Adhesive Hybrid SiO2.01C0.23Hx Nanoparticulate Coating on Polyethylene (PE) Separator by Roll-to-Roll Atmospheric Pressure Plasma
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
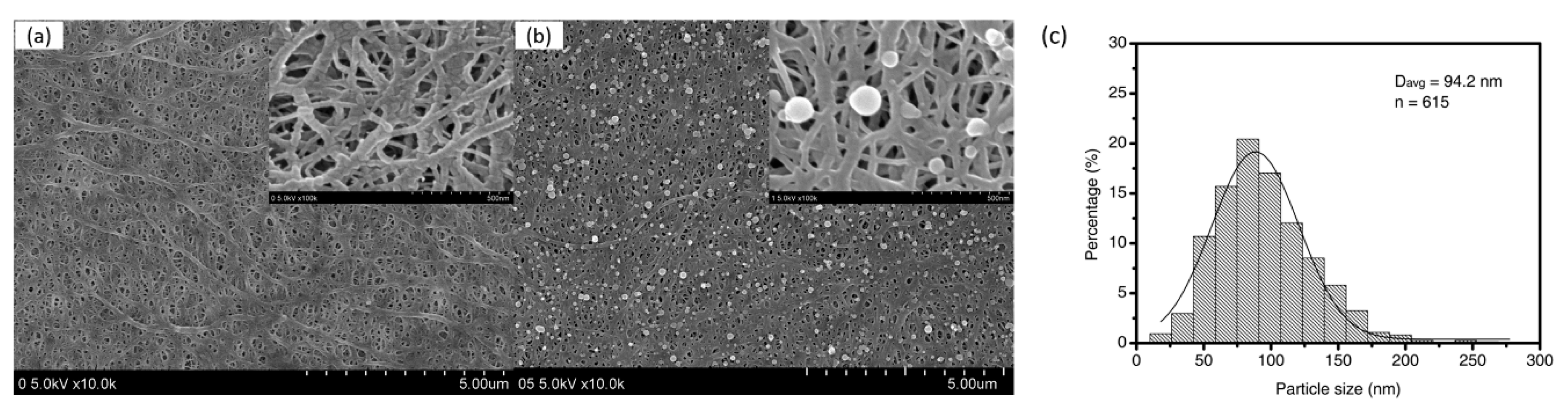
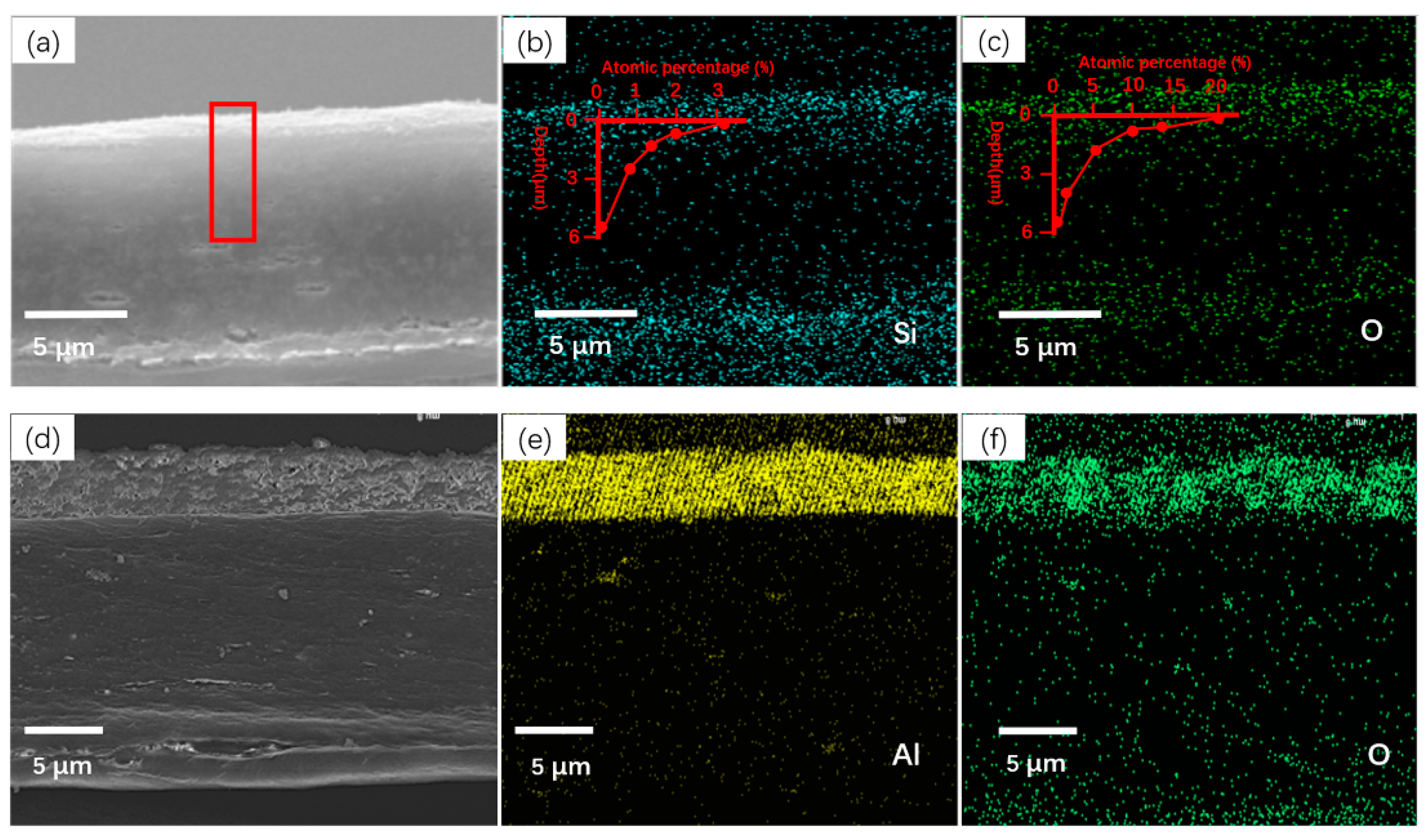
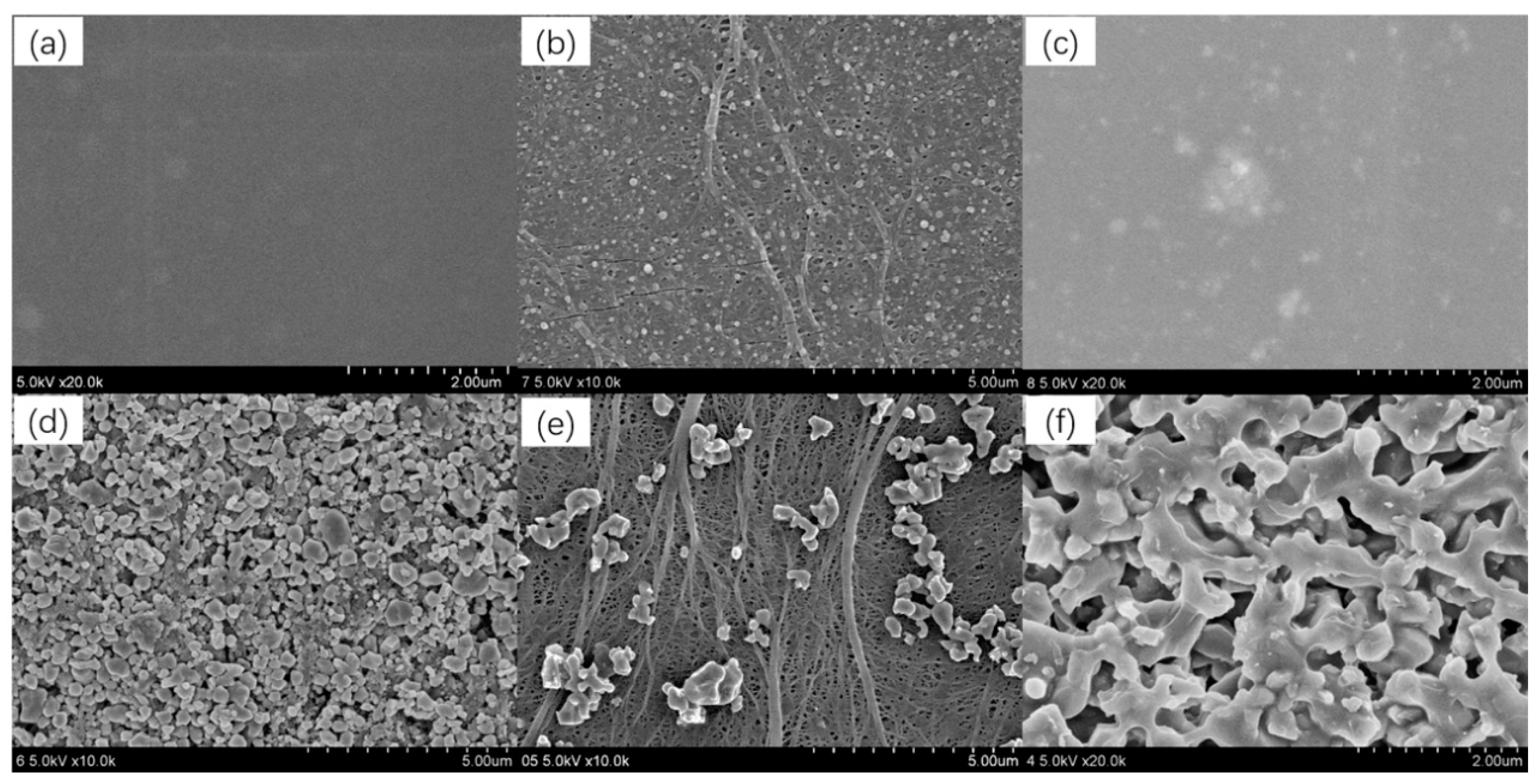
3.1. Morphological and Compositional Changes
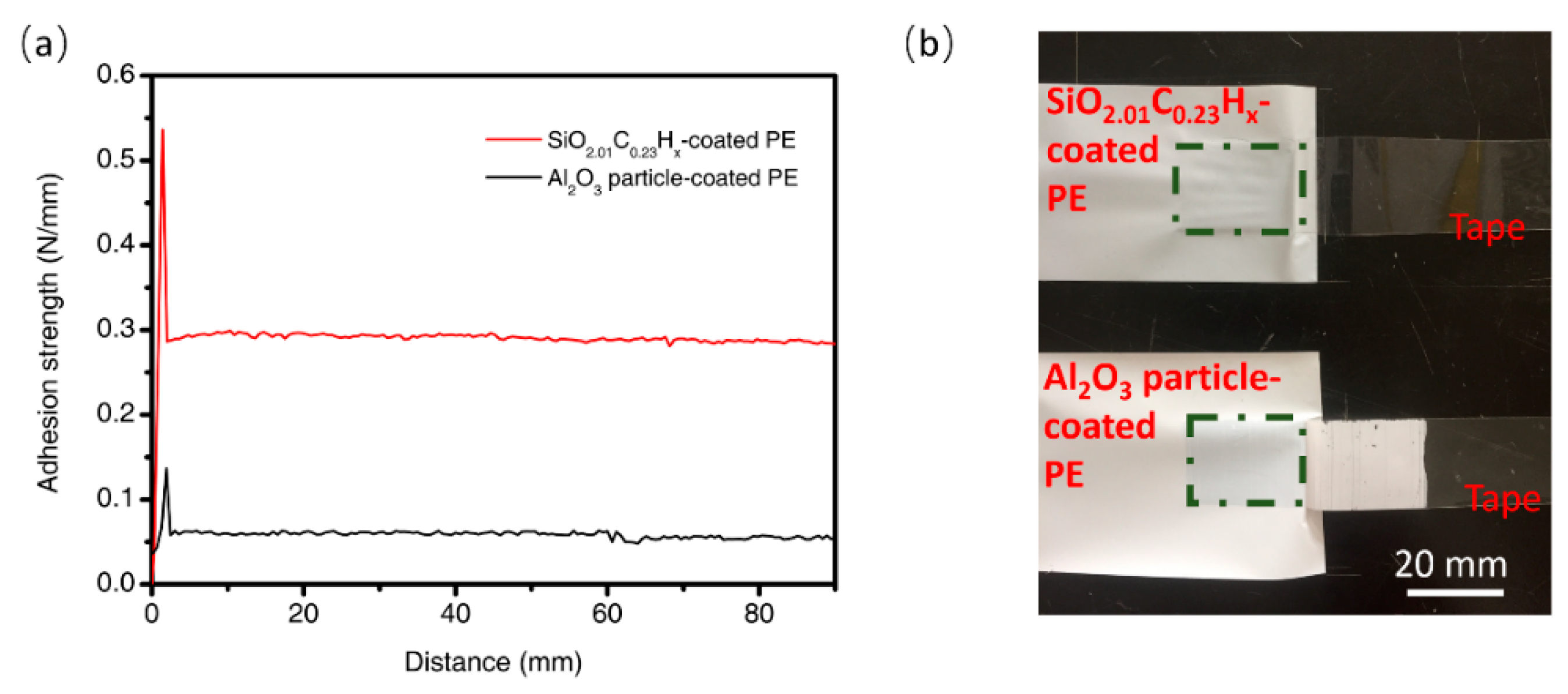
3.2. Adhesion Characteristics of the Coatings
3.3. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, M.-H.; Zhang, P.-P.; Gou, L.; Hou, Z.; Huang, B. Enhancement on the thermostability and wettability of lithium-ion batteries separator via surface chemical modification. Mater. Lett. 2017, 208, 98–101. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Shi, H.B.; Ding, J.Y.; Zhang, J.; Wang, H.; Wang, H. Fabrication of poly (vinylidene fluoride) separator with better thermostability and electrochemical performance for lithium ion battery by blending polyester. Mater. Lett. 2018, 228, 466–469. [Google Scholar] [CrossRef]
- Chawla, N.; Bharti, N.; Singh, S. Recent advances in non-flammable electrolytes for safer lithium-ion batteries. Batteries 2019, 5, 19. [Google Scholar] [CrossRef]
- Zhang, K.; Xiao, W.; Liu, J.; Yan, C. A novel self-binding composite separator based on poly(tetrafluoroethylene) coating for Li-ion batteries. Polymers 2018, 10, 1409. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Q.; Wang, J.; Zhang, P.; Zhu, Y.; Wu, G.; Lv, Y.; Lv, L.; Zhao, Y.; Yang, M. Effects of island-coated PVdF-HFP composite separator on the performance of commercial lithium-ion batteries. Coatings 2018, 8, 437. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Li, C.; Shen, X.; Peng, L.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A modified ceramic-coating separator with high-temperature stability for lithium-ion battery. Polymers 2017, 9, 159. [Google Scholar] [CrossRef]
- Lee, H.W.; Muralidharan, P.; Ruffo, R.; Mari, C.M.; Cui, Y.; Kim, D.K. Ultrathin spinel LiMn2O4 nanowires as high power cathode materials for Li-ion batteries. Nano Lett. 2010, 10, 3852. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Liu, J.; Qiao, S.; Lu, G.M.; Munroe, P.; Ahn, H. Mesoporous LiFePO4/C nanocomposite cathode materials for high power lithium ion batteries with superior performance. Adv. Mater. 2010, 22, 4944. [Google Scholar] [CrossRef]
- Kritzer, P.; Döring, H.; Emermacher, B. Improved safety for automotive lithium batteries: An innovative approach to include an emergency cooling element. Adv. Chem. Eng. Sci. 2014, 4, 197–207. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, X.; Liu, T.; Xie, Z.; Wang, Y.; Tian, K.; Bu, L.; Wang, H.; Gao, L.; Zhao, J. Ultrathin Al2O3 coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycleability at extended voltage ranges. Coatings 2019, 9, 92. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Tobishima, S.-I.; Yamaki, J.-I. A consideration of lithium cell safety. J. Power Sources 1999, 81, 882–886. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, H.; Gong, S.; Ryou, M.-H.; Yong, M.-L. A facile method to enhance the uniformity and adhesion properties of water-based ceramic coating layers on hydrophobic polyethylene separators. Appl. Surf. Sci. 2018, 427, 139–146. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, J.; Yang, C.; Gao, R.; Shi, C.; Zhao, J. A parallel bicomponent TPU/PI membrane with mechanical strength enhanced isotropic interfaces used as polymer electrolyte for lithium-ion battery. Polymers 2019, 11, 185. [Google Scholar] [CrossRef]
- Liu, J.C.; Yang, K.; Mo, Y.D.; Wang, S.J.; Han, D.M.; Xiao, M.; Meng, Y.Z. Highly safe lithium-ion batteries: High strength separator from polyformaldehyde/cellulose nanofibers blend. J. Power Sources 2018, 400, 502–510. [Google Scholar] [CrossRef]
- Starostin, S.A.; Premkumar, P.A.; Creatore, M.; de Vries, H.; Paffen, R.M.J.; van de Sanden, M.C.M. High current diffuse dielectric barrier discharge in atmospheric pressure air for the deposition of thin silica-like films. Appl. Phys. Lett. 2010, 96, 061502. [Google Scholar] [CrossRef]
- Alf, M.E.; Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Chelawat, H.; Ozaydin-Ince, G.; Petruczok, C.D.; Sreenivasan, R.; Tenhaeff, W.E.; Trujillo, N.J.; et al. Chemical vapor deposition of conformal, functional, and responsive polymer films. Adv. Mater. 2010, 22, 1993–2027. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Yang, J.; Mi, H.; Luo, S.; Deng, L.; Yan, C.; Zhang, P.; Lin, Z.; Ren, X.; et al. Robust SnO2−x nanoparticle-impregnated carbon nanofibers with outstanding electrochemical performance for advanced sodium-ion batteries. Nano Energy 2018, 43, 317. [Google Scholar] [CrossRef]
- Gawlik, G.; Ciepielewski, P.; Baranowski, J.M. Study of implantation defects in CVD graphene by optical and electrical methods. Appl. Sci. 2019, 9, 544. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, D.; Tu, R.; Yang, M.; Li, Q.; Han, M.; Zhang, S.; Zhang, L.; Goto, T. Structural controlling of highly-oriented polycrystal 3C-SiC bulks via halide CVD. Materials 2019, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Jäger, E.; Schmidt, J.; Pfuch, A.; Spang, S.; Beie, O.; Jäger, N.; Jantschner, O.; Daniel, R.; Mitterer, C. Antibacterial silicon oxide thin films doped with zinc and copper grown by atmospheric pressure plasma chemical vapor deposition. Nanomaterials 2019, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Min, J.; Lin, J.; Wan, H. Effect of atmospheric pressure plasma treatment on adhesive bonding of carbon fiber reinforced polymer. Polymers 2019, 11, 139. [Google Scholar] [CrossRef]
- Zhang, J.; Di, L.; Yu, F.; Duan, D.; Zhang, X. Atmospheric-pressure cold plasma activating Au/P25 for CO oxidation: effect of working gas. Nanomaterials 2018, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Dudina, D.V.; Bokhonov, B.B.; Olevsky, E.A. Fabrication of porous materials by spark plasma sintering: A review. Materials 2019, 12, 541. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Linh, N.N.; Ghimire, B.; Pengkit, A.; Sornsakdanuphap, J.; Lee, S.-J.; Choi, E.H. Plasma and nanomaterials: fabrication and biomedical applications. Nanomaterials 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, M.; Wang, C.; Jin, Y.; Yuan, N.; Wu, Z.; Zhang, J. Binder-free nanoparticulate coating of a polyethylene separator via a reactive atmospheric pressure plasma for lithium-ion batteries with improved performances. Adv. Mater. Interfaces 2018, 5, 1800579. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Q.; Guo, Y.; Liu, X.; Shi, J.; Zhang, J. One step growth of TiO2 crystal trees by atmospheric pressure plasma jet. Mater. Lett. 2011, 65, 2526–2529. [Google Scholar] [CrossRef]
- Jeon, H.; Jin, S.; Park, W.; Lee, H.; Kim, H.; Ryou, M.; Lee, Y. Plasma-assisted water-based Al2O3 ceramic coating for polyethylene-based microporous separators for lithium metalsecondary batteries. Electrochim. Acta 2016, 212, 649–656. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Yang, L.; Wang, X.; Liu, Z.; Chen, Q. Plasma modified polypropylene membranes as the lithium-ion battery separators. Plasma Sci. Technol. 2016, 18, 424. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Wang, C.; Yuan, N.; Ding, K.; Xu, Y.; Qin, S.; Wang, M.; Wu, Z.; Du, C.; Shi, J.; et al. Adhesive Hybrid SiO2.01C0.23Hx Nanoparticulate Coating on Polyethylene (PE) Separator by Roll-to-Roll Atmospheric Pressure Plasma. Coatings 2019, 9, 190. https://doi.org/10.3390/coatings9030190
Jin Y, Wang C, Yuan N, Ding K, Xu Y, Qin S, Wang M, Wu Z, Du C, Shi J, et al. Adhesive Hybrid SiO2.01C0.23Hx Nanoparticulate Coating on Polyethylene (PE) Separator by Roll-to-Roll Atmospheric Pressure Plasma. Coatings. 2019; 9(3):190. https://doi.org/10.3390/coatings9030190
Chicago/Turabian StyleJin, Yichao, Chaoliang Wang, Nana Yuan, Ke Ding, Yu Xu, Sicheng Qin, Ming Wang, Zhuangchun Wu, Chengran Du, Jianjun Shi, and et al. 2019. "Adhesive Hybrid SiO2.01C0.23Hx Nanoparticulate Coating on Polyethylene (PE) Separator by Roll-to-Roll Atmospheric Pressure Plasma" Coatings 9, no. 3: 190. https://doi.org/10.3390/coatings9030190
APA StyleJin, Y., Wang, C., Yuan, N., Ding, K., Xu, Y., Qin, S., Wang, M., Wu, Z., Du, C., Shi, J., & Zhang, J. (2019). Adhesive Hybrid SiO2.01C0.23Hx Nanoparticulate Coating on Polyethylene (PE) Separator by Roll-to-Roll Atmospheric Pressure Plasma. Coatings, 9(3), 190. https://doi.org/10.3390/coatings9030190



