1. Introduction
Technical textiles have a wide range of applications in many areas in which they are called upon to exhibit a wide variety of properties [
1]. These may include but are not limited to the mechanical resistance, chemical resilience, water-, air-, vapor permeability and others. The ever growing range of application brings with it new challenges that often includes antibacterial, antinfungal or in certain applications, such unusual characteristics as well controlled electrostatic behavior [
2,
3]. The textile wetting behavior describes their ability to enable or prevent the extent of wetting or coverage of their surface by the fluid of interest. Many of these behavioral properties can be effectively, albeit somewhat surprisingly, characterized by measuring the textile wetting behavior. Interestingly enough, the wetting behavior itself can be effectively and relatively easily fine tuned over a broad range of values. Once a proper and well defined relationship between the wetting characteristics and desirable property of interest has been established, that property itself can be adjusted to a required value by following the imparted changes in the wetting behavior. For example, hydrophobicity can be enhanced beyond the natural value or alternatively reduced as required.
Because these textiles can often find their way to a wide variety of applications representing a broad range of operating conditions, it is of interest to extend their range of applicability as much as possible [
4]. Moreover, should such an extension be prone to being readily realizable though a relatively facile and effective surface treatment method, it would make the chosen textile much more attractive owing to its extended applicability.
Before any surface treatment can be selected, it must be subjected to an exacting evaluation of its properties to ascertain that it can withstand the rigors of the operating environment and the native of the application. These requirements may include but not be limited to toxicity, biodegradability, chemical compatibility with the surrounding environment, as well as, and if required, thermal and temporal stability [
5]. It was not our intent in the present investigation to focus on any specific application, but to establish a proven methodology for the evaluation of the changes in the textile wetting behavior resulting from utilization of a selected surface active agent.
Because this was our first foray into surface treatment, we have decided to limit our initial investigations to a standard, commercially available glass fibre industrial material for simplicity. The main purpose was to achieve sufficient experiments with different coatings and coating processes in order to fully characterize the resulting changes in textile functional behavior. Our intent was to develop further understanding of the available analytical methods and the results obtained therefrom to property gauge the effectiveness to the treatment as applicable to the current investigations. Prior to pursuing the broader experimental program, it was necessary to confirm that the results obtained from these preliminary evaluations were sufficiently self explanatory and self consistent to allow their general utilization across the entire matrix of the investigated coatings. The main aim was to develop a methodology suitable for further discriminating characterization of surface treatments employed for applications to tune performance characteristics to allow the use of a select substrate or a relatively small number of substrates to cover as wide a range of applications as possible. Therein, we report several examples to describe how the surface of a simple substrate can be effectively modified to achieve a wide range of wetting behavior thereby broadening the range of applications for which the substrate could be deployed.
2. Materials and Methods
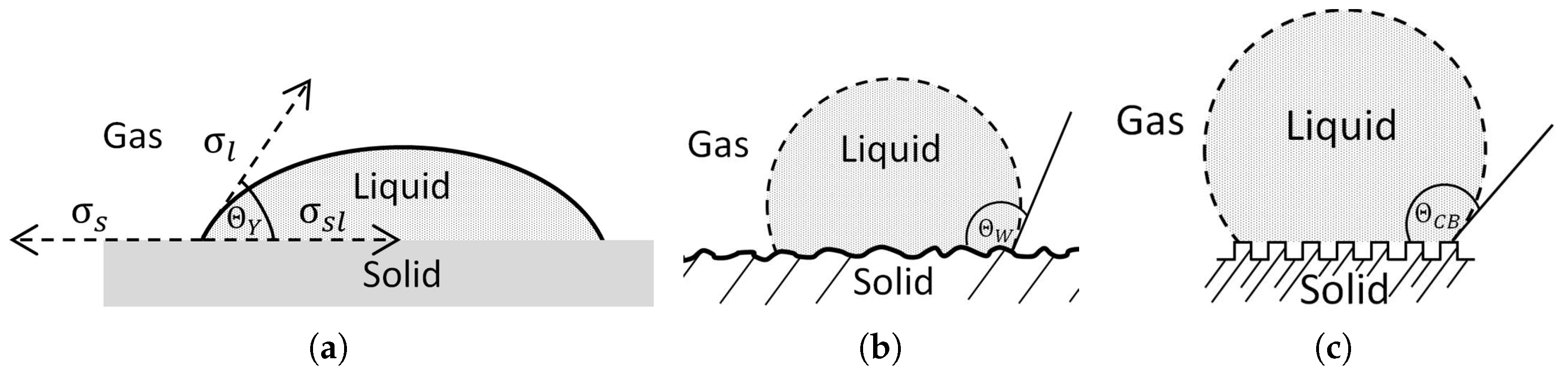
The wetting of a solid surface by a liquid, when both are surrounded by gas, can take generally different forms and depends on the relative energies of the two substances in contact. Lugscheider and Bobzin [
6] described the possible wetting states from complete spreading of a liquid over the solid surface through the shape of a flat lens to an almost spherical droplet in the case of complete non wetting behavior [
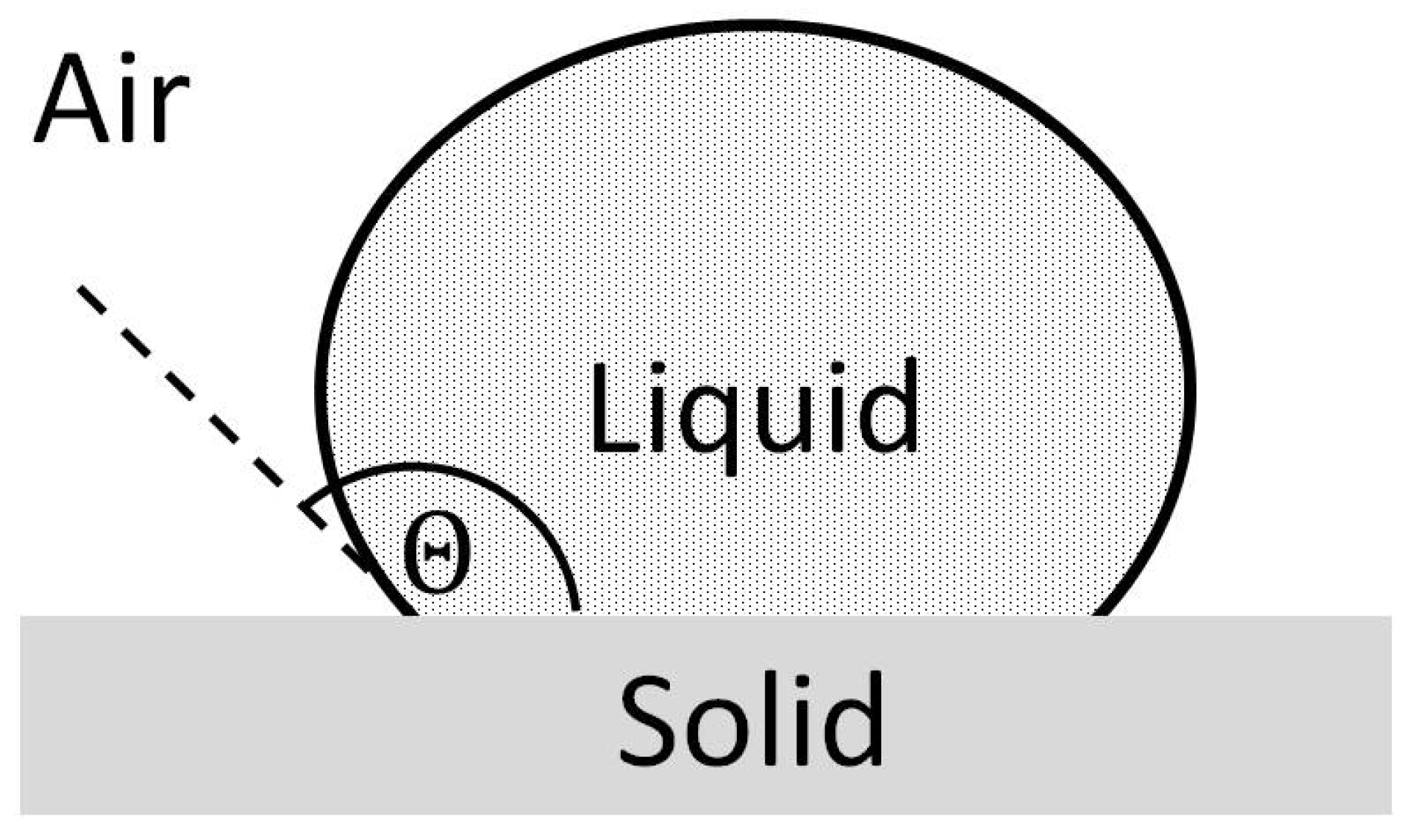
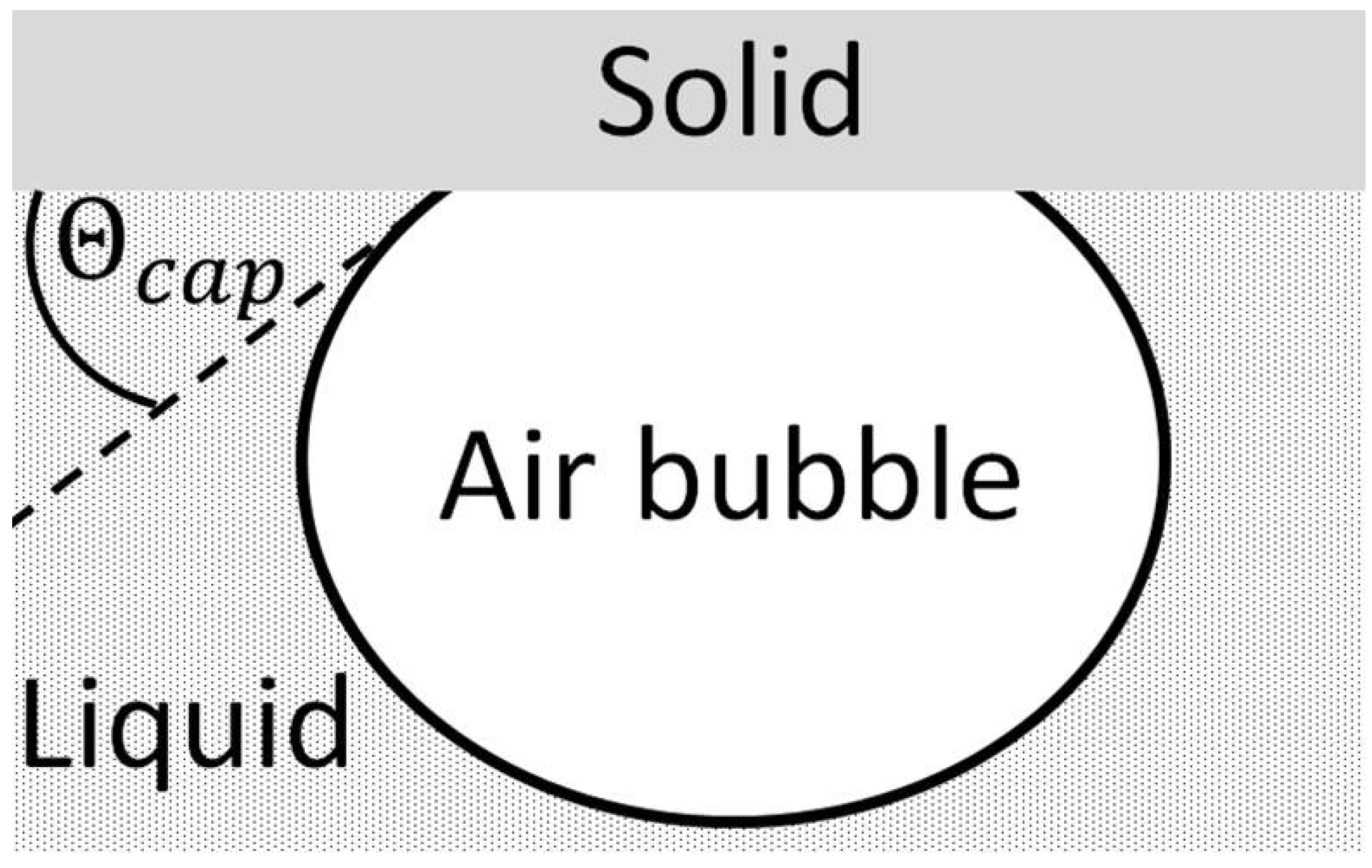
6]. The first mathematical relation of the wetting behavior and the equilibrium of forces at the three-phase contact line (
Figure 1a) was made in 1805 by Young [
7]
Here,
represents the contact angle;
and
the surface tensions of the solid and liquid;
the interfacial tension between the two. However, this relationship is only valid for smooth surfaces. This is because the contact angle can be strongly influenced by surface structure and surface chemical properties. In order to consider the influence of surface structure and compare different materials with different surfaces, suitable models were developed by Cassie-Baxter and Wenzel [
8,
9,
10,
11,
12]. For example, the ‘Wenzel state’ (
Figure 1b) describes the case of a liquid wetting the entire surface included with all its rough structures.
In the Wenzel model, the topographic property of the surface is accounted for the ‘r’ factor [
14]:
This describes the ratio between the actual contact surface and the projected contact area between the droplet and the substrate (
). For a perfectly smooth surface, the ratio is
because
. In contrast, when the liquid does not completely cover all surface features and air pockets form among them, the effective contact between the two phases is reduced to a minimum. This state is referred to as the Cassie–Baxter Model (
Figure 1c) and is often found on hydrophobic surfaces. The contact angle is then defined by [
13,
14]:
where
represents the ratio between the actual contact area and the projected contact area, respectively (
). Because the present work was carried out on textile samples of the same substrate, the surface roughness of all the samples was assumed to be the same. This assumption was further justified by the fact that the liquid droplet in the contact angle measurements was dimensioned to cover an appreciable area of the substrate surface and was born out by the experimental results [vide infra].
Fowkes wrote in his theoretical consideration of “Attractive Forces at Interfaces” that the total surface free energy (SFE) of a solid surface is the sum of the contributions from the various intermolecular forces at the surface. According to the Fowkes equation [
15], the surface tension of the respective phase can be divided into dispersive and polar components as follows:
Polar interactions at an interface between two liquids or a liquid and a solid result from dipole–dipole interaction, hydrogen bonding, Lewis acid/base interaction, or charge-transfer interaction. Dispersive interactions are due to the van der Waals forces [
16]. After Fowkes [
15] considered the dispersive and polar components, Owens and Wendt [
17] extended the Young equation for contacting solid and liquid phases as follows [
18]:
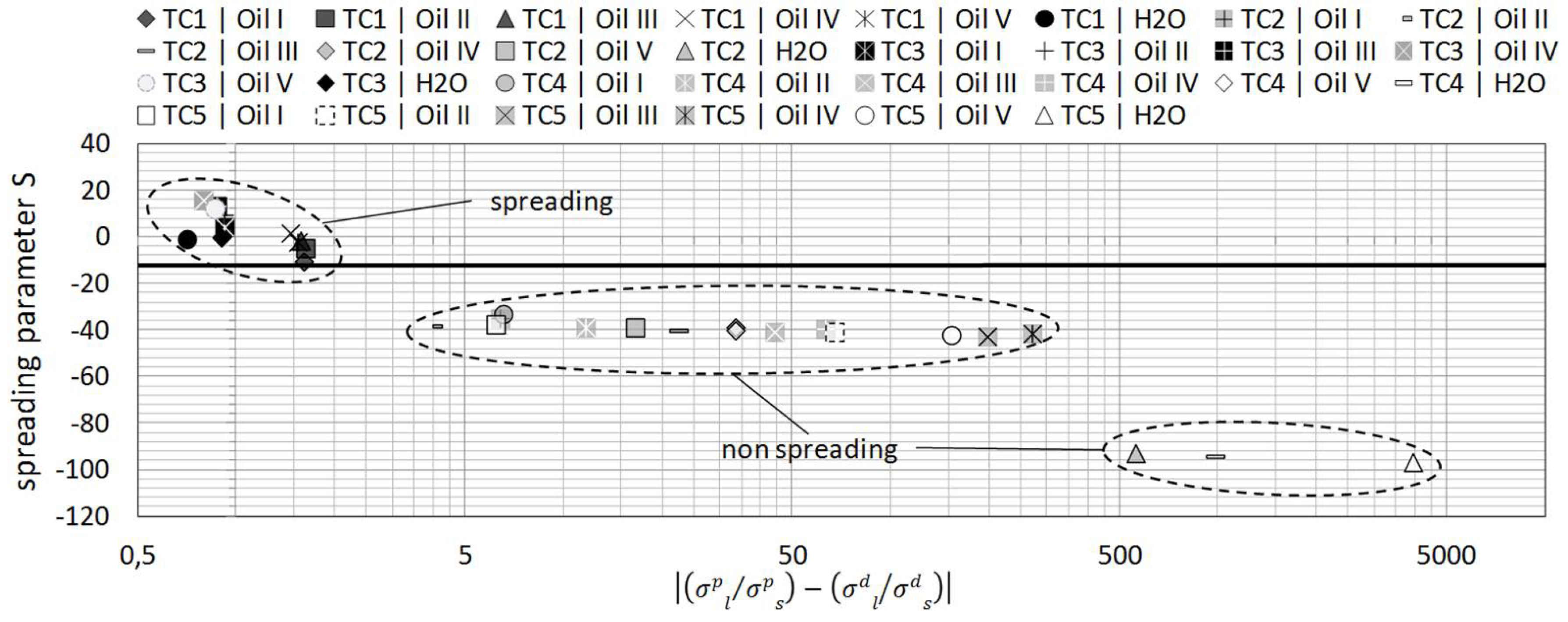
In order to be able to assess the wetting properties of liquids on textiles, the spreading tendency must be evaluated with a suitable parameter. For a mathematical prediction of the wetting behavior, the spreading parameter
S can be used [
6,
19].
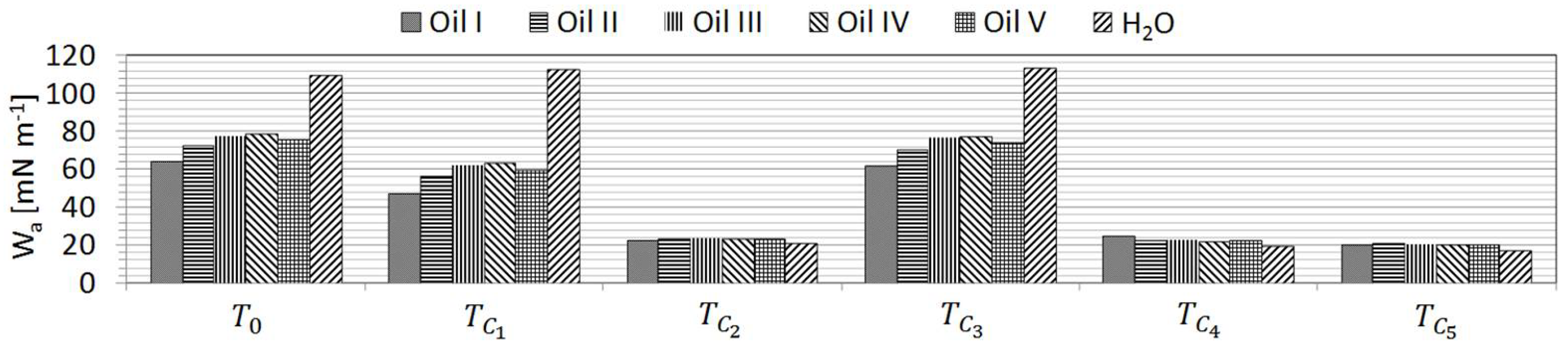
S is the difference between the work of adhesion and the work of cohesion, respectively, where [
15]:
When
, the liquid spreads on the surface and when
the wetting is incomplete [
6,
15]. The effect of various surface treatments determined by comparing the wetting behavior of a treated sample against the reference material. Samples of the same textile type were used in all experiments. The textile employed in the work consisted of a standard glass fiber non woven material with a well defined microfiber content. The surface treatment was carried out with five commercially available surface agents listed in
Table 1.
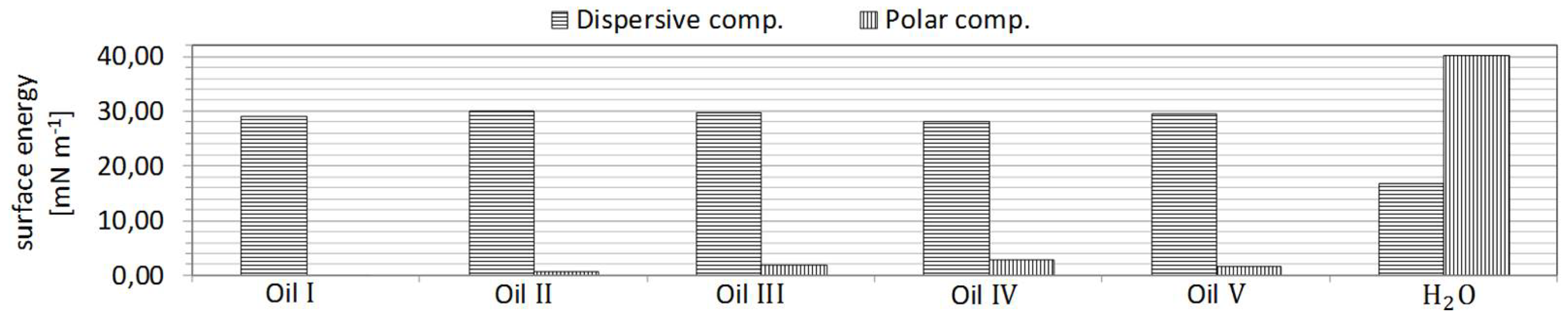
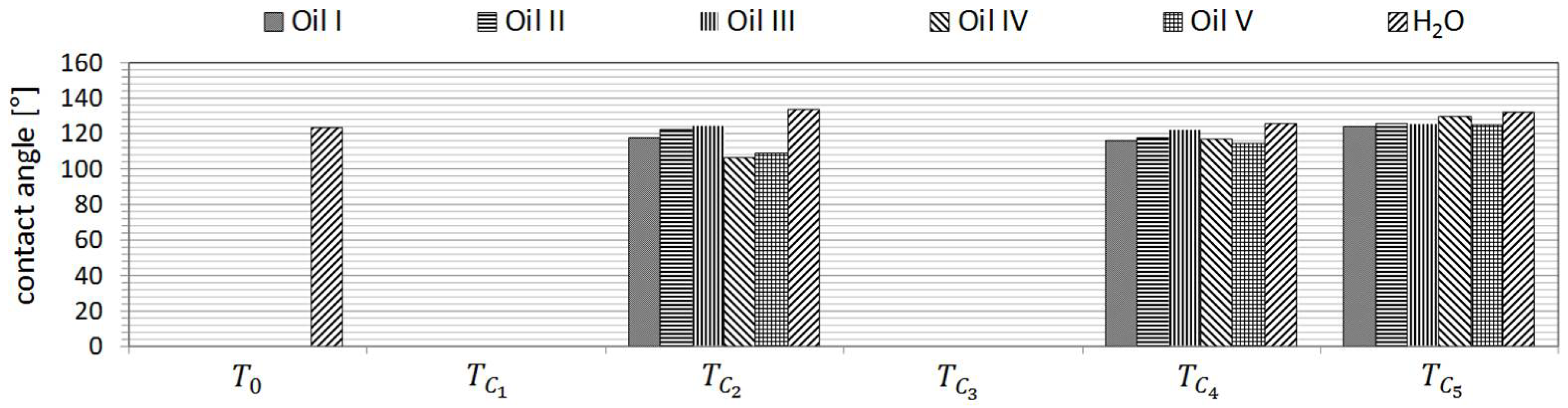
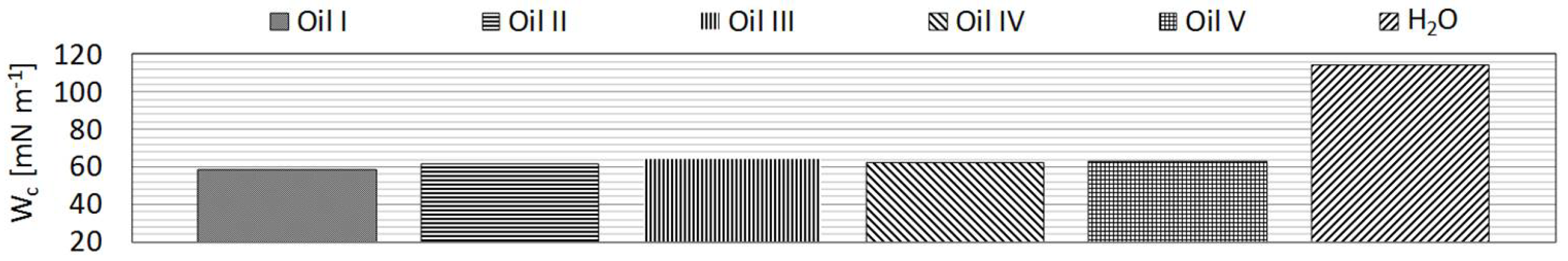
The wetting behavior of five commercially available industrial oils (
Table 2) on the treated samples was investigated to determine their response post treatment. Additionally, water (tap water) served as a representative of the highly polar liquids.
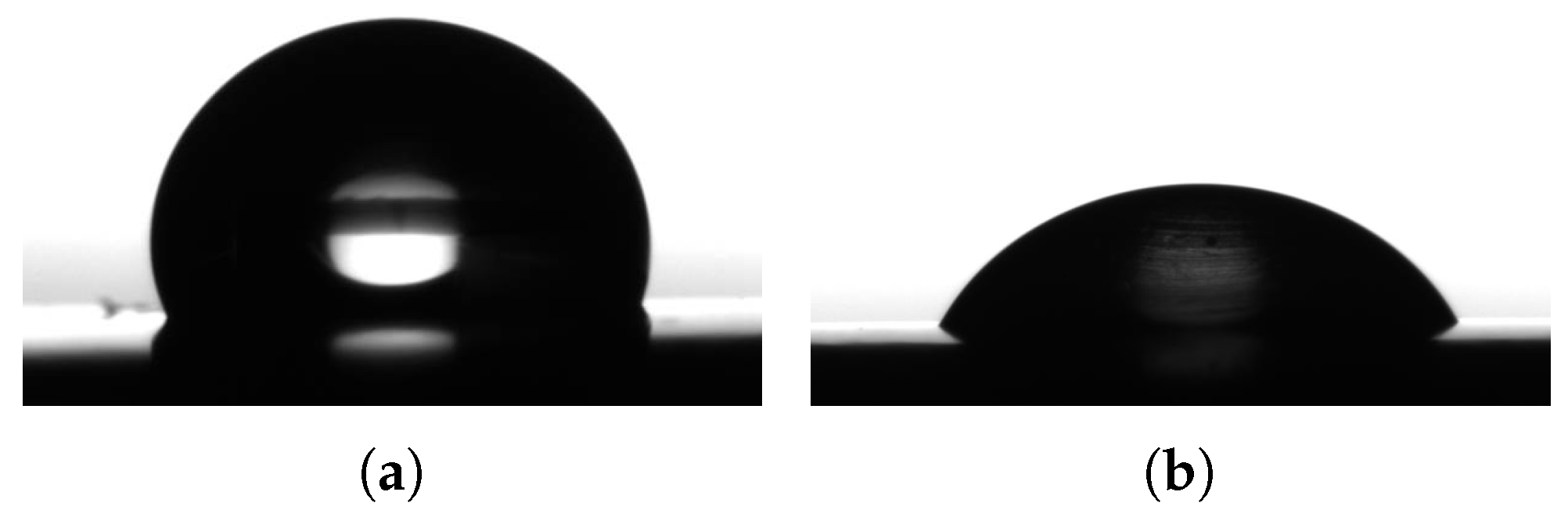
The surface energies of all samples were derived from contact angle measurements. The measurements were carried out a Krüss GmbH model DSA 30 (Hamburg, Germany) contact angle measurement device. The contact angle measurements were carried out with the aid of measuring liquids selected on the basis of their polarity as well as their respective polar and dispersive surface energy components (
Table 3). The range extended from complete non-polar (diiodo-methane) to highly polar (dist. water).
The dispersive and polar surface energy components of the test fluids were determined from the contact angle measurements on the non polar surface (as shown in
Figure 2a,b) and calculating from the combination of Young [
7] and Owens/Wendt [
17] equations. The total fluid surface tension was measured on a model KSV Sigma 702 tensiometer (Gothenburg, Sweden). The results showed good agreement with literature values and are listed in
Table 3.
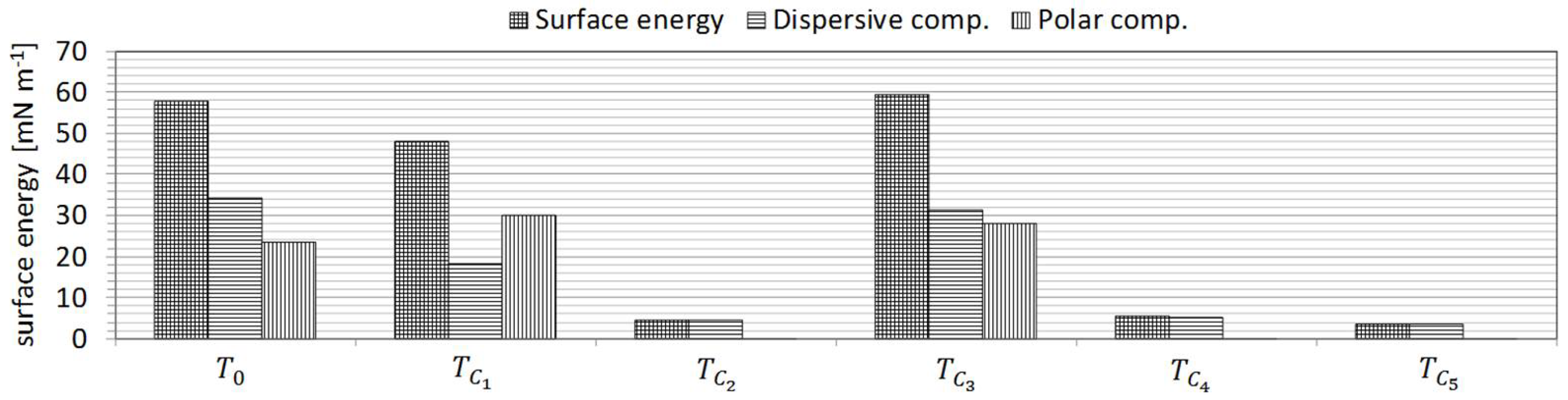
The same procedure was then employed for characterisation of surface tension properties of the functional fluids (
Figure 3). The density and kinematic viscosity of these fluids were also measured. These results are also displayed in
Table 4.
The characterisation of the select substrates was carried out by measuring the standard textile properties before and after coating. The results are listed in
Table 5.