Removal of Dyes and Cd2+ in Water by Kaolin/Calcium Alginate Filtration Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Kaolin/CaAlg Membrane
2.3. Characterizations
2.4. Mechanical Behaviors of the Kaolin/CaAlg Membrane
2.5. Evaluation of Anti-Fouling Properties of the Kaolin/CaAlg Membranes
2.6. Dyes Removal of Kaolin/CaAlg Membrane
2.7. Cd2+ Removal of Kaolin/CaAlg Membrane
3. Results and Discussions
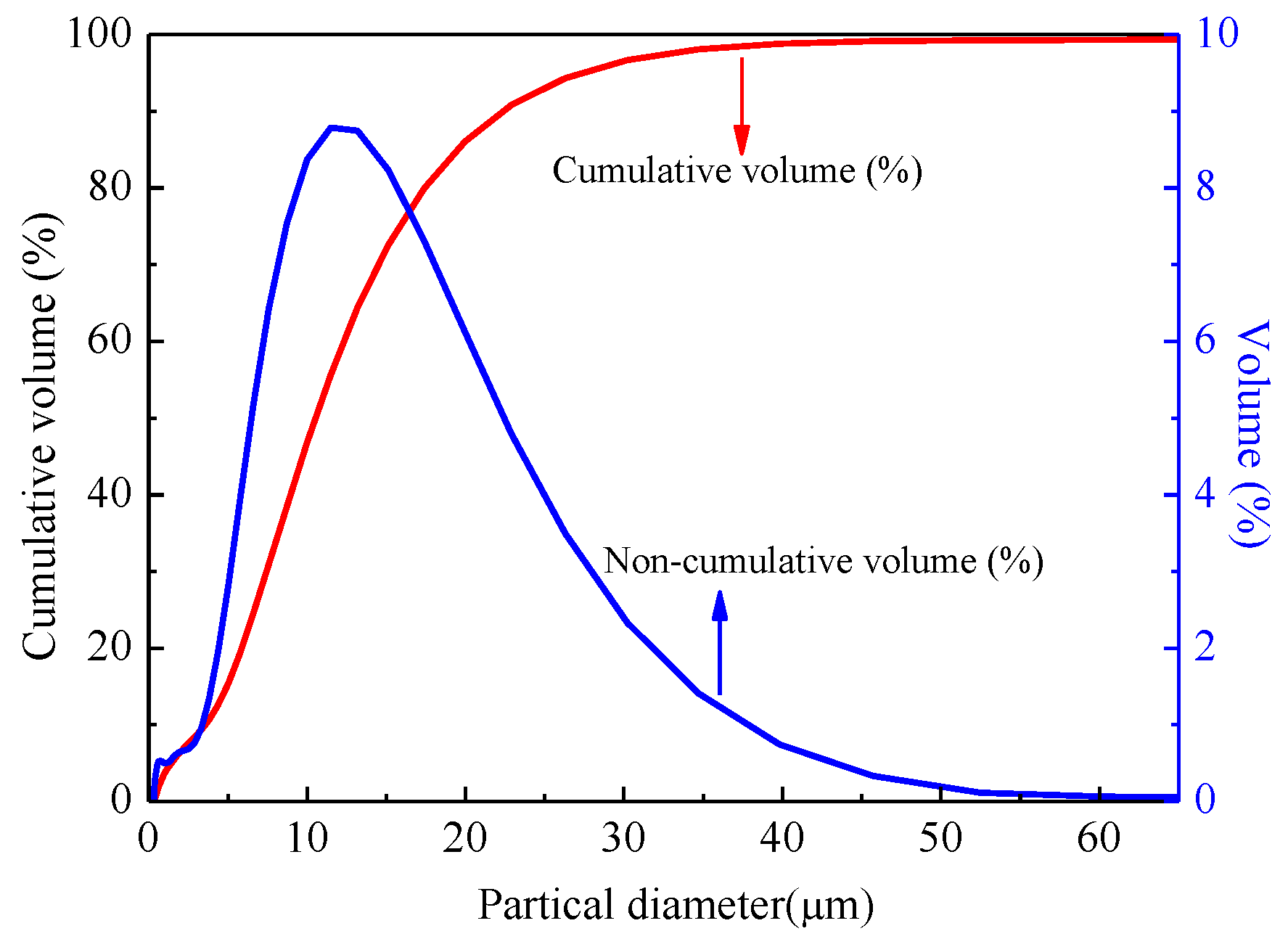
3.1. Granulometric Analysis of Kaolin Powders in Water
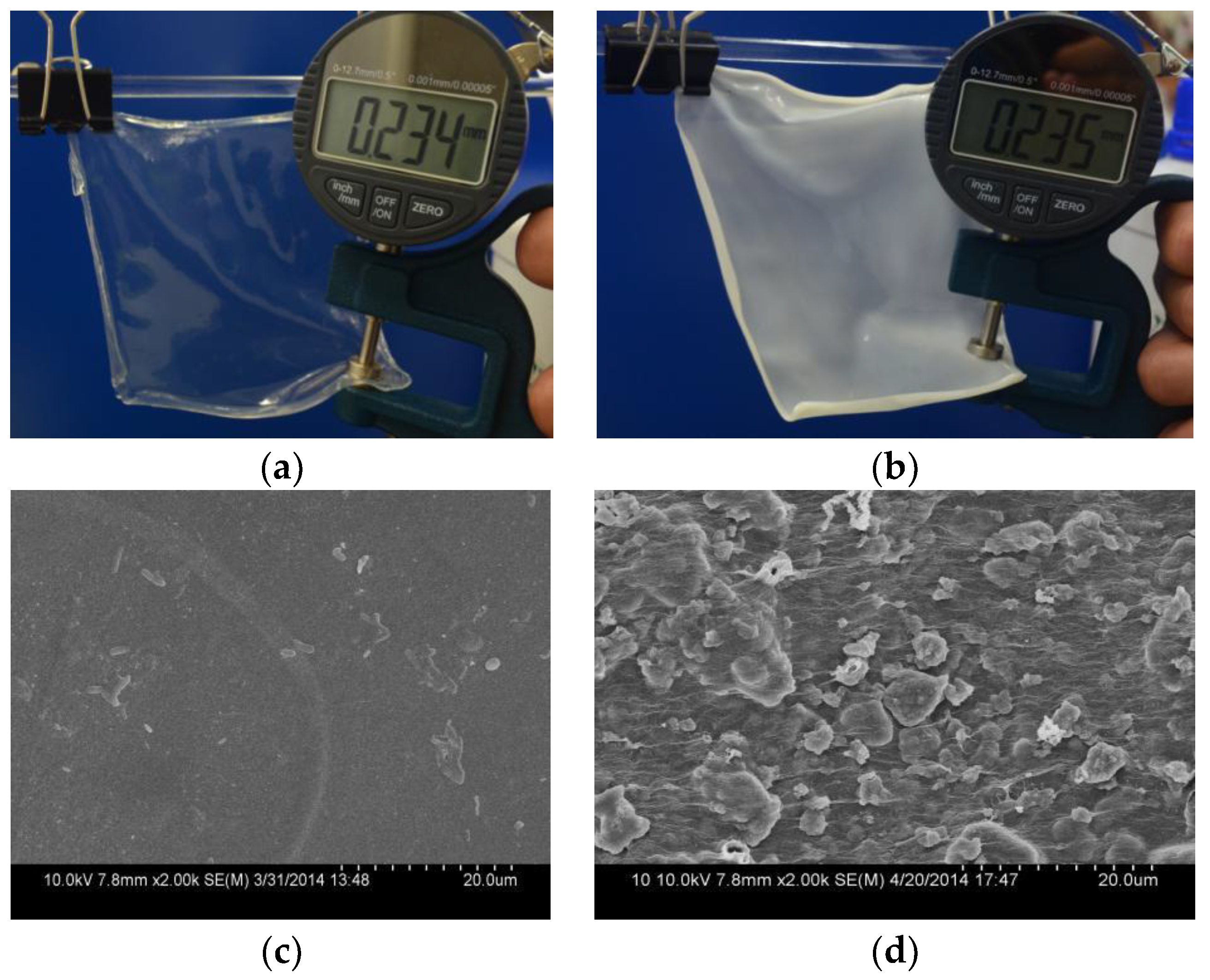
3.2. Morphology of CaAlg Filtration Membrane
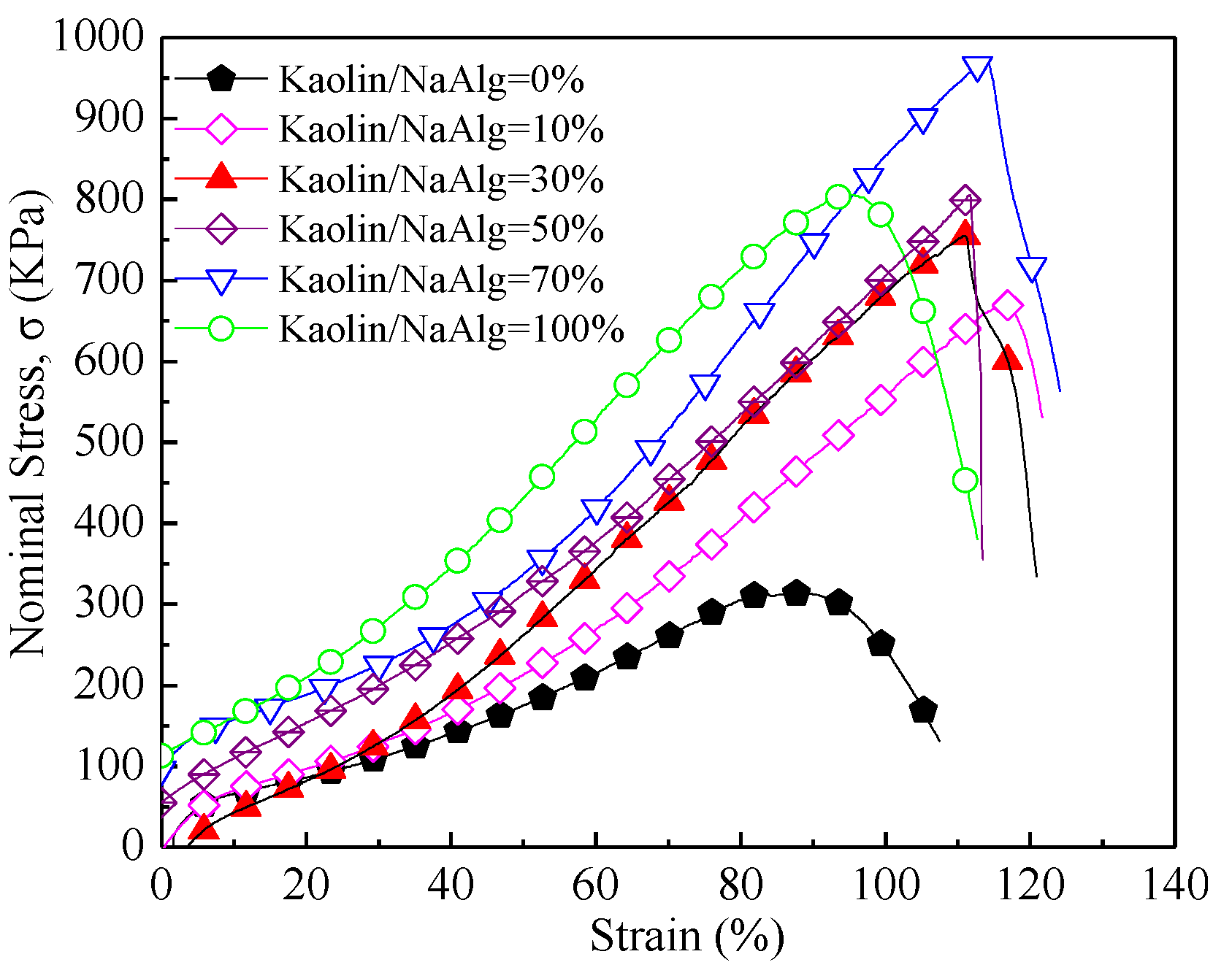
3.3. Effect of Kaolin Content on the Mechanical Properties of Kaolin/CaAlg Membranes
3.4. Pure Water Flux of Kaolin/CaAlg Membranes Prepared with Different Kaolin Contents
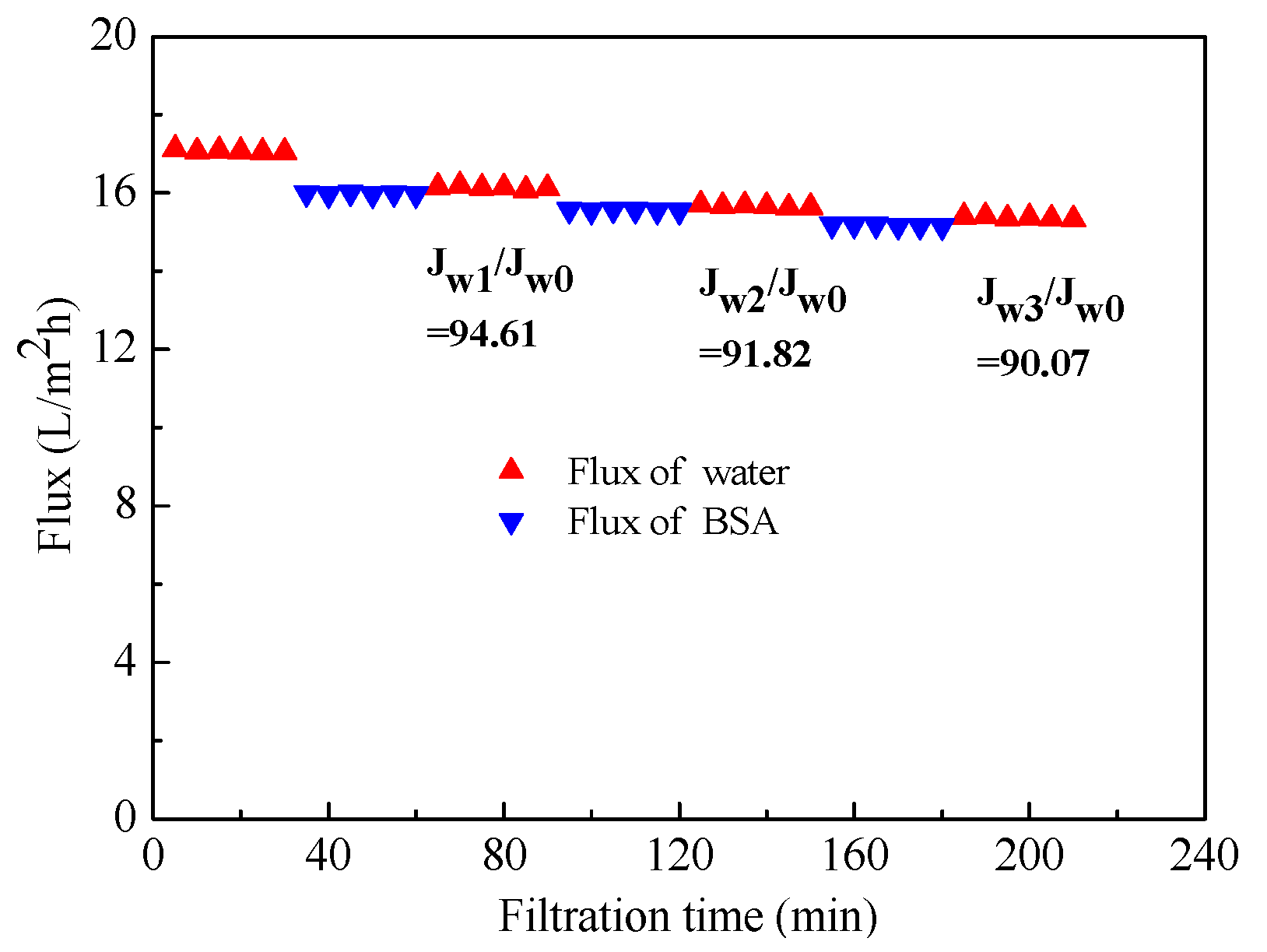
3.5. Anti-Fouling Properties of the Kaolin/CaAlg Filtration Membrane
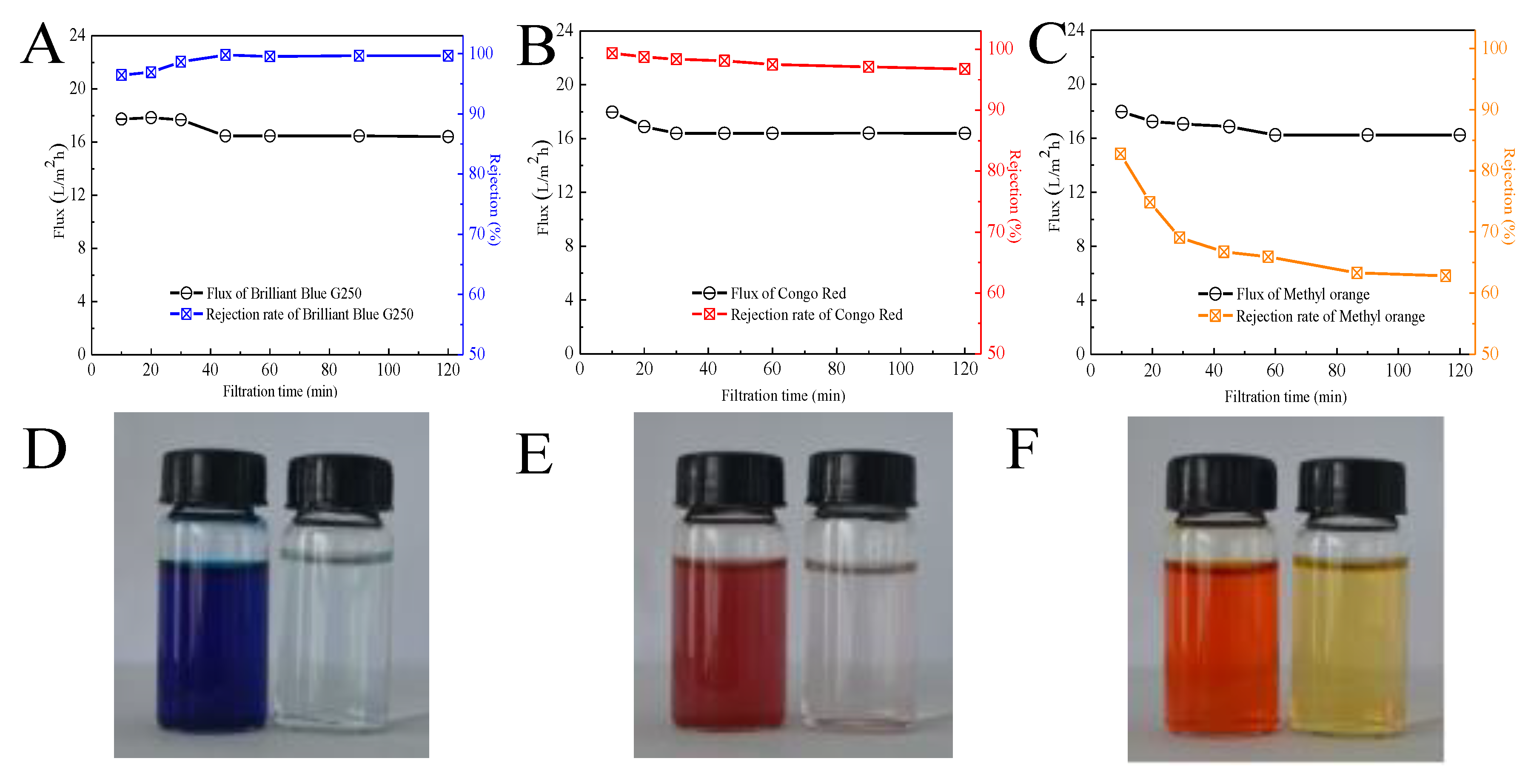
3.6. Dyes Removal of Kaolin/CaAlg Filtration Membrane
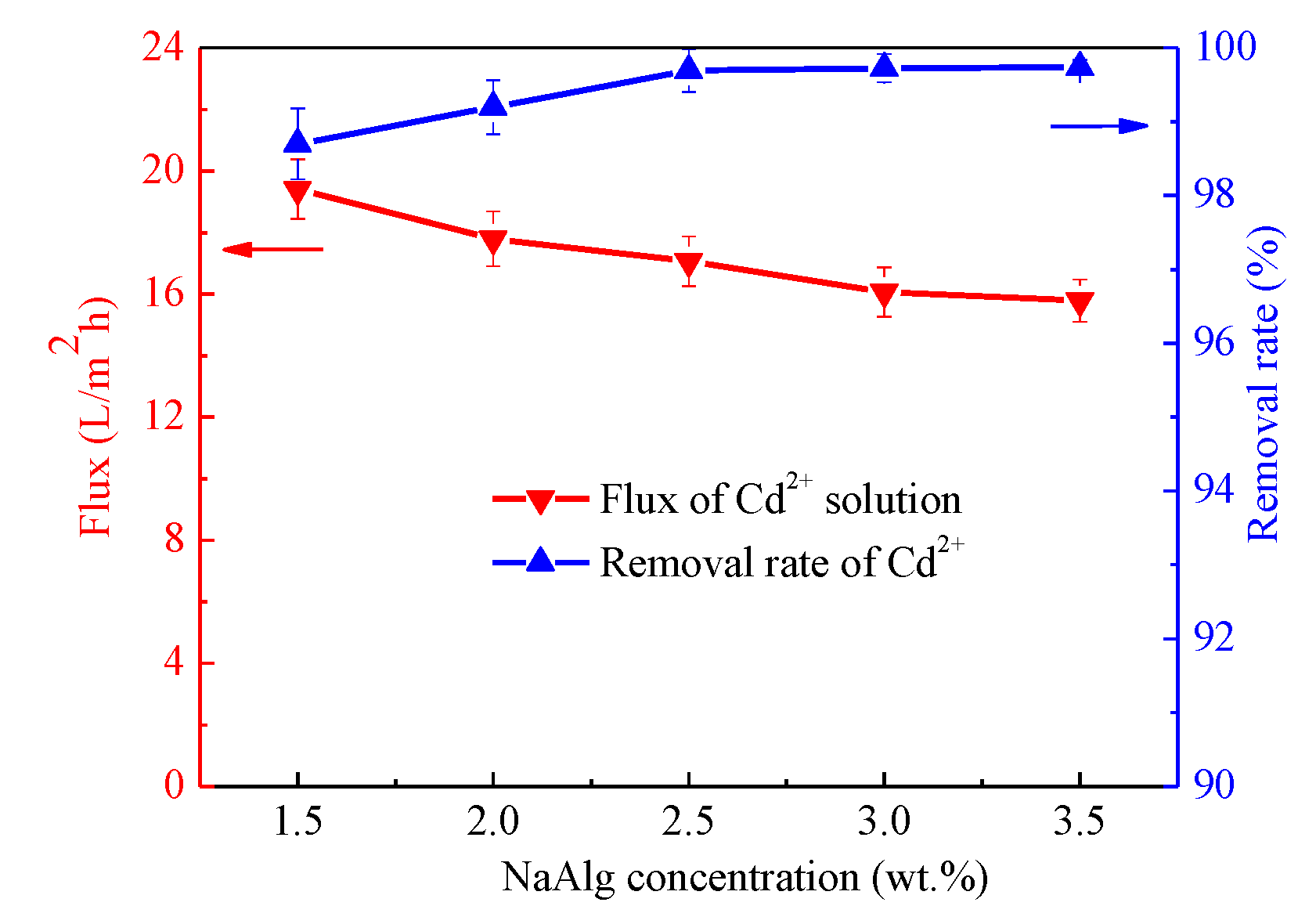
3.7. Removal of Cd2+ Ion by Kaolin/CaAlg Filtration Membrane
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Zhao, K.; Yang, B.; Chen, T.; Li, D.; Wu, H.; Wu, X. Adsorption of dibutyl phthalate in aqueous solution by mesoporous calcium silicate grafted non-woven polypropylene. Chem. Eng. J. 2016, 306, 452–459. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from spinifex as an effective adsorbent to remove cadmium(II) from water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Wang, X.; Bao, R.; Fu, J. The antagonistic effect of selenium on cadmium-induced damage and mRNA levels of selenoprotein genes and inflammatory factors in chicken kidney tissue. Biol. Trace Elem. Res. 2017, 181, 331–339. [Google Scholar] [CrossRef]
- Bassil, M.; Daou, F.; Hassan, H. Lead, cadmium and arsenic in human milk and their socio-demographic and lifestyle determinants in Lebanon. Chemosphere 2017, 191, 911–921. [Google Scholar] [CrossRef]
- Giusto, A.; Somma, L.A.; Ferrari, L. Cadmium toxicity assessment in juveniles of the Austral South America amphipod Hyalella curvispina. Ecotoxicol. Environ. Saf. 2012, 79, 163–169. [Google Scholar] [CrossRef]
- Anetor, J. Rising environmental cadmium levels in developing countries: Threat to genome stability and health. J. Physiol. Sci. 2012, 27, 103–115. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2017, 119, 157–184. [Google Scholar] [CrossRef]
- Shen, D.; Fan, J.; Zhou, W.; Gao, B.; Yue, Q.; Kang, Q. Adsorption kinetics and isotherm of anionic dyes onto organo-bentonite from single and multisolute systems. J. Hazard. Mater. 2009, 172, 99–107. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, K.; Yang, W.; Chen, G.; Zhang, X.; Zhao, Y.; Liu, L.; Chen, M. Efficient removal of Cd2+ ion from water by calcium alginate hydrogel filtration membrane. Water Sci. Technol. 2017, 75, 2322–2330. [Google Scholar] [CrossRef]
- Zhao, K.; Feng, L.; Lin, H.; Fu, Y.; Lin, B.; Cui, W.; Li, S.; Wei, J. Adsorption and photocatalytic degradation of methyl orange imprinted composite membranes using TiO2/calcium alginate hydrogel as matrix. Catal. Today 2014, 236, 127–134. [Google Scholar] [CrossRef]
- Zhao, K.; Feng, L.; Li, Z.; Fu, Y.; Zhang, X.; Wei, J.; Wei, S. Preparation, characterization and photocatalytic degradation properties of TiO2/calcium alginate composite film and the recovery of TiO2 nanoparticle. RSC Adv. 2014, 4, 51321–51329. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Cui, Z.; Yao, Y. Modeling of filtration characteristics during submerged hollow fiber membrane microfiltration of yeast suspension under aeration condition. J. Membr. Sci. 2016, 510, 455–465. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Chen, S.C.; Amy, G.L.; Chung, T.S. Membrane fouling and anti-fouling strategies using RO retentate from a municipal water recycling plant as the feed for osmotic power generation. Water. Res. 2016, 88, 144–155. [Google Scholar] [CrossRef]
- Kumar, R.; Isloor, A.M.; Ismail, A.F.; Matsuura, T. Synthesis and characterization of novel water soluble derivative of chitosan as an additive for polysulfone ultrafiltration membrane. J. Membr. Sci. 2013, 440, 140–147. [Google Scholar] [CrossRef]
- Wang, L.; Miao, R.; Wang, X.; Lv, Y.; Meng, X.; Yang, Y.; Huang, D.; Feng, L.; Liu, Z.; Ju, K. Fouling behavior of typical organic foulants in polyvinylidene fluoride ultrafiltration membranes: Characterization from microforces. Environ. Sci. Technol. 2013, 47, 3708–3714. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Xu, W.L.; Zhou, F.; Yoon, Y.; Yu, M. Graphene oxide: A novel 2-dimensional material in membrane separation for water purification. Adv. Mater. Interfaces 2017, 4, 1600918. [Google Scholar] [CrossRef]
- Khayet, M.; Seman, M.N.A.; Hilal, N. Response surface modeling and optimization of composite nanofiltration modified membranes. J. Membr. Sci. 2010, 349, 113–122. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Pothan, L.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable nanocomposite films based on sodium alginate and cellulose nanofibrils. Materials 2016, 9, 50. [Google Scholar] [CrossRef]
- Li, S.; Kan, B.; Zhao, K.; Ren, T.; Lin, B.; Wei, J.; Chen, T. Preparation of tricalcium phosphate-calcium alginate composite flat sheet membranes and their application for protein release. Polym. Compos. 2015, 36, 1899–1906. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, T.; Lin, B.; Cui, W.; Kan, B.; Yang, N.; Zhou, X.; Zhang, X.; Wei, J. Adsorption and recognition of protein molecular imprinted calcium alginate/polyacrylamide hydrogel film with good regeneration performance and high toughness. React. Funct. Polym. 2015, 87, 7–14. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, K.; Qi, M.; Li, S.; Xu, G.; Wei, J.; He, X. Preparation of protein molecular-imprinted polysiloxane membrane using calcium alginate film as matrix and its application for cell culture. Polymers 2018, 10, 170. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, X.; Wei, J.; Li, J.; Zhou, X.; Liu, D.; Li, J. Calcium alginate hydrogel filtration membrane with excellent anti-fouling property and controlled separation performance. J. Membr. Sci. 2015, 492, 536–546. [Google Scholar] [CrossRef]
- Tămăşan, M.; Radu, T.; Simon, V. Spectroscopic characterisation and in vitro behaviour of kaolinite polyvinyl alcohol nanocomposite. App. Clay Sci. 2013, 72, 147–154. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Hosseinzadeh, H.; Sadeghi, M. Synthesis, characterization and swelling behavior of gelatin-g-poly(sodium acrylate)/kaolin superabsorbent hydrogel composites. J. Compos. Mater. 2007, 41, 2057–2069. [Google Scholar] [CrossRef]
- Pinkas, O.; Zilberman, M. Effect of hemostatic agents on properties of gelatin-alginate soft tissue adhesives. J. Biomater. Sci. Polym. Ed. 2014, 25, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lin, B.; Cui, W.; Feng, L.; Chen, T.; Wei, J. Preparation and adsorption of bovine serum albumin-imprinted polyacrylamide hydrogel membrane grafted on non-woven polypropylene. Talanta 2014, 121, 256–262. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wang, X.; Fan, F.; Zhao, K.; Wei, J.; Zhang, L.; Zhu, D. Biologically inspired silk fibroin grafted polyacrylonitrile filtration membrane prepared in ZnCl2 aqueous solution. Chin. Chem. Lett. 2019, 30, 239–242. [Google Scholar] [CrossRef]
- Chang, J.H.; An, Y.U.; Cho, D.; Giannelis, E.P. Poly(lactic acid) nanocomposites: Comparison of their properties with montmorillonite and synthetic mica (II). Polymer 2003, 44, 3715–3720. [Google Scholar] [CrossRef]
- Panzavolta, S.; Gioffrè, M.; Bracci, B.; Rubini, K.; Bigi, A. Montmorillonite reinforced type A gelatin nanocomposites. J. Appl. Polym. Sci. 2014, 131, 40301. [Google Scholar] [CrossRef]
- Pinkas, O.; Goder, D.; Noyvirt, R.; Peleg, S.; Kahlon, M.; Zilberman, M. Structuring of composite hydrogel bioadhesives and its effect on properties and bonding mechanism. Acta Biomater. 2017, 51, 125–137. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, X.; Qi, M.; Bai, T.; Zhao, K.; Zhang, X. Removal of Dyes and Cd2+ in Water by Kaolin/Calcium Alginate Filtration Membrane. Coatings 2019, 9, 218. https://doi.org/10.3390/coatings9040218
Zhao Y, Liu X, Qi M, Bai T, Zhao K, Zhang X. Removal of Dyes and Cd2+ in Water by Kaolin/Calcium Alginate Filtration Membrane. Coatings. 2019; 9(4):218. https://doi.org/10.3390/coatings9040218
Chicago/Turabian StyleZhao, Yujie, Xiaowei Liu, Meng Qi, Tian Bai, Kongyin Zhao, and Xinxin Zhang. 2019. "Removal of Dyes and Cd2+ in Water by Kaolin/Calcium Alginate Filtration Membrane" Coatings 9, no. 4: 218. https://doi.org/10.3390/coatings9040218
APA StyleZhao, Y., Liu, X., Qi, M., Bai, T., Zhao, K., & Zhang, X. (2019). Removal of Dyes and Cd2+ in Water by Kaolin/Calcium Alginate Filtration Membrane. Coatings, 9(4), 218. https://doi.org/10.3390/coatings9040218



