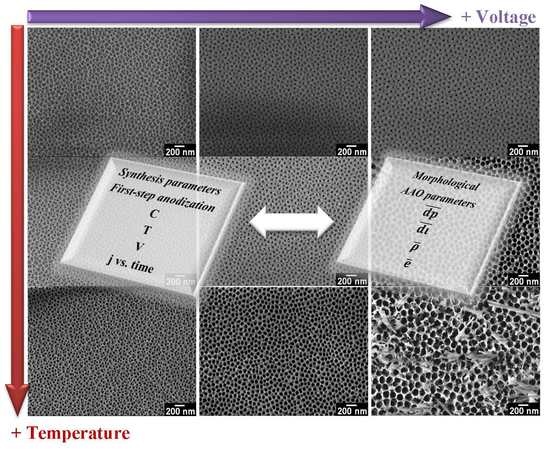
Synthesis and Morphological Characterization of Nanoporous Aluminum Oxide Films by Using a Single Anodization Step
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Material
2.2. Synthesis of AAO Coatings
2.2.1. Pre-Treatment of the Substrate Surface
2.2.2. Anodic Oxidation
2.3. Morphological Characterization of Coatings
3. Results and Discussion
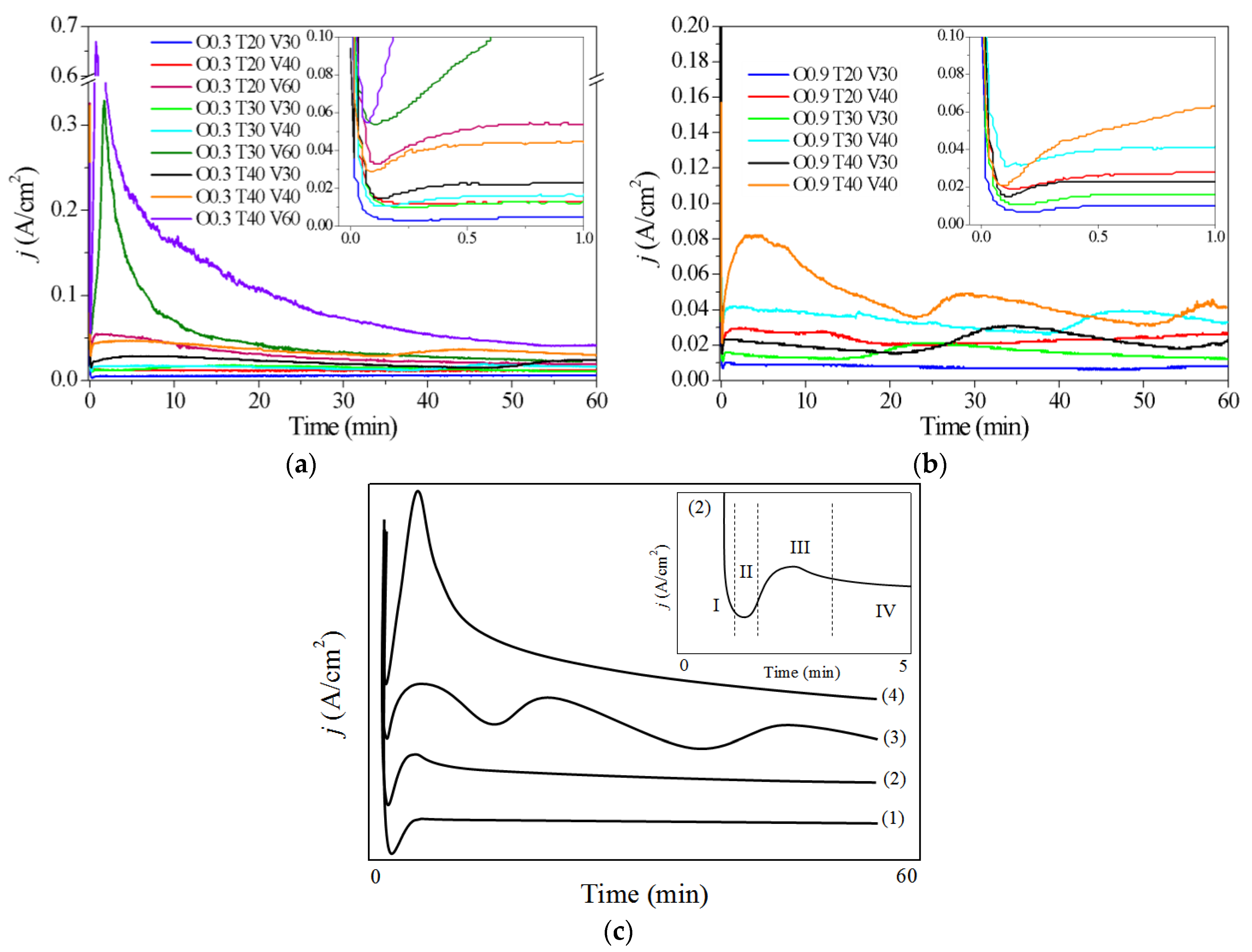
3.1. Influence of Variables (C, T and V) During the Synthesis of AAO Films—Current Density vs. Time Curves
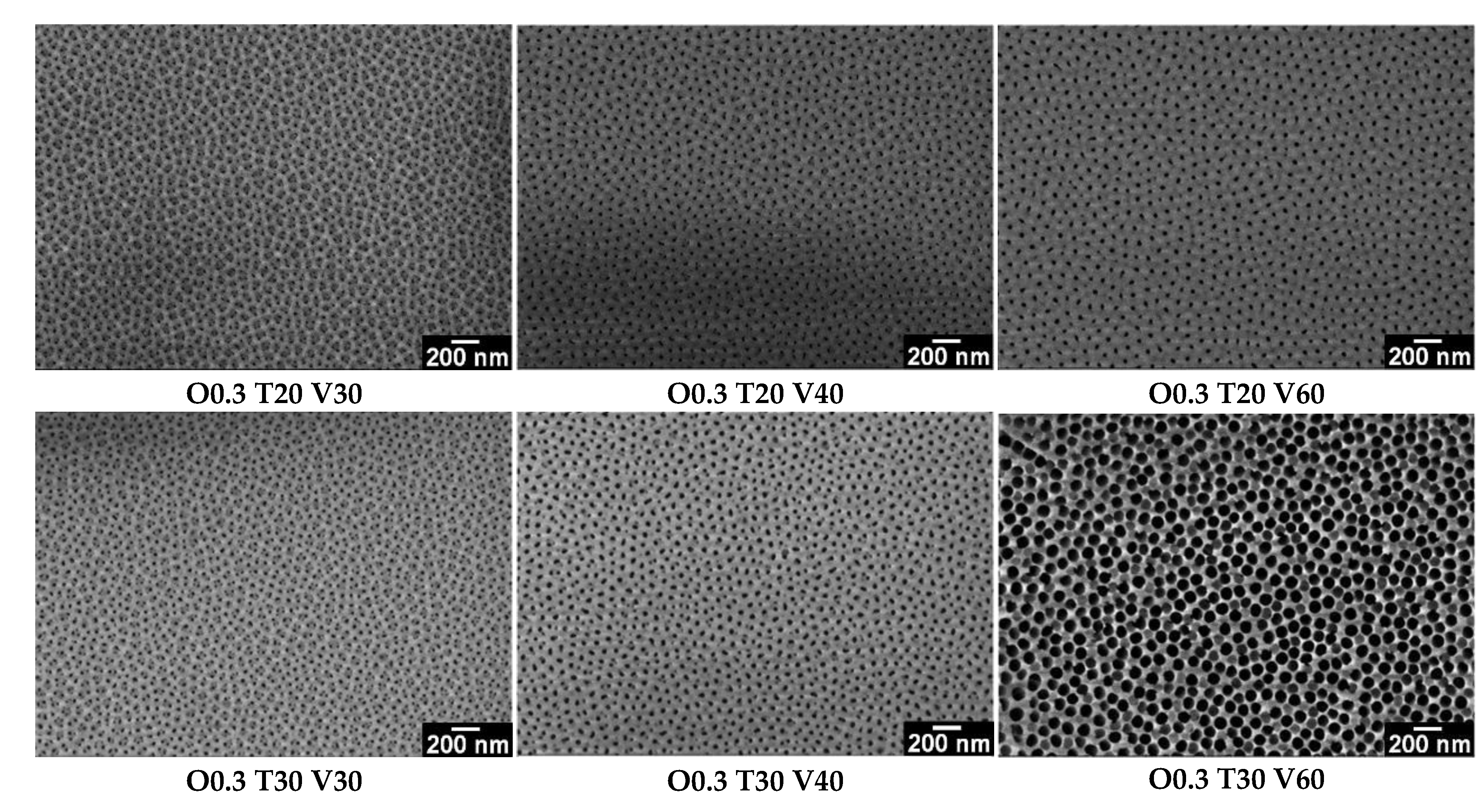
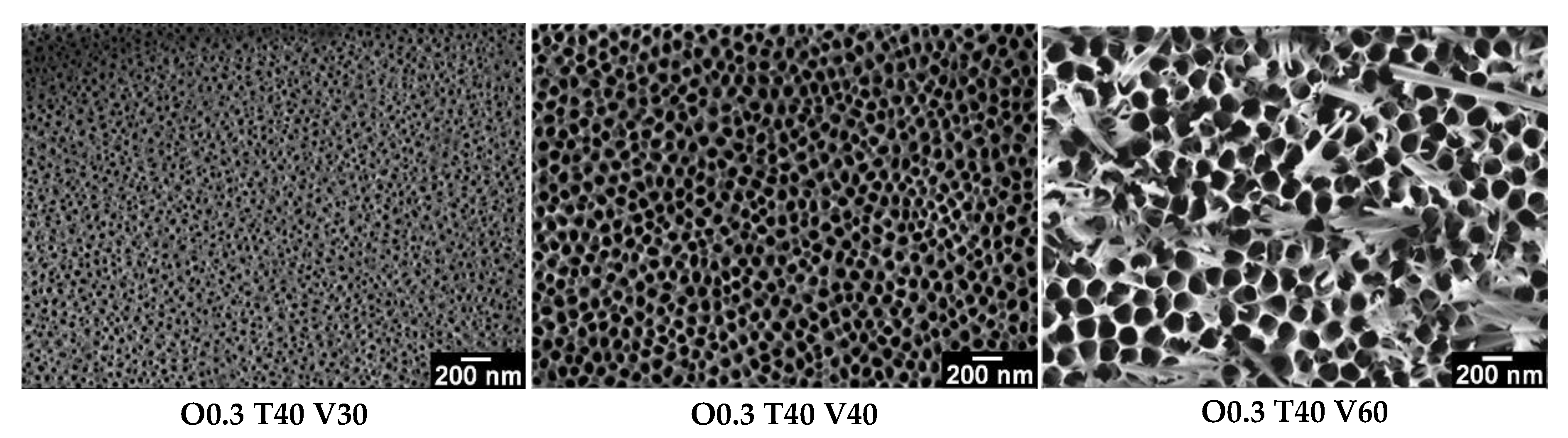
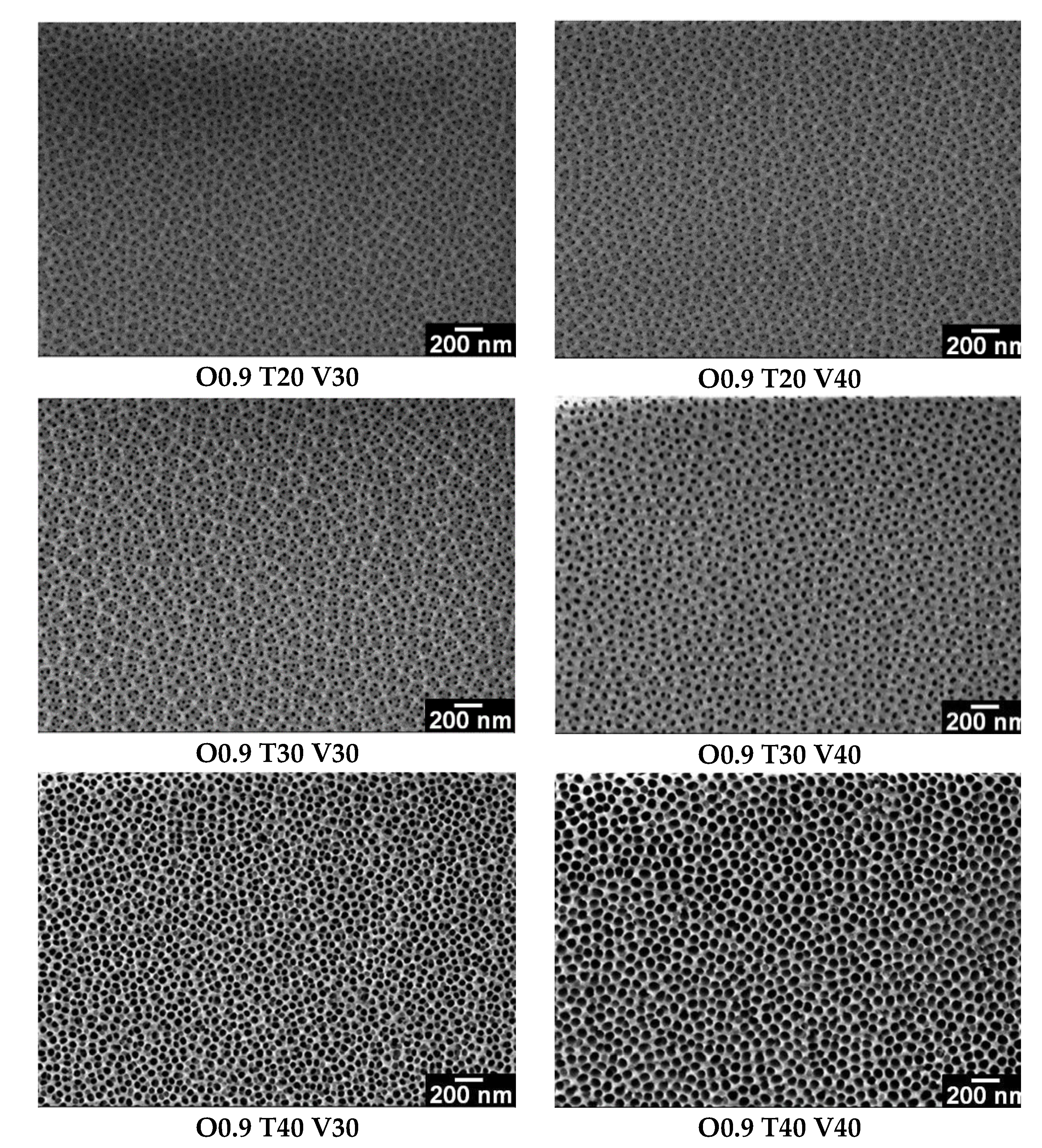
3.2. Influence of Variables (C, T and V) on the Morphology of Coatings
4. Conclusions
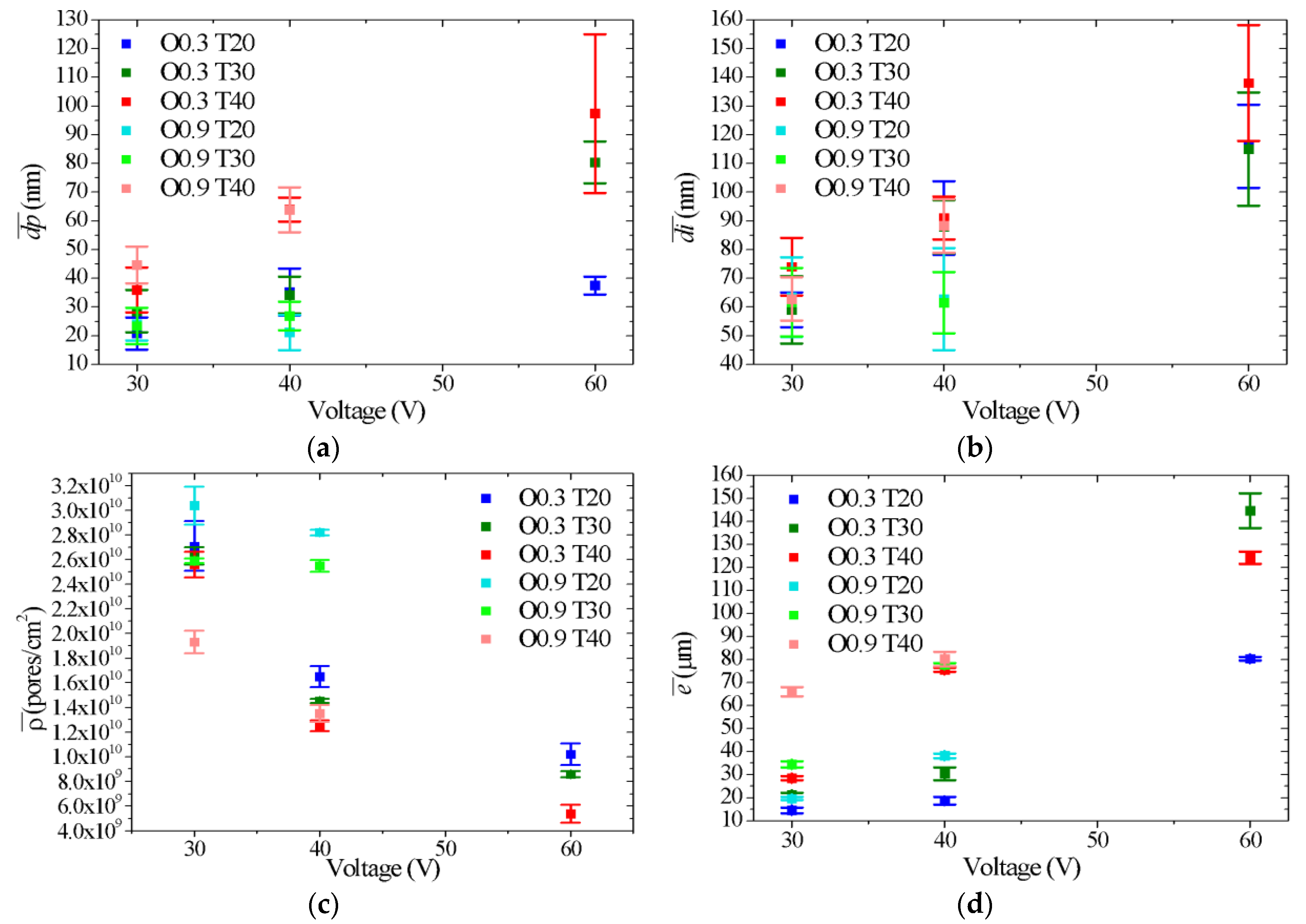
- By varying the oxalic acid concentration (0.3 and 0.9 M), the electrolyte temperature (20, 30 and 40 °C) and the voltage (30, 40 and 60 V), it is possible to obtain nanoporous AAO coatings with values between 21 and 97 nm, between 59 and 138 nm, between 2.8 × 1010 and 5.4 × 109 pores/cm2 and between 15 and 145 µm. In this way, selecting the appropriate combination of synthesis variables allows synthesizing AAO coatings with the morphological characteristics that best suit each particular application, mainly in catalysis, where pore sizes between 40 and 300 nm and thicknesses between 50–250 μm are commonly required.
- The pore diameter, interpore distance and pore density of anodic films vary significantly with the voltage and temperature of the electrolyte. In general, the highest values of and were obtained with the highest levels of voltage and temperature, whereas the highest values of were obtained with the lowest levels of voltage, temperature and concentration.
- Film thickness increased with increases in oxalic acid concentration, electrolyte temperature and voltage. However, certain anodic synthesis conditions favored the oxide dissolution process over the oxidation process, limiting the growth in thickness.
- The shape of the anodic curves strongly depended on the concentration of oxalic acid, temperature and anodizing voltage. These results coincide with those obtained from the characterization by optical microscopy and scanning microscopy, demonstrating the usefulness of the current density vs. time curves to predict some morphological characteristics of the oxides in a simple and fast way.
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Bai, A.; Hu, C.-C.; Yang, Y.-F.; Lin, C.-C. Pore diameter control of anodic aluminum oxide with ordered array of nanopores. Electrochimica Acta 2008, 53, 2258–2264. [Google Scholar] [CrossRef]
- Cheng, C. Electro-Chemo-Mechanics of Anodic Porous Alumina Nano-Honeycombs: Self-Ordered Growth and Actuation; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–166. [Google Scholar]
- Sulka, G.D. Highly ordered anodic porous alumina formation by self-organized anodizing. In Nanostructured Materials in Electrochemistry; WILEY-VCH: Weinheim, Germany, 2008; Volume 1, pp. 1–116. [Google Scholar]
- Jani, A.M.M.; Losic, D.; Voelcker, N.H.; Jani, A.M. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 636–704. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Vrublevsky, I.; Parkoun, V.; Sokol, V.; Schreckenbach, J.; Marx, G. The study of the volume expansion of aluminum during porous oxide formation at galvanostatic regime. Appl. Surf. Sci. 2004, 222, 215–225. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B.; Gösele, U. Self-ordering regimes of porous alumina: The 10% porosity rule. Nano Lett. 2002, 2, 677–680. [Google Scholar] [CrossRef]
- Li, A.P.; Müller, F.; Birner, A.; Nielsch, K.; Gösele, U. Hexagonal pore arrays with a 50–420 nm interpore distance formed by self-organization in anodic alumina. J. Appl. Phys. 1998, 84, 6023–6026. [Google Scholar] [CrossRef]
- Belwalkar, A.; Grasing, E.; Van Geertruyden, W.; Huang, Z.; Misiolek, W.Z. Effect of processing parameters on pore structure and thickness of anodic aluminum oxide (AAO) tubular membranes. J. Membr. Sci. 2008, 319, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Nasirpouri, F.; Saedi, A. On the growth sequence of highly ordered nanoporous anodic aluminium oxide. Mater. Des. 2006, 27, 983–988. [Google Scholar] [CrossRef]
- Han, X.Y.; Shen, W.Z. Improved two-step anodization technique for ordered porous anodic aluminum membranes. J. Electroanal. Chem. 2011, 655, 56–64. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.; Pan, C.; Zhu, J. Nano-porous anodic aluminum oxide membranes with 6–19 nm pore diameters formed by a low-potential anodizing process. Nanotechnology 2007, 18, 345302. [Google Scholar] [CrossRef]
- Chung, C.K.; Liao, M.W.; Chang, H.C.; Lee, C.T. Effects of temperature and voltage mode on nanoporous anodic aluminum oxide films by one-step anodization. Thin Solid Films 2011, 520, 1554–1558. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Bojar, Z. Synthesis of anodic aluminum oxide (AAO) at relatively high temperatures. Study of the influence of anodization conditions on the alumina structural features. Surf. Coat. Technol. 2011, 206, 265–272. [Google Scholar] [CrossRef]
- Jessensky, O.; Müller, F.; Gösele, U. Self-organized formation of hexagonal pore arrays in anodic alumina. Appl. Phys. Lett. 1998, 72, 1173–1175. [Google Scholar] [CrossRef]
- Na, H.C.; Sung, T.J.; Yoon, S.H.; Hyun, S.K.; Kim, M.S.; Lee, Y.G.; Shin, S.H.; Choi, S.M.; Yi, S. Formation of unidirectional nanoporous structures in thickly anodized aluminum oxide layer. Trans. Nonferrous Met. Soc. China 2009, 19, 1013–1017. [Google Scholar] [CrossRef]
- Pardo-Saavedra, D.C; Londoño-Calderón, C.L.; Menchaca-Nal, S.; Pampillo, L.G.; Martínez García, R.; Socolovsky, L.M. Morfological study of pore widening process in anodized alumina films. An. AFA 2013, 25, 68–71. [Google Scholar] [CrossRef]
- Zaraska, L.; Sulka, G.D.; Szeremeta, J.; Jaskuła, M. Porous anodic alumina formed by anodization of aluminum alloy (AA1050) and high purity aluminum. Electrochim. Acta 2010, 55, 4377–4386. [Google Scholar] [CrossRef]
- Londoño Calderón, C.L.; Menchaca Nal, S.; Pardo Saavedra, D.C.; Silveyra, J.M.; Socolovsky, L.M.; Pampillo, L.G.; Martínez García, R. Low cost fabrication of porous anodic alumina: A comparative study of the morphology produced by one- and two-steps of anodization. RevistMatéria 2016, 21, 677–690. [Google Scholar]
- Nasirpouri, F.; Abdollahzadeh, M.; Almasi, M.J.; Parvini-Ahmadi, N. A comparison between self-ordering of nanopores in aluminium oxide films achieved by two- and three-step anodic oxidation. Curr. Appl. Phys. 2009, 9, S91–S94. [Google Scholar] [CrossRef]
- Hurtado, M.J.; Capitán, M.J.; Alvarez, J.; Fatás, E.; Herrasti, P. The anodic oxidation of aluminum: Fabrication and characterization. Portugaliae Electrochim. Acta 2007, 25, 153–162. [Google Scholar] [CrossRef]
- Ingham, C.J.; ter Maat, J.; de Vos, W.M. Where bio meets nano: The many uses for nanoporous aluminum oxide in biotechnology. Biotechol. Adv. 2012, 30, 1089–1099. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in nano-engineered anodic aluminum oxide membrane development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef]
- Stair, P.C.; Marshall, C.; Xiong, G.; Feng, H.; Pellin, M.J.; Elam, J.W.; Curtiss, L.; Iton, L.; Kung, H.; Kung, M.; et al. Novel, uniform nanostructured catalytic membranes. Top. Catal. 2006, 39, 181–186. [Google Scholar] [CrossRef]
- Dotzauer, D.M.; Dai, J.; Sun, L.; Bruening, M.L. Catalytic membranes prepared using layer-by-layer adsorption of polyelectrolyte/metal nanoparticle films in porous supports. Nano Lett. 2006, 6, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Ganley, J.C.; Riechmann, K.L.; Seebauer, E.G.; Masel, R.I. Porous anodic alumina optimized as a catalyst support for microreactors. J. Catal. 2004, 227, 26–32. [Google Scholar] [CrossRef]
- Milka, P.; Krest, I.; Keusgen, M. Immobilization of alliinase on porous aluminum oxide. Biotechnol. Bioeng. 2000, 69, 344–348. [Google Scholar] [CrossRef]
- Itaya, K.; Sugawara, S.; Arai, K.; Saito, S. Properties of porous anodic aluminum oxide films as membranes. J. Chem. Eng. Jpn. 1984, 17, 514–520. [Google Scholar] [CrossRef]
- Thormann, A.; Teuscher, N.; Pfannmçller, M.; Rothe, U.; Heilmann, A. Nanoporous aluminum oxide membranes for filtration and biofunctionalization. Small 2007, 3, 1032–1040. [Google Scholar] [CrossRef]
- Osmanbeyoglu, H.U.; Hur, T.B.; Kim, H.K. Thin alumina nanoporous membranes for similar size biomolecule separation. J. Membr. Sci. 2009, 343, 1–6. [Google Scholar] [CrossRef]
- Sulka, G.D.; Stepniowski, W.J. Structural features of self-organized nanopore arrays formed by anodization of aluminum in oxalic acid at relatively high temperatures. Electrochim. Acta 2009, 54, 3683–3691. [Google Scholar] [CrossRef]
- Hodgson, D.E.; Wu, M.H.; Biermann, R.J. ASM Handbook, Volume 2, Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Novelty, OH, USA, 1990; pp. 1–1328. [Google Scholar]
- Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Selección de pretratamientos superficiales para la síntesis de recubrimientos anódicos nanoestructurados de Al 1050. In 6° Encuentro de Jóvenes Investigadores en Ciencia y Tecnología de Materiales—JIM 2017, Buenos Aires, Argentina, 17–18 August 2017; Argentinian Association of Crystallography: Buenos Aires, Argentina, 2017; pp. 547–550. (In Spanish) [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Vojkuvka, L.; Marsal, L.F.; Ferré-Borrull, J.; Formentin, P.; Pallarés, J. Self-ordered porous alumina membranes with large lattice constant fabricated by hard anodization. Superlattices Microstruct. 2008, 44, 577–582. [Google Scholar] [CrossRef]
- Sulka, G.D.; Parkola, K.G. Anodising potential influence on well-ordered nanostructures formed by anodisation of aluminium in sulphuric acid. Thin Solid Films 2006, 515, 338–345. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, K.M.; Ahmed, N.M.; Hashim, M.R.; Elfadill, N.G.; Maryam, W.; Ahmad, M.A.; Bououdina, M. Effects of the voltage and time of anodization on modulation of the pore dimensions of AAO films for nanomaterials synthesis. Superlattices Microstruct. 2015, 88, 489–500. [Google Scholar] [CrossRef]
- Cheng, C.; Ng, K.Y.; Ngan, A.H.W. Quantitative characterization of acid concentration and temperature dependent self-ordering conditions of anodic porous alumina. AIP Adv. 2011, 1, 042113. [Google Scholar] [CrossRef]
| Element | Al | Fe | Si | Cu | Zn | Ti | V | Mg | Mn |
|---|---|---|---|---|---|---|---|---|---|
| Min. | 99.50 | 0.12 | 0.07 | – | – | – | – | – | – |
| Max. | – | 0.30 | 0.20 | 0.051 | 0.05 | 0.03 | 0.05 | 0.05 | 0.05 |
| Sample | C (M) | T (°C) | V (V) | S | (pores/cm2) | (μm) | ||
|---|---|---|---|---|---|---|---|---|
| O0.3 T20 V30 | 0.3 | 20 | 30 | A | 20.8 ± 5.6 | 58.9 ± 6.0 | 2.7 × 1010 ± 2.0 × 109 | 14.6 ± 1.2 |
| O0.3 T20 V40 | 0.3 | 20 | 40 | B | 35.2 ± 8.2 | 90.9 ± 12.8 | 1.7 × 1010 ± 8.4 × 108 | 18.7 ± 1.6 |
| O0.3 T20 V60 | 0.3 | 20 | 60 | B | 37.5 ± 3.1 | 116.0 ± 14.5 | 1.0 × 1010 ± 8.9 × 108 | 80.3 ± 0.8 |
| O0.3 T30 V30 | 0.3 | 30 | 30 | A | 28.5 ± 7.3 | 59.2 ± 11.8 | 2.6 × 1010 ± 7.1 × 108 | 21.3 ± 0.9 |
| O0.3 T30 V40 | 0.3 | 30 | 40 | B | 34.1 ± 6.4 | 88.2 ± 9.2 | 1.5 × 1010 ± 1.6 × 108 | 30.3 ± 2.8 |
| O0.3 T30 V60 | 0.3 | 30 | 60 | C | 80.5 ± 7.3 | 115.1 ± 19.7 | 8.6 × 109 ± 2.5 × 108 | 144.6 ± 7.5 |
| O0.3 T40 V30 | 0.3 | 40 | 30 | A | 35.9 ± 7.9 | 73.6 ± 10.0 | 2.6 × 1010 ± 1.0 × 109 | 28.4 ± 0.8 |
| O0.3 T40 V40 | 0.3 | 40 | 40 | C | 64.0 ± 4.2 | 91.3 ± 7.5 | 1.3 × 1010 ± 4.3 × 108 | 75.5 ± 1.0 |
| O0.3 T40 V60 | 0.3 | 40 | 60 | C | 97.4 ± 27.6 | 138 ± 20.1 | 5.4 × 109 ± 7.2 × 108 | 124.2 ± 2.8 |
| O0.9 T20 V30 | 0.9 | 20 | 30 | A | 24.1 ± 5.7 | 63.4 ± 13.8 | 3.0 × 1010 ± 1.5 × 109 | 19.7 ± 0.6 |
| O0.9 T20 V40 | 0.9 | 20 | 40 | A | 21.1 ± 6.1 | 62.7 ± 17.8 | 2.8 × 1010 ± 2.3 × 108 | 38.2 ± 1.0 |
| O0.9 T30 V30 | 0.9 | 30 | 30 | A | 23.4 ± 6.3 | 61.7 ± 11.9 | 2.6 × 1010 ± 2.0 × 108 | 34.4 ± 1.4 |
| O0.9 T30 V40 | 0.9 | 30 | 40 | B | 26.9 ± 5.0 | 61.5 ± 10.7 | 2.6 × 1010 ± 4.8 × 108 | 77.8 ± 0.7 |
| O0.9 T40 V30 | 0.9 | 40 | 30 | C | 44.6 ± 6.4 | 62.8 ± 7.5 | 1.9 × 1010 ± 9.1 × 108 | 66.0 ± 2.0 |
| O0.9 T40 V40 | 0.9 | 40 | 40 | C | 63.8 ± 7.8 | 88.3 ± 9.3 | 1.4 × 1010 ± 6.8 × 108 | 80.2 ± 3.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Synthesis and Morphological Characterization of Nanoporous Aluminum Oxide Films by Using a Single Anodization Step. Coatings 2019, 9, 115. https://doi.org/10.3390/coatings9020115
Bruera FA, Kramer GR, Vera ML, Ares AE. Synthesis and Morphological Characterization of Nanoporous Aluminum Oxide Films by Using a Single Anodization Step. Coatings. 2019; 9(2):115. https://doi.org/10.3390/coatings9020115
Chicago/Turabian StyleBruera, Florencia Alejandra, Gustavo Raúl Kramer, María Laura Vera, and Alicia Esther Ares. 2019. "Synthesis and Morphological Characterization of Nanoporous Aluminum Oxide Films by Using a Single Anodization Step" Coatings 9, no. 2: 115. https://doi.org/10.3390/coatings9020115
APA StyleBruera, F. A., Kramer, G. R., Vera, M. L., & Ares, A. E. (2019). Synthesis and Morphological Characterization of Nanoporous Aluminum Oxide Films by Using a Single Anodization Step. Coatings, 9(2), 115. https://doi.org/10.3390/coatings9020115