A Study on the Characteristics of Cu–Mn–Dy Alloy Resistive Thin Films
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Cu–Mn–Dy Thin Film
2.2. Analysis
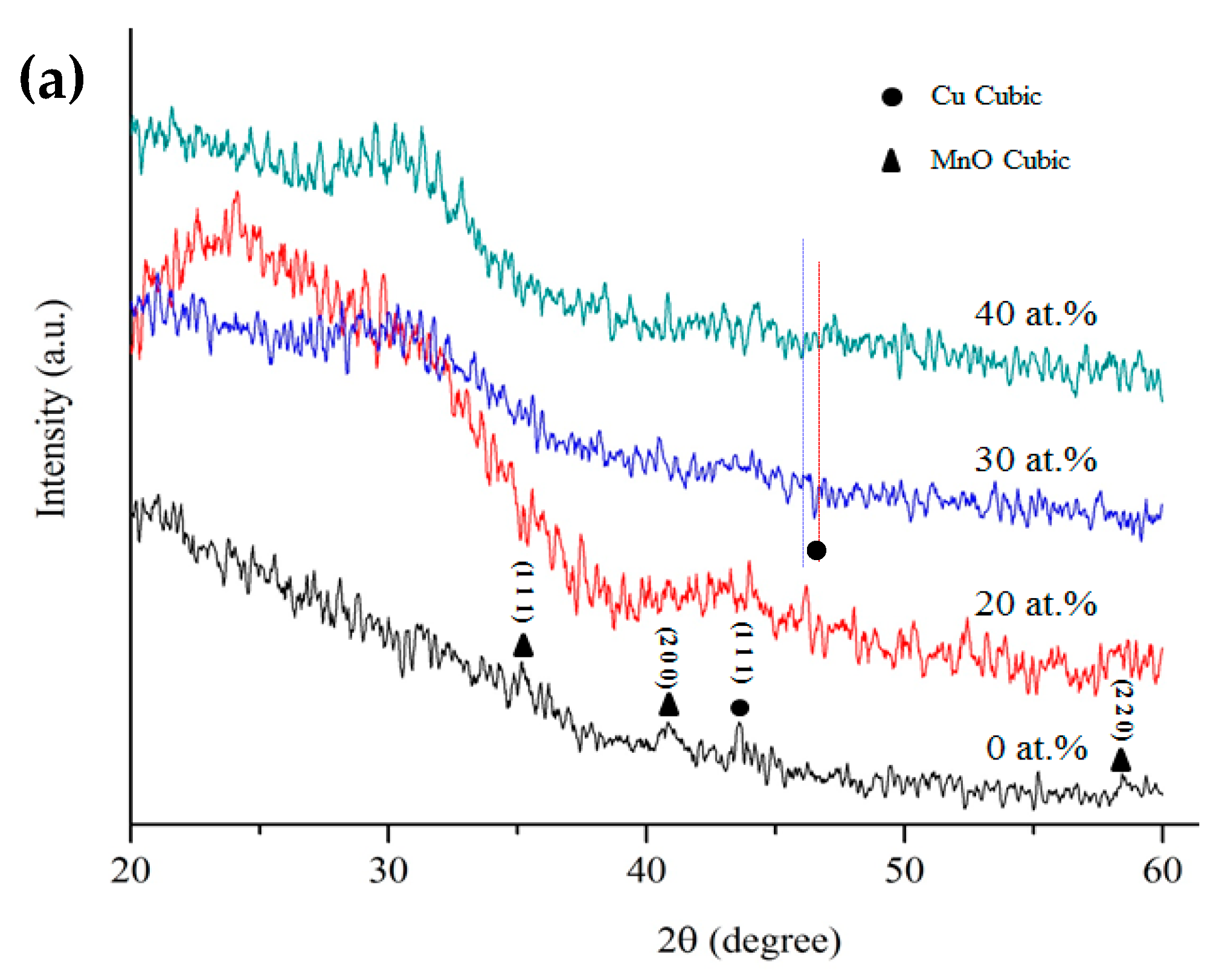
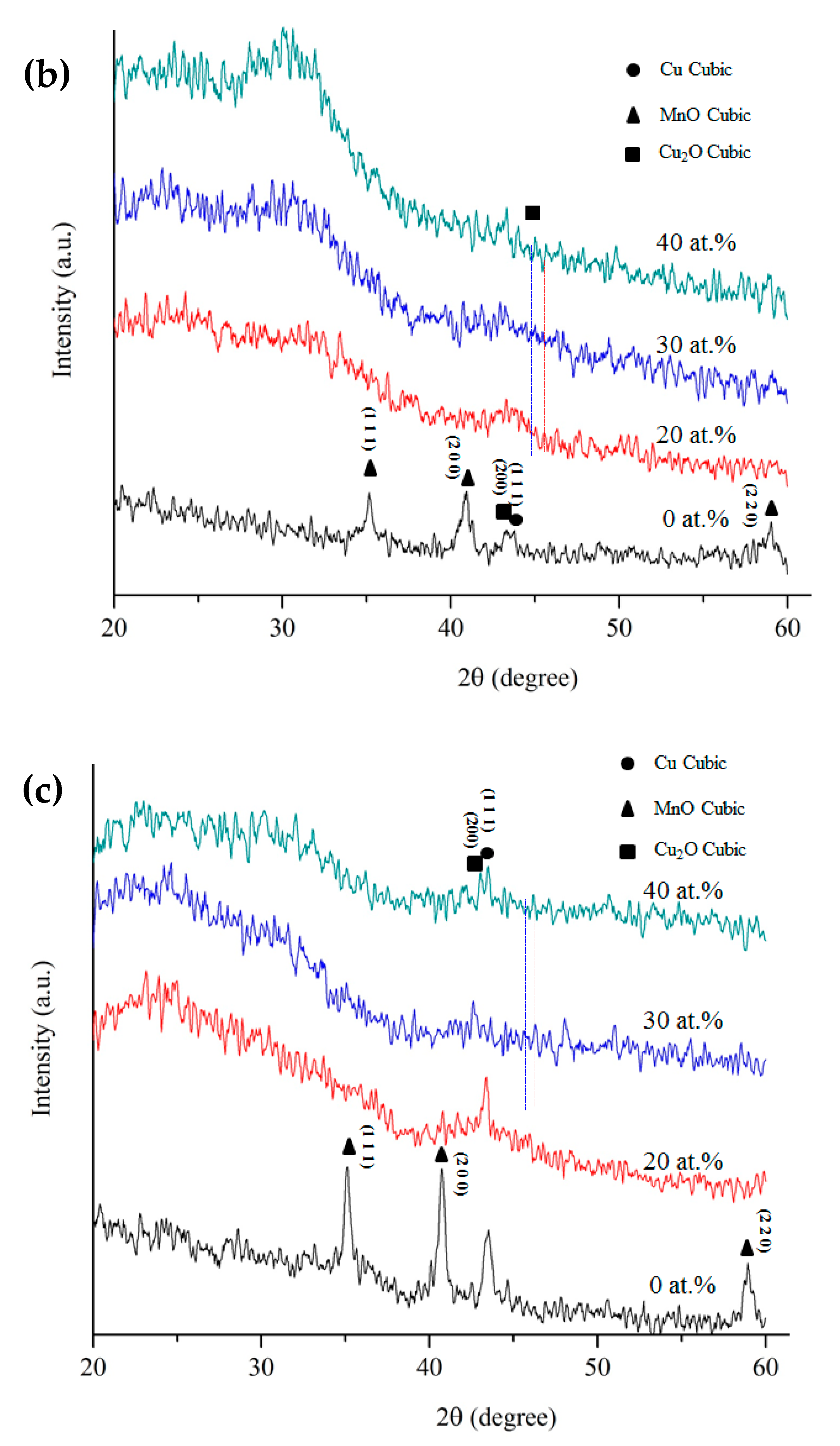
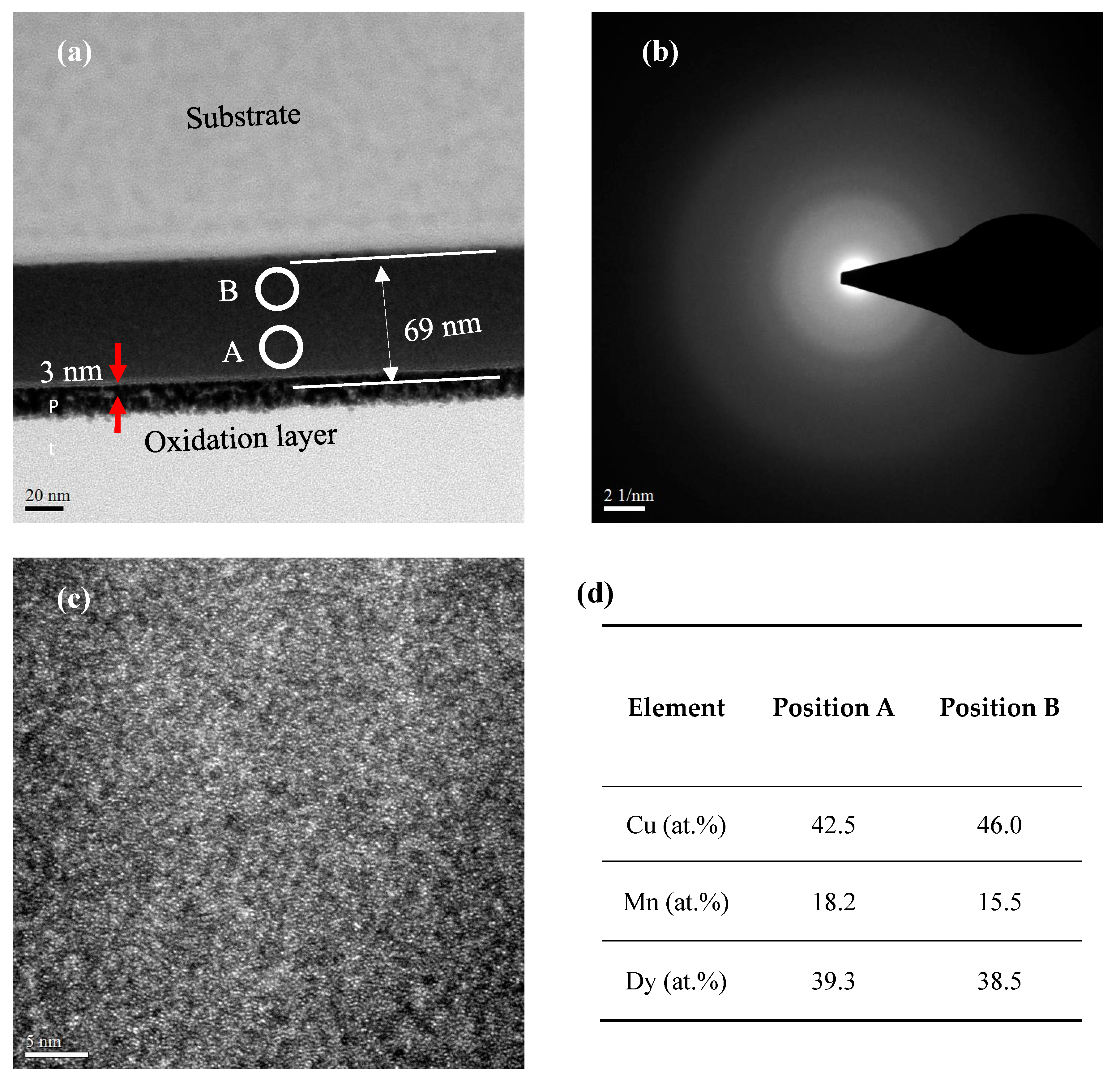
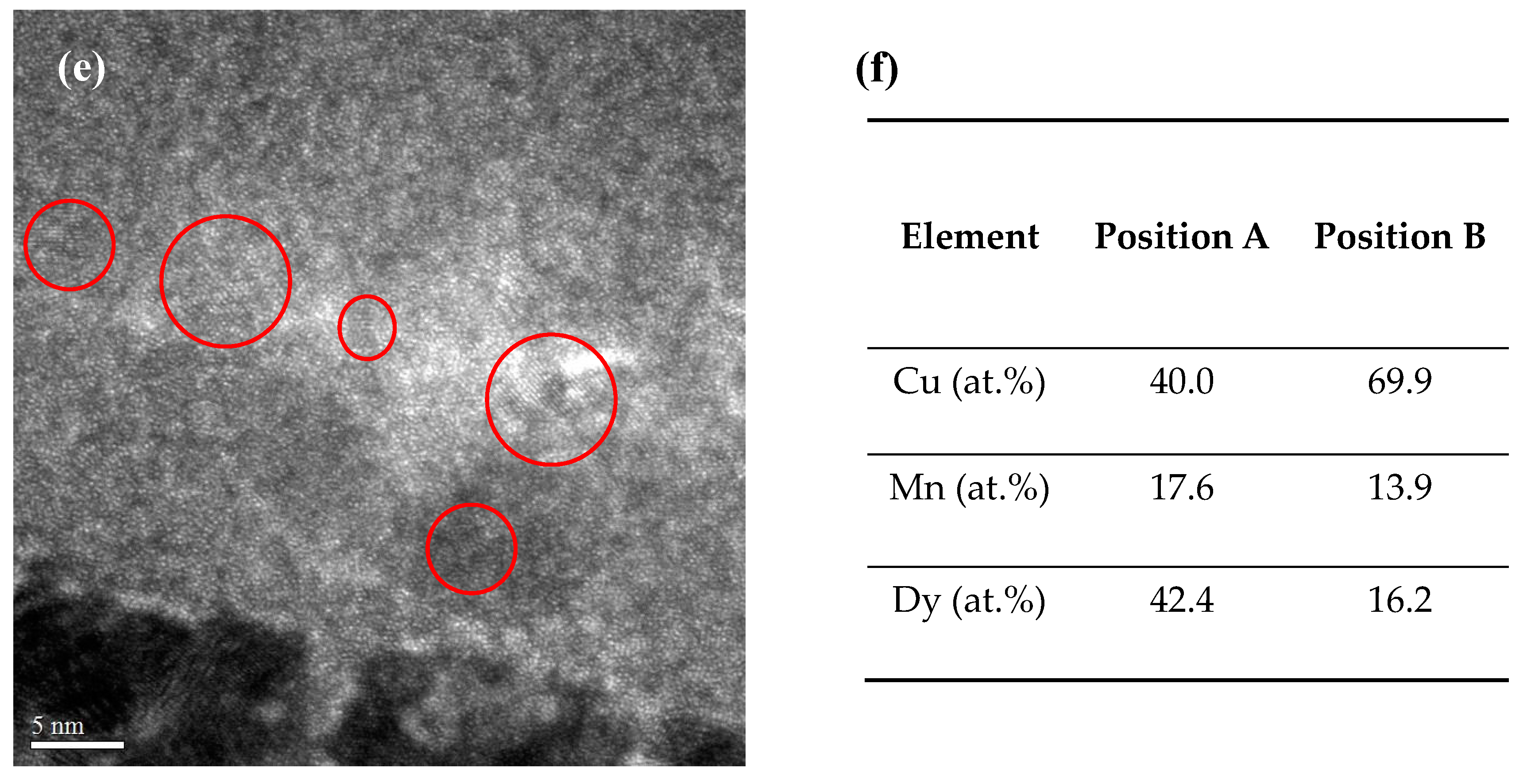
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chuang, N.C.; Lin, J.T.; Chen, H.R. TCR control of Ni–Cr resistive film deposited by DC magnetron sputtering. Vacuum 2015, 119, 200–203. [Google Scholar] [CrossRef]
- Matsuda, K.; Sato, K.; Doi, T.; Ogata, K.; Konishi, K. Super precision metal film resistors. National Tech. Rep. 1980, 26, 283–288. [Google Scholar]
- Wang, X.Y.; Zhang, Z.S.; Bai, T. Investigation on powder metallurgy Cr–Si–Ta–Al alloy target for high-resistance thin film resistors with low temperature coefficient of resistance. Mater. Des. 2010, 31, 1302–1307. [Google Scholar] [CrossRef]
- Kim, E.M.; Kim, S.C.; Lee, S. Electrical Properties of Cu/Mn Alloy Resistor with Low Resistance and Thermal Stability. J. Korean Inst. Electr. Electron. Mater. Eng. 2016, 29, 365–369. [Google Scholar]
- Pearson, W.B. A Handbook of Lattice Parameters and Structures in Metals and Alloys; Pergamon Press: London, UK, 1958; Volume 1. [Google Scholar]
- Iijima, J.; Fujii, Y.; Neishi, K.; Koikea, J. Resistivity reduction by external oxidation of Cu–Mn alloy films for semiconductor interconnect application. J. Vac. Sci. Technol. B 2009, 27, 1963–1968. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wu, C.T.; Lee, W.H.; Chang, S.C.; Wang, Y.L. A study on annealing mechanisms with different manganese contents in CuMn alloy. J. Alloys Comp. 2012, 542, 118–123. [Google Scholar] [CrossRef]
- Misjak, F.; Nagy, K.H.; Lobotka, P.; Radnoczi, G. Electron scattering mechanisms in Cu–Mn films for interconnect applications. J. Appl. Phys. 2014, 116, 083507. [Google Scholar] [CrossRef]
- Govindarajan, S.; Boscke, T.S.; Sivasubramani, P.; Kirsch, P.D.; Lee, B.H.; Tseng, H.H.; Jammy, R.; Schroder, U.; Ramanathan, S.; Gnade, B.E. Higher permittivity rare earth doped HfO2 for sub-45-nm metal-insulator-semiconductor devices. Appl. Phys. Lett. 2007, 91, 062906. [Google Scholar] [CrossRef]
- Du, Q.; Huang, Z.; Wu, Z.; Meng, X.; Yin, G.; Gao, F.; Wang, L. Facile preparation and bifunctional imaging of Eu-doped GdPO4 nanorods with MRI and cellular luminescence. Dalton Trans. 2015, 44, 3934–3940. [Google Scholar] [CrossRef]
- Gu, D.; Sun, Z.; Zhou, X.; Guo, R.; Wang, T.; Jiang, Y. Effect of yttrium-doping on the microstructures and semiconductor-metal phase transition characteristics of polycrystalline VO2 thin films. Appl. Surf. Sci. 2015, 359, 819–825. [Google Scholar] [CrossRef]
- Asanuma, H.; Suzuki, T.; Kusunoki, T. An investigation of optimal interfacial film condition for Cu–Mn alloy based source/drain electrodes in hydrogenated amorphous silicon thin film transistors. AIP Adv. 2012, 2, 022147. [Google Scholar] [CrossRef]
- Javed, A.; Durrani, H.G.; Zhu, C. The effect of vacuum annealing on the microstructure, mechanical and electrical properties of tantalum films. Int. J. Refract. Met. Hard Mater. 2016, 54, 154–158. [Google Scholar] [CrossRef]
- Liu, L.; Gong, H.; Wang, Y.; Wang, J.; Wee, A.T.S.; Liu, R. Annealing effects of tantalum thin films sputtered on [001] silicon substrate. Mater. Sci. Eng. C 2001, 16, 85–89. [Google Scholar] [CrossRef]
- Lee, S.-K.; Hsu, H.C.; Tuan, W.H. Oxidation Behavior of Copper at a Temperature below 300 °C and the Methodology for Passivation. Mater. Res. 2016, 19, 51–56. [Google Scholar] [CrossRef]
- Xu, C.H.; Woo, C.H.; Shi, S.Q. Formation of CuO nanowires on Cu foil. Chem. Phys. Lett. 2004, 399, 62–66. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, J.C. Temperature effect on the Cu2O oxide morphology created by oxidation of Cu (001) as investigated by in situ UHV TEM. Appl. Surf. Sci. 2003, 210, 165–170. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, C.; Liu, L.; Wang, L.; Ding, J.; Zhao, W. Synthesis of CuxO(x = 1,2)/Amorphous Compounds by Dealloying and Spontaneous Oxidation Method. Mater. Res. 2014, 17, 33–37. [Google Scholar] [CrossRef]
- Otsuka, Y.; Koike, J.; Sako, H.; Ishibashi, K.; Kawasaki, N.; Chung, S.M.; Tanaka, I. Graded composition and valence states in self-forming barrier layers at Cu–Mn/SiO2 interface. Appl. Phys. Lett. 2010, 96, 012101. [Google Scholar] [CrossRef]
- Lozano, J.G.; Lozano-Perez, S.; Bogan, J.; Wang, Y.C.; Brennan, B.; Nellist, P.D.; Hughes, G. Interdiffusion and barrier layer formation in thermally evaporated Mn/Cu heterostructures on substrates. Appl. Phys. Lett. 2011, 98, 123112. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Tsai, M.; Wang, C.; Tsai, C.; Shen, W.; Ye, J.; Wu, J.; We, G. Thermal stability and performance of NbSiTaTiZr high-entropy alloy barrier for copper metallization. J. Electrochem. Soc. 2011, 158, H1161. [Google Scholar] [CrossRef]
- Dong, X.; Wu, J. Study on the crystallization of amorphous Cr–Si–Ni thin films using in situ X-ray diffraction. J. Mater. Sci. Technol. 2001, 17, s43–s46. [Google Scholar]
- Cheng, H.Y.; Chen, Y.C.; Li, P.J.; Yang, C.F.; Huang, H.H. Effect of Annealing Process on the Properties of Ni(55%)Cr(40%)Si(5%) Thin-Film Resistors. Materials 2015, 8, 6752–6760. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Lee, D.C. Electrical Properties of Sputtered Ni–Cr–Al–Cu Thin Film Resistors with Ni and Cr Contents. J. Kor. Phys. Soc. 2002, 40, 339–343. [Google Scholar]
- Vinayak, S.; Vyas, H.P.; Vankar, V.D. Microstructure and electrical characteristics of Ni–Cr thin films. Thin Solid Films 2007, 515, 7109–7116. [Google Scholar] [CrossRef]


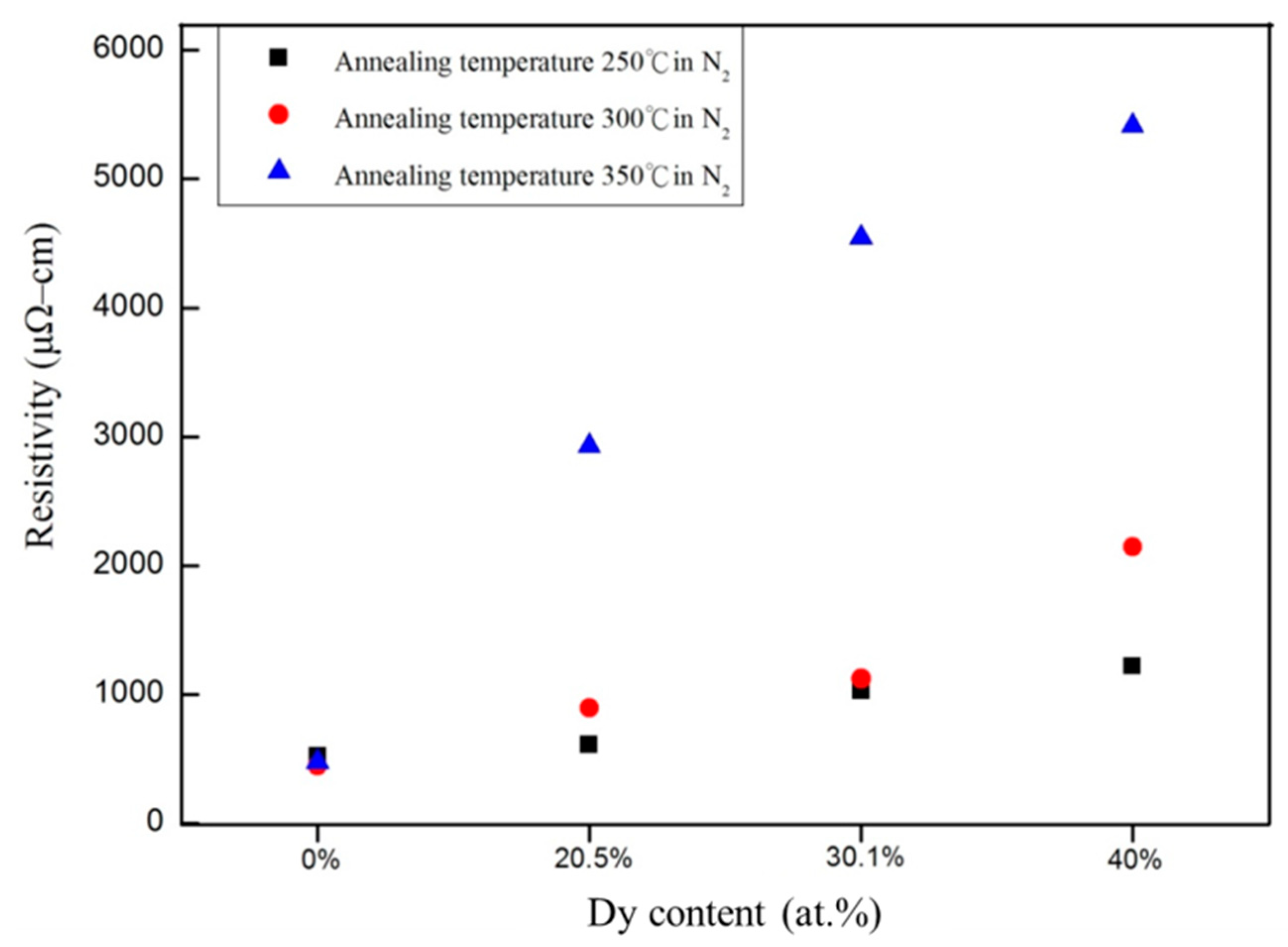
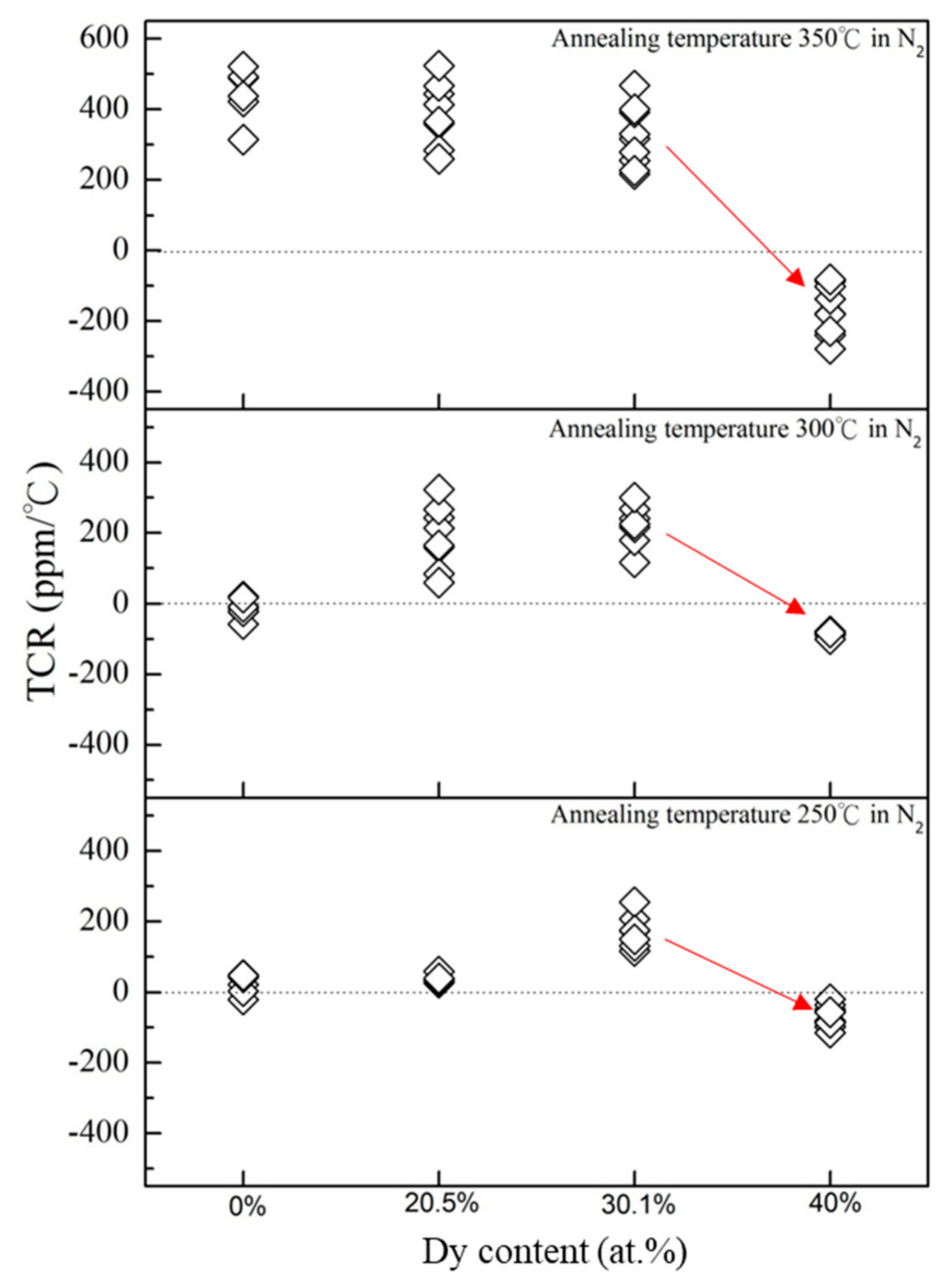
| Power (W) | DC/RF | 50/40 | 50/55 | 50/70 |
|---|---|---|---|---|
| Element (at.%) | Cu | 52.3 | 45.5 | 37 |
| Mn | 27.2 | 24.5 | 23 | |
| Dy | 20.5 | 30 | 40 |
| Film Position | Surface | Internal | |
|---|---|---|---|
| Element | |||
| Cu (at.%) | 8.8 | 47.6 | |
| Mn (at.%) | 7.4 | 9.3 | |
| Dy (at.%) | 14.9 | 41.5 | |
| O (at.%) | 68.9 | 1.6 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; He, C.-W.; Lee, Y.-C.; Wu, D.-C. A Study on the Characteristics of Cu–Mn–Dy Alloy Resistive Thin Films. Coatings 2019, 9, 118. https://doi.org/10.3390/coatings9020118
Lee H-Y, He C-W, Lee Y-C, Wu D-C. A Study on the Characteristics of Cu–Mn–Dy Alloy Resistive Thin Films. Coatings. 2019; 9(2):118. https://doi.org/10.3390/coatings9020118
Chicago/Turabian StyleLee, Ho-Yun, Chi-Wei He, Ying-Chieh Lee, and Da-Chuan Wu. 2019. "A Study on the Characteristics of Cu–Mn–Dy Alloy Resistive Thin Films" Coatings 9, no. 2: 118. https://doi.org/10.3390/coatings9020118
APA StyleLee, H.-Y., He, C.-W., Lee, Y.-C., & Wu, D.-C. (2019). A Study on the Characteristics of Cu–Mn–Dy Alloy Resistive Thin Films. Coatings, 9(2), 118. https://doi.org/10.3390/coatings9020118




