Review of Micro–Nanoscale Surface Coatings Application for Sustaining Dropwise Condensation
Abstract
:1. Introduction
- Industrial-based condenser is mostly shell and tube exchangers and requires a high heat transfer area, which indicates higher footprints [14].
- In addition, a huge amount of cooling water is required, which reflects in more pumping power and intake/outfall footprints.
- The heat absorbed by cooling water from the condenser is dumped back into the water source. In some plants, such as desalination, the condenser is used as a preheater, but approximately one part out of ten is used as feed and the remaining nine parts are pumped back to the sea [15].
- Rejected cooling water has higher temperature and may affect the environment and marine life [16].
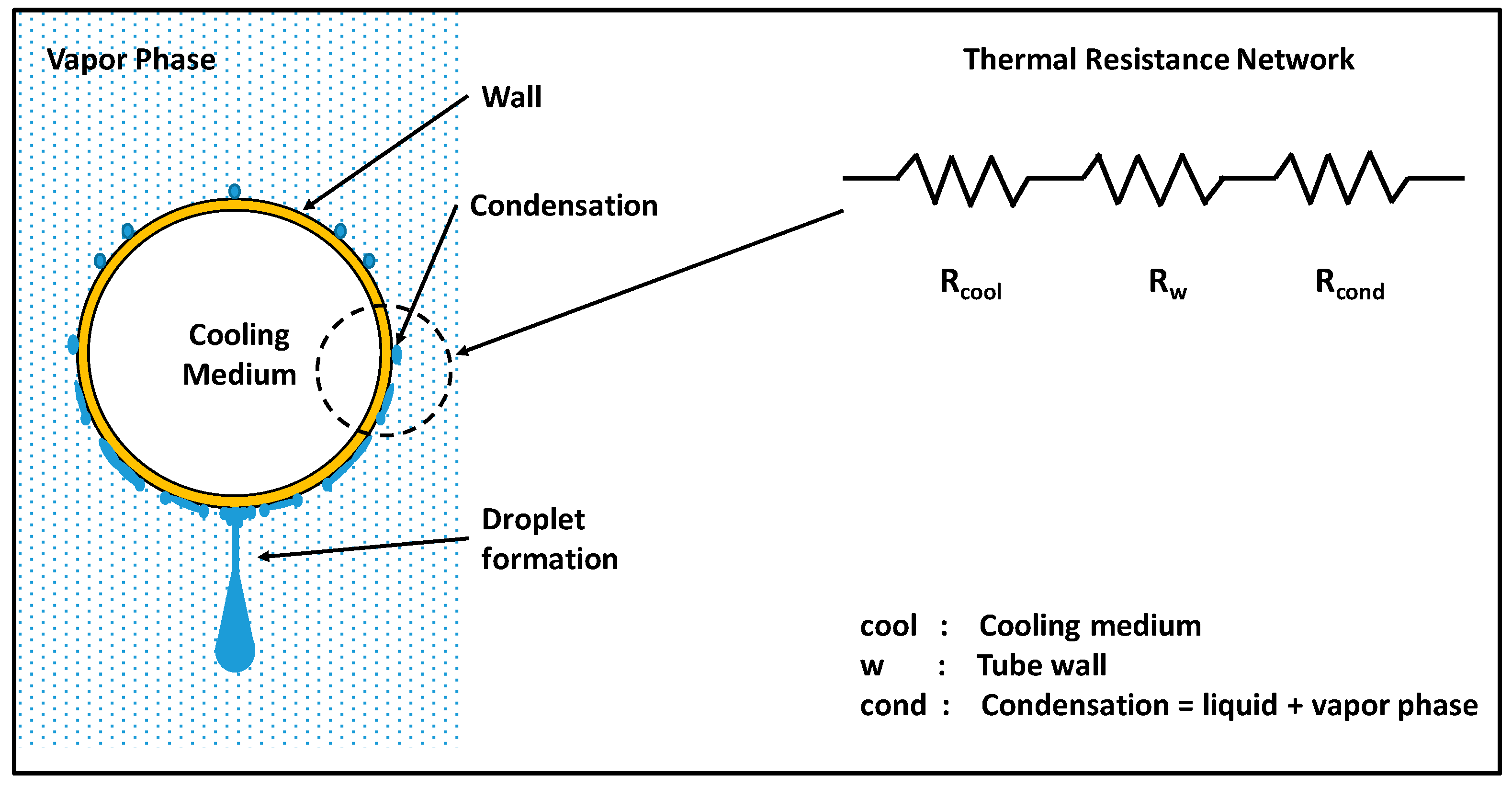
2. Condensation: Phase Change Heat Transfer Process
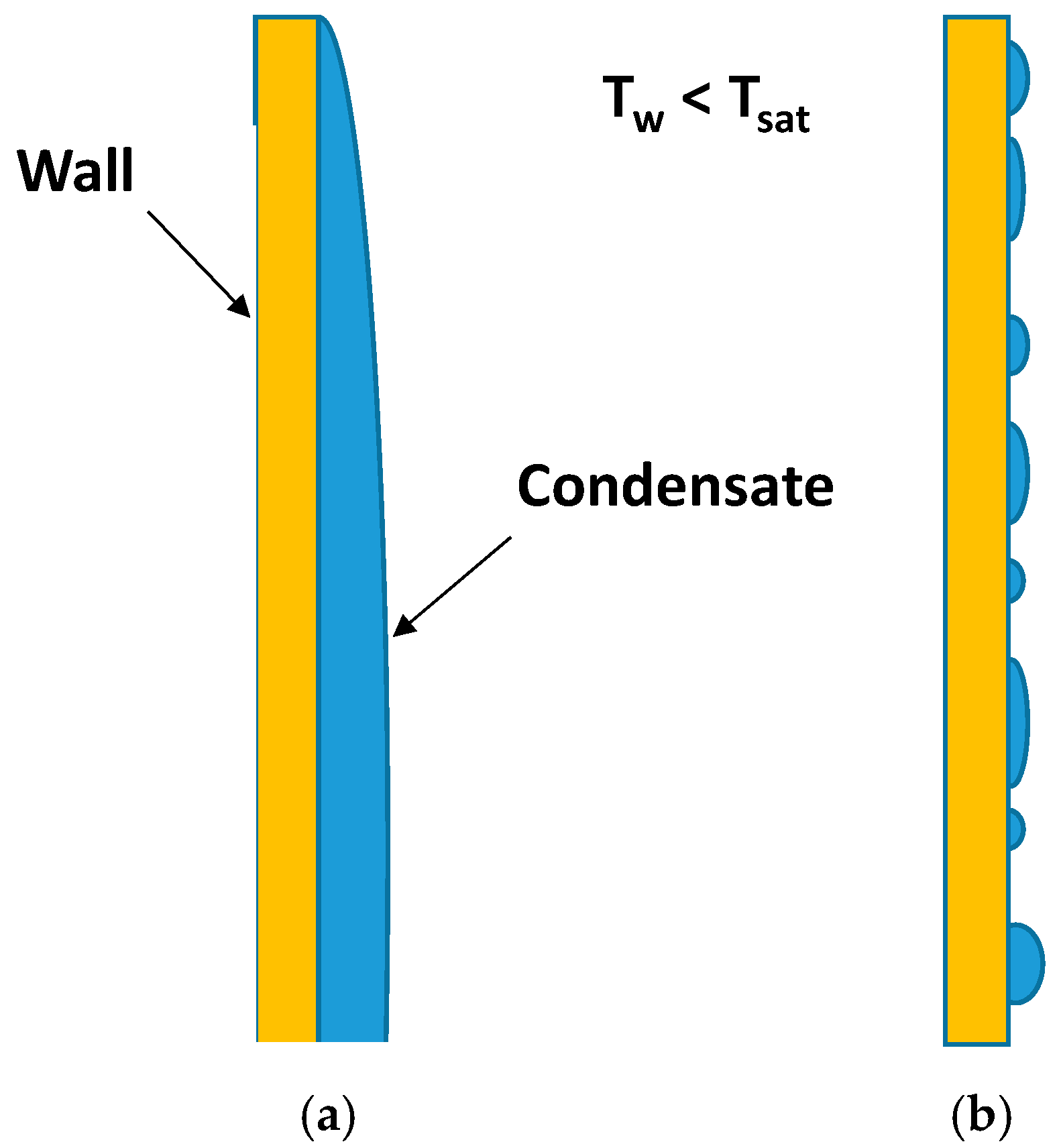
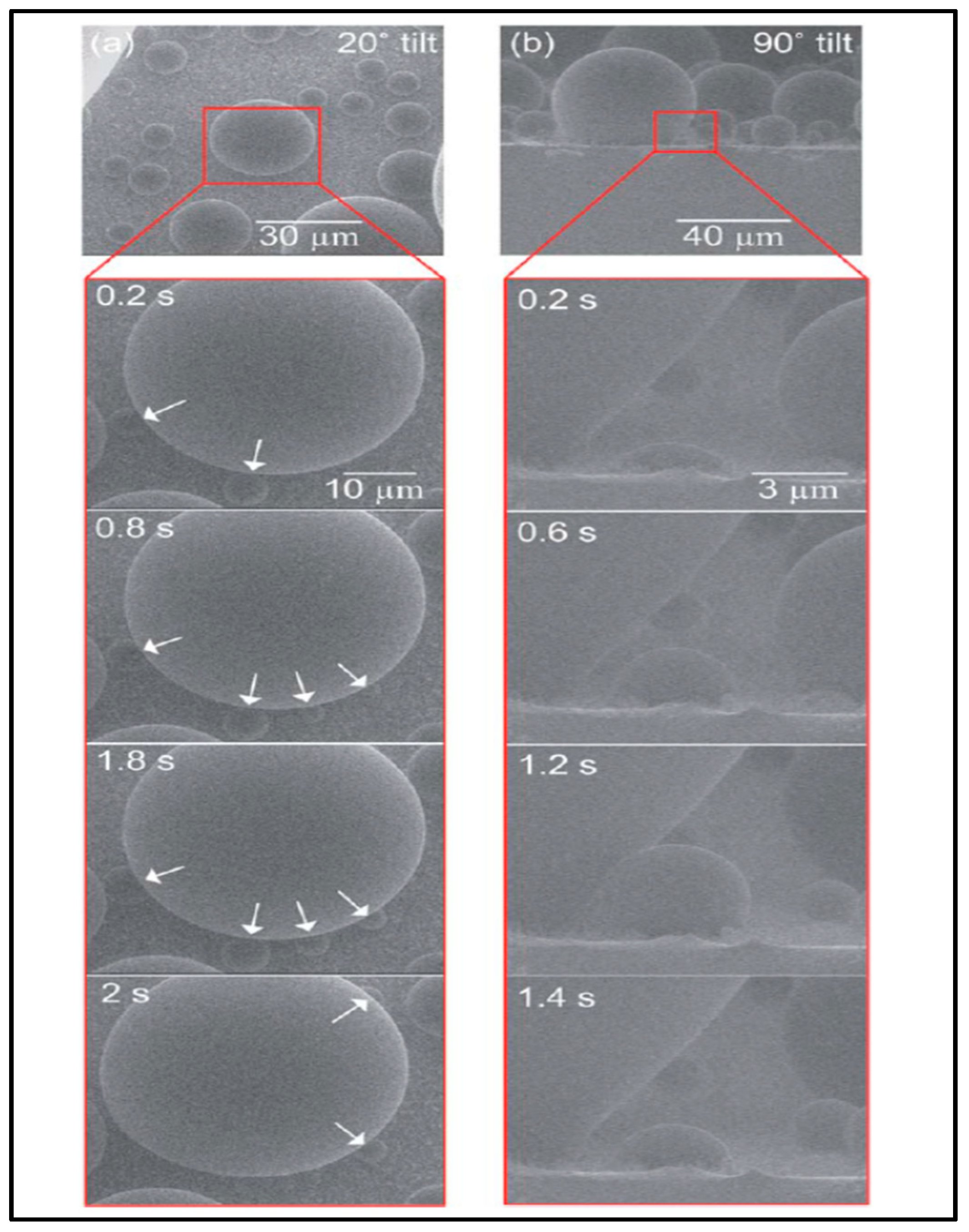
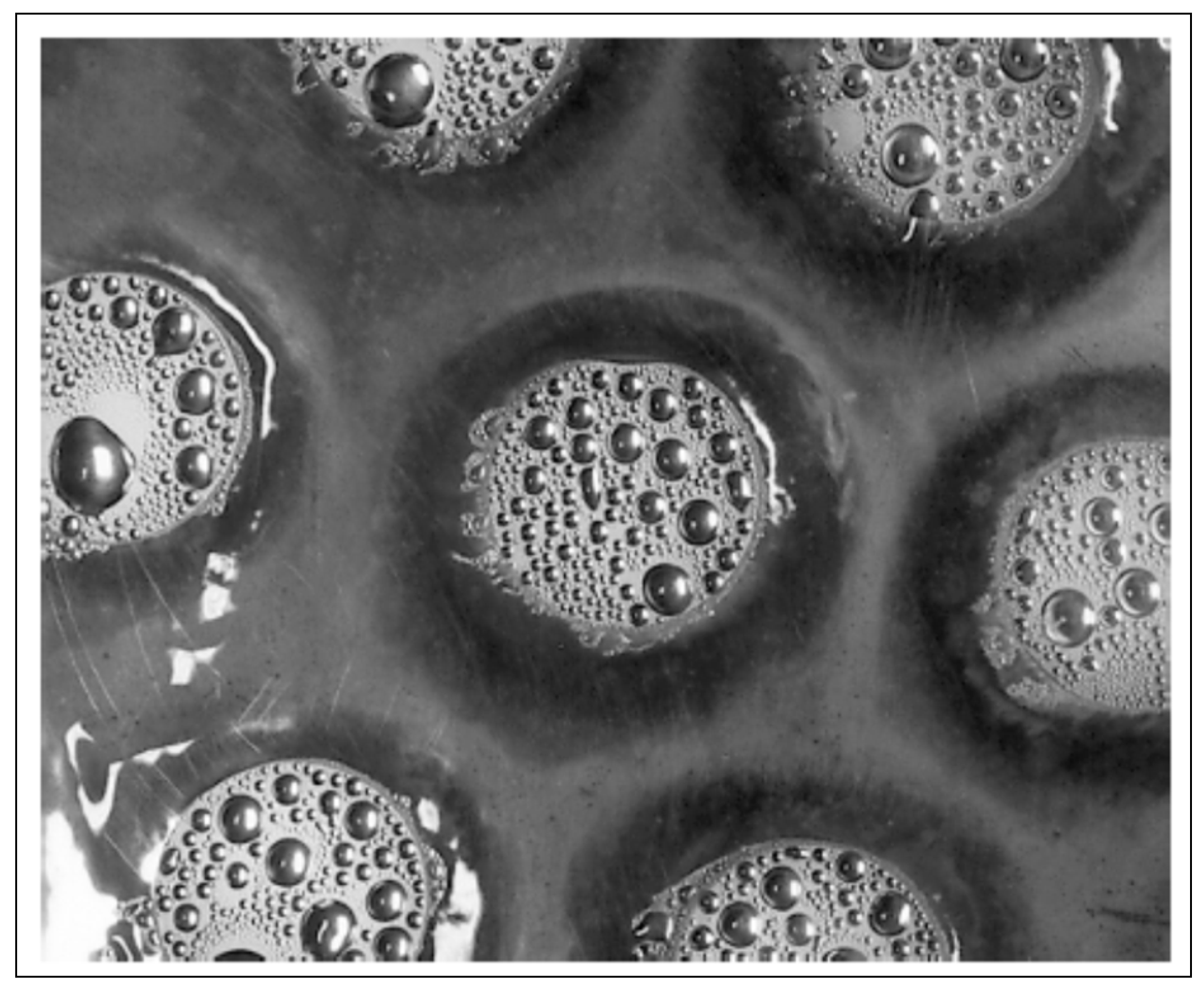
2.1. Dropwise Condensation (DWC)
2.2. Filmwise Condensation (FWC)
- The density of nucleation sites increases with a rise in temperature difference between the vapor and cool substrate. These drops merge with neighboring drops and after a certain limit, form a liquid film on the surface.
- Higher heat flux results in increased droplet size, which leads toward film condensation [47].
2.3. Noncondensable Gases
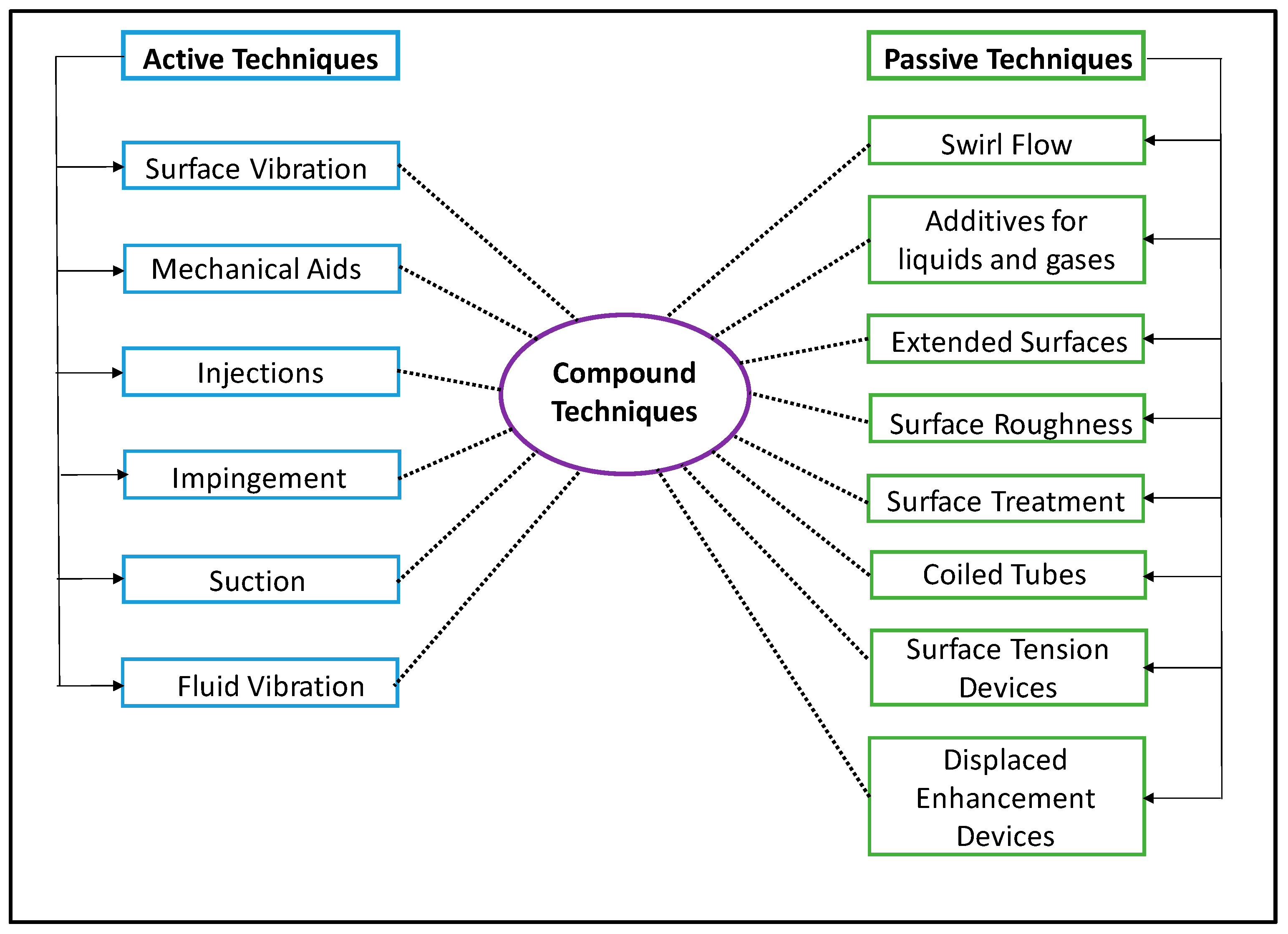
2.4. Optimal Surface Conditioning
- Changing surface geometry, i.e., via roughening, fins, and external modifications on condenser tubes [66];
- Use of external forces to enhance condensing phenomena (active method).
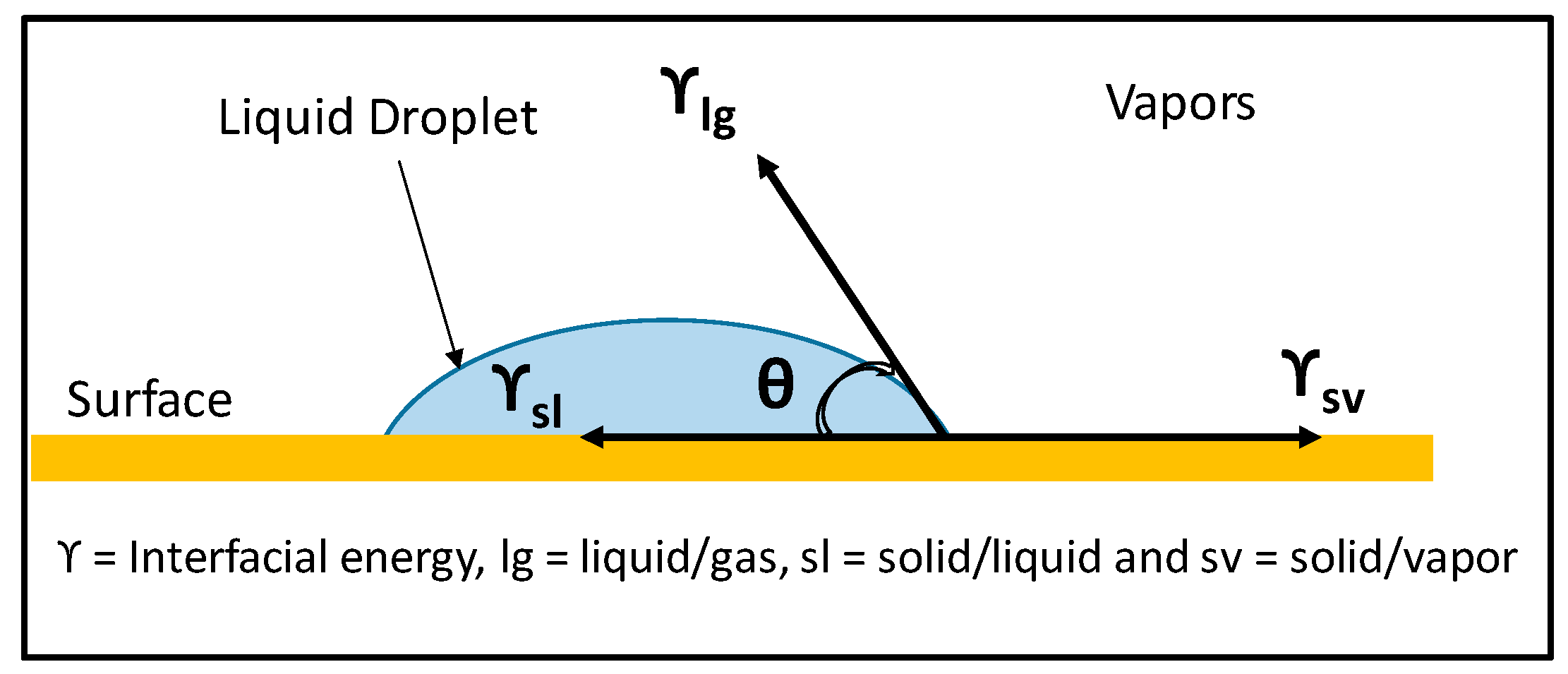
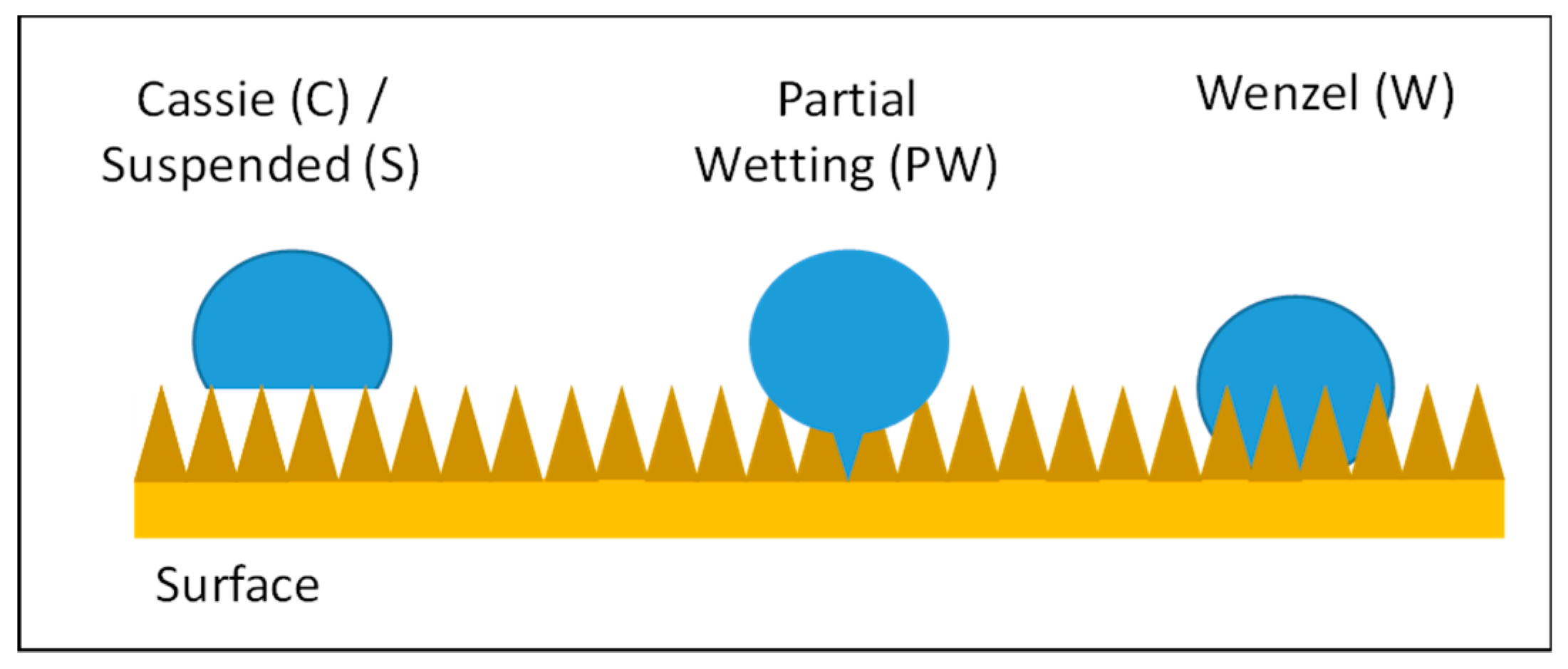
2.5. Surface Roughness: from Young’s to Wenzel and Cassie States
2.6. Wettability
- Encouraging droplet nucleation and early removal, and hence maximizing htc;
- Ensuring drainage of condensate before film formation;
- Sustaining surface quality to maintain its performance for long time.
3. Condensation Enhancement by Surface Coatings
3.1. Noble Metals
3.2. Rare Earth Oxides (REOs)
3.3. Ion Implantation
3.4. Polymer Coatings
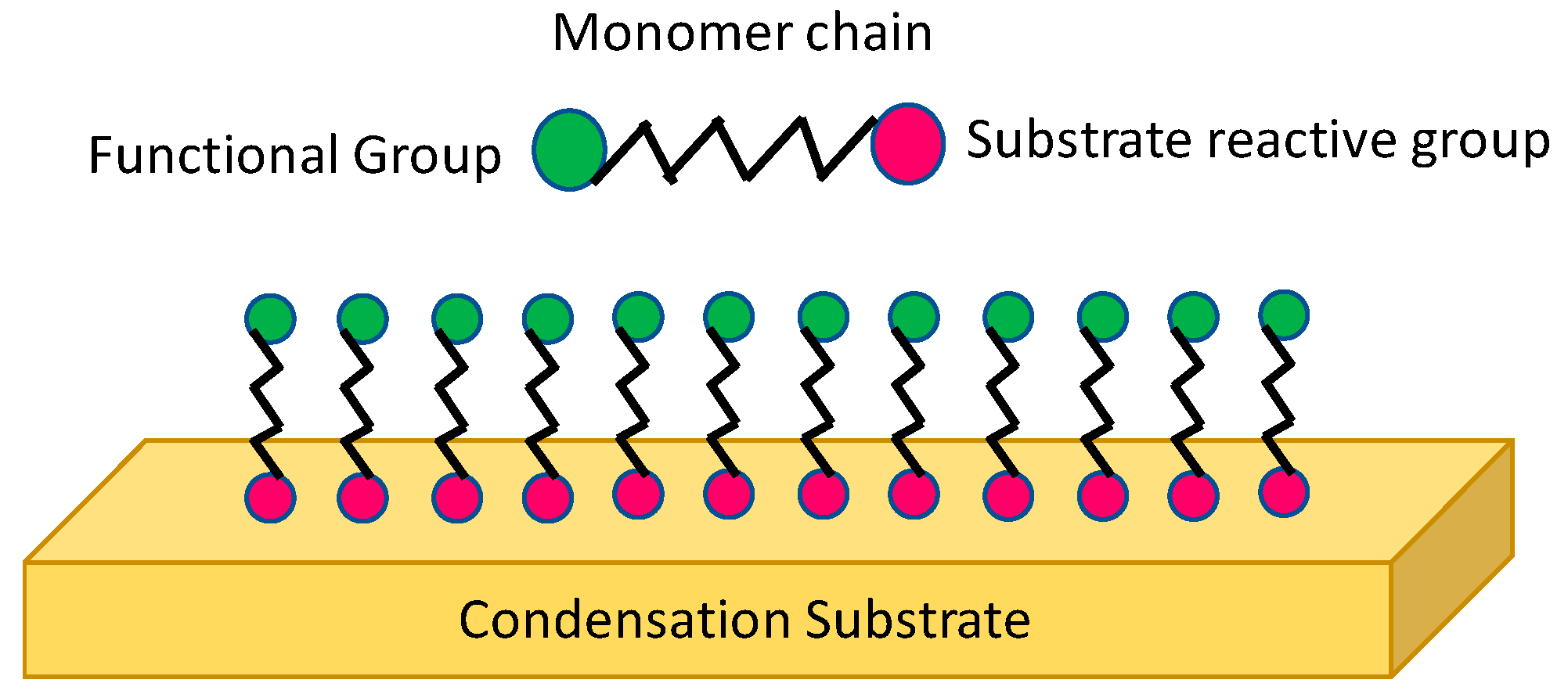
3.5. Self-Assembled Monolayers (SAMs)
3.6. Lubricant-Infused Surfaces (LIS)
3.7. Graphene, Carbon Nanotubes (CNT), and Nanostructured Surfaces
3.8. Porous Coatings
4. Discussion and Challenges
- Erb and Thelen [92,93] reported a total cost of 0.07–0.20 $/m2 for electrodeposited noble metals and 0.01–0.02 $/m2 using their experimental data. In addition, Preston et al. [65] also used experimental data and calculated the cost for scalable graphene as 11.98 $/m2. Diezel et al. [112] used simulations to evaluate ion-implanted condenser cost reduction, which was around 35.4%. Although different studies accounted for cost analysis, they are inconclusive as the techno-economic comparison depends on several interdependent factors. Detailed economic analysis is needed for accurate cost estimation and to be used for the comparison of different coating techniques. In addition, novel manufacturing techniques need to be studied to achieve cost-effectiveness of these coatings.
- Toxicity and compatibility with condensing medium should be investigated for industrial applications [171]. For the LIS coating system, the lubricant may be deteriorated with condensing steam and affect steam quality.
- Kim et al. [144] studied multiwalled CNT and Fe coatings for refrigerant R-134a condensation, which is widely used in the refrigeration industry. However, most of the condensation experiments in the literature are for steam and at low pressure and temperature. For higher pressure and temperature, experiments need to be carried in order to facilitate the studies for different condensing liquids as per industrial applications.
- Standardized lifetime test needs to be developed for performance and lifetime measurement to make fair comparison of these coating techniques. The long-term experimental data is required that can provide information on the durability, performance, and shortcomings of surface coating techniques. These kinds of experiments can help in identifying the root causes of physical mechanisms of failures of these surfaces and in implementing surface-coated condenser at the industrial scale. In addition, corrosion, erosion, and adhesion problems of micro–nano surface coatings need to be addressed.
- For the past few years, more emphasis has been given to nanostructured coatings, CNT, and graphene coatings. As these types of coatings are new and in development stages, as compared to other coating techniques, certain issues need to be resolved for their practical application. The main drawbacks of these coatings are durability and scalability. Furthermore, to ensure droplet-jumping phenomenon and sustained DWC, controlled methods for nanostructured coating needs to be considered.
- Industrial applications are not reported in the literature for structured hydrophobic coatings. Further investigation is required for deterioration, erosion, and maintenance requirements for this type of coating. In addition, projected area is normally used for heat transfer calculations rather than actual surface area, which may lead to inaccurate and overestimated results.
5. Conclusions
- Ion implantation and noble metals offer better hydrophobicity, but the cost of developing coatings is high and their hydrophobicity is not stable as compared to polymer coatings.
- REOs’ cost is less than 1% of noble metal coatings and thermal conductivity is approximately 50 times higher than fluoropolymers. Nevertheless, the heat transfer studies on REOs are limited, so more in-depth analysis is needed before commercialization.
- Polymer coatings can provide stable DWC mode and durability if the coating’s thickness is in the order of microns. Nevertheless, higher thickness leads to increased thermal resistance. On the contrary, low coating thickness can be effective and may achieve 10 times higher htc. The challenges associated with thin coatings are uniformity and durability.
- SAM coatings’ thickness is very low (~10 nm) and uniformity can be maintained; the thermal resistance is negligible, which is beneficial for heat transfer. Despite these favorable properties, its thermal stability in hot streams limits its practical implementation.
- LIS can be an effective alternative for obtaining stable DWC mode, but with the passage of time, DWC mode changes to hybrid/FWC mode due to lubricant deterioration. The lubricant durability still needs to be improved. In addition, in applications where quality of condensate is critical, the removal of these lubricants and contaminating condensate makes its practical applications inadequate.
- Emerging surface coatings such as CNTs, CF, graphene, and nanostructured surfaces can be promising alternatives for stable DWC mode, but the development of these coatings is in early stages and needs maturity for its real-world implementations.
- Hybridization of different techniques should be applied to study the possibilities of enhanced heat transfer results, such as combination of nanoparticles and structured porous coatings using durable materials, coating processes, and combination of structured porous and hydrophilic and hydrophobic coatings.
- Despite the significant improvements in condensation heat transfer performance with newly developed coating materials and processes, their industrial application is still not realized practically.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Heat transfer area |
| a | Constant |
| C | Cassie state |
| CAPEX | Capital expenditure |
| CF | Carbon fiber |
| CHF | Critical Heat Flux (W/m2) |
| CNT | Carbon nanotubes |
| CVD | Chemical vapor deposition |
| DLC | Diamond-like carbon |
| DWC | Dropwise condensation |
| E | Modulus of elasticity (N/m2) |
| e | Surface energy |
| FWC | Filmwise condensation |
| g | Acceleration due to gravity (m/s2) |
| HT | Heat transfer |
| h | Enthalpy (J/kg) |
| htc | Heat transfer coefficient (W/m2·K) |
| k | Thermal conductivity (W/m·K) |
| L | Length (m) |
| LIS | Lubricant-infused surfaces |
| OPEX | Operating expenditure |
| PHCT | Phase change heat transfer |
| PTFE | Polytetrafluoroethylene |
| PVD | Physical vapor deposition |
| PW | Partial wetting |
| Q | Heat transfer rate (W) |
| q | Heat flux (W/m2) |
| R | Thermal resistance (m2·K/W) |
| REOs | Rare earth oxides |
| r | Roughness ratio (total surface area divided by projected area) |
| S | Suspended state |
| SAMs | Self-assembled monolayers |
| SLIPS | Slippery liquid-infused porous surfaces |
| T | Temperature (°C/K) |
| U | Overall heat transfer coefficient (W/m2·K) |
| W | Wenzel state |
| Greek Letters | |
| Δ | Difference |
| µ | Viscosity (kg/m·s) |
| ρ | Density (kg/m3) |
| θ | Contact angle (degrees) |
| γ | Interfacial energy |
| φ | Ratio of contacting solid area to the projected area of the droplet |
| Subscripts | |
| c | Cassie state |
| cond | Condensation |
| cool | Cooling medium |
| int | Interface |
| l | Liquid phase |
| s | Surface |
| sat | Saturation |
| sub | Substrate |
| tot | Total/overall |
| v | Vapor phase |
| w | Wall |
| wz | Wenzel state |
References
- Khan, S.; Atieh, M.; Koç, M. Micro-nano scale surface coating for nucleate boiling heat transfer: A critical review. Energies 2018, 11, 3189. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Ma, R.; Hua, M.; Koratkar, N.; Yao, S.; Wang, Z. Nanograssed micropyramidal architectures for continuous dropwise condensation. Adv. Funct. Mater. 2011, 21, 4617–4623. [Google Scholar] [CrossRef]
- Khan, S.A.; Bicer, Y.; Koç, M. Design and analysis of a multigeneration system with concentrating photovoltaic thermal (CPV/T) and hydrogen storage. Int. J. Hydrog. Energy 2018, in press. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.-M. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Scott, J.H.J.; Rajauria, S.; Chinn, J.; Chinn, A.M.; Jones, W. Three dimensional aspects of droplet coalescence during dropwise condensation on superhydrophobic surfaces. Soft Matter 2011, 7, 8749. [Google Scholar] [CrossRef]
- Human Development Report 2006; United Nations Development Programme: New York, NY, USA, 2006.
- World Energy Outlook 2010; International Energy Agency: Paris, France, 2010.
- Khan, Z.; Shah, R.; Islam, S.; Jan, H.; Jan, B.; Rasheed, H.-U.; Khan, A. MHD flow and heat transfer analysis in the wire coating process using elastic-viscous. Coatings 2017, 7, 15. [Google Scholar] [CrossRef]
- Lara, J.R.; Holtzapple, M.T. Experimental investigation of dropwise condensation on hydrophobic heat exchangers part I: Dimpled-sheets. Desalination 2011, 278, 165–172. [Google Scholar] [CrossRef]
- Wi, Y.; Kim, J.; Lee, J.; Lee, J. Optimal patterned wettability for microchannel flow boiling using the lattice Boltzmann method. Coatings 2018, 8, 288. [Google Scholar] [CrossRef]
- Bonner, R.W. Dropwise condensation on surfaces with graded hydrophobicity. In Proceedings of the ASME 2009 Heat Transfer Summer Conference collocated with the InterPACK09 and 3rd Energy Sustainability Conferences, San Francisco, CA, USA, 19–23 July 2009; pp. 491–495. [Google Scholar]
- Wang, Q.; Xie, H.; Hu, Z.; Liu, C. The impact of the electric field on surface condensation of water vapor: Insight from molecular dynamics simulation. Nanomaterials 2019, 9, 64. [Google Scholar] [CrossRef]
- Islam, A.; Sun, A.; Sepehrnoori, K. An efficient computational scheme for two-phase steam condensation in the presence of CO2 for wellbore and long-distance flow. ChemEngineering 2019, 3, 4. [Google Scholar] [CrossRef]
- Mabrouk, A.; Abotaleb, A.; Tahir, F.; Darwish, M.; Aini, R.; Koc, M.; Abdelrashid, A. High performance MED desalination plants Part I: Novel design MED evaporator. In Proceedings of the IDA 2017 World Congress on Water Reuse and Desalination and Water Science, Sao Paulo, Brazil, 15–20 October 2017. [Google Scholar]
- Tahir, F.; Atif, M.; Antar, M.A. The effect of fouling on performance and design aspects of multiple-effect desalination systems. In Recent Progress in Desalination, Environmental and Marine Outfall Systems; Baawain, M.S., Choudri, B.S., Ahmed, M., Purnama, A., Eds.; Springer International Publishing: New York, NY, USA, 2015; pp. 35–52. [Google Scholar]
- Mabrouk, A.; Abotaleb, A.; Abdelrehim, H.; Tahir, F.; Koc, M.; Abdelrashid, A.; Nasralla, A. High performance MED desalination plants, Part II: Novel integration MED-absorption vapor compression. In Proceedings of the IDA 2017 World Congress on Water Reuse and Desalination and Water Science, Sao Paulo, Brazil, 15–20 October 2017. [Google Scholar]
- Bonner, R.W., III. Dropwise condensation life testing of self assembled monolayers. In Proceedings of the 2010 14th International Heat Transfer Conference, Washington, DC, USA, 8–13 August 2010. [Google Scholar]
- Phadnis, A.; Rykaczewski, K. Dropwise condensation on soft hydrophobic coatings. Langmuir 2017, 33, 12095–12101. [Google Scholar] [CrossRef] [PubMed]
- Manglik, R.M. Heat transfer enhancement. In Heat Transfer Handbook; Bejan, A., Kraus, A.D., Eds.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ullah, A.; Alzahrani, E.; Shah, Z.; Ayaz, M.; Islam, S. Nanofluids thin film flow of Reiner-Philippoff fluid over an unstable stretching surface with Brownian motion and thermophoresis effects. Coatings 2018, 9, 21. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Zhang, L.; Liu, Q.; Wang, Y.; Zhang, W.; Zheng, J. Preparation of nano-hydroxyapatite coated carbon nanotube reinforced hydroxyapatite composites. Coatings 2018, 8, 357. [Google Scholar] [CrossRef]
- Zarei, S.; Talesh Bahrami, H.R.; Saffari, H. Effects of geometry and dimension of micro/nano-structures on the heat transfer in dropwise condensation: A theoretical study. Appl. Therm. Eng. 2018, 137, 440–450. [Google Scholar] [CrossRef]
- Attinger, D.; Frankiewicz, C.; Betz, A.R.; Schutzius, T.M.; Ganguly, R.; Das, A.; Kim, C.; Megaridis, C.M. Surface engineering for phase change heat transfer: A review. MRS Energy Sustain. 2014, 1. [Google Scholar] [CrossRef]
- Kim, D.E.; Yu, D.I.; Jerng, D.W.; Kim, M.H.; Ahn, H.S. Review of boiling heat transfer enhancement on micro/nanostructured surfaces. Exp. Therm. Fluid Sci. 2015, 66, 173–196. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. Oxidative acid treatment of carbon nanotubes. Surf. Interfaces 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. Stabilization of the aqueous dispersion of carbon nanotubes using different approaches. Therm. Sci. Eng. Prog. 2018, 8, 411–417. [Google Scholar] [CrossRef]
- Wu, J.M.; Zhao, J. A review of nanofluid heat transfer and critical heat flux enhancement—Research gap to engineering application. Prog. Nucl. Energy 2013, 66, 13–24. [Google Scholar] [CrossRef]
- Bisetto, A.; Torresin, D.; Tiwari, M.K.; Del Col, D.; Poulikakos, D. Dropwise condensation on superhydrophobic nanostructured surfaces: Literature review and experimental analysis. J. Phys. Conf. Ser. 2014, 501, 012028. [Google Scholar] [CrossRef]
- Orejon, D.; Shardt, O.; Gunda, N.S.K.; Ikuta, T.; Takahashi, K.; Takata, Y.; Mitra, S.K. Simultaneous dropwise and filmwise condensation on hydrophilic microstructured surfaces. Int. J. Heat Mass Transf. 2017, 114, 187–197. [Google Scholar] [CrossRef]
- Su, J.; Inalpolat, M.; Ge, T.; Esmaeilzadeh, H.; Sun, H. Experimental study and analysis of dropwise condensation using quartz. In Proceedings of the ASME 2016 Heat Transfer Summer Conference collocated with the ASME 2016 Fluids Engineering Division Summer Meeting and the ASME 2016 14th International Conference on Nanochannels, Microchannels, and Minichannels, Washington, DC, USA, 10–14 July 2016. [Google Scholar]
- Gusarov, A.V.; Smurov, I. Gas-dynamic boundary conditions of evaporation and condensation: Numerical analysis of the Knudsen layer. Phys. Fluids 2002, 14, 4242–4255. [Google Scholar] [CrossRef]
- Zhu, T.; Ye, W. Theoretical and numerical studies of noncontinuum gas-phase heat conduction in micro/nano devices. Numer. Heat Transf. Part B Fundam. 2010, 57, 203–226. [Google Scholar] [CrossRef]
- Liu, H.; Xu, K.; Zhu, T.; Ye, W. Multiple temperature kinetic model and its applications to micro-scale gas flows. Comput. Fluids 2012, 67, 115–122. [Google Scholar] [CrossRef]
- Zheng, S.; Eimann, F.; Fieback, T.; Xie, G.; Gross, U. Numerical investigation of convective dropwise condensation flow by a hybrid thermal lattice Boltzmann method. Appl. Therm. Eng. 2018, 145, 590–602. [Google Scholar] [CrossRef]
- Gore, G.B.; Sali, N.V.; Ghodake, A.B. Of dropwise condensation heat transfer enhancement on silver coated copper surface using n-Heptane as surfactant additive. Int. Adv. Res. J. Sci. Eng. Technol. 2016, 3, 291–294. [Google Scholar]
- Kashchiev, D. Nucleation: Basic Theory with Applications; Butterworth Heinemann: Oxford, UK, 2000. [Google Scholar]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Lv, C.; Hao, P.; Zhang, X.; He, F. Dewetting transitions of dropwise condensation on nanotexture-enhanced superhydrophobic surfaces. ACS Nano 2015, 9, 12311–12319. [Google Scholar] [CrossRef]
- Chen, L.-H.; Chen, C.-Y.; Lee, Y.-L. Nucleation and growth of clusters in the process of vapor deposition. Surf. Sci. 1999, 429, 150–160. [Google Scholar] [CrossRef]
- Glassford, A.P.M. Practical model for molecular contaminant deposition kinetics. J. Thermophys. Heat Transf. 1992, 6, 656–664. [Google Scholar] [CrossRef]
- Westwater, J.W. Gold surfaces for condensation heat transfer. Gold Bull. 1981, 14, 95–101. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Chen, C.-H. Self-propelled dropwise condensate on superhydrophobic surfaces. Phys. Rev. Lett. 2009, 103, 184501. [Google Scholar] [CrossRef]
- Miljkovic, N.; Wang, E.N. Condensation heat transfer on superhydrophobic surfaces. MRS Bull. 2013, 38, 397–406. [Google Scholar] [CrossRef]
- Rose, J.W.; Spalding, D.B. Interphase matter transfer, the condensation coefficient and dropwise condensation. Proc. R. Soc. Lond. A 1987, 1841, 305–311. [Google Scholar] [CrossRef]
- Carey, V.P. Liquid-Vapor Phase-Change Phenomena, 2nd ed.; Taylor & Francis Group: New York, NY, USA, 2008. [Google Scholar]
- Mikic, B.B. On mechanism of dropwise condensation. Int. J. Heat Mass Transf. 1969, 12, 1311–1323. [Google Scholar] [CrossRef]
- Rose, J.W. Dropwise condensation theory and experiment: A review. Proc. Inst. Mech. Eng. Part A J. Power Energy 2002, 216, 115–128. [Google Scholar] [CrossRef]
- Rose, J.W. Condensation overview. In A-to-Z Guide to Thermodynamics, Heat and Mass Transfer, and Fluids Engineering; Begell House: Danbury, CT, USA, 2011. [Google Scholar]
- Leipertz, A.; Fröba, A.P. Improvement of condensation heat transfer by surface modifications. Heat Transf. Eng. 2008, 29, 343–356. [Google Scholar] [CrossRef]
- Schmidt, E.; Schurig, W.; Sellschopp, W. Versuche über die Kondensation von Wasserdampf in Film- und Tropfenform. Tech. Mech. Thermodyn. 1930, 1, 53–63. [Google Scholar] [CrossRef]
- Tanasawa, I. Advances in condensation heat transfer. Adv. Heat Transf. 1991, 21, 55–139. [Google Scholar]
- Al-Shammari, S.B.; Webb, D.R.; Heggs, P. Condensation of steam with and without the presence of non-condensable gases in a vertical tube. Desalination 2004, 169, 151–160. [Google Scholar] [CrossRef]
- Fu, W.; Li, X.; Wu, X.; Corradini, M.L. Numerical investigation of convective condensation with the presence of non-condensable gases in a vertical tube. Nucl. Eng. Des. 2016, 297, 197–207. [Google Scholar] [CrossRef]
- Kroger, D.G.; Rohsenow, W.M. Condensation heat transfer in the presence of a non-condensable gas. Int. J. Heat Mass Transf. 1968, 11, 15–26. [Google Scholar] [CrossRef]
- Martín-Valdepeñas, J.M.; Jiménez, M.A.; Martín-Fuertes, F.; Benítez, J.A.F. Comparison of film condensation models in presence of non-condensable gases implemented in a CFD Code. Heat Mass Transf. 2005, 41, 961–976. [Google Scholar] [CrossRef]
- Citakoglu, E.; Rose, J.W. Dropwise condensation—some factors influencing the validity of heat-transfer measurements. Int. J. Heat Mass Transf. 1968, 11, 523–537. [Google Scholar] [CrossRef]
- Le Fevre, E.J.; Rose, J.W. An experimental study of heat transfer by dropwise condensation. Int. J. Heat Mass Transf. 1965, 8, 1117–1133. [Google Scholar] [CrossRef]
- Lee, J.; Shao, B.; Won, Y. Two-level copper oxide nanostructured surfaces for condensation heat transfer. In Proceedings of the 2018 17th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), San Diego, CA, USA, 29 May–1 June 2018; pp. 12–18. [Google Scholar]
- Lee, Y.A.; Kuo, L.S.; Su, T.W.; Hsu, C.C.; Chen, P.H. Orientation effects of nanoparticle-modified surfaces with interlaced wettability on condensation heat transfer. Appl. Therm. Eng. 2016, 98, 1054–1060. [Google Scholar] [CrossRef]
- Niu, D.; Tang, G.H. The effect of surface wettability on water vapor condensation in nanoscale. Sci. Rep. 2016, 6, 19192. [Google Scholar] [CrossRef]
- Jaikumar, A.; Gupta, A.; Kandlikar, S.G.; Yang, C.Y.; Su, C.Y. Scale effects of graphene and graphene oxide coatings on pool boiling enhancement mechanisms. Int. J. Heat Mass Transf. 2017, 109, 357–366. [Google Scholar] [CrossRef]
- Enright, R.; Miljkovic, N.; Alvarado, J.L.; Kim, K.; Rose, J.W. Dropwise condensation on micro- and nanostructured surfaces. Nanoscale Microscale Thermophys. Eng. 2014, 18, 223–250. [Google Scholar] [CrossRef]
- Mondal, B.; Mac Giolla Eain, M.; Xu, Q.; Egan, V.M.; Punch, J.; Lyons, A.M. Design and fabrication of a hybrid superhydrophobic–hydrophilic surface that exhibits stable dropwise condensation. ACS Appl. Mater. Interfaces 2015, 7, 23575–23588. [Google Scholar] [CrossRef]
- Zhang, B.J.; Kuok, C.; Kim, K.J.; Hwang, T.; Yoon, H. Dropwise steam condensation on various hydrophobic surfaces: Polyphenylene sulfide (PPS), polytetrafluoroethylene (PTFE), and self-assembled micro/nano silver (SAMS). Int. J. Heat Mass Transf. 2015, 89, 353–358. [Google Scholar] [CrossRef]
- Preston, D.J.; Mafra, D.L.; Miljkovic, N.; Kong, J.; Wang, E.N. Scalable graphene coatings for enhanced condensation heat transfer. Nano Lett. 2015, 15, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.W.; Tang, G.H.; Niu, D. Experimental investigation of condensation heat transfer on hybrid wettability finned tube with large amount of noncondensable gas. Int. J. Heat Mass Transf. 2015, 85, 513–523. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1832, 95, 171–172. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Alwazzan, M.; Egab, K.; Peng, B.; Khan, J.; Li, C. Condensation on hybrid-patterned copper tubes (I): Characterization of condensation heat transfer. Int. J. Heat Mass Transf. 2017, 112, 991–1004. [Google Scholar] [CrossRef]
- Xiao, R.; Miljkovic, N.; Enright, R.; Wang, E.N. Immersion condensation on oil-infused heterogeneous surfaces for enhanced heat transfer. Sci. Rep. 2013, 3, 1988. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Kilty, H.P.; Marto, P.J.; Andeen, G.B.; Kumar, A. The use of an organic self-assembled monolayer coating to promote dropwise condensation of steam on horizontal tubes. J. Heat Transf. 2000, 122, 278–286. [Google Scholar] [CrossRef]
- Torresin, D.; Tiwari, M.K.; Del Col, D.; Poulikakos, D. Flow condensation on copper-based nanotextured superhydrophobic surfaces. Langmuir 2013, 29, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Qin, Z.; Yao, S. Factors affecting the spontaneous motion of condensate drops on superhydrophobic copper surfaces. Langmuir 2012, 28, 6067–6075. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Zhang, D.C.; Leipertz, A. Condensation of steam on the surface of hard coated copper discs. Heat Mass Transf. 1997, 32, 149–156. [Google Scholar] [CrossRef]
- Holden, K.M.; Wanniarachchi, A.S.; Marto, P.J.; Boone, D.H.; Rose, J.W. The use of organic coatings to promote dropwise condensation of steam. J. Heat Transf. 1987, 109, 768–774. [Google Scholar] [CrossRef]
- Marto, P.J.; Looney, D.J.; Rose, J.W.; Wanniarachchi, A.S. Evaluation of organic coatings for the promotion of dropwise condensation of steam. Int. J. Heat Mass Transf. 1986, 29, 1109–1117. [Google Scholar] [CrossRef]
- Sezer, N.; Koc, M. Dispersion stability of CNT and CNT/Metal-based nanofluids. In Proceedings of the ICTEA 2018: 11th International Conference on Thermal Engineering, Doha, Qatar, 25–28 February 2018. [Google Scholar]
- Mori, K.; Fujita, N.; Horie, H.; Mori, S.; Miyashita, T.; Matsuda, M. Heat transfer promotion of an aluminum-brass cooling tube by surface treatment with triazinethiols. Langmuir 1991, 7, 1161–1166. [Google Scholar] [CrossRef]
- Erb, R.A. Wettability of metals under continuous condensing conditions. J. Phys. Chem. 1965, 69, 1306–1309. [Google Scholar] [CrossRef]
- Erb, R.A. Dropwise condensation on gold. Gold Bull. 1973, 6, 2–6. [Google Scholar] [CrossRef]
- Wilkins, D.G.; Bromley, L.A.; Read, S.M. Dropwise and filmwise condensation of water vapor on gold. AIChE J. 1973, 19, 119–123. [Google Scholar] [CrossRef]
- Erb, R.; Thelen, E. Promoting permanent dropwise condensation. Ind. Eng. Chem. 1965, 57, 49–52. [Google Scholar] [CrossRef]
- Woodruff, D.W.; Westwater, J.W. Steam condensation on electroplated gold: effect of plating thickness. Int. J. Heat Mass Transf. 1979, 22, 629–632. [Google Scholar] [CrossRef]
- Woodruff, D.W.; Westwater, J.W. Steam condensation on various gold surfaces. J. Heat Transf. 1981, 103, 685–692. [Google Scholar] [CrossRef]
- Smith, T. The hydrophilic nature of a clean gold surface. J. Colloid Interface Sci. 1980, 75, 51–55. [Google Scholar] [CrossRef]
- O’neill, G.A.; Westwater, J.W. Dropwise condensation of steam on electroplated silver surfaces. Int. J. Heat Mass Transf. 1984, 27, 1539–1549. [Google Scholar] [CrossRef]
- Ge, M.; Wang, S.; Zhao, J.; Zhao, Y.; Liu, L. Effects of extended surface and surface gold plating on condensation characteristics of steam with large amount of CO2. Exp. Therm. Fluid Sci. 2018, 92, 13–19. [Google Scholar] [CrossRef]
- Habib, M.A.; Tahir, F.; Nemitallah, M.A.; Ahmed, W.H.; Badr, H.M. Experimental and numerical analysis of oxy-fuel combustion in a porous plate reactor. Int. J. Energy Res. 2015, 39, 1229–1240. [Google Scholar] [CrossRef]
- Thiel, G.P.; Lienhard, J.H. Entropy generation in condensation in the presence of high concentrations of noncondensable gases. Int. J. Heat Mass Transf. 2012, 55, 5133–5147. [Google Scholar] [CrossRef]
- Erb, R.A.; Thelen, E. Dropwise condensation. In Proceedings of the First International Sympposium on Water Desalination, Washington, DC, USA, 3–9 October 1965. [Google Scholar]
- Di Luzio, F.C.; Sieder, E.N.; Cadwallader, E.A. Research and Development Progress Report No. 184 Dropwise Condensation Characteristics of Permanent Hydrophobic Systems; Franklin Institute: Philadelphia, PA, USA, 1966. [Google Scholar]
- Azimi, G.; Kwon, H.-M.; Varanasi, K.K. Superhydrophobic surfaces by laser ablation of rare-earth oxide ceramics. MRS Commun. 2014, 4, 95–99. [Google Scholar] [CrossRef]
- Zenkin, S.; Kos, Š.; Musil, J. Hydrophobicity of thin films of compounds of low-electronegativity metals. J. Am. Ceram. Soc. 2014, 97, 2713–2717. [Google Scholar] [CrossRef]
- Azimi, G.; Dhiman, R.; Kwon, H.-M.; Paxson, A.T.; Varanasi, K.K. Hydrophobicity of rare-earth oxide ceramics. Nat. Mater. 2013, 12, 315–320. [Google Scholar] [CrossRef]
- Khan, S.; Azimi, G.; Yildiz, B.; Varanasi, K.K. Role of surface oxygen-to-metal ratio on the wettability of rare-earth oxides. Appl. Phys. Lett. 2015, 106, 061601. [Google Scholar] [CrossRef]
- Preston, D.J.; Miljkovic, N.; Sack, J.; Enright, R.; Queeney, J.; Wang, E.N. Effect of hydrocarbon adsorption on the wettability of rare earth oxide ceramics. Appl. Phys. Lett. 2014, 105, 011601. [Google Scholar] [CrossRef]
- Lundy, R.; Byrne, C.; Bogan, J.; Nolan, K.; Collins, M.N.; Dalton, E.; Enright, R. Exploring the role of adsorption and surface state on the hydrophobicity of rare earth oxides. ACS Appl. Mater. Interfaces 2017, 9, 13751–13760. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.K. Hydrophobicity of Rare-Earth Oxide Ceramics and Their Application in Promoting Sustained Drop-Wise Condensation. Ph.D. Thesis, Massachusetts Institute of Technology (MIT), Cambridge, MA, USA, February 2016. [Google Scholar]
- Shim, J.; Seo, D.; Oh, S.; Lee, J.; Nam, Y. Condensation heat-transfer performance of thermally stable superhydrophobic cerium-oxide surfaces. ACS Appl. Mater. Interfaces 2018, 10, 31765–31776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lin, Z.; Lin, J. New surface materials for dropwise condensation. In Proceedings of the 8th International Heat Transfer Conference, San Francisco, CA, USA, 17–22 August 1986. [Google Scholar]
- Zhao, Q.; Zhang, D.; Lin, J. Surface material with dropwise condensation made by ion implantation technology. Int. J. Heat Mass Transf. 1991, 34, 2833–2835. [Google Scholar]
- Zhao, Q.; Burnside, B.M. Dropwise condensation of steam on ion implanted condenser surfaces. Heat Recovery Syst. CHP 1994, 14, 525–534. [Google Scholar] [CrossRef]
- Rausch, M.H.; Leipertz, A.; Fröba, A.P. On the characteristics of ion implanted metallic surfaces inducing dropwise condensation of steam. Langmuir 2010, 26, 5971–5975. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.H.; Leipertz, A.; Fröba, A.P. Dropwise condensation of steam on ion implanted titanium surfaces. Int. J. Heat Mass Transf. 2010, 53, 423–430. [Google Scholar] [CrossRef]
- Rausch, M.H.; Leipertz, A.; Fröba, A.P. Experimental study on the origin of dropwise condensation of steam on ion implanted metallic surfaces. In Proceedings of the 2010 14th International Heat Transfer Conference, Washington, DC, USA, 8–13 August 2010; pp. 19–24. [Google Scholar]
- Do, S.C.; Kim, K.W.; Jeong, J.H. The variation of hydrophobicity of aluminum alloy by nitrogen and argon ion implantation. Heat Mass Transf. 2015, 51, 487–495. [Google Scholar] [CrossRef]
- Bani Kananeh, A.; Rausch, M.H.; Fröba, A.P.; Leipertz, A. Experimental study of dropwise condensation on plasma-ion implanted stainless steel tubes. Int. J. Heat Mass Transf. 2006, 49, 5018–5026. [Google Scholar] [CrossRef]
- Guo, Y.-C.; Wang, Z.X. Amorphous Physics; Science: Beijing, China, 1984. (In Chinese) [Google Scholar]
- Kim, K.; Lee, Y.; Jeong, J.H. Dropwise condensation induced on chromium ion implanted aluminum surface. Nucl. Eng. Technol. 2019, 51, 84–94. [Google Scholar] [CrossRef]
- Diezel, L.L.; Fröba, A.P.; Lukić, N.; Leipertz, A. Optimierung einer auf dem Verfahren der mechanischen Brüdenverdichtung basierenden Meerwasserentsalzungsanlage. Chem. Ing. Tech. 2007, 79, 459–467. [Google Scholar] [CrossRef]
- Lukic, N.; Diezel, L.L.; Fröba, A.P.; Leipertz, A. Economical aspects of the improvement of a mechanical vapour compression desalination plant by dropwise condensation. Desalination 2010, 264, 173–178. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Xu, D.; Lin, J.; Ren, C.; Long, Z. Influence of processing conditions of polymer film on dropwise condensation heat transfer. Int. J. Heat Mass Transf. 2002, 45, 3405–3411. [Google Scholar] [CrossRef]
- Haraguchi, T.; Shimada, R.; Kumagai, S.; Takeyama, T. The effect of polyvinylidene chloride coating thickness on promotion of dropwise steam condensation. Int. J. Heat Mass Transf. 1991, 34, 3047–3054. [Google Scholar] [CrossRef]
- Miljkovic, N.; Preston, D.J.; Enright, R.; Wang, E.N. Electrostatic charging of jumping droplets. Nat. Commun. 2013, 4, 2517. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Kapur, V.; Pinkerton, N.M.; Gleason, K.K. Initiated chemical vapor deposition (iCVD) of conformal polymeric nanocoatings for the surface modification of high-aspect-ratio pores. Chem. Mater. 2008, 20, 1646–1651. [Google Scholar] [CrossRef]
- Paxson, A.T.; Yagüe, J.L.; Gleason, K.K.; Varanasi, K.K. Stable dropwise condensation for enhancing heat transfer via the initiated chemical vapor deposition (iCVD) of grafted polymer films. Adv. Mater. 2014, 26, 418–423. [Google Scholar] [CrossRef]
- Uçar, I.O.; Erbil, H.Y. Droplet condensation on polymer surfaces: A review. Turkish J. Chem. 2013, 37, 643–674. [Google Scholar]
- Haque, M.R.; Zhu, C.; Qu, C.; Kinzel, E.C.; Betz, A.R. Experimental investigation of condensation phenomenon on hydrophilic and hydrophobic titanium (Ti) pillared glass surfaces. In Proceedings of the 6th Micro Nano Flows Conference, Atlanta, GA, USA, 9–12 September 2018. [Google Scholar]
- Ji, X.; Zhou, D.; Dai, C.; Xu, J. Dropwise condensation heat transfer on superhydrophilic-hydrophobic network hybrid surface. Int. J. Heat Mass Transf. 2019, 132, 52–67. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Manabe, K.; Gaudelet, T.; Moriya, T.; Suwabe, K.; Tenjimbayashi, M.; Kyong, K.H.; Gillot, F.; Shiratori, S. Improvement of heat transfer by promoting dropwise condensation using electrospun polytetrafluoroethylene thin films. New J. Chem. 2017, 41, 982–991. [Google Scholar] [CrossRef]
- Huang, D.-J.J.; Leu, T.-S.S. Condensation heat transfer enhancement by surface modification on a monolithic copper heat sink. Appl. Therm. Eng. 2015, 75, 908–917. [Google Scholar] [CrossRef]
- Schroeder-Richter, D.; Yildiz, S.; Bartsch, G. Effect of porous coating on critical heat flux. Int. Commun. Heat Mass Transf. 1996, 23, 463–471. [Google Scholar] [CrossRef]
- Yang, Q.; Gu, A. Dropwise condensation on SAM and electroless composite coating surfaces. J. Chem. Eng. Japan 2006, 39, 826–830. [Google Scholar] [CrossRef]
- Vemuri, S.; Kim, K.J.; Wood, B.D.; Govindaraju, S.; Bell, T.W. Long term testing for dropwise condensation using self-assembled monolayer coatings of n-octadecyl mercaptan. Appl. Therm. Eng. 2006, 26, 421–429. [Google Scholar] [CrossRef]
- Chen, L.; Liang, S.; Yan, R.; Cheng, Y.; Huai, X.; Chen, S. n-Octadecanethiol self-assembled monolayer coating with microscopic roughness for dropwise condensation of steam. J. Therm. Sci. 2009, 18, 160–165. [Google Scholar] [CrossRef]
- Chandekar, A.; Sengupta, S.K.; Whitten, J.E. Thermal stability of thiol and silane monolayers: A comparative study. Appl. Surf. Sci. 2010, 256, 2742–2749. [Google Scholar] [CrossRef]
- Xue, C.-H.; Ma, J.-Z. Long-lived superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 4146–4161. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Sun, J. Bioinspired self-healing superhydrophobic coatings. Angew. Chemie Int. Ed. 2010, 49, 6129–6133. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Paxson, A.T.; Dhiman, R.; Smith, J.D.; Varanasi, K.K. Enhanced condensation on lubricant-impregnated nanotextured surfaces. ACS Nano 2012, 6, 10122–10129. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Lafuma, A.; Quéré, D. Slippery pre-suffused surfaces. EPL (Europhys. Lett.) 2011, 96, 56001. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. 2012, 109, 13182–13187. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, V.; Nosonovsky, M. Wetting transitions in two-, three-, and four-phase systems. Langmuir 2012, 28, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Chen, S.; Li, J.; Cheng, P. Enhanced dropwise condensation by oil infused nano-grass coatings on outer surface of a horizontal copper tube. Int. Commun. Heat Mass Transf. 2018, 91, 11–16. [Google Scholar] [CrossRef]
- Weisensee, P.B.; Wang, Y.; Qian, H.; Schultz, D.; King, W.P.; Miljkovic, N. Condensate droplet size distribution on lubricant-infused surfaces. Int. J. Heat Mass Transf. 2017, 109, 187–199. [Google Scholar] [CrossRef]
- Daniel, D.; Mankin, M.N.; Belisle, R.A.; Wong, T.-S.; Aizenberg, J. Lubricant-infused micro/nano-structured surfaces with tunable dynamic omniphobicity at high temperatures. Appl. Phys. Lett. 2013, 102, 231603. [Google Scholar] [CrossRef]
- Preston, D.J.; Lu, Z.; Song, Y.; Zhao, Y.; Wilke, K.L.; Antao, D.S.; Louis, M.; Wang, E.N. Heat transfer enhancement during water and hydrocarbon condensation on lubricant infused surfaces. Sci. Rep. 2018, 8, 540. [Google Scholar] [CrossRef]
- Chen, X.; Akinwande, D.; Lee, K.-J.; Close, G.F.; Yasuda, S.; Paul, B.C.; Fujita, S.; Kong, J.; Wong, H.-S.P. Fully integrated graphene and carbon nanotube interconnects for gigahertz high-speed CMOS electronics. IEEE Trans. Electron Devices 2010, 57, 3137–3143. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef]
- Chen, C.-H.; Cai, Q.; Tsai, C.; Chen, C.-L.; Xiong, G.; Yu, Y.; Ren, Z. Dropwise condensation on superhydrophobic surfaces with two-tier roughness. Appl. Phys. Lett. 2007, 90, 173108. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ada, K.A.L.; Kwon, O.K.; Park, C.W. A comparison of condensation heat transfer performance of MWCNT/Fe composite coatings on steel substrate. J. Mech. Sci. Technol. 2014, 28, 1589–1596. [Google Scholar] [CrossRef]
- Koch, G.; Kraft, K.; Leipertz, A. Parameter study on the performance of dropwise condensation. Revue Générale de Thermique 1998, 37, 539–548. [Google Scholar] [CrossRef]
- Preston, D.J.; Wang, E.N. Jumping droplets push the boundaries of condensation heat transfer. Joule 2018, 2, 205–207. [Google Scholar] [CrossRef]
- Ko, T.-J.; Her, E.K.; Shin, B.; Kim, H.-Y.; Lee, K.-R.; Hong, B.K.; Kim, S.H.; Oh, K.H.; Moon, M.-W. Water condensation behavior on the surface of a network of superhydrophobic carbon fibers with high-aspect-ratio nanostructures. Carbon 2012, 50, 5085–5092. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, D.C.; Lin, J.F.; Wang, G.M. Dropwise condensation on L-B film surface. Chem. Eng. Process. Process Intensif. 1996, 35, 473–477. [Google Scholar] [CrossRef]
- Quan, X.; Wang, D.; Cheng, P. An experimental investigation on wettability effects of nanoparticles in pool boiling of a nanofluid. Int. J. Heat Mass Transf. 2017, 108, 32–40. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, Y.; Hu, S.; Yin, G.; Ma, X. Droplet regulation and dropwise condensation heat transfer enhancement on hydrophobic-superhydrophobic hybrid surfaces. Heat Transf. Eng. 2018, 39, 1543–1554. [Google Scholar] [CrossRef]
- Dong, J.; Dong, H.; Jin, Y.; Sun, L.; Ye, S. Nanograssed Micro-V-Groove architectures for continuous dropwise condensation and droplet directional movement. Adv. Mater. Interfaces 2018, 5, 1800202. [Google Scholar] [CrossRef]
- Park, K.-C.; Kim, P.; Grinthal, A.; He, N.; Fox, D.; Weaver, J.C.; Aizenberg, J. Condensation on slippery asymmetric bumps. Nature 2016, 531, 78–82. [Google Scholar] [CrossRef]
- Bahrami, H.R.T.; Saffari, H. Theoretical study of stable dropwise condensation on an inclined micro/nano-structured tube. Int. J. Refrig. 2017, 75, 141–154. [Google Scholar] [CrossRef]
- Cheng, J. Resistant energy analysis of dropwise condensation on superhydrophobic surfaces with hierarchical roughness. In Proceedings of the Fluids Engineering Division Summer Meeting 2017, Waikoloa, HI, USA, 30 July–3 August 2017; p. V01CT24A001. [Google Scholar]
- Parin, R.; Martucci, A.; Sturaro, M.; Bortolin, S.; Bersani, M.; Carraro, F.; Del Col, D. Nano-structured aluminum surfaces for dropwise condensation. Surf. Coat. Technol. 2018, 348, 1–12. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, Y.; Tian, J.; Li, J.; Gao, X. Clustered ribbed-nanoneedle structured copper surfaces with high-efficiency dropwise condensation heat transfer performance. ACS Appl. Mater. Interfaces 2015, 7, 10660–10665. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, J.; Li, X.; Liu, H. Dropwise condensation on superhydrophobic nanostructure surface, Part I: Long-term operation and nanostructure failure. Int. J. Heat Mass Transf. 2019, 129, 86–95. [Google Scholar] [CrossRef]
- Parin, R.; Del Col, D.; Bortolin, S.; Martucci, A. Dropwise condensation over superhydrophobic aluminium surfaces. J. Phys. Conf. Ser. 2016, 745, 032134. [Google Scholar] [CrossRef]
- Chen, X.; Weibel, J.A.; Garimella, S.V. Exploiting microscale roughness on hierarchical superhydrophobic copper surfaces for enhanced dropwise condensation. Adv. Mater. Interfaces 2015, 2, 1400480. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Y.; Zhu, J.; Li, J.; Gao, X. Copper-based ultrathin nickel nanocone films with high-efficiency dropwise condensation heat transfer performance. ACS Appl. Mater. Interfaces 2015, 7, 11719–11723. [Google Scholar] [CrossRef]
- Wen, R.; Li, Q.; Wu, J.; Wu, G.; Wang, W.; Chen, Y.; Ma, X.; Zhao, D.; Yang, R. Hydrophobic copper nanowires for enhancing condensation heat transfer. Nano Energy 2017, 33, 177–183. [Google Scholar] [CrossRef]
- Qu, M.; Liu, J.; He, J. Fabrication of copper-based ZnO nanopencil arrays with high-efficiency dropwise condensation heat transfer performance. RSC Adv. 2016, 6, 59405–59409. [Google Scholar] [CrossRef]
- Lu, M.-C.; Lin, C.-C.; Lo, C.-W.; Huang, C.-W.; Wang, C.-C. Superhydrophobic Si nanowires for enhanced condensation heat transfer. Int. J. Heat Mass Transf. 2017, 111, 614–623. [Google Scholar] [CrossRef]
- Wen, R.; Xu, S.; Ma, X.; Lee, Y.-C.; Yang, R. Three-dimensional superhydrophobic nanowire networks for enhancing condensation heat transfer. Joule 2018, 2, 269–279. [Google Scholar] [CrossRef]
- Preston, D.J.; Lu, Z.; Zhao, Y.; Antao, D.; Wilke, K.; Wang, E.N. Optimal design of slippery liquid-infused porous surfaces for enhanced condensation of low surface tension fluids. In Proceedings of the APS March Meeting 2017, New Orleans, LA, USA, 13–17 March 2017. [Google Scholar]
- Tsuchiya, H.; Tenjimbayashi, M.; Moriya, T.; Yoshikawa, R.; Sasaki, K.; Togasawa, R.; Yamazaki, T.; Manabe, K.; Shiratori, S. Liquid-infused smooth surface for improved condensation heat transfer. Langmuir 2017, 33, 8950–8960. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, S.H.; Bonner, R.W., III. Dropwise condensation on hydrophobic microporous powder and the transition to intrapowder droplet removal. In Proceedings of the 3rd Thermal and Fluids Engineering Conference (TFEC), Fort Lauderdale, FL, USA, 4–7 March 2018; pp. 643–653. [Google Scholar]
- Hoenig, S.H.; Bonner, R.W. Dropwise condensation on superhydrophobic microporous wick structures. J. Heat Transf. 2018, 140, 071501. [Google Scholar] [CrossRef]
- Zhu, L.; Xiu, Y.; Xu, J.; Tamirisa, P.A.; Hess, D.W.; Wong, C.-P. Superhydrophobicity on two-tier rough surfaces fabricated by controlled growth of aligned carbon nanotube arrays coated with fluorocarbon. Langmuir 2005, 21, 11208–11212. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, M.; Buck-Emden, A.; Bart, H.-J. Is dropwise condensation feasible? A review on surface modifications for continuous dropwise condensation and a profitability analysis. J. Adv. Res. 2018, in press. [Google Scholar] [CrossRef]
- Watson, R.C.H.; Birt, D.C.P.; Honour, C.W.; Ash, B.W. The promotion of dropwise condensation by montan wax. I. Heat transfer measurements. J. Appl. Chem. 1962, 12, 539–546. [Google Scholar] [CrossRef]
- Ma, X.; Rose, J.W.; Xu, D.; Lin, J.; Wang, B. Advances in dropwise condensation heat transfer: Chinese research. Chem. Eng. J. 2000, 78, 87–93. [Google Scholar] [CrossRef]

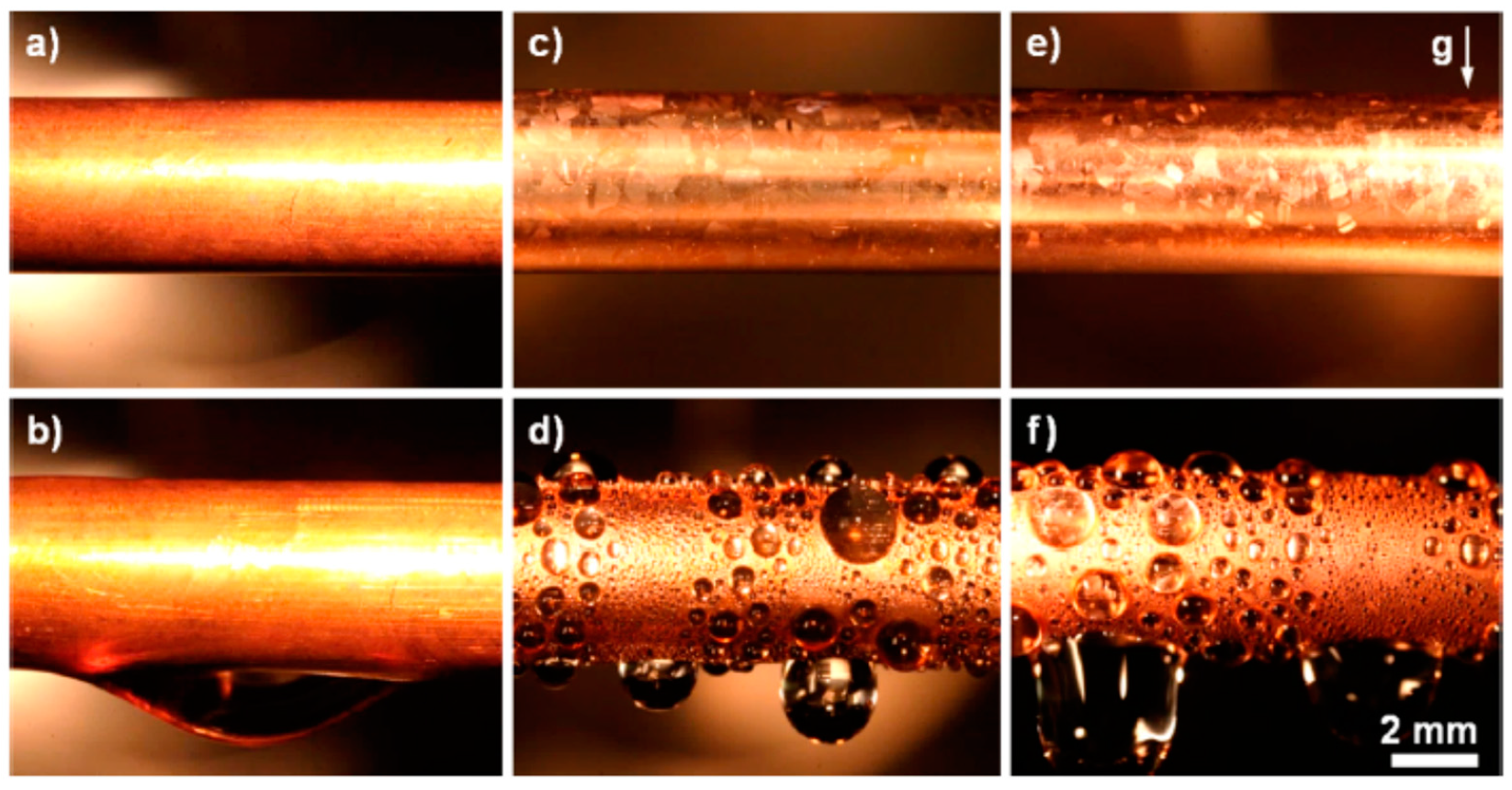
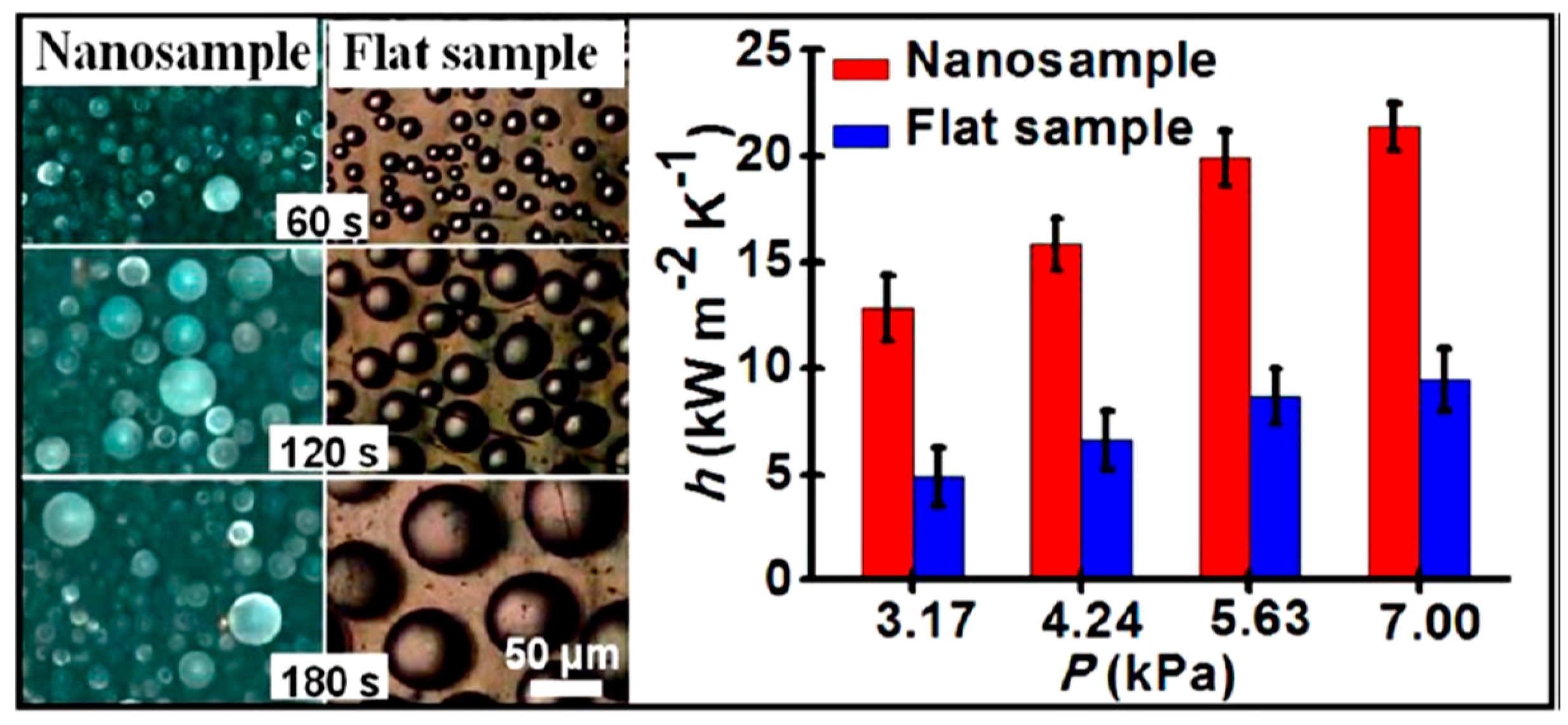
| Substrate | Coated Metal | Coating Method | Condensate/Environment | Effective Life of Coating (h) | Effective Coating Thickness (nm) | Enhancement in htc (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Cu | Ag | Electroplating | Steam | 2,400 | 300 nm | 550% max | [88] |
| Cu | Ag (with n-heptane as surfactant) | − | Steam | − | − | 57% | [35] |
| SS | Au | Electroplating | Steam/CO2 | − | 15,000–50,000 nm | Negligible (with rich CO2 environment) | [89] |
| Cu | Au | Electroplating | Steam | − | 100 nm | ~1000% max as compared to FWC | [86] |
| Substrate | Coated Metal | Coating Method | Effective Life of Coating (h) | Effective Coating Thickness (nm) | Enhancement in htc | Ref. |
|---|---|---|---|---|---|---|
| Cu tubes | N, Ar, He, H, and Cr | Ion implantation | − | Thin layer (~1 nm) | 3 times than that of noble metals | [103] |
| Cu tubes | Cr and O | Ion implantation | 15,600 (continued) | Thin layer (~1 nm) | − | [110] |
| polytetrafluoroethylene (PTFE) coated surfaces | Cr+ | Ion implantation | − | 5 µm PTFE with Cr+ ion implantation | 1.8 times | [104] |
| Stainless steel tubes | N+ | Plasma ion implantation | − | Thin layer (~1 nm) | 3.2 times | [109] |
| Titanium | N+ | Ion beam implantation | − | Thin layer (~1 nm) | 5.5 times | [106] |
| Al | Cr | Plasma ion implantation | − | 9–13 nm | 2 times | [111] |
| Substrate | Coated Metal | Coating Method | Effective Life of Coating (h) | Effective Coating Thickness (nm) | Enhancement in htc | Ref. |
|---|---|---|---|---|---|---|
| Cu, Cu–Ni/Au, Ti, brass | Organic coatings (parylene D/N, fluoroacrylic) | − | >1200 | 2000–3000 (fluoroacrylic) 500–1000 (parylene N/D) | 3–6 times | [78] |
| Cu, brass, Stainless steel (SS) and carbon steel (CS) tubes | PTFE | Ion-beam mixed implantation | >1000 | − | 1.6–28.6 times | [114] |
| Cu block | Polyvinylidene chloride | − | >21,568 | 50–500 | 20 times | [115] |
| Cu condenser coil and Al | Ultrathin copolymer films: poly-(1H,1H,2H,2H- perfluorodecyl acrylate)-co-divinyl benzene p(PFDA-co-DVB) | Initiated CVD | >48 | 40 | 7 times | [118] |
| Glass | Teflon-coated titanium (Ti) pillars | Spray coating | − | 200 (Ti pillars) | − | [120] |
| SS | PTFE+SiO2 | Spray coating | − | − | 2.7 and 3.4 times that of hydrophilic and hydrophobic surfaces, respectively | [121] |
| Cu substrate | Needles soldered superhydrophobic surface | Impaling superhydrophobic film | >2.9 | 200,000 | 4 times | [63] |
| Al, Cu | Polytetrafluoroethylene (PTFE) thin films | Electrospun | − | 1000 | 1.64 times | [122] |
| Cu | fluorosilane polymer (EGC-1720) | Spin-coating | >3 | − | >2.1 times | [123] |
| Substrate | Coated Metal | Effective Life of Coating (h) | Enhancement in htc | Ref. |
|---|---|---|---|---|
| Cu and Au | heptadecafluoro-1-decanethiol | >6480 | – | [17] |
| Cu and Cu–Ni | hexadecylthiol | − | 5 times | [73] |
| Cu | n-octadecanethiol | − | 1.7–2.1 times | [127] |
| Cu | stearic acid solution and n-octadecyl mercaptan solution | >2600 | 1.8 times | [126] |
| Cu | aqueous silver nitrate solution and 1-dodecanethiol was spray-coated | >500 | 4.18 times | [64] |
| Cu tube | docosanoic acid | − | 10 times | [125] |
| Substrate | Coated Metal | Coating Method | Condensate | Effective Life of Coating (h) | Enhancement in htc | Ref. |
|---|---|---|---|---|---|---|
| Cu | Graphene | Low- and atmospheric-pressure CVD | Steam | >336 | 4 times | [65] |
| Si | CNT with Cr–Ni as catalyst | Plasma-enhanced CVD | Steam | − | − | [143] |
| Carbon steel | Composite of multiwalled CNTs and Fe | Electrostatic spraying followed by sintering at 900 °C | R-134a | − | 1.6 times | [144] |
| Cu | Barium stearate monomolecular film | Langmuir–Blodgett (L–B) built-up film technology | Steam | − | 30 times | [148] |
| Cu | 1H,1H,2H,2H-perfluorooctyltriethoxysilane | Wet oxidation + self-organization of macromolecules | Steam | >168 | Decreased on 3rd day | [157] |
| Al | Trichloro (1H,1H,2H,2H-perfluorooctyl) silane | Spin-coated | Steam | ~1 | 8 times | [155] |
| Cu | Cu nanoneedles | In situ grown | Steam | − | 1.25 times | [156] |
| Si | CNT on Si micropillars | CNT growth | Humid air | − | Self-propelled jumping motion was investigated | [42] |
| Al | flurorosilane film | Etching and flurorosilane film | Steam | − | 4 times | [158] |
| Cu | Cu micro–nanostructured surface | Spin coating followed by soft-baking at 100 °C | Humid air | − | Droplet growth was compared with only nanocoated surface | [159] |
| Cu | Nickle nanocone film | In situ growth | Steam | − | 89% | [160] |
| Cu | Copper nanowires | porous anodic alumina template-assisted electrodeposition | Steam | − | 100% higher heat flux | [161] |
| Cu | ZnO | ZnO nanopencil array | Steam | − | 140% increase | [162] |
| Si | Si nanowires | Electroless etching | Steam | − | 155% increase | [163] |
| Cu | Cu nanowires | 3D porous anodic alumina oxide templates | Steam | − | 100% increase | [164] |
| Surface Coating Technique | Advantages | Disadvantages |
|---|---|---|
| Noble metals | DWC increases due to the absorption of impurities and hydrocarbons. | High price of the material limits its practical application. |
| REOs | Possesses hydrophobic nature, which promotes DWC. | Low thermal conductivity. Lack of heat transfer studies. |
| Ion implantation | Enhances heat transfer capability at low subcooling. Low-temperature process. | Performance decreases with increase of subcooling after a particular level. Expensive, which limits its scalability. |
| Polymer coating | Effective method from both cost and performance aspects. Increases DWC and htc. | Durability of thin coating needs to be addressed. |
| SAMs | Negligible thermal resistance. High htc. | Robustness and durability for SAM needs to be improved for industrial-based applications. |
| Lubricant-infused surfaces (LIS) | Effective for condensation enhancement. Advantages include self-healing, self-cleaning, antifouling, and omniphobic properties. | The removal of lubricants and contaminating condensate makes practical application inadequate. |
| Nanostructured Surfaces | htc enhancement for condensation. Offers chemical unitability, robustness, and low thermal resistivity. | Long-term durability issues. Limits scalability at the industrial level. |
| Porous coating | Increases overall surface area. Quick condensation process. | Liquid trapped has a negative effect on the overall heat transfer from gas phase to the substrate. Unlike boiling, this property makes it less feasible for condensation. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.A.; Tahir, F.; Baloch, A.A.B.; Koc, M. Review of Micro–Nanoscale Surface Coatings Application for Sustaining Dropwise Condensation. Coatings 2019, 9, 117. https://doi.org/10.3390/coatings9020117
Khan SA, Tahir F, Baloch AAB, Koc M. Review of Micro–Nanoscale Surface Coatings Application for Sustaining Dropwise Condensation. Coatings. 2019; 9(2):117. https://doi.org/10.3390/coatings9020117
Chicago/Turabian StyleKhan, Shoukat Alim, Furqan Tahir, Ahmer Ali Bozdar Baloch, and Muammer Koc. 2019. "Review of Micro–Nanoscale Surface Coatings Application for Sustaining Dropwise Condensation" Coatings 9, no. 2: 117. https://doi.org/10.3390/coatings9020117
APA StyleKhan, S. A., Tahir, F., Baloch, A. A. B., & Koc, M. (2019). Review of Micro–Nanoscale Surface Coatings Application for Sustaining Dropwise Condensation. Coatings, 9(2), 117. https://doi.org/10.3390/coatings9020117





