1. Introduction
Food and pharmaceutical packaging plays an important role in keeping the quality and freshness of the products and prolonging their shelf life. The selection of suitable materials with optimum environmental and utilization properties belongs among the key factors in developing an effective coating fulfilling the industrial and consumer’s requirements [
1,
2,
3]. Polysaccharides represent an important category of polymers with good biodegradability and biocompatibility, which are essential properties required from biomaterials. In this group, chitosan, comprising glucosamine and
N-acetylglucosamine, ranks in a high position. This natural, nontoxic, biodegradable cationic polysaccharide is usually obtained through deacetylation of chitin and is available in various forms—solution, flakes, fibers, films, etc. [
4,
5]. Chitosan is applied in a range of industrial areas, e.g., as a chelating agent, flocculant for wastewater treatment, and a fungicidal agent for crop protection in agriculture. Other potential applications include cosmetics and skin care, and antimicrobial or anticoagulant agents. Chitosan has the ability to promote wound-healing processes because of its ability to support tissue regeneration and stimulate hemostasis. In addition, chitosan itself has proven antimicrobial effects, which can also be effectively used in biomedical research [
6]. The film-forming properties of chitosan can be exploited in tissue engineering, drug delivery systems, and the production of active food packaging [
3,
7].
These films and coatings can also serve as carriers of different active substances that include antimicrobials, antioxidants, or flavors. Among antimicrobial agents, bacteriocins, enzymes, organic acids, and/or essential oils can be mentioned [
2]. It is known that essential oils represent attractive natural-based substances possessing antimicrobial properties that are strongly affected by the composition and functional groups of these biologically active compounds [
8,
9]. Ecological and geographical aspects, as well as harvest conditions, are the predominant factors here. The highest efficiency was shown in oils based on thyme, cinnamon, oregano, and clove [
10,
11]. Wang et al. [
7] investigated effects of different essential oils in combination with chitosan film. The study revealed that antimicrobial properties were enhanced when cinnamon and clove bud oils were incorporated, and other physical properties were modified strongly depending on the specific oil type. Thyme essential oil is characteristic for a high content of biologically active compounds, such as thymol, carvacrol, p-cymene, and γ-terpinene. It was proven that carvacrol and thymol exhibit not only significant bacteriostatic and bactericidal properties, but also high antioxidant capacity [
8,
12,
13]. Altiok et al. [
8] prepared chitosan films enriched with thyme oil for potential application in healing wounds. The minimum inhibition concentration of thyme oil was set to 1.2% (
v/
v). Moreover, hydrophobic essential oils can improve not only the antimicrobial properties, but also the water-barrier properties of polymers [
14]. In a study by Perdones et al. [
15], chitosan systems with basil and thyme essential oils in combination with oleic acid were investigated. Water vapor permeability was improved in all samples. Barrier properties were also enhanced in chitosan films containing carvacrol and grape-seed extract [
16].
Since the incorporation of essential oils may lower the system stability and cause phase separation, other substances are usually required to control the stability, homogeneity, and surface properties of polymer films. Among these, various amphiphilic compounds with both a hydrophilic and a hydrophobic part are worth mentioning. Hydrophilic–lipophilic balance (HLB) is an important factor determining the final functional properties and applications of a surface-active molecule [
17]. The class of polysorbate non-ionic surfactants (commercially known as Tweens) comprise ethoxylated sorbitan attached to a hydrophobic chain based on a fatty-acid residue [
18]. Tween 80 was applied as an emulsifier into chitosan solutions enriched with carvacrol and grape-seed extract [
16]. The effects of Tween 20 and Tween 80 were compared in the study of Tongnuanchan et al. [
14], who evaluated their impact on the structural and thermal properties of films based on fish skin gelatin incorporated with basil and citronella oils. A strong effect of surfactant type on microstructural and thermal properties of prepared systems was proven in Reference [
17]. Tween 80 and Span 80 surfactants were added to the chitosan film-forming solution with lemon essential oil to evaluate their effects on structural, optical, and physical properties. While a more hydrophilic Tween 80 enhanced the compatibility and stability of essential oil in the chitosan matrix, Span 80 supported the oil transport to the film surface.
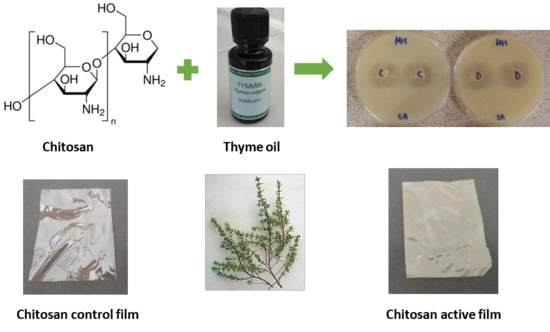
The objective of this work was to evaluate the physical and antimicrobial properties of chitosan systems prepared with various emulsifiers and enriched with thyme essential oil, with regard to their potential application as active packaging to enhance the quality and shelf life of food products, such as meat or vegetables.
2. Materials and Methods
2.1. Materials
Low-molecular-weight chitosan was provided by Sigma-Aldrich (Prague, Czech Republic). Two types of thyme essential oils were used, supplied by Nobilis Tilia s.r.o. (Krásná Lípa, Czech Republic) and Cosmetics Atok International s.r.o. (Trmice, Czech Republic). Emulsifiers Tween 20, 80, and 85 (T20, T80, T85) were supplied by Sigma-Aldrich. Microorganisms (Escherichia coli CCM 3954, Salmonella enterica subsp. Enterica ser. Typhimurium CCM 7205, Staphylococcus aureus CCM 3953, Bacillus cereus CCM 2010, Candida albicans CCM 8215, and Aspergillus niger CCM 8155) were obtained from the Czech Collection of Microorganisms (CCM, Brno, Czech Republic). Mueller–Hinton Agar (Himedia Laboratories, Telangana, India) at 37 °C (24 h) and Sabouraud Agar (Himedia Laboratories) at 20 °C (72 h) were used for the growth of bacterial and fungal cultures, respectively.
2.2. Analysis of Essential Oils by Gas Chromatography
Gas chromatography (GC) of essential oils was carried out on a DANI GC Master Fast Gas Chromatograph (Cologno Monzese, Italy) equipped with a Zebron™ ZB-5MS (Aschaffenburg, Germany) capillary column (30 m × 0.25 mm × 0.5 μm) and flame ionization detector (FID). The initial column temperature was set to 50 °C and then gradually increased to 120 °C (4 °C·min−1), after which it was raised to 230 °C at 15 °C·min−1 (left for 15 min). The nitrogen was used as the carrier gas (flow rate 1 mL/min). The injection port was heated and maintained at 200 °C, and the detector temperature was 270 °C. The volume of diluted samples of the essential oils in 1 mL of methanol was 1 μL (split ratio of 1:25)
2.3. Preparation of Chitosan Dispersions and Films
The dispersions were prepared by dissolving 1 g of chitosan in 100 mL of acetic acid (1%
v/
v) under continuous stirring at ambient temperature for 24 h. After chitosan solution filtration, a mixture of emulsifier (20%) and thyme oil (80%), marked as TEO, was added in concentrations ranging from 0.5 wt.% to 5 wt.%. The dispersions were subjected to premixing on a Vortex V-1 Plus device (Biosan, Riga, Latvia) for 1 min and then homogenization with Ultra Turrax IKA
® T-25 (IKA, Staufen, Germany) for 5 min at 15,600 rpm. After homogenization, 25 mL of the solutions were cast on sterile Petri dishes (9 cm in diameter) and dried at 35 °C in an air-circulated oven. Prior to further testing, the dried films were stored at 25 °C and 60% relative humidity. The description of samples is specified in
Table 1.
2.4. Particle Size and Zeta Potential of Film-Forming Solutions
The measurement was carried out on a Zetasizer Nano ZS device (Malvern Instruments, Ltd., Malvern, UK) after diluting the samples using filtrated distilled water (VWR® syringe filter 0.2 µm, Stříbrná Skalice, Czech Republic). The particle size was gauged via laser diffractometry (90° scattering angle, 1.33 refractive index, 0.001 absorption (Malvern Instruments, Ltd.). Zeta potential was analyzed using the Smoluchowski model. All measurements were carried out at 25 ± 1 °C.
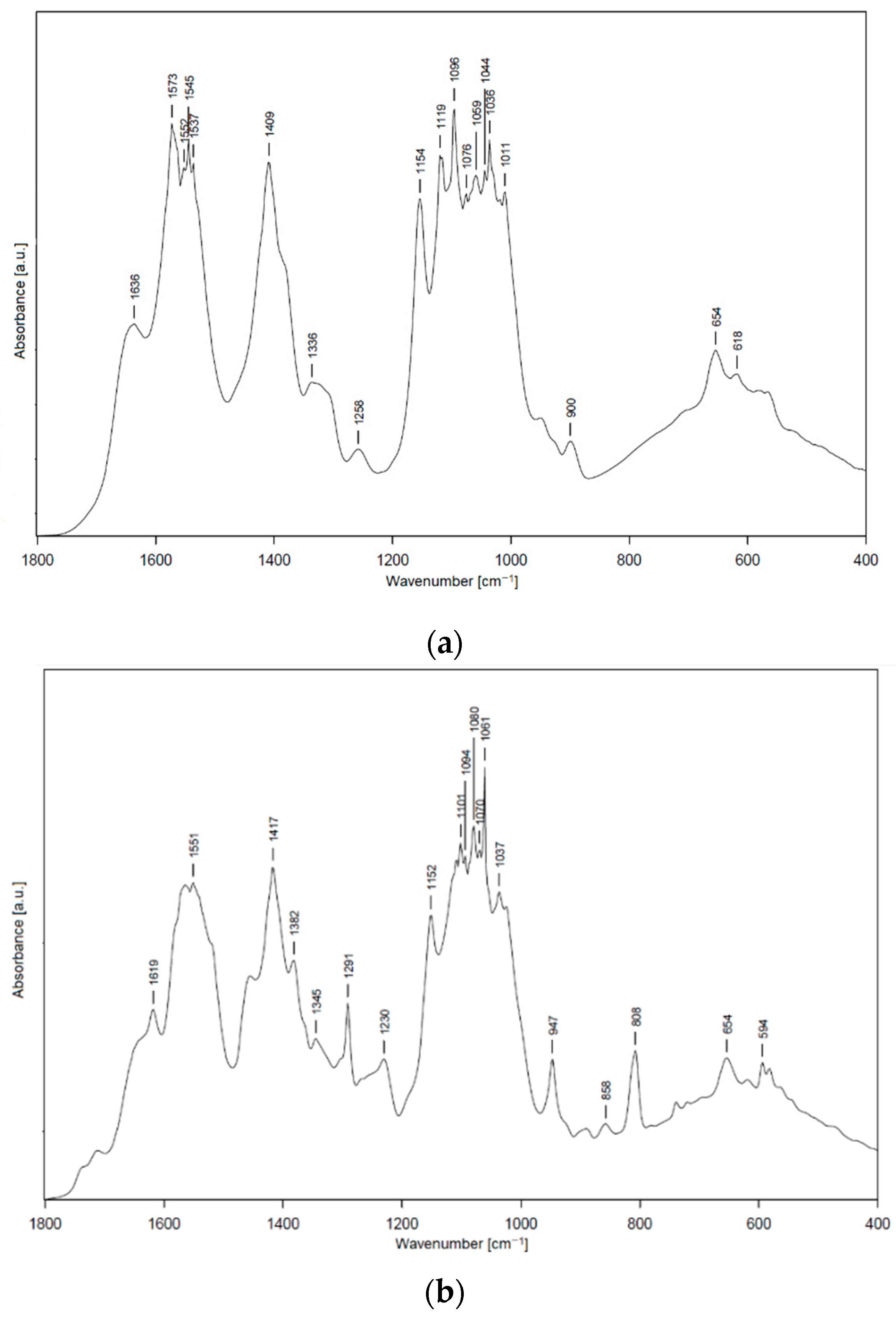
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
FTIR spectra were recorded on an Alpha-T FTIR spectrometer (Bruker, Billerica, MA, USA). The essential oils were measured using the KBr disc method (1 mg of thyme oil was ground with 150 mg of KBr (FTIR purity Acros Organics)). The films were measured without any previous treatment. The spectrum was recorded in the spectral range from 400 to 4000 cm−1 with a resolution of 4 cm−1 (16 scans). Evaluation of the spectra was performed using the OPUS program (version 7.5).
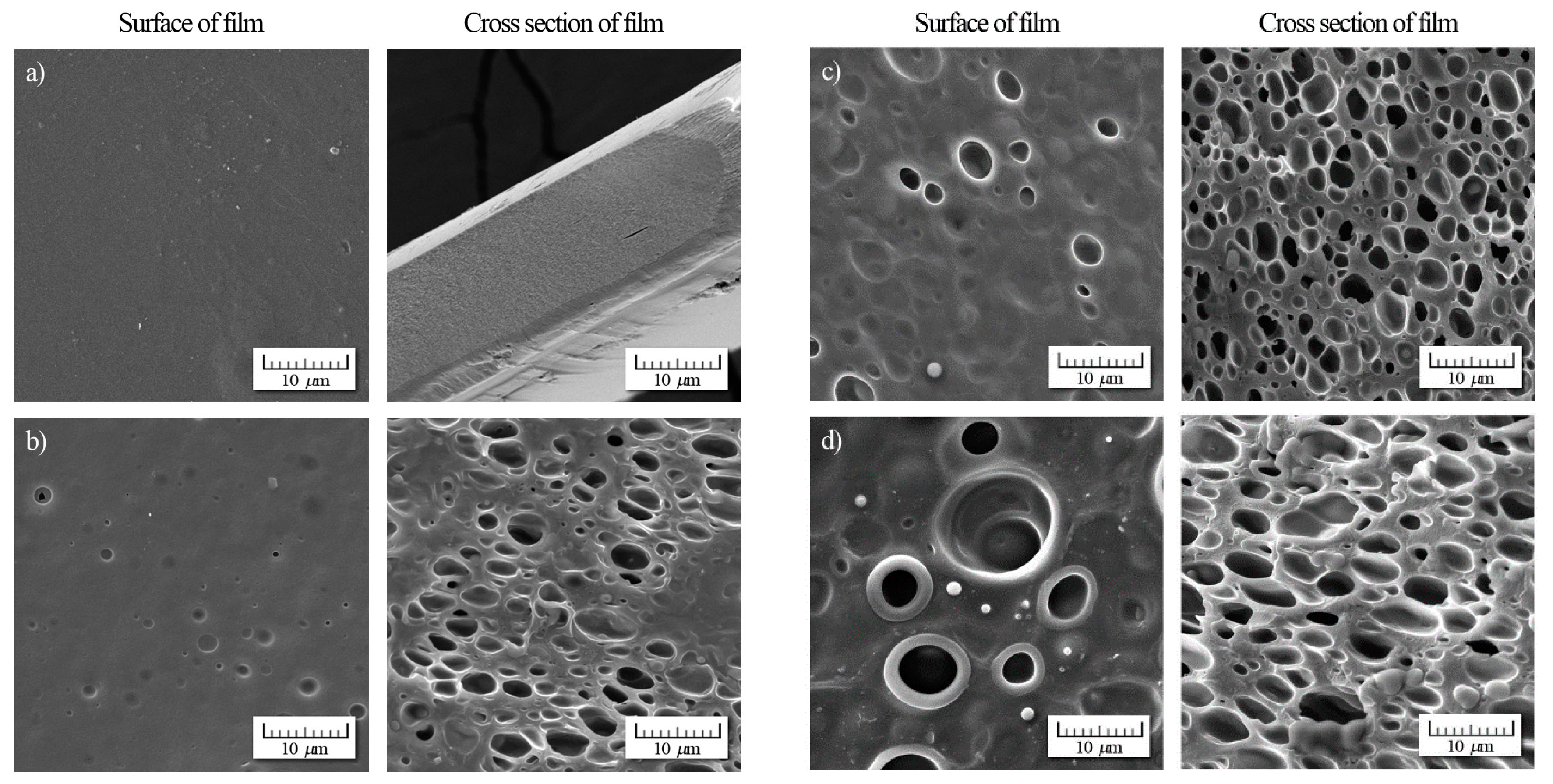
2.6. Morphological Characterization by Scanning Electron Microscopy
Morphology of the surfaces and cross-sections of the chitosan films was visualized using a Vega 3 high-resolution scanning electron microscope (Tescan, Brno, Czech Republic). The samples were sputtered with a conductive coating layer prior to visualization.
2.7. Film Thickness
The film thickness was gauged with a digital micrometer (Schut, Trossingen, Germany) as an average of ten measurements from each film at an accuracy of ±0.006 mm.
2.8. Contact Angle
The wettability of the chitosan films was analyzed via measuring the contact angles using the sessile drop method on a Surface Energy Evaluation System by Advex Instruments (Brno, Czech Republic) at ambient temperature. The final values were calculated as an average from three measurements. Demineralized water was used as the reference liquid; the volume of each deposited droplet was 5 µL.
2.9. Mechanical Properties
Prior to the measurements, the samples were conditioned at 25 °C and 50% relative humidity for 48 h. Mechanical properties were determined on a texture analysis machine TA1 Series (AMETEK Test & Calibration Instruments, Largo, FL, USA) using a NEXYGENPlus texture analysis software (version 4.0.1.184). For the puncture test, the films were cut to square-shaped samples (40 × 40 mm
2), which were placed between two plates with a circular hole (20 mm in diameter) and secured with a clamping device. A cylindrical probe (2 mm in diameter) was pressed through the center of the sample at a speed of 1 mm/s. The puncture strength (PS) in N·mm
−1 was then calculated from four measurements according to Equation (1).
where
Fmax is the maximum puncture strength (N), and
T is the average thickness of the film sample (mm). Puncture deformation (PD) in mm was evaluated from the distance when the probe contacted the specimen and the break point.
Tensile strength (TS) of the films was measured on samples of 10 mm × 60 mm size. The ends of the strips were mounted into the tensile grips so that the exposed specimen area was 10 mm × 40 mm. The crosshead speed was set to 1 mm·s
−1. Tensile strength (TS) was then evaluated by the NEXYGENPlus software (version 4.0.1.184) from the maximum stretching strength (N), thickness, and width of the specimen (mm), according to Equation (2).
where
Ft is the maximum tensile strength (N),
T is the average thickness of the film sample (mm), and
W is the width of the film sample (mm).
Elongation at break (E) was determined as a percentage by dividing the elongation at the break point by the initial specimen length multiplied by 100. The resultant values were calculated from four measurements.
2.10. Moisture Content and Water Solubility of Films
Chitosan samples (2 × 2 cm
2) were weighed (initial weight
M1) and then dried at 105 °C until constant weight (dry sample weight
M2). A moisture content was calculated as a percentage according to Equation (3).
The dried film pieces were then immersed in distilled water and, after agitation (for 24 h at ambient temperature), they were carefully rinsed with water and transferred into an oven to dry at 105 °C until constant weight (
M3). The solubility in water was calculated according to Equation (4). All experiments were carried out in triplicate.
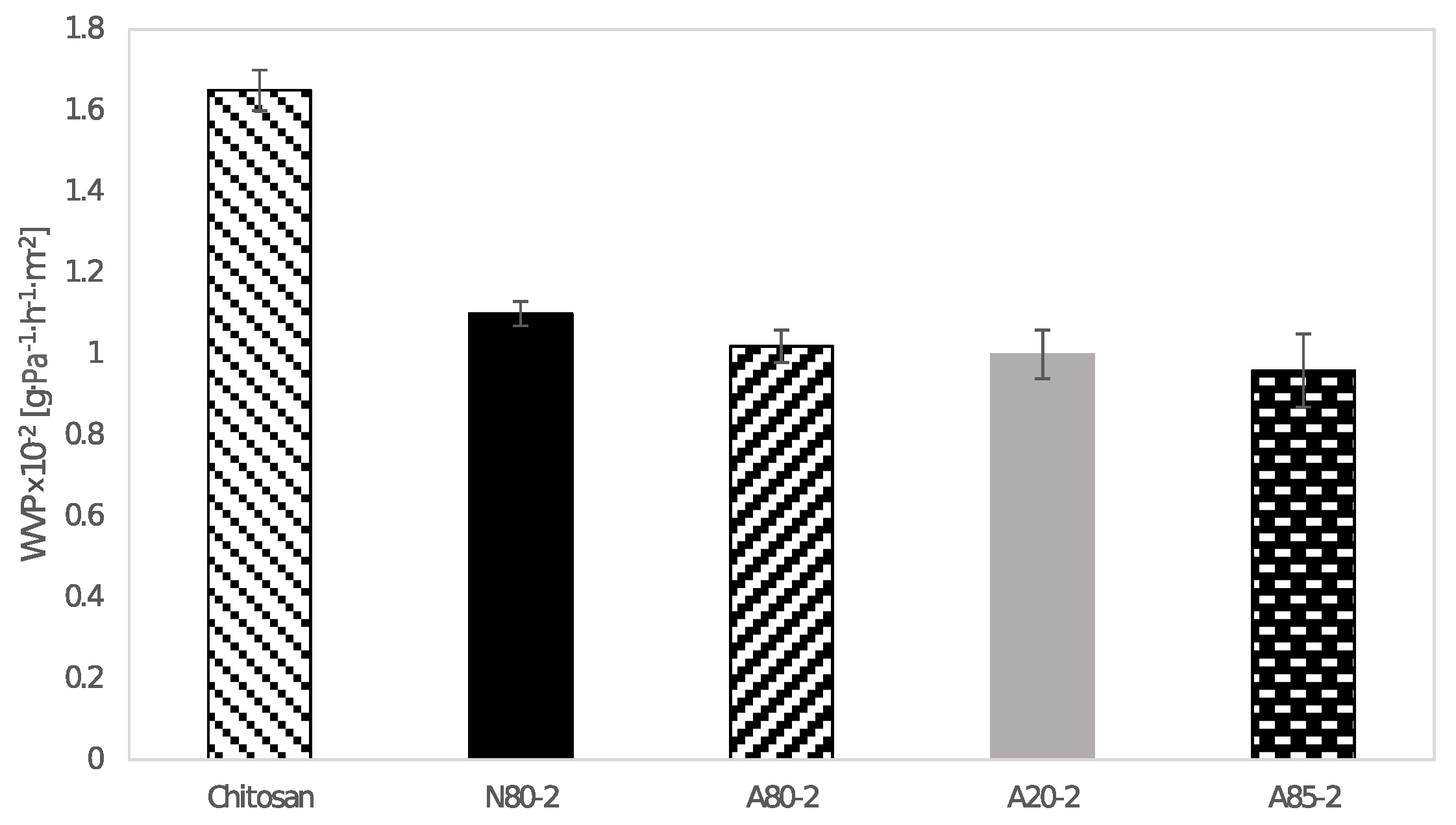
2.11. Water Vapour Barrier Properties of Films
Barrier properties were studied through to the measurement of water vapor permeability (WVP) according to ASTM E96–95 [
19]. The films were sealed at the mouth of a test dish filled with distilled water (100% relative humidity) that was placed in a desiccator containing silica gel (0% relative humidity). The periodic weighing of dishes was carried out as a function of time every 2 h until changes in values did not differ in more than 0.001 g. The obtained values were used for the calculation of the water vapor transmission rate (WVT) in g·h
−1·m
−2 and the water vapor permeability (WVP) in g·Pa
−1·h
−1·m
−2 according to Equations (5) and (6).
where
is weight loss vs. time (g·h
−1),
A is the test area in m
2 (the mouth area of the test dish), ∆
p is the difference in water vapor pressure (Pa),
S is the saturation pressure of vapor at the tested temperature,
R1 is relative humidity in the test dish expressed as a fraction, and
R2 is relative humidity at the vapor sink expressed as a fraction.
2.12. Antimicrobial Effectiveness of Essential Oils and Films
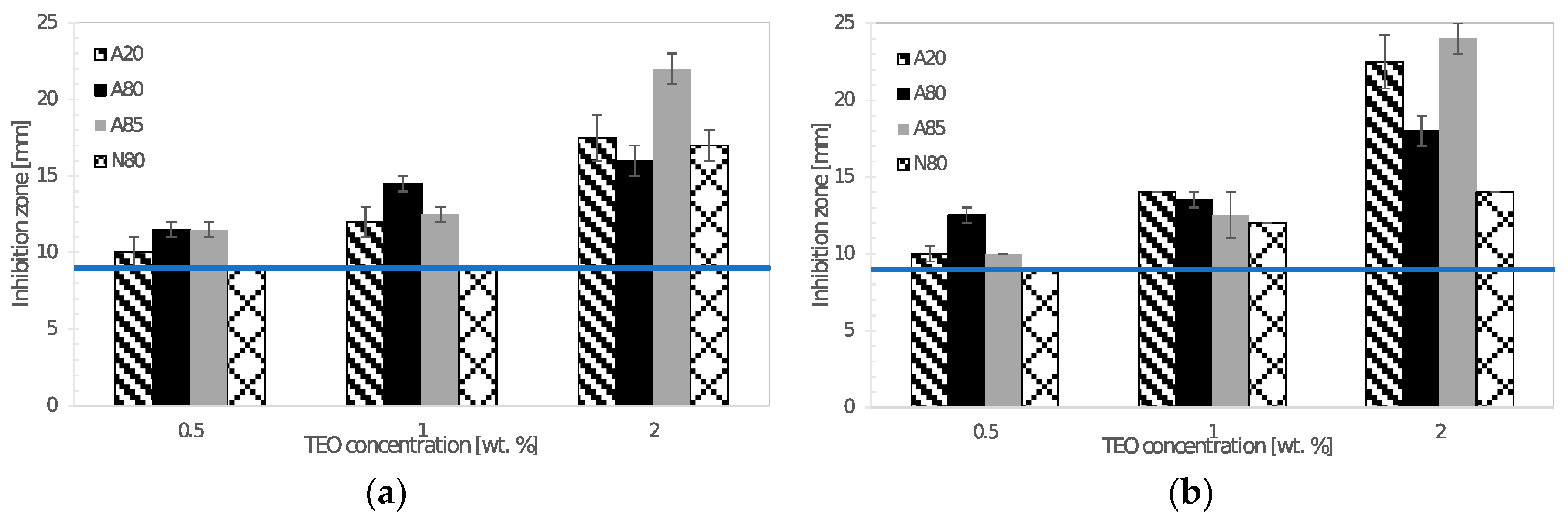
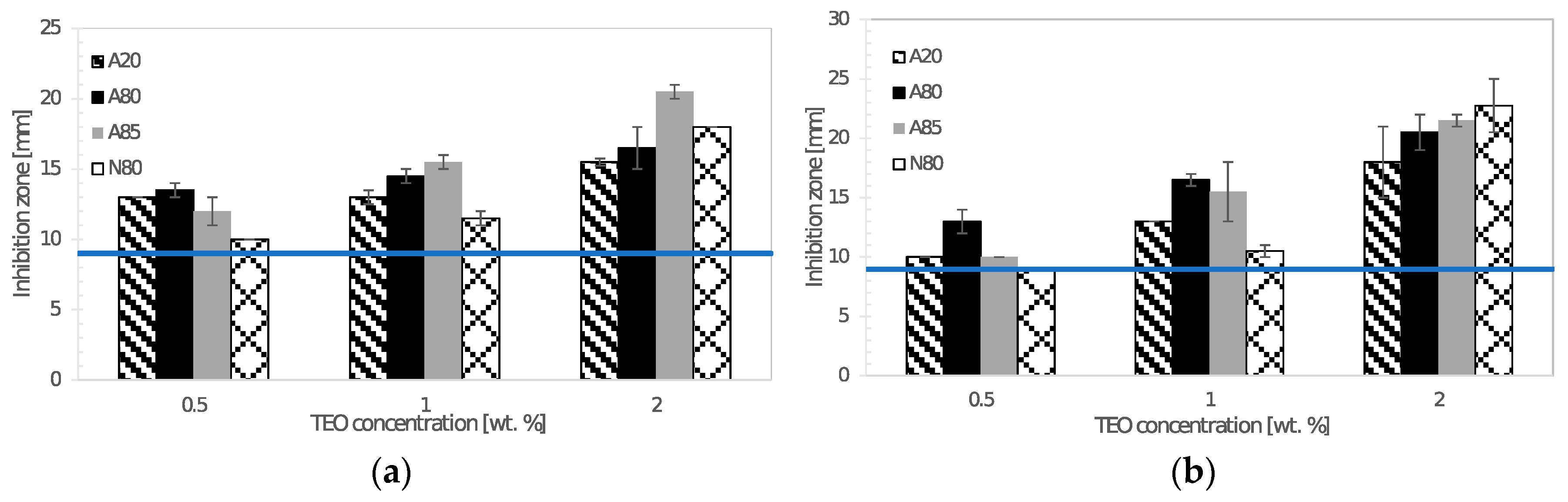
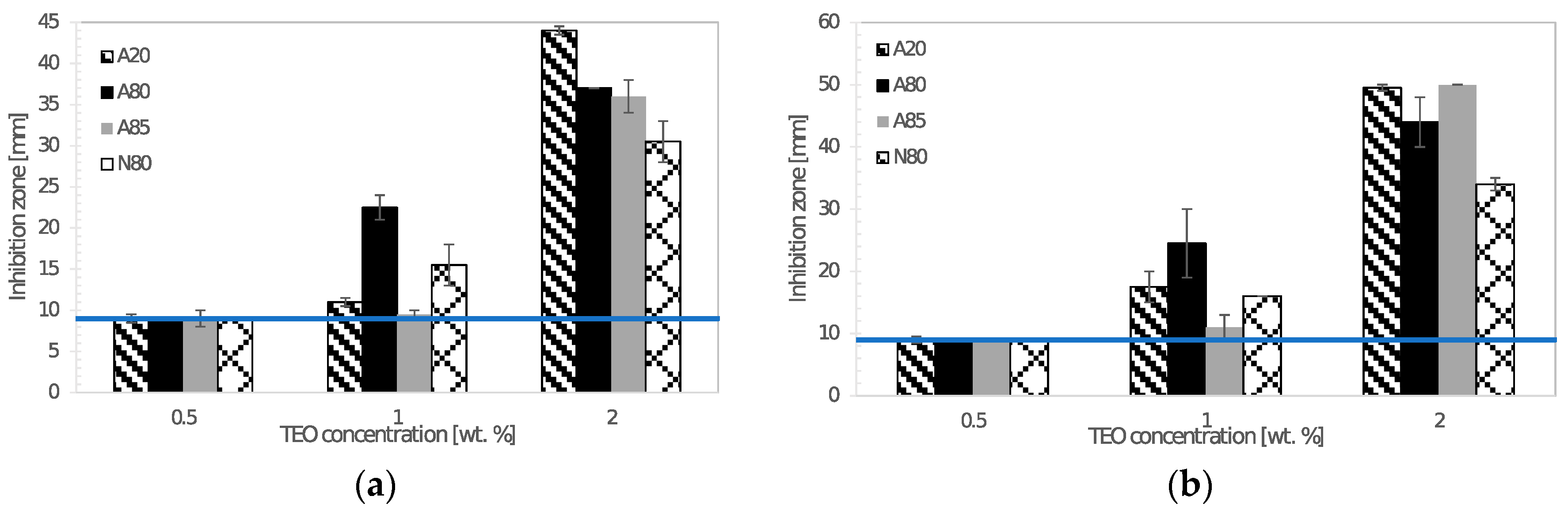
The agar diffusion method was used for testing the antimicrobial activity of chitosan films incorporated with thyme essential oil. The discs of chitosan films (9 mm in diameter) were put on agar plates previously inoculated with 1 mL of 0.5 McF turbid microbial suspension prepared in sterile saline solution. After incubation at given conditions, the diameters of inhibition zones around the discs were recorded. Antimicrobial testing was carried out in triplicate.
2.13. Statistical Analysis
The statistical evaluation of the experimental data was carried through one-way analysis of variance (ANOVA), using Statistica software (version 10, StatSoft, Inc., Tulsa, OK, USA), at the significance level of p < 0.05.
4. Conclusions
Low-molecular-weight chitosan was enriched with stabilizing agents of different HLB values (Tween 20, 80, and 85) and thyme essential oil to evaluate the final physical and antimicrobial properties. A predominant role of stabilizer was shown during the measurement of zeta potential of polymer dispersions; systems with Tween 20 and 80 revealed values reaching the zeta potential of unmodified chitosan solution (+58.7 mV), while, on the other hand, the sample with Tween 80 showed a significantly lower value (about +30 mV), regardless of thyme oil used.
Prepared films exhibited a sufficient antimicrobial activity against tested Gram-negative and Gram-positive bacteria, yeasts, and molds. Atok thyme oil proved to be more effective at the lowest tested concentrations, which was probably due to the higher thymol and carvacrol content that was observed by GC analysis. FTIR spectra of modified chitosan films revealed the presence of new peaks that could signal the interaction between polymer and active substance, which was successfully incorporated into the chitosan matrix. The results of mechanical testing of modified samples are satisfying, even though a decrease in tensile and puncture strength was ascertained when compared to the control sample. The microstructural study revealed relatively homogeneously distributed TEO droplets of various size depending on the type of emulsifier used, whereby the most noticeable particles were observed in the sample containing Tween 85 with the lowest HLB value. With regard to a comprehensive evaluation of the final properties, Tween 80 and Atok thyme essential oil seems to be the optimum choice for stabilizing and active agent, respectively. The modification of chitosan with TEO mixture provided stable polymer films with improved water-barrier characteristics and efficient antimicrobial properties, which could serve as a cost-effective and environmentally favorable active coating for food preservation.