.2.3. TBARS
3.2.4. pH
3.2.5. Color
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teasdale, A.; Jahn, M.; Bailey, S.; Feilden, A.; Taylor, G.; Corcoran, M.L.; Malick, R.; Jenke, D.; Stults, C.L.; Nagao, L.M. Controlled Extraction Studies Applied to Polyvinyl Chloride and Polyethylene Materials: Conclusions from the ELSIE Controlled Extraction Pilot Study. AAPS PharmSciTech. 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Dai, H.; Dai, P. Non-Petroleum Based Biodegradable Plastic with the Development of Ecologicalization in Food Packaging Materials. Packag. J. 2015, 7, 1–6. [Google Scholar]
- Zhang, H. Preparation Technology of Compound Packaging Film from Degradable Soy Protein Isolate. J. Chin. Cereals Oils Assoc. 2009, 24, 32–37. [Google Scholar]
- Bourlieu, C.; Guillard, V.; Vallès-Pamiès, B.; Guilbert, S.; Gontard, N. Edible Moisture Barriers: How to Assess of their Potential and Limits in Food Products Shelf-Life Extension? Crit. Rev. Sci. Nutr. 2009, 49, 474–499. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Chakraborty, M.; Chen, S. Impact of reaction conditions on the simultaneous production of polysaccharides and bio-oil from heterotrophically grown Chlorella sorokiniana by a unique sequential hydrothermal liquefaction process. Bioresour. Technol. 2012, 110, 617–627. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, J.; Wang, Z.; Hang, H.; Zhao, W.; Zhuang, Y.; Chu, J. Kinetic analysis of curdlan production by Alcaligenes faecalis with maltose, sucrose, glucose and fructose as carbon sources. Bioresour. Technol. 2018, 259, 319–324. [Google Scholar] [CrossRef]
- Nakao, Y.; Konno, A.; Taguchi, T.; Tawada, T.; Kasai, H.; Toda, J.; Terasaki, M. Curdlan: Properties and Application to Foods. J. Food Sci. 2010, 56, 769–772. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Anand, M.; Kalaivani, R.; Maruthupandy, M.; Kumaraguru, A.K.; Suresh, S. Extraction and Characterization of Chitosan from Marine Crab and Squilla Collected from the Gulf of Mannar Region, South India. J. Chitin Chitosan Sci. 2014, 2, 280–287. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Boil. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Poshina, D.N.; Raik, S.V.; Poshin, A.N.; Skorik, Y.A. Accessibility of chitin and chitosan in enzymatic hydrolysis: A review. Polym. Degrad. Stab. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhou, X.Y.; Tian, Z.H.; Li, H.; Wang, S.S. Application of Modified Chitosan in Fruit Juice Clarification. Appl. Mech. Mater. 2014, 651, 211–214. [Google Scholar] [CrossRef]
- Pushkala, R.; Raghuram, P.K.; Srividya, N. Chitosan based powder coating technique to enhance phytochemicals and shelf life quality of radish shreds. Boil. Technol. 2013, 86, 402–408. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhao, Y. Effect of Molecular Weight, Acid, and Plasticizer on the Physicochemical and Antibacterial Properties of β-Chitosan Based Films. J. Sci. 2012, 77, E127–E136. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Kim, S.; Yang, G.Y.; Lee, M.J.; Liao, J.; Chung, J.Y.; Ho, C.T. Inhibition of carcinogenesis by tea: Bioavailability of tea polyphenols and mechanisms of actions. Proc. Soc. Exp. Biol. Med. 2010, 220, 213–217. [Google Scholar]
- Zhang, J.; Jiang, Y.W. Preparation of the Tea Polyphenols by Resin Chromatography. Chin. Agric. Sci. Bull. 2012, 28, 282–287. [Google Scholar]
- Parker, C. Effect of Extraction Solvents on Phenolic, Flavonoid and Antioxidant activities of Three Nigerian Medicinal Plants. Nat. Sci. 2011, 9, 7. [Google Scholar]
- Zhu, M.X.; Huang, C.X. Effect of Tea Polyphenols on Properties of Chitosan/Polyvinyl Alcohol Composite Films. Packag. Eng. 2018, 39, 110–114. [Google Scholar]
- Kumudavally, K.; Phanindrakumar, H.; Tabassum, A.; Radhakrishna, K.; Bawa, A. Green tea—A potential preservative for extending the shelf life of fresh mutton at ambient temperature (25±2 °C). Food Chem. 2008, 107, 426–433. [Google Scholar] [CrossRef]
- Tang, S.; Sheehan, D.; Buckley, D.J.; Morrissey, P.A.; Kerry, J.P. Anti-oxidant activity of added tea catechins on lipid oxidation of raw minced red meat, poultry and fish muscle. Int. J. Food Sci. Technol. 2010, 36, 685–692. [Google Scholar] [CrossRef]
- Liu, W.R.; Yuan, Q. Study on Modeling of Fresh Meat Product Cold Chain Logistics Process Based on Temporal Petri Nets. Logist. Technol. 2012, 31, 289–291. [Google Scholar]
- Zhou, G.; Xu, X.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Baltic´, T.; Baltic´, B. The influence of vacuum packaging on microbiological status and sensory properties of fresh beef. Tehnol. Mesa 2012, 53, 103–111. [Google Scholar] [CrossRef]
- Choi, I.; Lee, S.E.; Chang, Y.; Lacroix, M.; Han, J. Effect of oxidized phenolic compounds on cross-linking and properties of biodegradable active packaging film composed of turmeric and gelatin. LWT-Food Sci. Technol. 2018, 93, 427–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, Y.; Wang, Y.; Jiang, P.; Liu, X. Improvement of application properties of chitosan-based food packaging films by lavender essential oil. Trans. Chin. Soc. Agric. Eng. 2014, 58, 1907–1914. [Google Scholar]
- Kanatt, S.R.; Chander, R.; Sharma, A. Effect of irradiated chitosan on the rancidity of radiation-processed lamb meat. Int. J. Sci. Technol. 2004, 39, 997–1003. [Google Scholar] [CrossRef]
- El-Fattah, H.A.A.; El-Mahallawi, I.S.; Shazly, M.H.; Khalifa, W.A.; El-Fattah, H.A. Optical Properties and Microstructure of TiNxOy and TiN Thin Films before and after Annealing at Different Conditions. Coatings 2019, 9, 22. [Google Scholar] [CrossRef]
- Park, M.; Jin, J.S.; Wilson, L.S. A new texture analysis method for classification of interstitial lung abnormalities in chest radiography. PLoS ONE 2014, 9, 239–244. [Google Scholar]
- Kwak, Y.S.; Park, S.W.; Lee, Y.H.; Kim, H.D. Preparation and properties of waterborne polyurethanes for water-vapor-permeable coating materials. J. Appl. Polym. Sci. 2010, 89, 123–129. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Shi, F.Y.; Yang, J.; Li, F.-F. Study on changes of sensory, physical and chemical properties and the total number of colonies, and the mutual relationship between them during meat storage. Food Sci. Technol. 2009, 7, 1–6. [Google Scholar]
- Li, E.-W. Three kinds of food colony of the total number of testing methods. Chin. J. Health Lab. Technol. 2010, 20, 1940–1941. [Google Scholar]
- Dainty, R.H.; Mackey, B.M. The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. J. Appl. Microbiol. 2010, 73, 103s–114s. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Q.; Wan, X.; Zhao, J. Determination of total volatile basic nitrogen (TVB-N) content and Warner–Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chem. 2011, 126, 1354–1360. [Google Scholar] [CrossRef]
- Rey, A.I.; Kerry, J.P.; Lynch, P.B.; López-Bote, C.J.; Buckley, D.J.; Morrissey, P.A. Effect of dietary oils and alpha-tocopheryl acetate supplementation on lipid (TBARS) and cholesterol oxidation in cooked pork. J. Anim. Sci. 2001, 79, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Sigalas, M.P. A DFT Study on the Radical Scavenging Activity of Maritimetin and Related Aurones. J. Phys. Chem. A 2008, 112, 12196–12202. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Liu, F.Z. The effect of dietary VC on growth performance, meat quality, immune function and antioxidant capacity of broilers. J. Northwest A F Univ. 2010, 38, 1–7. [Google Scholar]
- Cheng, F.-Y.; Huang, C.-W.; Tominaga, K.; Lin, L.-C.; Sakata, R.; Wan, T.-C. The Effects of Chicken Leg Bone Extract on Antioxidative Properties under Different Heating Condition. Asian-Aust. J. Anim. Sci. 2008, 21, 1815–1820. [Google Scholar] [CrossRef]
- Zhao, J.W.; Huang, X.W.; Zou, X.B. Research on Discrimination of Dominant Spoilage Bacteria in Pork Based on Porphyrins and pH Indicators Gas-Sensing Material. J. Food Sci. Technol. 2013, 31, 9–13. [Google Scholar]
- GB/T 9959.2-2008. Fresh and Frozen Pork Lean, Cuts; China National Administration for Quality Supervision and Inspection and Quarantine: Beijing, China, 2008. [Google Scholar]
- Xiong, Z.; Sun, D.-W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituricacid reactive substances (TBARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Comprehensive Reviews in Food Science and Food Safety; Wiley-Blackwell: Hoboken, NJ, USA, 2013.
- Chen, X.L.; Wang, S.P.; Liu, H. Rapid Analysis and Evaluation of Freshness of Chilled Pork Based on Color Difference. Food Sci. 2012, 33, 204–208. [Google Scholar]
- Ristić, M.; Damme, K. Significance of pH-value for meat quality of broilers: Influence of breed lines. VET GLASNIK 2013, 67, 67–73. [Google Scholar] [CrossRef]
- Du, M.; Ahn, D.; Nam, K.; Sell, J. Influence of dietary conjugated linoleic acid on volatile profiles, color and lipid oxidation of irradiated raw chicken meat. Meat Sci. 2000, 56, 387–395. [Google Scholar] [CrossRef]
| Group | Content of Tea Polyphenols(w/w) |
|---|---|
| control | blank control |
| L.0 | 0% |
| L.1 | 0.6% |
| L.2 | 1.8% |
| L.3 | 3% |
| Content of Tea Polyphenols | TS | EB | WVP × 10−11 (g·m−1·s−1·Pa−1) | MC % |
|---|---|---|---|---|
| 0% | 40.02 ± 0.16a | 20.03 ± 0.19a | 12.0 ± 1.0a | 30.29 ± 1.64a |
| 0.6% | 20.24 ± 0.35c | 20.76 ± 0.24a | 5.3 ± 0.2c | 14.0 ± 0.7c |
| 1.8% | 25.73 ± 1.05b | 14.9 ± 0.7b | 5.6 ± 0.3b | 14.3 ± 0.8b |
| 3.0% | 23.45 ± 0.63c | 6.0 ± 0.6c | 4.4 ± 0.3c | 14.1 ± 0.6c |
| Evaluation Project | Processing Group | Storage Time/d | ||||
|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | ||
| logarithm of the total colony number | Control | 4.02a | 4.85a | 5.68a | 6.25a | 7.15a |
| L.0 | 3.51a | 4.25e | 4.84c | 5.25d | 6.24a | |
| L.1 | 3.25b | 3.43e | 3.75c | 4.25a | 5.25a | |
| L.2 | 3.31c | 3.42e | 3.81d | 4.16b | 4.85a | |
| L.3 | 3.42c | 3.55d | 3.62a | 4.08e | 4.67d | |
| pH | Control | 6.15a | 6.13a | 6.20a | 6.29a | 6.65a |
| L.0 | 6.05c | 5.93b | 6.11e | 6.20c | 6.37b | |
| L.1 | 5.90b | 5.78c | 5.92d | 6.03d | 6.15e | |
| L.2 | 6.08c | 6.12e | 6.08d | 6.13d | 6.14a | |
| L.3 | 6.08c | 6.06b | 6.08a | 6.03e | 6.05d | |
| lightness (L*) | Control | 35.41a | 34.13a | 33.54a | 33.48a | 33.45a |
| L.0 | 35.30d | 34.52d | 54.51e | 34.02a | 33.71b | |
| L.1 | 34.75c | 34.08a | 34.00b | 33.71c | 33.45e | |
| L.2 | 34.32b | 33.75c | 33.79e | 33.25c | 32.75e | |
| L.3 | 34.23a | 33.95b | 33.69b | 33.68e | 33.31c | |
| red degree (a*) | Control | 10.63a | 10.25a | 9.01a | 11.05a | 13.52a |
| L.0 | 10.81e | 11.53c | 9.92c | 12.90b | 14.07a | |
| L.1 | 11.52c | 12.02d | 10.74a | 12.21c | 12.15c | |
| L.2 | 11.34d | 12.19d | 11.28c | 10.57a | 12.18c | |
| L.3 | 9.66c | 10.09d | 10.13e | 10.88b | 11.49c | |
| yellow degree (b*) | Control | 6.15a | 6.65a | 7.72a | 8.19a | 7.22a |
| L.0 | 5.75a | 6.15c | 6.65b | 7.49d | 6.92e | |
| L.1 | 6.28e | 6.43c | 7.02b | 7.22d | 6.65e | |
| L.2 | 6.14d | 6.38e | 6.75d | 7.23a | 6.65c | |
| L.3 | 5.88d | 6.25a | 6.65c | 6.67c | 6.65d | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Xu, T.; Zhang, Y.; Zhang, C.; Lu, Z.; Lu, F.; Zhao, H. Effect of Tea Polyphenols on Curdlan/Chitosan Blending Film Properties and Its Application to Chilled Meat Preservation. Coatings 2019, 9, 262. https://doi.org/10.3390/coatings9040262
Zhou Y, Xu T, Zhang Y, Zhang C, Lu Z, Lu F, Zhao H. Effect of Tea Polyphenols on Curdlan/Chitosan Blending Film Properties and Its Application to Chilled Meat Preservation. Coatings. 2019; 9(4):262. https://doi.org/10.3390/coatings9040262
Chicago/Turabian StyleZhou, Ying, Tonglin Xu, Yu Zhang, Chong Zhang, Zhaoxin Lu, Fengxia Lu, and Haizhen Zhao. 2019. "Effect of Tea Polyphenols on Curdlan/Chitosan Blending Film Properties and Its Application to Chilled Meat Preservation" Coatings 9, no. 4: 262. https://doi.org/10.3390/coatings9040262
APA StyleZhou, Y., Xu, T., Zhang, Y., Zhang, C., Lu, Z., Lu, F., & Zhao, H. (2019). Effect of Tea Polyphenols on Curdlan/Chitosan Blending Film Properties and Its Application to Chilled Meat Preservation. Coatings, 9(4), 262. https://doi.org/10.3390/coatings9040262







