Super-Hydrophobic Co–Ni Coating with High Abrasion Resistance Prepared by Electrodeposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Surface Morphology of the As-Deposited Co–Ni Coating
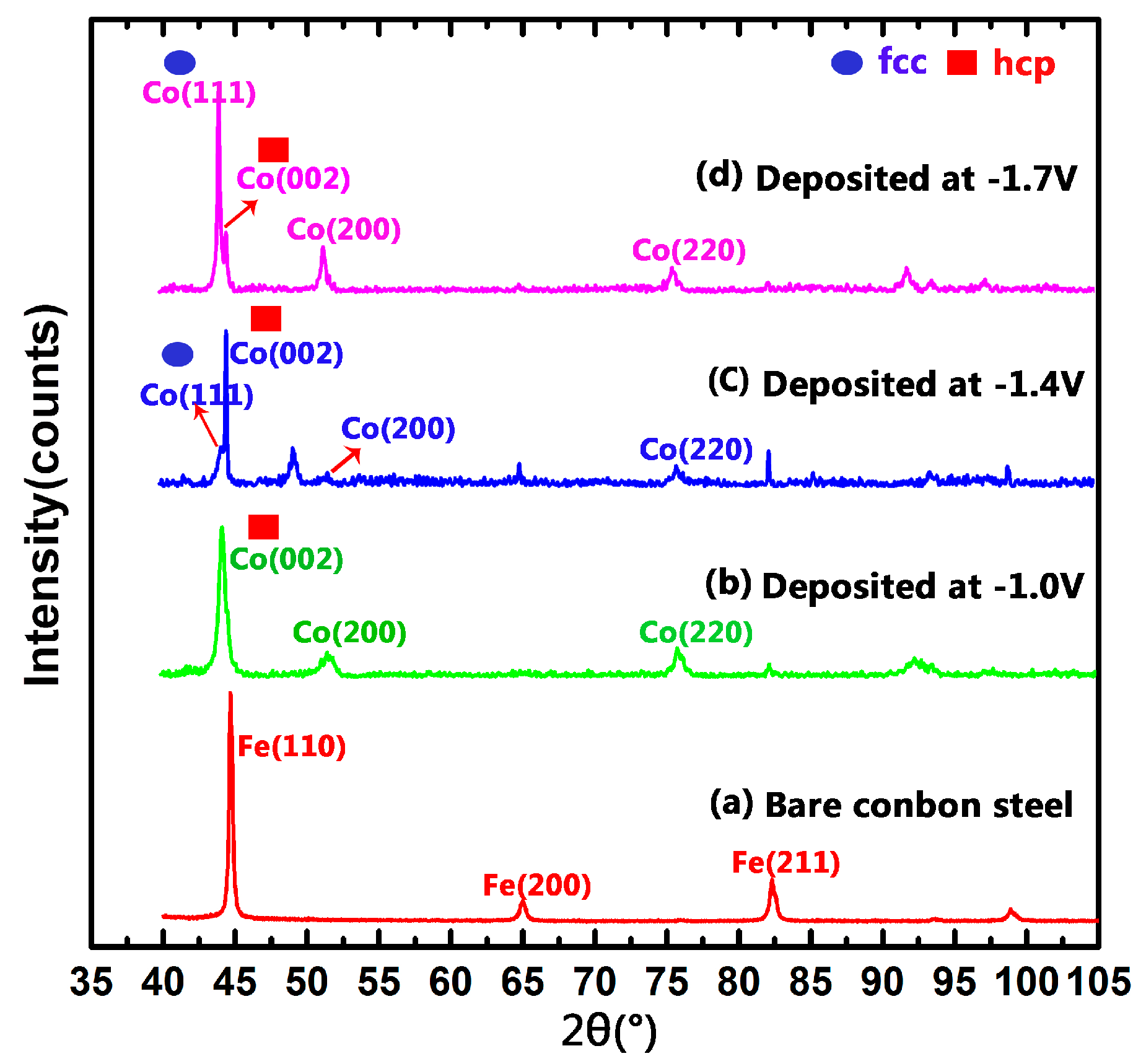
3.2. Structure of As-Deposited Co–Ni Coating
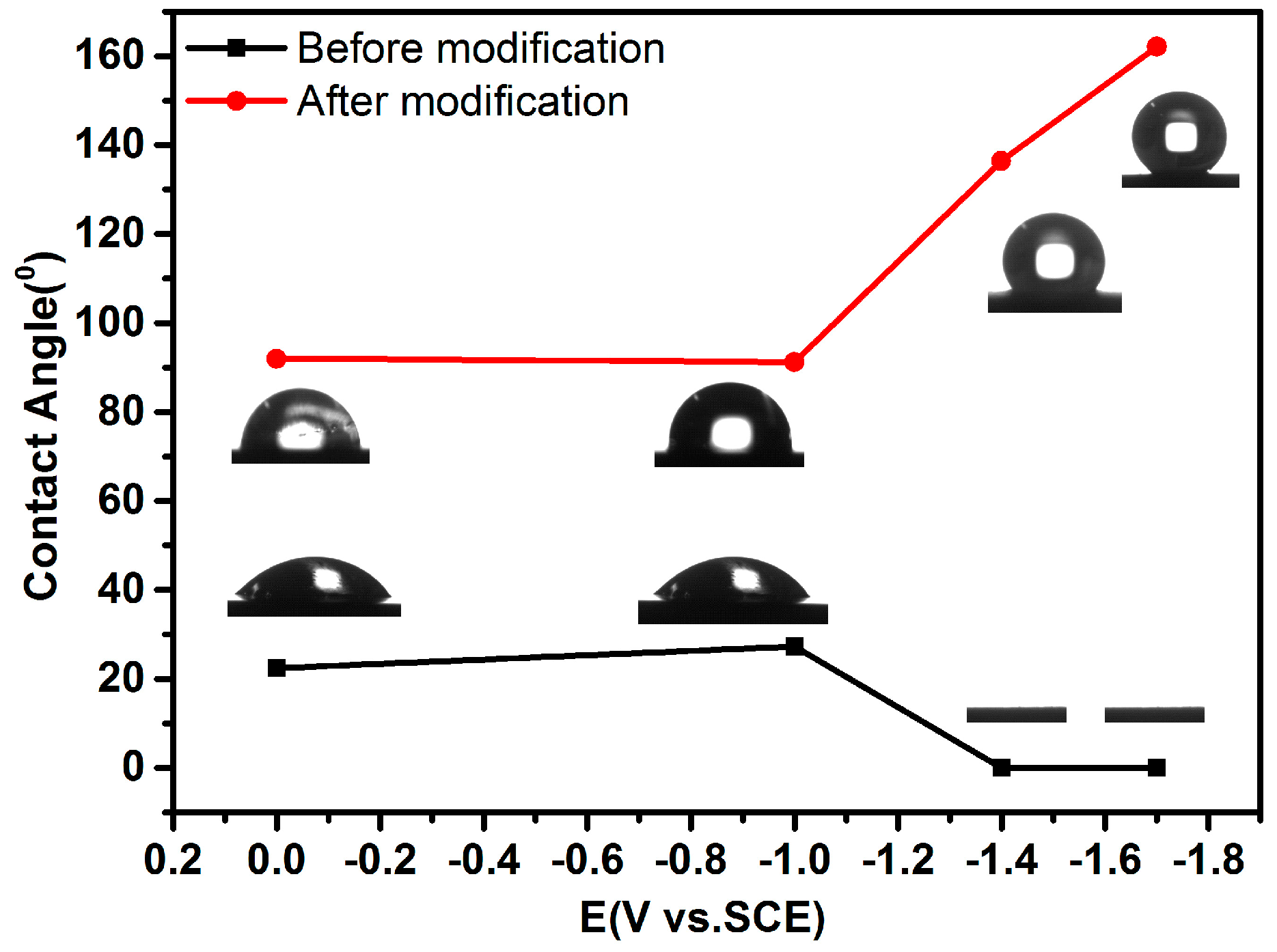
3.3. Surface Wetting Property of As-Deposited Co–Ni Coatings
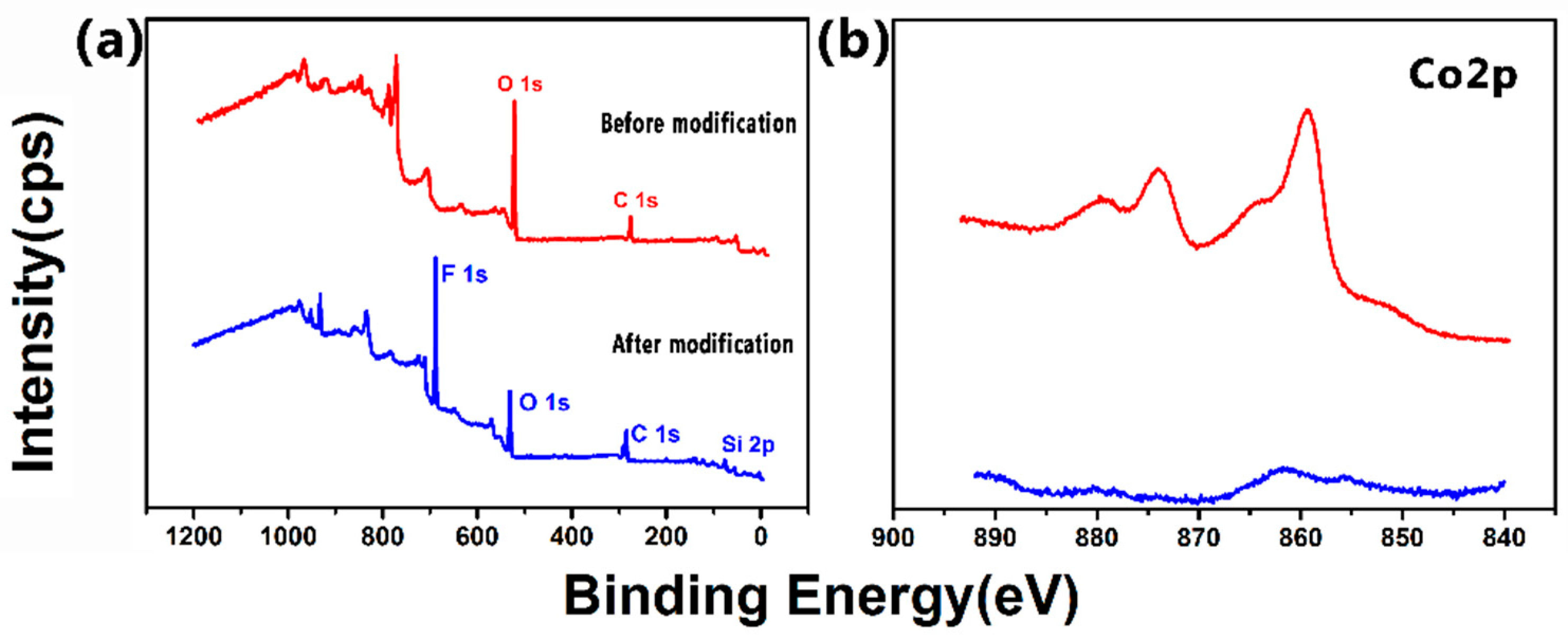
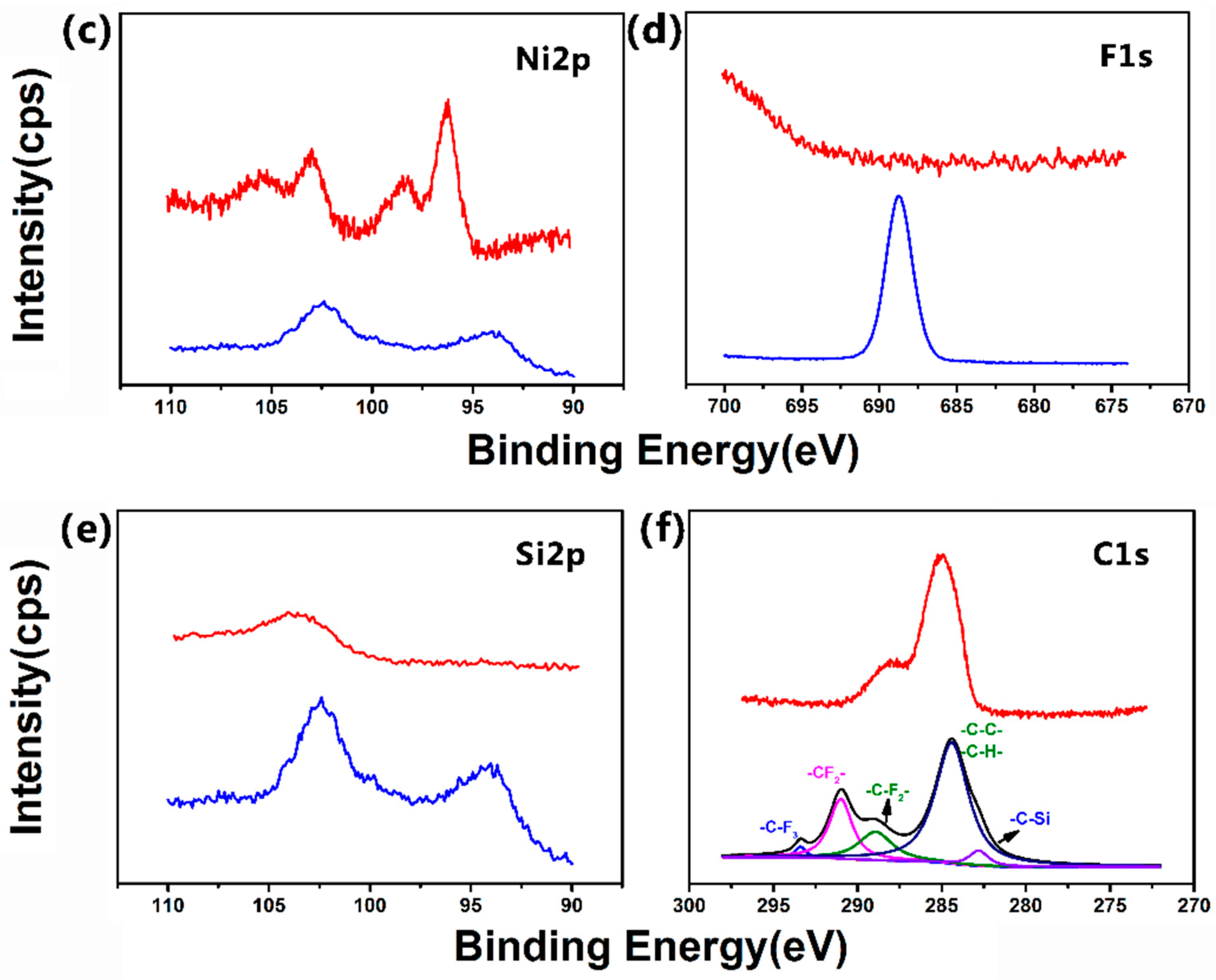
3.4. Surface Composition of the Super-Hydrophobic Co–Ni Coating
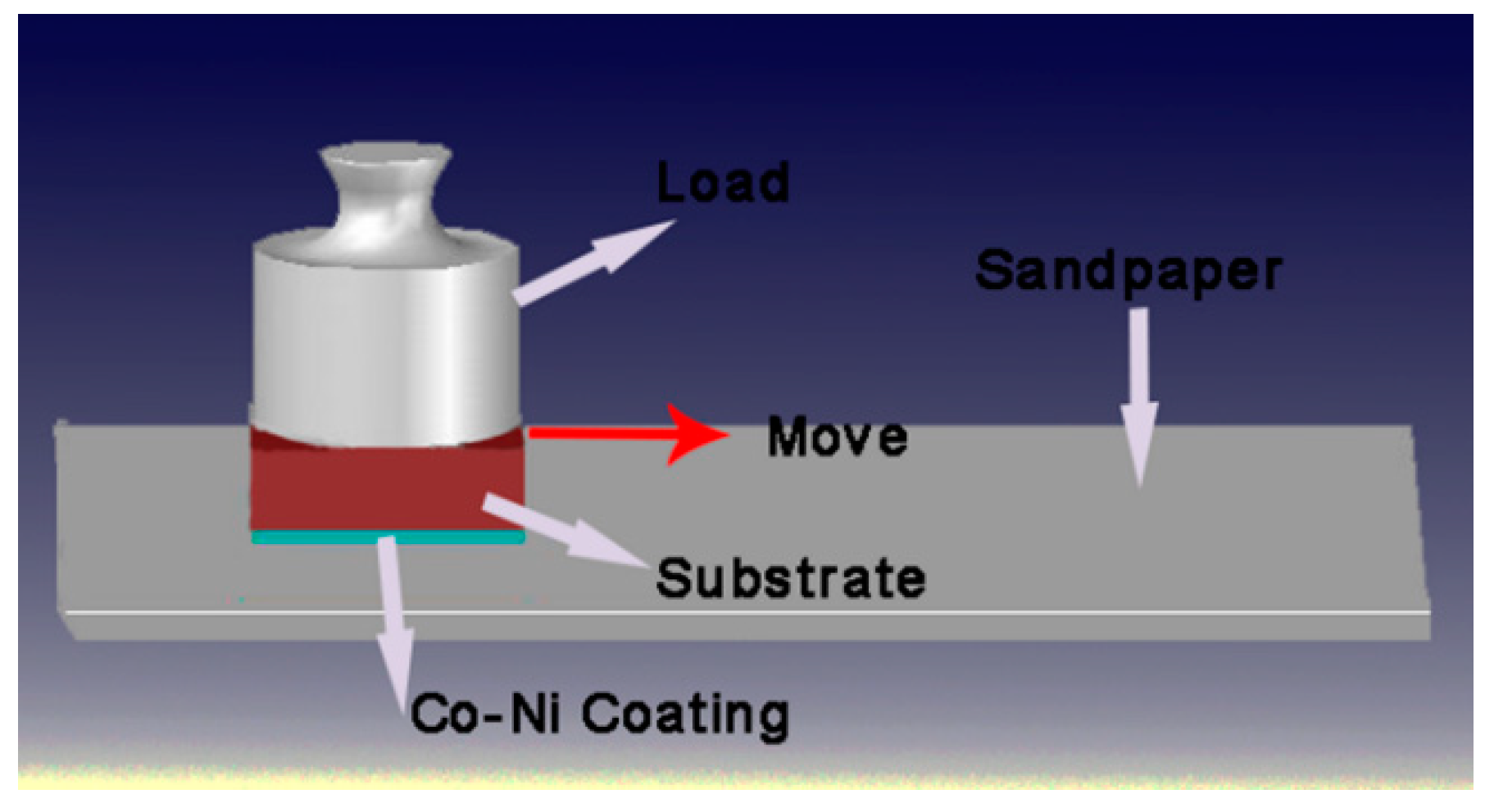
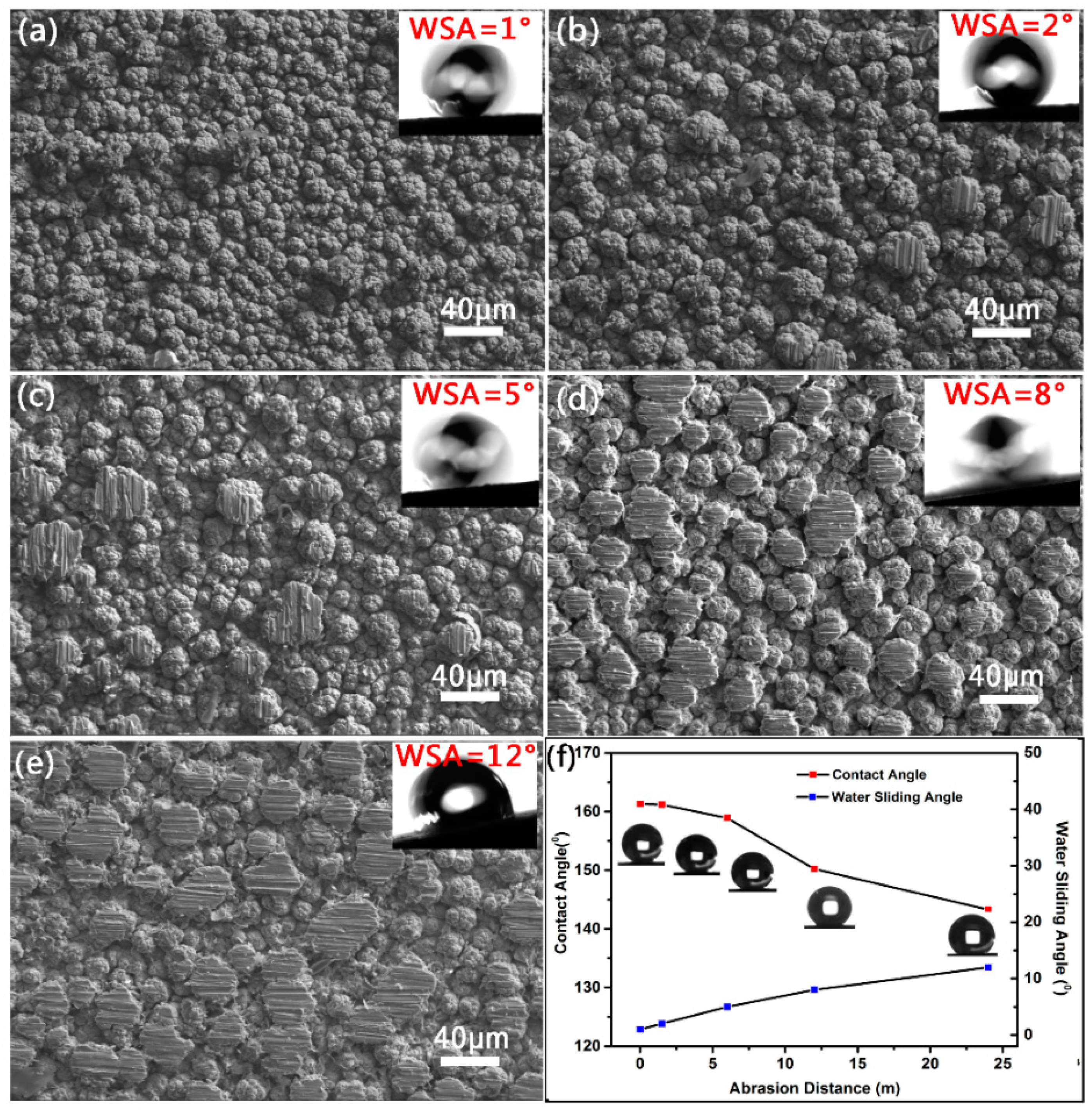
3.5. Abrasion Resistance of the Super-Hydrophobic Co–Ni Coating
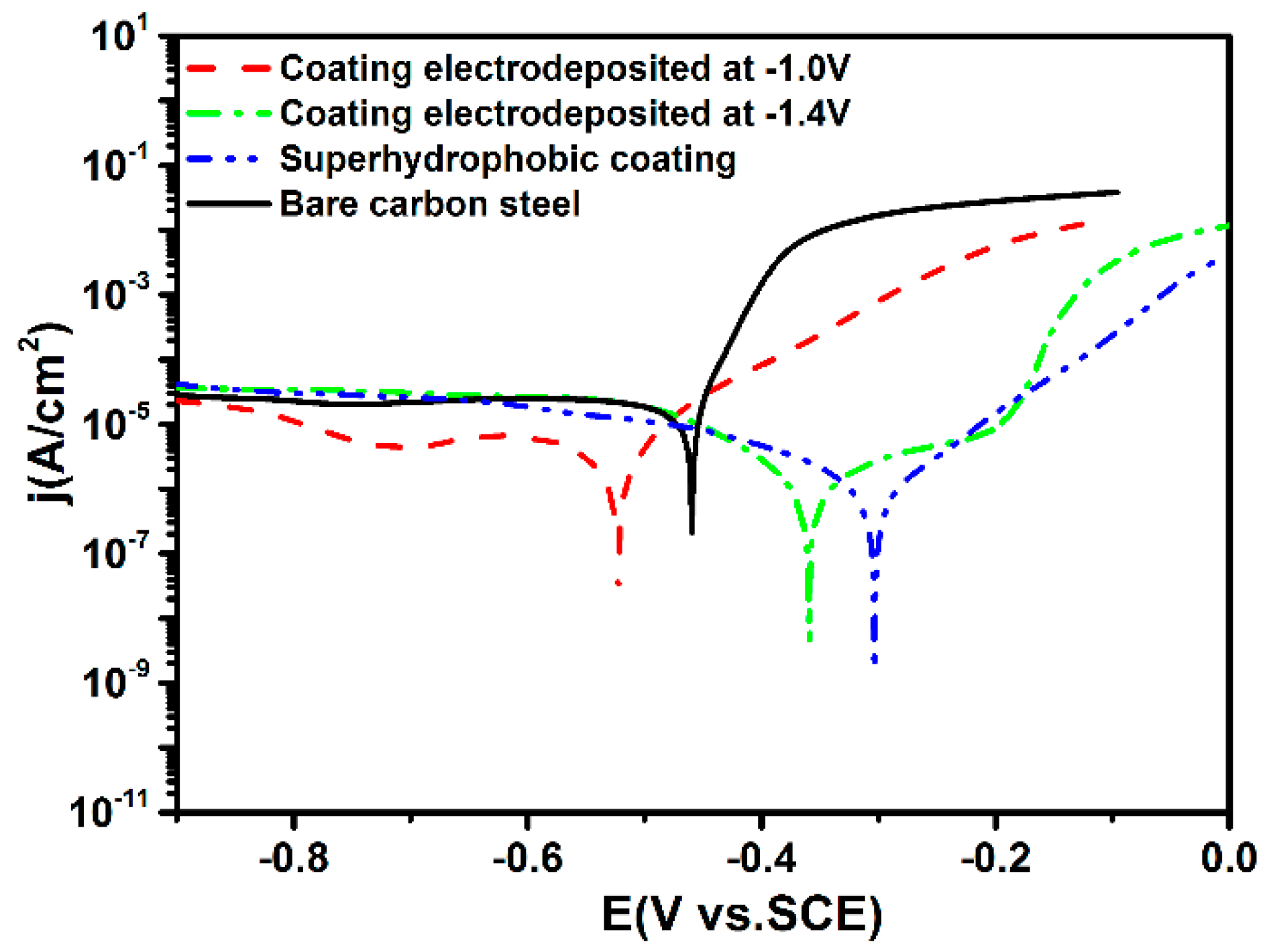
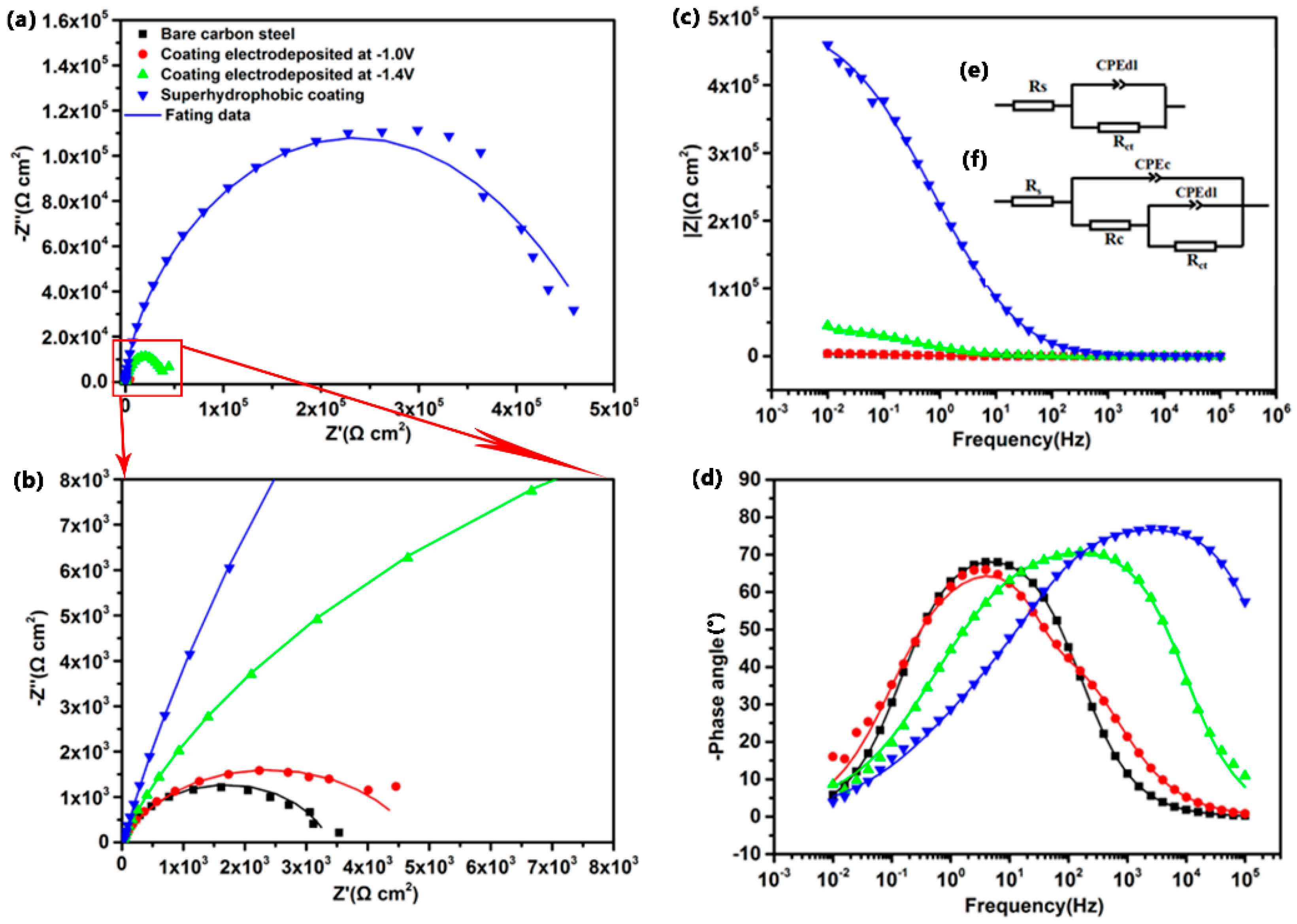
3.6. Corrosion Resistance of the Super-Hydrophobic Co–Ni Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sanchez, C.; Arribart, H.; Guille, M.M.G. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Serrà, A.; Zhang, Y.; Sepúlveda, B.; Gómez, E.; Nogués, J.; Michler, J.; Philippe, L. Highly active ZnO-based biomimetic fern-like microleaves for photocatalytic water decontamination using sunlight. Appl. Catal. B Environ. 2019, 248, 129–146. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-inspired self-cleaning surfaces. Annu. Rev. Mater. Res. 2012, 42, 231–263. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240. [Google Scholar] [CrossRef] [PubMed]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Eberle, P.; Tiwari, M.K.; Maitra, T.; Poulikakos, D. Rational nanostructuring of surfaces for extraordinary icephobicity. Nanoscale 2014, 6, 4874–4881. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability for selective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef]
- Pu, X.; Li, G.; Huang, H. Preparation, anti-biofouling and drag-reduction properties of a biomimetic shark skin surface. Biol. Open 2016, 5, 389–396. [Google Scholar] [CrossRef]
- Liao, R.; Zuo, Z.; Guo, C.; Yuan, Y.; Zhuang, A. Fabrication of superhydrophobic surface on aluminum by continuous chemical etching and its anti-icing property. Appl. Surf. Sci. 2014, 317, 701–709. [Google Scholar] [CrossRef]
- Long, J.; Fan, P.; Gong, D.; Jiang, D.; Zhang, H.; Li, L.; Zhong, M. Superhydrophobic surfaces fabricated by femtosecond laser with tunable water adhesion: From lotus leaf to rose petal. ACS Appl. Mater. Interfaces 2015, 7, 9858–9865. [Google Scholar] [CrossRef]
- Yu, J.; Qin, L.; Hao, Y.; Kuang, S.; Bai, X.; Chong, Y.; Zhang, W.; Wang, E. Vertically aligned boron nitride nanosheets: Chemical vapor synthesis, ultraviolet light emission, and superhydrophobicity. ACS Nano 2010, 4, 414–422. [Google Scholar] [CrossRef]
- Jeong, C.; Choi, C.H. Single-step direct fabrication of pillar-on-pore hybrid nanostructures in anodizing aluminum for superior superhydrophobic efficiency. ACS Appl. Mater. Interfaces 2012, 4, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kwak, G.; Yong, K. Wettability control of ZnO nanoparticles for universal applications. ACS Appl. Mater. Interfaces 2011, 3, 3350–3356. [Google Scholar] [CrossRef]
- Tam, J.; Palumbo, G.; Erb, U. Recent advances in superhydrophobic electrodeposits. Materials 2016, 9, 151. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef]
- Peng, C.; Chen, Z.; Tiwari, M.K. All-organic superhydrophobic coatings with mechanochemical robustness and liquid impalement resistance. Nat. Mater. 2018, 17, 355–360. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Li, Q.; Wang, Z.; Li, L.; Chen, F.; Zhou, J. Researching the fabrication of anticorrosion superhydrophobic surface on magnesium alloy and its mechanical stability and durability. Chem. Eng. J. 2013, 228, 415–424. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, D.; Kang, Z. One-step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Yao, K. Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef]
- Jain, R.; Pitchumani, R. Facile fabrication of durable copper-based superhydrophobic surfaces via electrodeposition. Langmuir 2018, 34, 3159–3169. [Google Scholar] [CrossRef]
- Tam, J.; Jiao, Z.; Lau, J.C.F.; Erb, U. Wear stability of superhydrophobic nano Ni–PTFE electrodeposits. Wear 2017, 374, 1–4. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Zhao, G.; Taleb, A.; Jin, Y. Fabrication of Ni–Co coating by electrochemical deposition with high super-hydrophobic properties for corrosion protection. Surf. Coat. Technol. 2019, 363, 352–361. [Google Scholar] [CrossRef]
- Tian, X.; Verho, T.; Ras, R.H.A. Moving superhydrophobic surfaces toward real-world applications. Science 2016, 352, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.K.; Chang, W.T. Effect of pulse frequency and current density on anomalous composition and nanomechanical property of electrodeposited Ni–Co films. Thin Solid Films 2009, 517, 4800–4804. [Google Scholar] [CrossRef]
- Maksimović, V.M.; Lačnjevac, U.Č.; Stoiljković, M.M.; Pavlović, M.G.; Jović, V.D. Morphology and composition of Ni–Co electrodeposited powders. Mater. Charact. 2011, 62, 1173–1179. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Electrolytic deposition of Ni–Co–SiC nano-coating for erosion-enhanced corrosion of carbon steel pipes in oilsand slurry. Surf. Coat. Technol. 2011, 205, 3198–3204. [Google Scholar] [CrossRef]
- Rafailović, L.D.; Minić, D.M.; Karnthaler, H.P.; Wosik, J.; Trišović, T.; Nauer, G.E. Study of the dendritic growth of Ni–Co alloys electrodeposited on Cu substrates. J. Electrochem. Soc. 2010, 157, D295. [Google Scholar] [CrossRef]
- Jain, R.; Pitchumani, R. Fractal model for wettability of rough surfaces. Langmuir 2017, 33, 7181–7190. [Google Scholar] [CrossRef]
- ASM Handbook: Volume 3: Alloy Phase Diagrams, 10th ed.; ASM International: Novelty, OH, USA, 1992.
- Wang, N.; Hang, T.; Shanmugam, S.; Li, M. Preparation and characterization of nickel–cobalt alloy nanostructures array fabricated by electrodeposition. CrystEngComm 2014, 16, 6937–6943. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Q.; Li, Y.; Hou, B. Facile fluorine-free one step fabrication of superhydrophobic aluminum surface towards self-cleaning and marine anticorrosion. Chem. Eng. J. 2018, 352, 625–633. [Google Scholar] [CrossRef]
- Wang, P.; Li, T.; Zhang, D. Fabrication of non-wetting surfaces on zinc surface as corrosion barrier. Corros. Sci. 2017, 128, 110–119. [Google Scholar] [CrossRef]
- Li, W.; Kang, Z. Fabrication of corrosion resistant superhydrophobic surface with self-cleaning property on magnesium alloy and its mechanical stability. Surf. Coat. Technol. 2014, 253, 205–213. [Google Scholar] [CrossRef]
- Tan, C.; Li, Q.; Cai, P.; Yang, N.; Xi, Z. Fabrication of color-controllable superhydrophobic copper compound coating with decoration performance. Appl. Surf. Sci. 2015, 328, 623–631. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Yu, Z.; Zhang, Z.; Hu, Q.; Liu, C. Large-area fabrication of superhydrophobic zinc surface with reversible wettability switching and anticorrosion. J. Electrochem. Soc. 2016, 163, D385–D391. [Google Scholar] [CrossRef]

| Potentials | Coating | |
|---|---|---|
| Ni (at.%) | Co (at.%) | |
| −1.0 V | 6.2 | 93.8 |
| −1.4 V | 19.0 | 81.0 |
| −1.7 V | 22.3 | 77.7 |
| Materials | Pressure (kPa) | Abrasion Length (m) | Initial WCA (°) | Final WCA (°) | Initial WSA (°) | Final WSA (°) |
|---|---|---|---|---|---|---|
| Co–Ni coating (this work) | 5.0 | 12.0 | 161 | 150 | 1 | 8 |
| Ni–PTFE [21] | 2.0 | 50.0 | 156 | 150 | 3 | 52 |
| Cu [20] | 3.0 | 2.0 | 162 | 143 | 3 | 18 |
| Ni [19] | 4.8 | 1.0 | 162 | 150 | 3 | 15 |
| Mg−Mn−Ce [18] | 1.3 | 0.4 | 160 | 150 | 2 | Not given |
| Co [33] | 1.5 | 1.1 | 156 | 148 | 1 | 40 |
| Cu compound [34] | 1.2 | 0.7 | 163 | 140 | 1 | Not given |
| Sample | Ecorr (mV) | Icorr (A/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|
| Bare carbon steel | −459.4 | 1.23 × 10−5 | 0.137 |
| Co–Ni coating at −1.0 V | −522.7 | 5.08 × 10−6 | 0.057 |
| Co–Ni coating at −1.4 V | −359.4 | 1.50× 10−6 | 0.017 |
| Super−hydrophobic coating | −303.3 | 5.87 × 10−7 | 0.0066 |
| Fitted Parameters | Rs | CPEdl | ndl | Rct | CPEc | nc | Rc |
|---|---|---|---|---|---|---|---|
| Bare carbon steel | 8.42 | 1.08 × 10−3 | 0.659 | 2.40 | − | − | − |
| Co–Ni coating deposited at −1.0 V | 10.75 | 3.59 × 10−5 | 0.983 | 4.65 | 2.83 × 10−4 | 0.729 | 0.116 |
| Co–Ni coating deposited at −1.4 V | 11.68 | 3.06 × 10−5 | 0.629 | 26.4 | 1.00 × 10−5 | 0.816 | 16.1 |
| Super−hydrophobic coating | 23.82 | 1.43 × 10−6 | 0.582 | 369.1 | 1.78 × 10−7 | 0.868 | 98.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Wang, S.; Bi, P.; Zhao, G.; Jin, Y. Super-Hydrophobic Co–Ni Coating with High Abrasion Resistance Prepared by Electrodeposition. Coatings 2019, 9, 232. https://doi.org/10.3390/coatings9040232
Xue Y, Wang S, Bi P, Zhao G, Jin Y. Super-Hydrophobic Co–Ni Coating with High Abrasion Resistance Prepared by Electrodeposition. Coatings. 2019; 9(4):232. https://doi.org/10.3390/coatings9040232
Chicago/Turabian StyleXue, Yanpeng, Shuqiang Wang, Peng Bi, Guochen Zhao, and Ying Jin. 2019. "Super-Hydrophobic Co–Ni Coating with High Abrasion Resistance Prepared by Electrodeposition" Coatings 9, no. 4: 232. https://doi.org/10.3390/coatings9040232
APA StyleXue, Y., Wang, S., Bi, P., Zhao, G., & Jin, Y. (2019). Super-Hydrophobic Co–Ni Coating with High Abrasion Resistance Prepared by Electrodeposition. Coatings, 9(4), 232. https://doi.org/10.3390/coatings9040232







