Photocatalytic and Photostability Behavior of Ag- and/or Al-Doped ZnO Films in Methylene Blue and Rhodamine B under UV-C Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
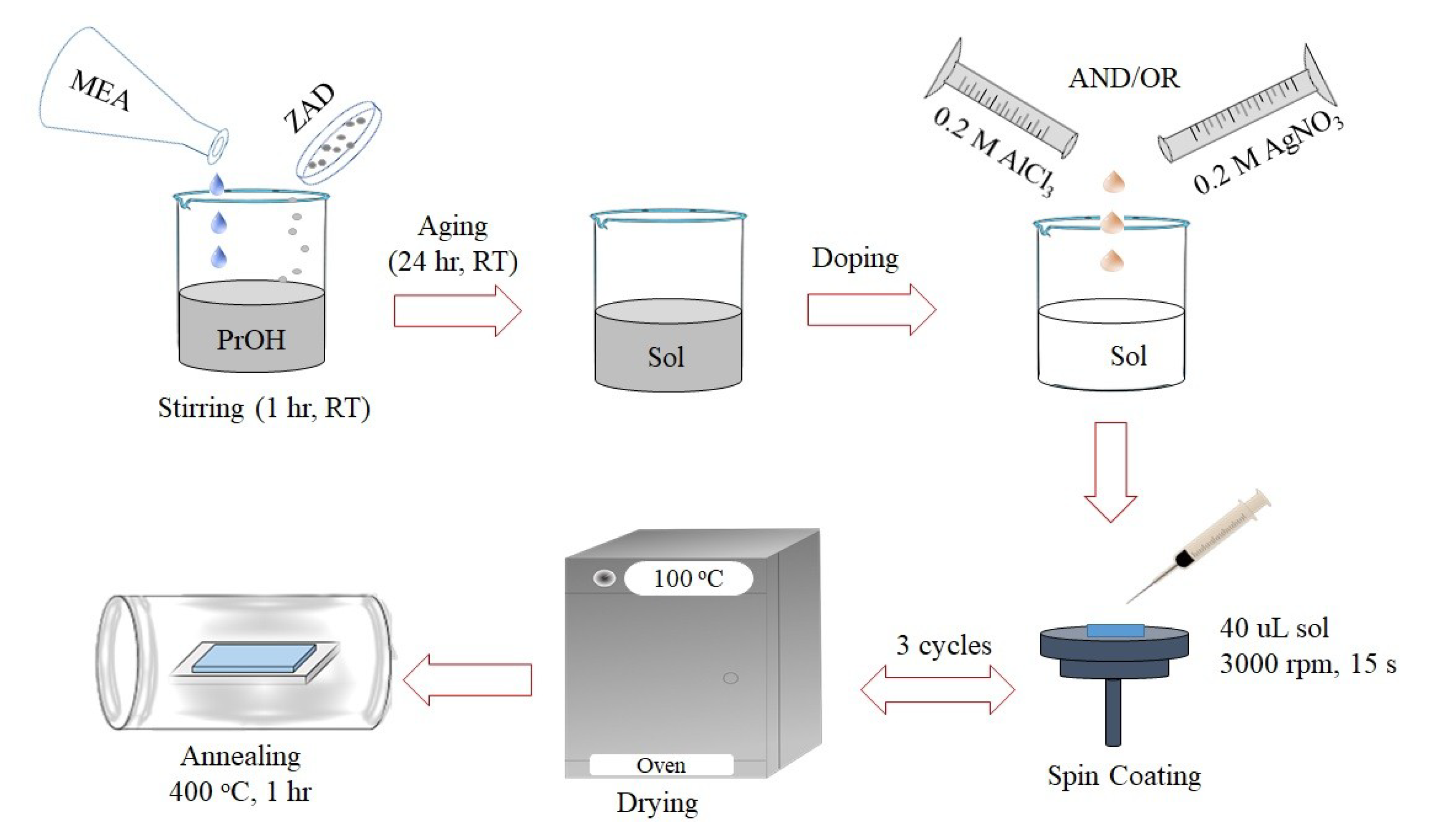
2.2. Precursor Sol Preparation and Film Deposition
2.3. Photocatalysis of Methylene Blue (MB) and Rhodamine B (RhB)
2.4. Characterization and Data Analysis
3. Results and Discussion
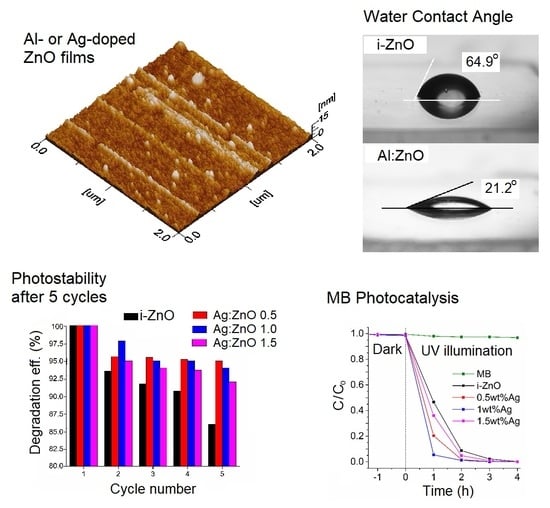
3.1. Morphological and Wettability Studies
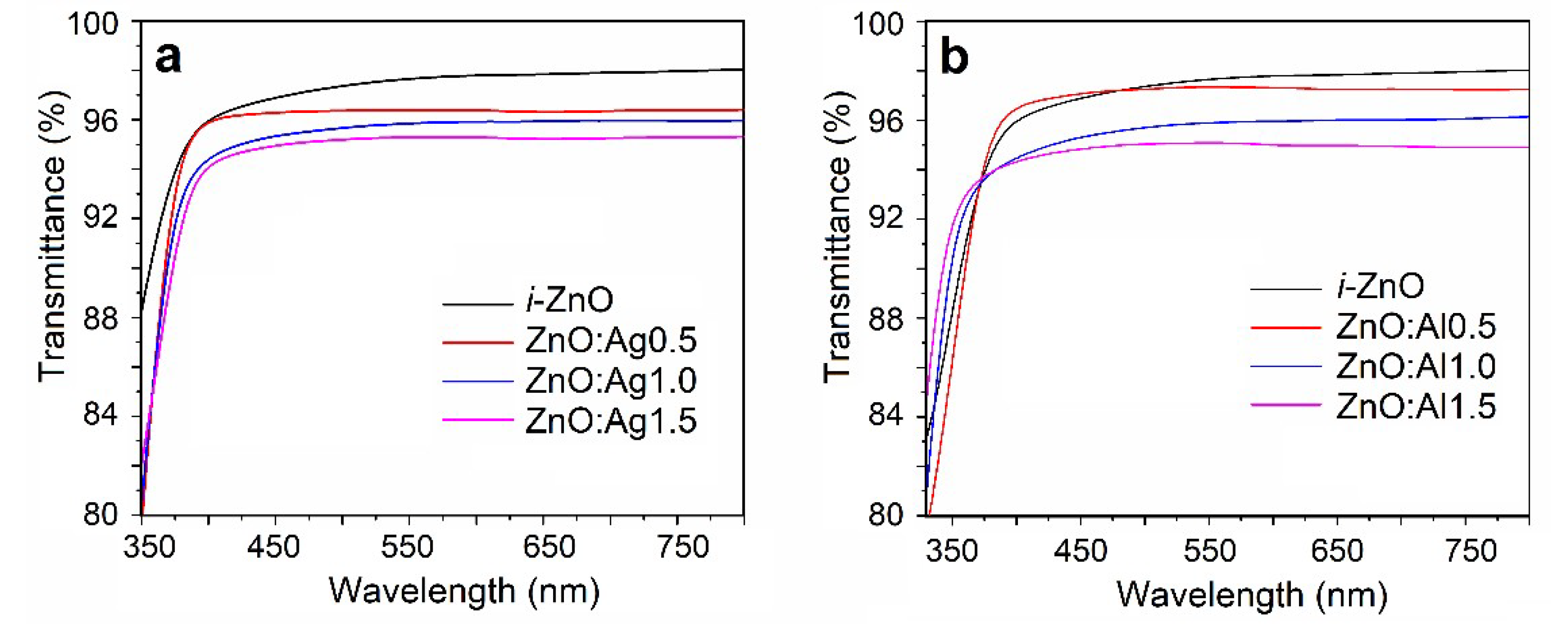
3.2. Optical Transmittance and Band Gap Measurements
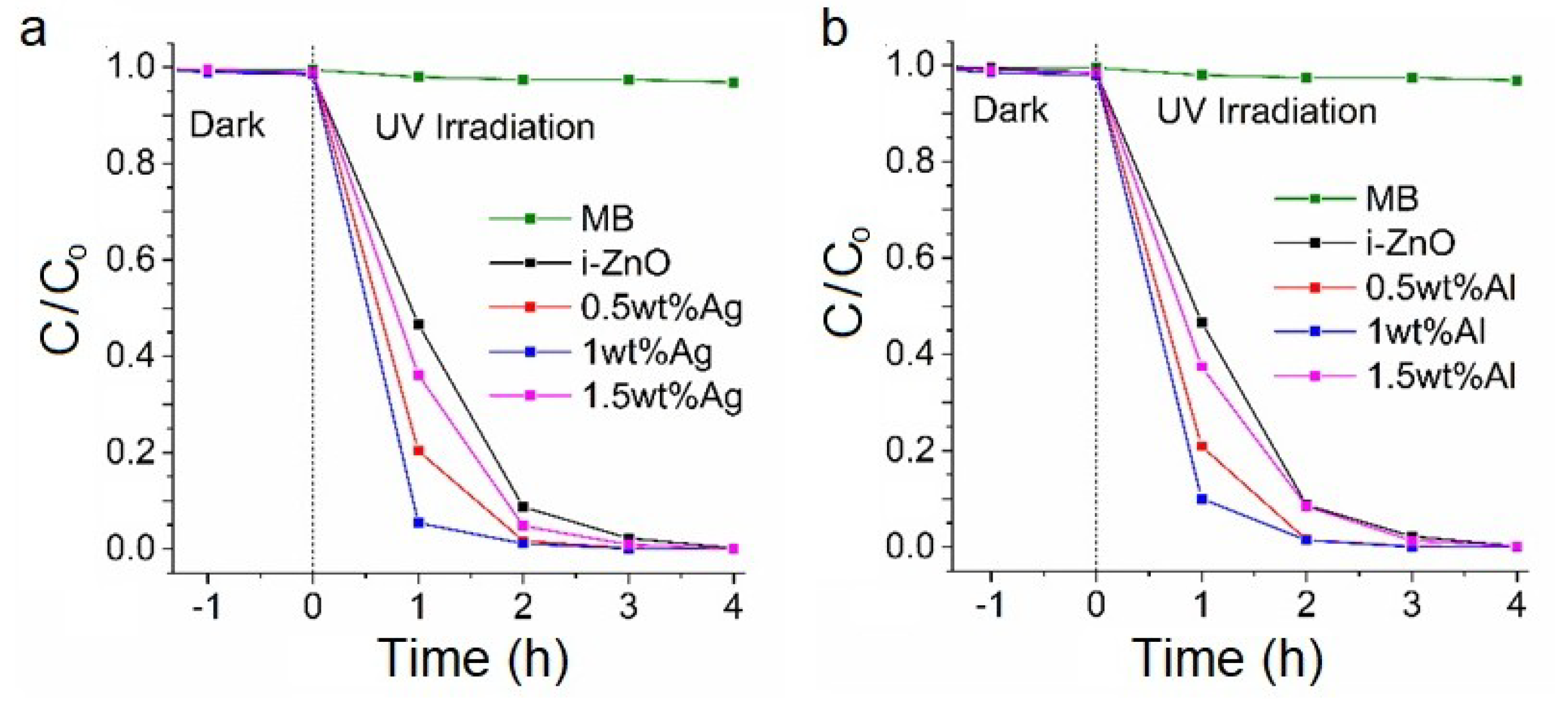
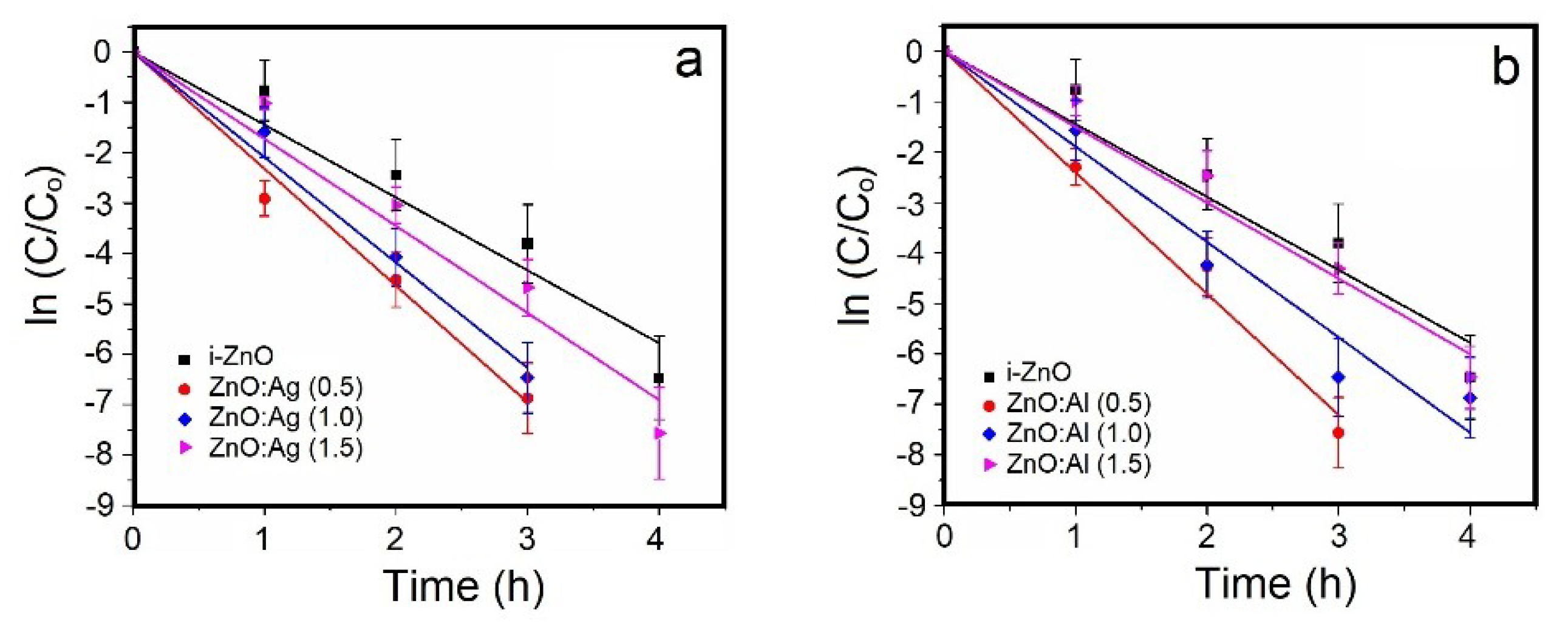
3.3. Methylene Blue (MB) Photocatalytic Decomposition
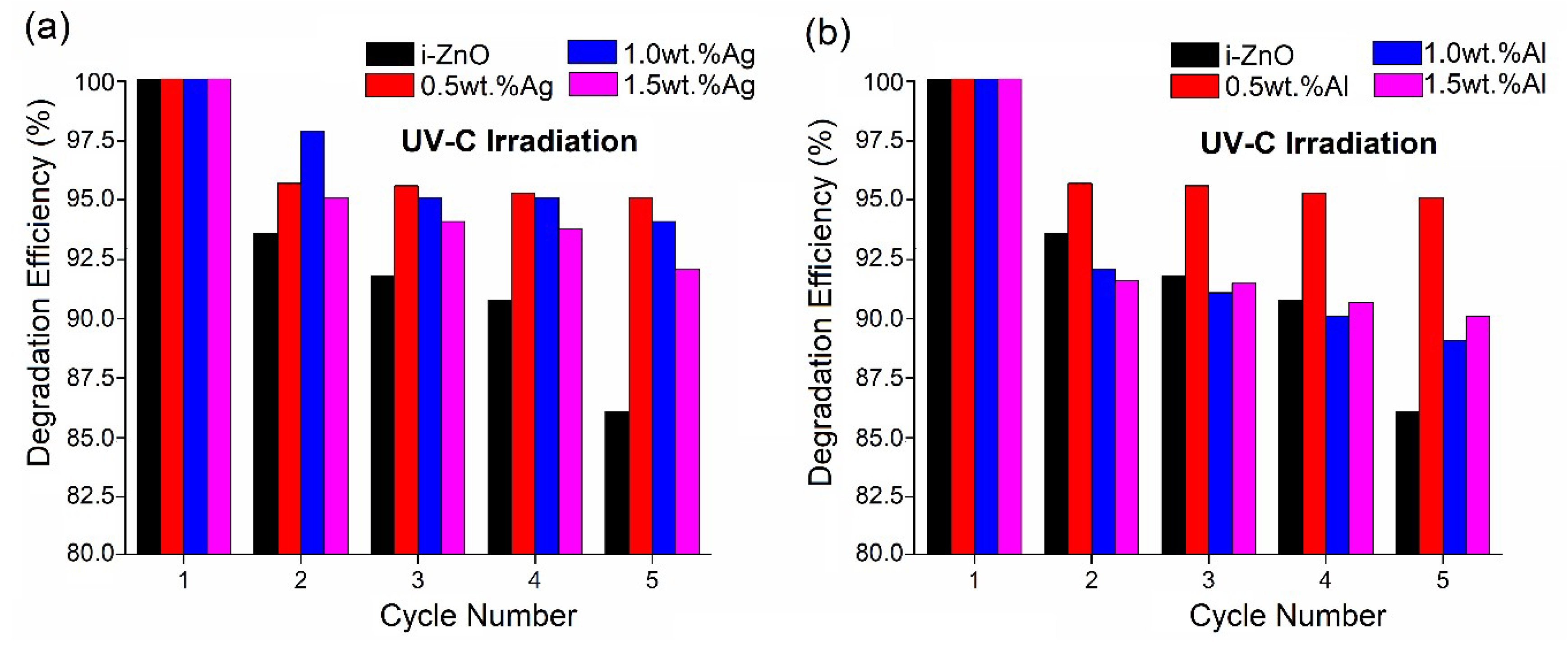
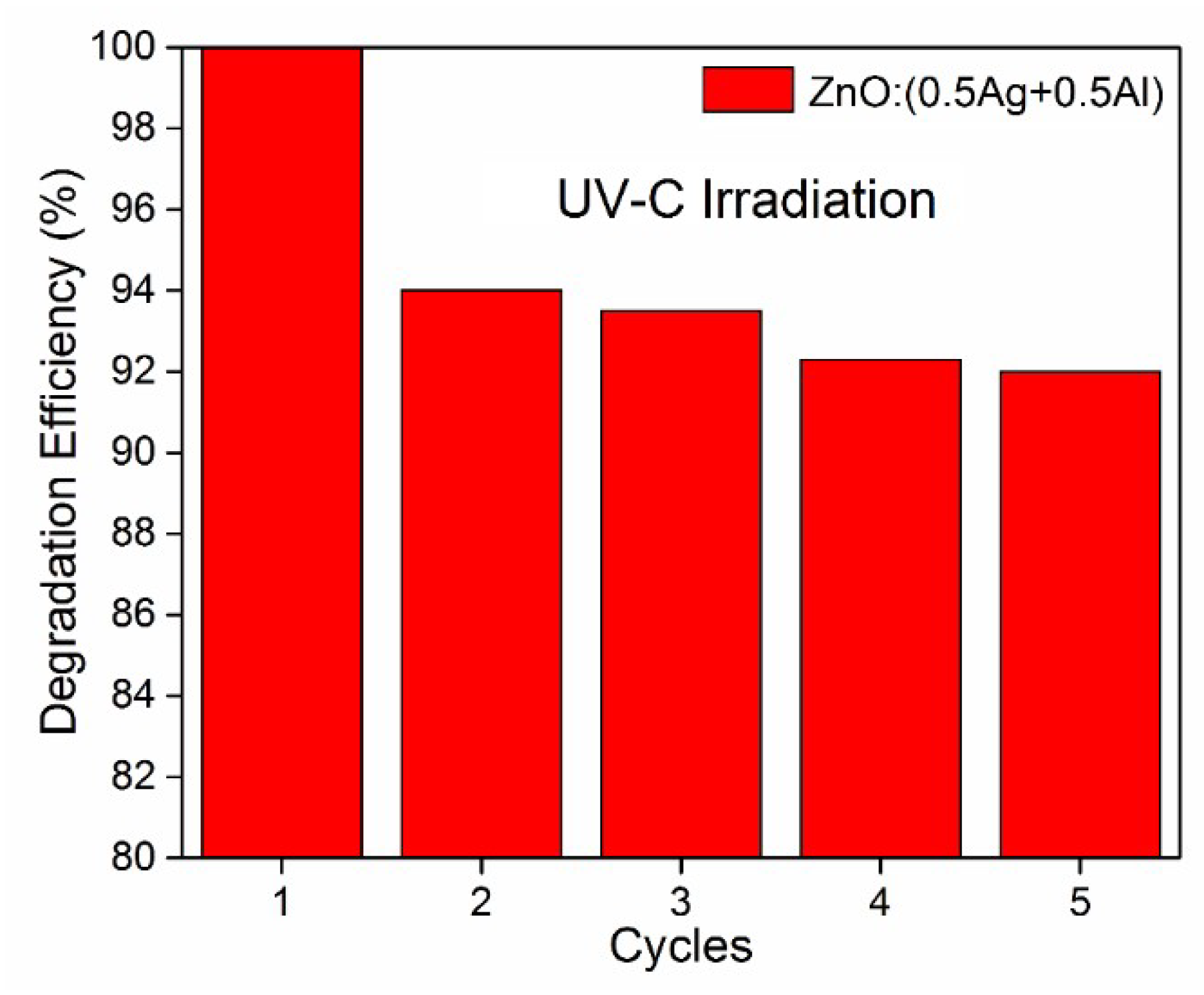
3.4. Photostability Evaluation of the Doped ZnO Films
3.5. Photocatalytic Effect of ZnO:(Ag + Al) Film in Rhodamine B Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parkin, I.P.; Palgrave, R.G. Self-cleaning coatings. J. Mater. Chem. 2005, 15, 1689–1695. [Google Scholar] [CrossRef]
- Ragesh, P.; Ganesh, V.A.; Nair, S.V.; Nair, A.S. A review on ‘self-cleaning and multifunctional materials’. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Mellott, N.P.; Durucan, C.; Pantano, C.G.; Guglielmi, M. Commercial and laboratory prepared titanium dioxide thin films for self-cleaning glasses: Photocatalytic performance and chemical durability. Thin Solid Films 2006, 502, 112–120. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Elliott, N.; Bhopal, S.; Parkin, I.P.; O’neill, S.A. Characterisation of the photocatalyst pilkington activ™: A reference film photocatalyst? J. Photochem. Photobiol. A Chem. 2003, 160, 213–224. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, L.; Meng, W.; Zhong, C.; Wei, S.; Dong, B.; Xu, Z.; Wan, L.; Wang, S. Tungsten-doped vanadium dioxide thin films as smart windows with self-cleaning and energy-saving functions. J. Alloy. Compd. 2017, 694, 124–131. [Google Scholar] [CrossRef]
- Thi, V.H.T.; Lee, B.-K. Development of multifunctional self-cleaning and UV blocking cotton fabric with modification of photoactive ZnO coating via microwave method. J. Photochem. Photobiol. A Chem. 2017, 338, 13–22. [Google Scholar] [CrossRef]
- Baudys, M.; Krýsa, J.; Mills, A. Smart inks as photocatalytic activity indicators of self-cleaning paints. Catal. Today 2017, 280, 8–13. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Demirel, A.L.; Avcı, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef]
- Sutha, S.; Suresh, S.; Raj, B.; Ravi, K. Transparent alumina based superhydrophobic self–cleaning coatings for solar cell cover glass applications. Sol. Energy Mater. Sol. Cells 2017, 165, 128–137. [Google Scholar] [CrossRef]
- Pathokoti, K.; Manubolu, M.; Hwang, H.-M. Nanotechnology applications for environmental industry. In Handbook of Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 894–907. [Google Scholar]
- Kalyanasundaram, K. Photochemical applications of solar energy: Photocatalysis and photodecomposition of water. Photochemistry 2013, 41, 182–265. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Hussein, M.Z.; Erfani, M.; Abedini, A.; Bahmanrokh, G.; Navasery, M.; Vaziri, P. Visible light-induced degradation of methylene blue in the presence of photocatalytic ZnS and CdS nanoparticles. Int. J. Mol. Sci. 2012, 13, 12242–12258. [Google Scholar] [CrossRef]
- Intarasuwan, K.; Amornpitoksuk, P.; Suwanboon, S.; Graidist, P. Photocatalytic dye degradation by ZnO nanoparticles prepared from X2C2O4 (X = H, Na and NH4) and the cytotoxicity of the treated dye solutions. Sep. Purif. Technol. 2017, 177, 304–312. [Google Scholar] [CrossRef]
- Badawy, M.I.; Mahmoud, F.; Abdel-Khalek, A.A.; Gad-Allah, T.A.; Abdel Samad, A. Solar photocatalytic activity of sol–gel prepared Ag-doped ZnO thin films. Desalin. Water Treat. 2014, 52, 2601–2608. [Google Scholar] [CrossRef]
- Sun, J.-H.; Dong, S.-Y.; Wang, Y.-K.; Sun, S.-P. Preparation and photocatalytic property of a novel dumbbell-shaped ZnO microcrystal photocatalyst. J. Hazard. Mater. 2009, 172, 1520–1526. [Google Scholar] [CrossRef]
- Trandafilović, L.; Jovanović, D.; Zhang, X.; Ptasińska, S.; Dramićanin, M. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO:Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Sun, W.; Huang, J.; Xie, H.; Zhao, X. Surface modification of ZnO with Ag improves its photocatalytic efficiency and photostability. J. Photochem. Photobiol. A Chem. 2010, 216, 149–155. [Google Scholar] [CrossRef]
- Manzoor, U.; Kim, D.K.; Islam, M.; Bhatti, A.S. Removal of micrometer size morphological defects and enhancement of ultraviolet emission by thermal treatment of Ga-doped ZnO nanostructures. PLoS ONE 2014, 9, e86418. [Google Scholar] [CrossRef]
- Alam, M.; Islam, M.; Achour, A.; Hayat, A.; Ahsan, B.; Rasheed, H.; Salam, S.; Mujahid, M. Solution processing of cadmium sulfide buffer layer and aluminum-doped zinc oxide window layer for thin films solar cells. Surf. Rev. Lett. 2014, 21, 1450059. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef]
- Akram, M.A.; Javed, S.; Islam, M.; Mujahid, M.; Safdar, A. Arrays of czts sensitized ZnO/ZnS and ZnO/ZnSe core/shell nanorods for liquid junction nanowire solar cells. Sol. Energy Mater. Sol. Cells 2016, 146, 121–128. [Google Scholar] [CrossRef]
- Akram, M.A.; Javed, S.; Xu, J.; Mujahid, M.; Lee, C.-S. Arrays of ZnO/CuInxGa1−xSe2 nanocables with tunable shell composition for efficient photovoltaics. J. Appl. Phys. 2015, 117, 205306. [Google Scholar] [CrossRef]
- Shahid, M.; Deen, K.; Ahmad, A.; Akram, M.; Aslam, M.; Akhtar, W. Formation of Al-doped ZnO thin films on glass by sol–gel process and characterization. Appl. Nanosci. 2016, 6, 235–241. [Google Scholar] [CrossRef]
- Chopra, K.; Paulson, P.; Dutta, V. Thin-film solar cells: An overview. Prog. Photovolt. Res. Appl. 2004, 12, 69–92. [Google Scholar] [CrossRef]
- Suchea, M.; Christoulakis, S.; Moschovis, K.; Katsarakis, N.; Kiriakidis, G. ZnO transparent thin films for gas sensor applications. Thin Solid Films 2006, 515, 551–554. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.; Park, J.; Bao, X.; Lo, Y.-H.; Wang, D. ZnO nanowire UV photodetectors with high internal gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Subasri, R.; Asha, M.; Hembram, K.; Rao, G.; Rao, T. Microwave sintering of doped nanocrystalline ZnO and characterization for varistor applications. Mater. Chem. Phys. 2009, 115, 677–684. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Javed, F.; Javed, S.; Akram, M.A.; Mujahid, M.; Islam, M.; Bhatti, A.S. Surface plasmon mediated optical properties of ZnO/Au/TiO2 nanoheterostructure rod arrays. Mater. Sci. Eng. B 2018, 231, 32–39. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Kenanakis, G.; Giannakoudakis, Z.; Vernardou, D.; Savvakis, C.; Katsarakis, N. Photocatalytic degradation of stearic acid by ZnO thin films and nanostructures deposited by different chemical routes. Catal. Today 2010, 151, 34–38. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Chen, Y.-Y. Comparison of the optical and electrical properties of Al-doped ZnO films using a lorentz model. Coatings 2019, 9, 4. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Hu, Z.; Zhao, G.; Zhao, Y. Effective light trapping enhanced near-UV/blue light absorption in inverted polymer solar cells via sol–gel textured Al-doped ZnO buffer layer. Sol. Energy Mater. Sol. Cells 2014, 121, 28–34. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Peer, A.; Joshi, P.H.; Biswas, R.; Dalal, V.L. Blue photon management by inhouse grown ZnO:Al cathode for enhanced photostability in polymer solar cells. Sol. Energy Mater. Sol. Cells 2018, 179, 95–101. [Google Scholar] [CrossRef]
- Ajala, F.; Hamrouni, A.; Houas, A.; Lachheb, H.; Megna, B.; Palmisano, L.; Parrino, F. The influence of Al doping on the photocatalytic activity of nanostructured ZnO: The role of adsorbed water. Appl. Surf. Sci. 2018, 445, 376–382. [Google Scholar] [CrossRef]
- Baradaran, M.; Ghodsi, F.; Bittencourt, C.; Llobet, E. The role of Al concentration on improving the photocatalytic performance of nanostructured ZnO/ZnO:Al/ZnO multilayer thin films. J. Alloy. Compd. 2019, 788, 289–301. [Google Scholar] [CrossRef]
- Dakhel, A.; Bououdina, M.; Jaafar, A.; Khan, W.; Azam, S.; Minar, J.; Kanoun, M.; Goumri-Said, S. Effect of (Cd, Al) co-doping and hydrogenation on the long-range ferromagnetic ordering of ZnO: Experimental and DFT studies. J. Alloy. Compd. 2018, 753, 813–820. [Google Scholar] [CrossRef]
- Benzitouni, S.; Zaabat, M.; Aida, M.; Ebothe, J.; Michel, J.; Boudine, B.; Mansouri, L.; Saidani, T. Morphology and photocatalytic activity of porous (In, Mg) co-doped ZnO nanoparticles. Optik 2018, 156, 949–960. [Google Scholar] [CrossRef]
- Ghomri, R.; Shaikh, M.N.; Ahmed, M.; Song, W.; Cai, W.; Bououdina, M.; Ghers, M. Pure and (Er, Al) co-doped ZnO nanoparticles: Synthesis, characterization, magnetic and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2018, 29, 10677–10685. [Google Scholar] [CrossRef]
- Thejaswini, T.; Prabhakaran, D.; Maheswari, M.A. Soft synthesis of potassium co-doped Al–ZnO nanocomposites: A comprehensive study on their visible-light driven photocatalytic activity on dye degradation. J. Mater. Sci. 2016, 51, 8187–8208. [Google Scholar] [CrossRef]
- Salam, S.; Islam, M.; Akram, A. Sol–gel synthesis of intrinsic and aluminum-doped zinc oxide thin films as transparent conducting oxides for thin film solar cells. Thin Solid Films 2013, 529, 242–247. [Google Scholar] [CrossRef]
- Jongnavakit, P.; Amornpitoksuk, P.; Suwanboon, S.; Ndiege, N. Preparation and photocatalytic activity of Cu-doped ZnO thin films prepared by the sol–gel method. Appl. Surf. Sci. 2012, 258, 8192–8198. [Google Scholar] [CrossRef]
- Rudd, A.L.; Breslin, C.B. Photo-induced dissolution of zinc in alkaline solutions. Electrochim. Acta 2000, 45, 1571–1579. [Google Scholar] [CrossRef]

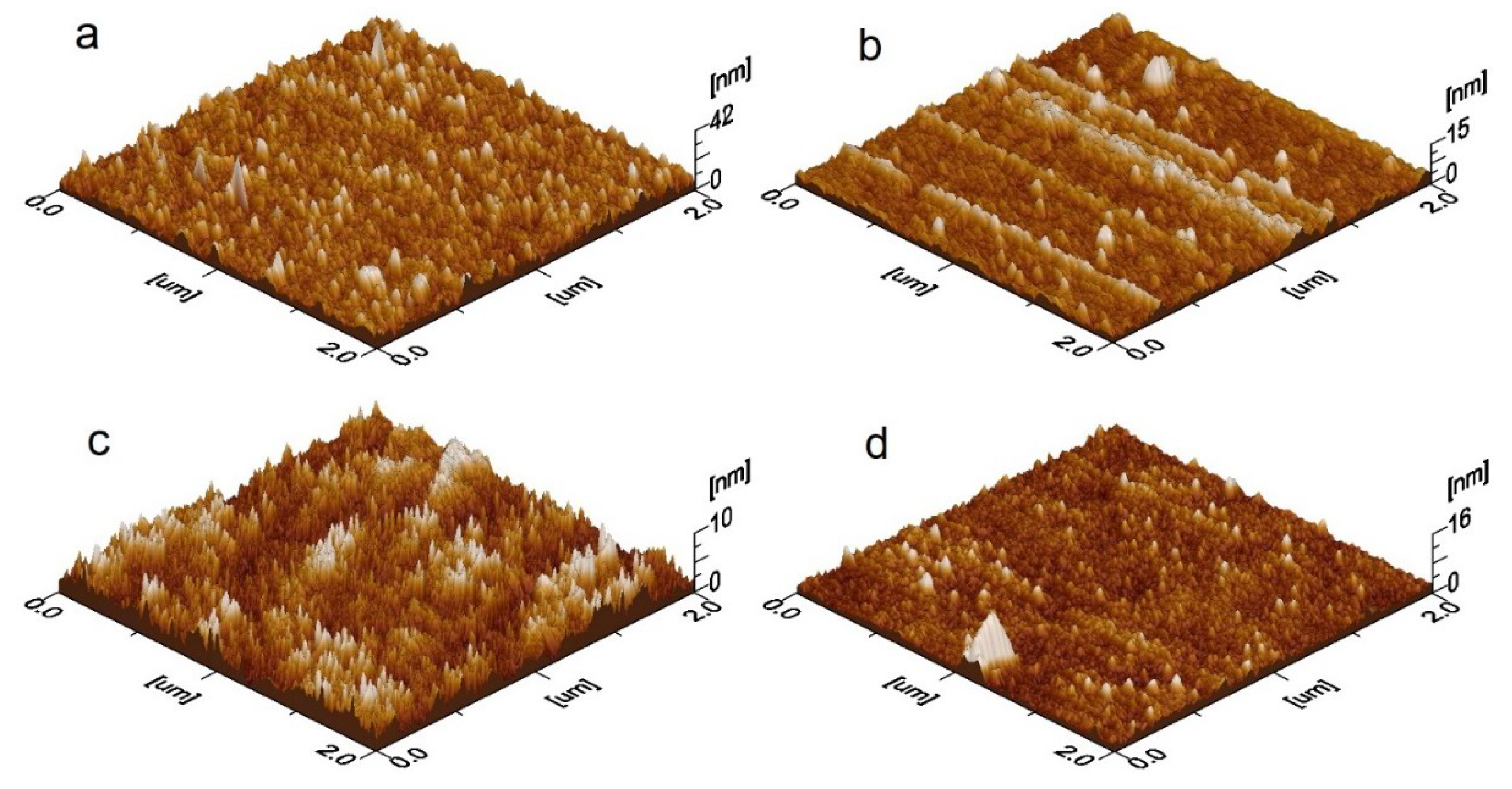
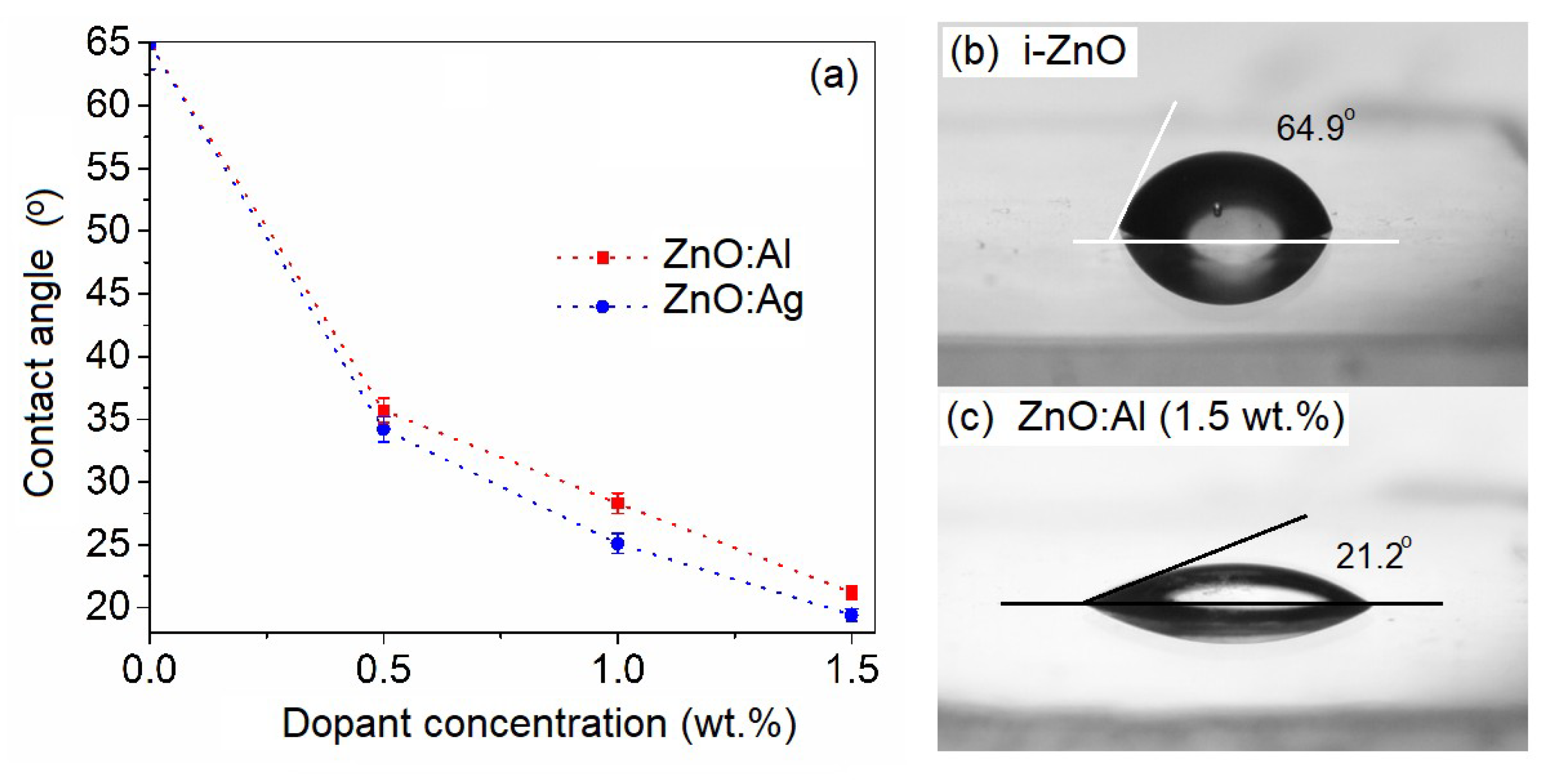


| Sample ID | Description | Ra (nm) | Rq (nm) | Bandgap Eg (eV) | Contact Angle θ (°) | Rate Constant kapp (h−1) |
|---|---|---|---|---|---|---|
| i-ZnO | Intrinsic ZnO | 2.37 | 3.13 | 3.27 | 64.9 | 1.44 |
| ZnO:Ag0.5 | ZnO with 0.5 wt.% Ag | 2.09 | 2.97 | 3.26 | 34.2 | 2.32 |
| ZnO:Ag1.0 | ZnO with 1.0 wt.% Ag | 1.56 | 1.85 | 3.24 | 25.1 | 2.09 |
| ZnO:Ag1.5 | ZnO with 1.5 wt.% Ag | 1.47 | 1.80 | 3.21 | 19.4 | 1.73 |
| ZnO:Al0.5 | ZnO with 0.5 wt.% Al | 1.05 | 1.34 | 3.32 | 35.7 | 2.40 |
| ZnO:Al1.0 | ZnO with 1.0 wt.% Al | 0.94 | 1.15 | 3.36 | 28.3 | 1.89 |
| ZnO:Al1.5 | ZnO with 1.5 wt.% Al | 0.87 | 1.09 | 3.40 | 21.2 | 1.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, A.; Ashraf, A.; Taimoor, H.; Javed, S.; Akram, M.A.; Islam, M.; Mujahid, M.; Ahmad, I.; Saeed, K. Photocatalytic and Photostability Behavior of Ag- and/or Al-Doped ZnO Films in Methylene Blue and Rhodamine B under UV-C Irradiation. Coatings 2019, 9, 202. https://doi.org/10.3390/coatings9030202
Riaz A, Ashraf A, Taimoor H, Javed S, Akram MA, Islam M, Mujahid M, Ahmad I, Saeed K. Photocatalytic and Photostability Behavior of Ag- and/or Al-Doped ZnO Films in Methylene Blue and Rhodamine B under UV-C Irradiation. Coatings. 2019; 9(3):202. https://doi.org/10.3390/coatings9030202
Chicago/Turabian StyleRiaz, Adeel, Amna Ashraf, Hymna Taimoor, Sofia Javed, Muhammad Aftab Akram, Mohammad Islam, Mohammad Mujahid, Iftikhar Ahmad, and Khalid Saeed. 2019. "Photocatalytic and Photostability Behavior of Ag- and/or Al-Doped ZnO Films in Methylene Blue and Rhodamine B under UV-C Irradiation" Coatings 9, no. 3: 202. https://doi.org/10.3390/coatings9030202
APA StyleRiaz, A., Ashraf, A., Taimoor, H., Javed, S., Akram, M. A., Islam, M., Mujahid, M., Ahmad, I., & Saeed, K. (2019). Photocatalytic and Photostability Behavior of Ag- and/or Al-Doped ZnO Films in Methylene Blue and Rhodamine B under UV-C Irradiation. Coatings, 9(3), 202. https://doi.org/10.3390/coatings9030202