Corrosion Resistant TiTaN and TiTaAlN Thin Films Grown by Hybrid HiPIMS/DCMS Using Synchronized Pulsed Substrate Bias with No External Substrate Heating
Abstract
1. Introduction
2. Experimental Procedures
2.1. Film Growth
2.2. Film Characterization
2.2.1. Film Compositions
2.2.2. Film Microstructure
2.2.3. Electrochemical Measurements
3. Results and Discussion
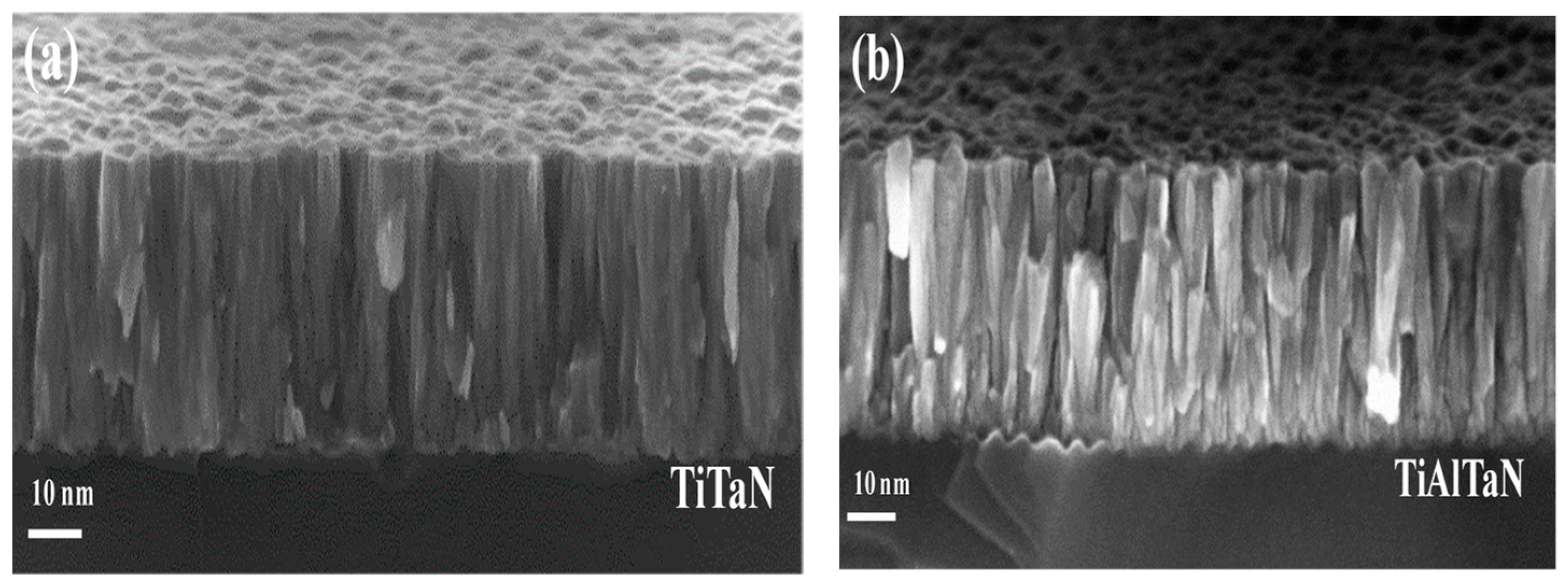
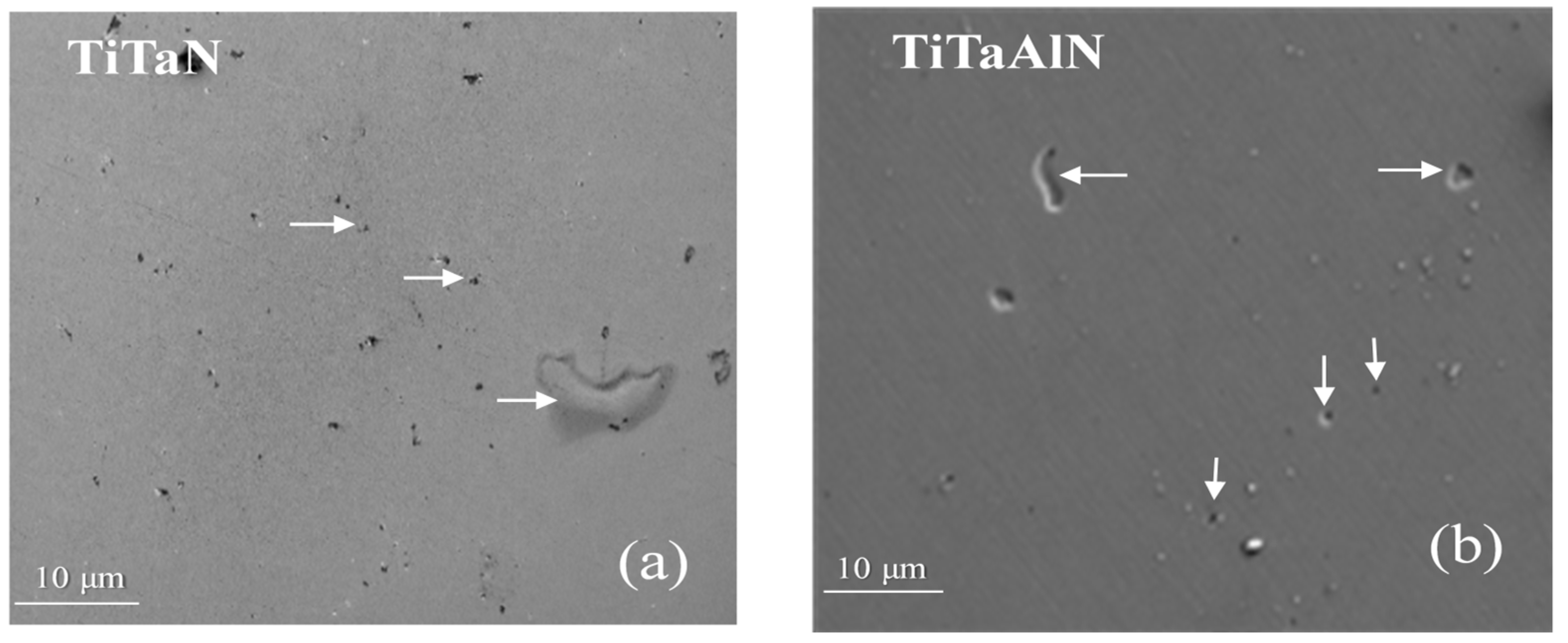
3.1. Film Microstructure
3.2. Electrochemical Behavior
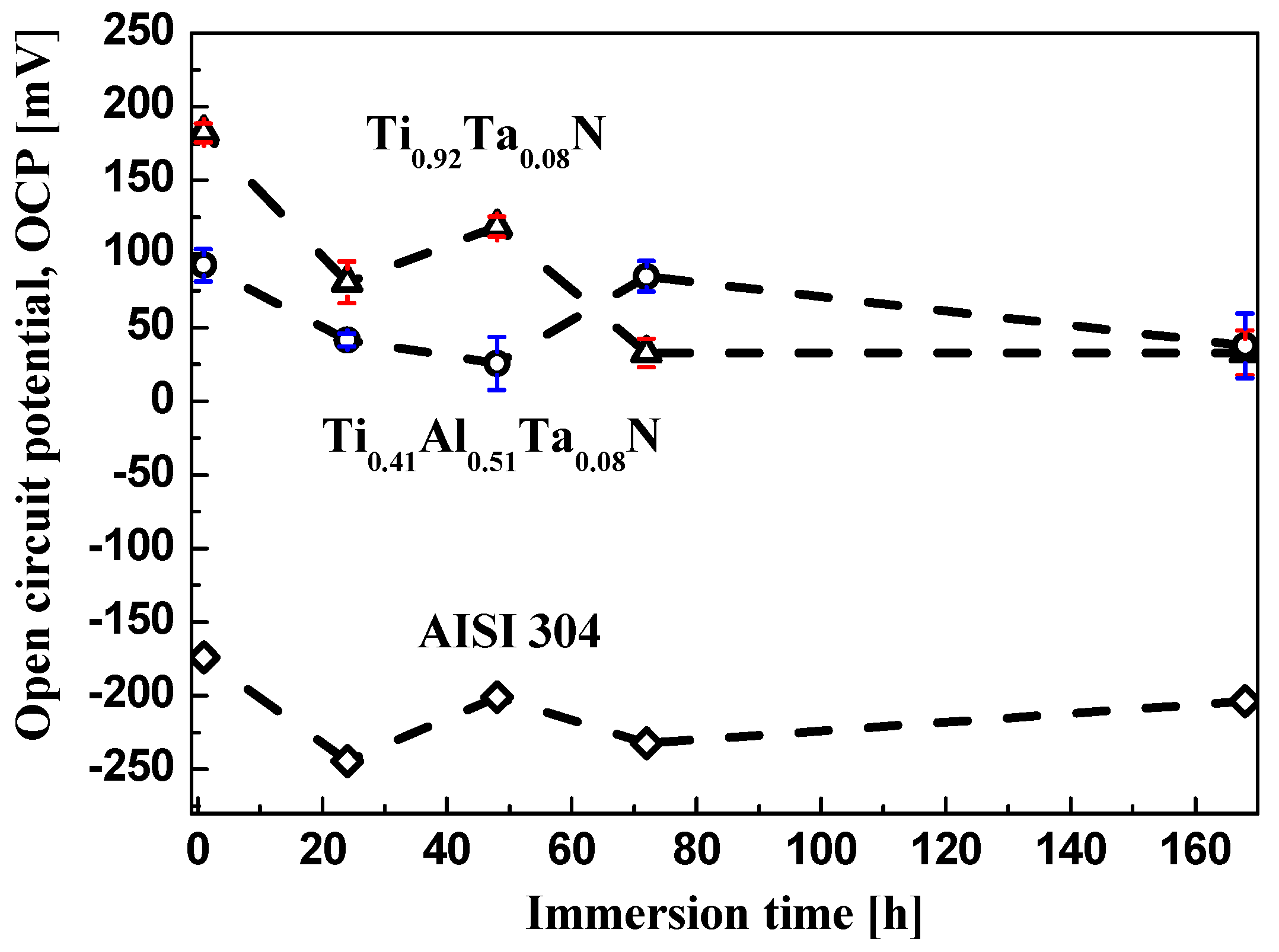
3.2.1. Potentiostatic Corrosion Measurements
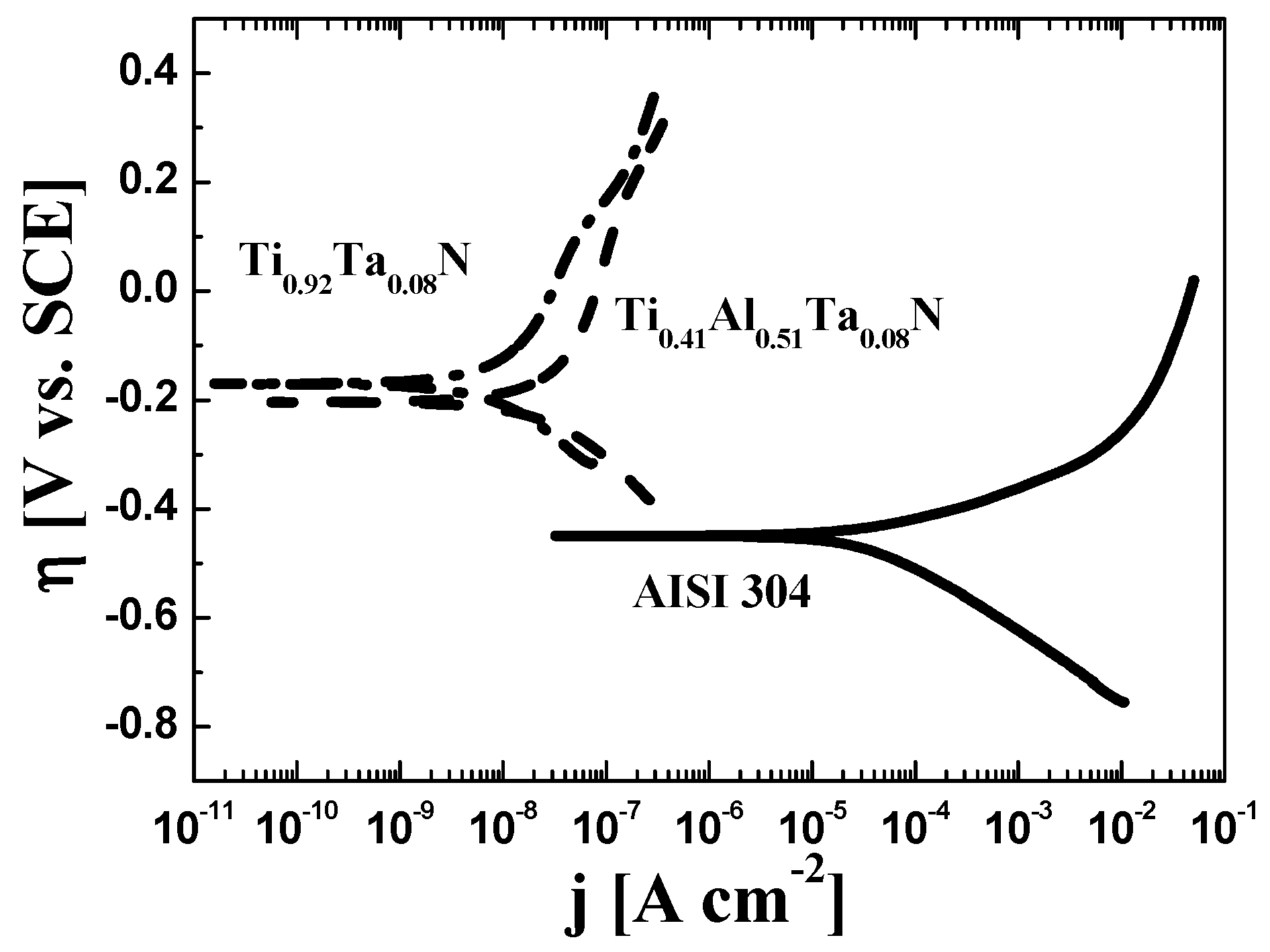
3.2.2. Potentiodynamic Corrosion Measurements
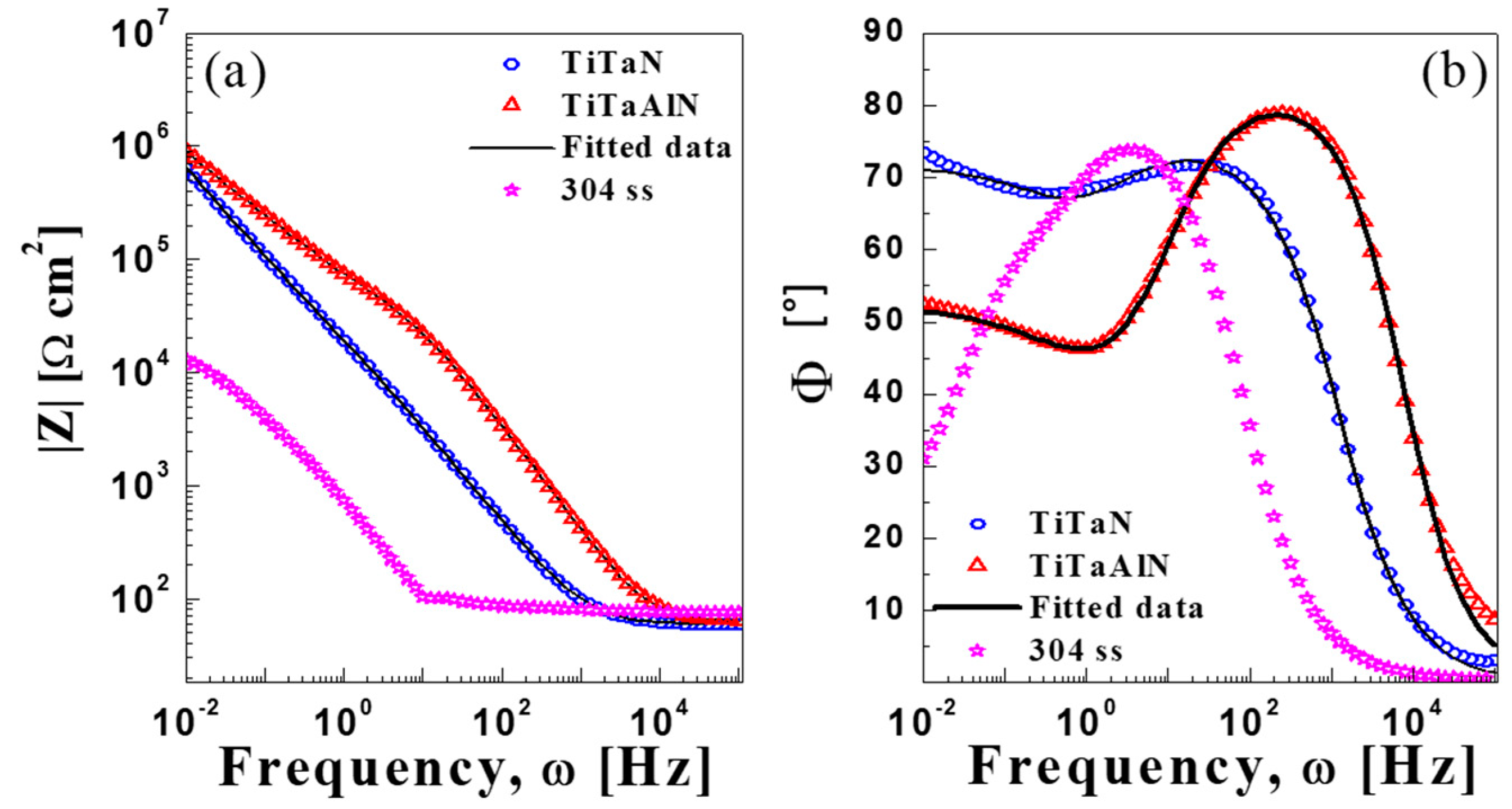
3.2.3. Impedance Measurements
3.3. XPS Depth Profiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Helmersson, U.; Todorova, S.; Barnett, S.A.; Sundgren, J.-E.; Markert, L.C.; Greene, J.E. Growth of single-crystal TiN/VN strained-layer superlattice with extremely high mechanical hardness. J. Appl. Phys. 1987, 62, 481. [Google Scholar] [CrossRef]
- Sundgren, J.-E.; Birch, J.; Håkansson, G.; Hultman, L.; Helmersson, U. Growth, structural characterization and properties of hard and wear-protective layered materials. Thin Solid Films 1990, 193, 818–831. [Google Scholar] [CrossRef]
- Shin, C.-S.; Gall, D.; Hellgren, N.; Patscheider, J.; Petrov, I.; Greene, J.E. Vacancy hardening in single-crystal TiNx(001) layers. J. Appl. Phys. 2003, 93, 6025. [Google Scholar] [CrossRef]
- Kindlund, H.; Sangiovanni, D.G.; Lu, J.; Jensen, J.; Chirita, V.; Petrov, I.; Greene, J.E.; Hultman, L. Effect of WN content on toughness enhancement in V1−xWxN/MgO(001) thin film. J. Vac. Sci. Technol. 2014, 32, 030603. [Google Scholar] [CrossRef]
- Lee, T.; Ohmori, K.; Shin, C.-S.; Cahill, D.G.; Petrov, I.; Greene, J.E. Elastic constants of single-crystal TiNx(001) (0.67 ≤ x ≤ 1.0) determined as a function of x by picosecond ultrasonic measurements. Phys. Rev. B 2005, 71, 144106. [Google Scholar] [CrossRef]
- Hedenqvist, P.; Bromark, M.; Olsson, M.; Hogmark, S.; Bergmann, E. Mechacanical and tribological characterization of low-temperature deposited by PVD TiN coatings. Surf. Coat. Technol. 1994, 63, 115–122. [Google Scholar] [CrossRef]
- Polcar, T.; Kubart, T.; Novák, R.; Kopecký, L.; Široký, P. Comparison of tribological behavior of TiN, TiCN and CrN at elevated temperatures. Surf. Coat. Technol. 2005, 193, 192–199. [Google Scholar] [CrossRef]
- McIntyre, D.; Greene, J.E.; Håkansson, G.; Sundgren, J.-E.; Münz, W.-J. Oxidation of metastable single-phase polycrystalline Ti0.5Al0.5N films: Kinetics and mechanisms. Appl. Phys. 1990, 67, 1542. [Google Scholar] [CrossRef]
- Sánchez-López, J.C.; Martínez-Martínez, D.; López-Cartes, C.; Fernández, A.; Brizuela, M.; García-Luis, A.; Oñate, J.I. Mechanical behavior and oxidation resistance of Cr(Al)N coatings. J. Vac. Sci. Technol. 2005, 23, 681. [Google Scholar] [CrossRef]
- Donohue, L.A.; Smith, I.J.; Münz, W.-D.; Petrov, I.; Greene, J.E. Microstructure and oxidation-resistance of Ti1−x−y−zAlxCryYzN layers grown by combined steered-arc/unbalanced-magnetron-sputter deposition. Surf. Coat. Technol. 1997, 94, 226–231. [Google Scholar] [CrossRef]
- Mei, A.B.; Tuteja, M.; Sangiovanni, D.G.; Haasch, R.T.; Rockett, A.; Hultman, L.; Petrov, I.; Greene, J.E. Growth, nanostructure, and optical properties of epitaxial VNx/MgO (001) (0.80 ≤ x ≤ 1.00) layers deposited by reactive magnetron sputtering. J. Mater. Chem. C 2016, 4, 7924. [Google Scholar] [CrossRef]
- Gall, D.; Petrov, I.; Greene, J.E. Epitaxial Sc1−xTixN(001): Optical and electronic transport properties. J. Appl. Phys. 2001, 89, 401. [Google Scholar] [CrossRef]
- Mei, A.B.; Rockett, A.; Hultman, L.; Petrov, I.; Greene, J.E. Electron/phonon coupling in group-IV transition-metal and rare-earth nitride. J. Appl. Phys. 2013, 114, 193708. [Google Scholar] [CrossRef]
- Mei, A.B.; Howe, B.M.; Zhang, C.; Sardela, M.; Eckstein, J.N.; Hultman, L.; Rockett, A.; Petrov, I.; Greene, J.E. Physical properties of epitaxial ZrN/MgO(001) layers grown by reactive magnetron sputtering. J. Vac. Sci. Technol. A 2013, 31, 061516. [Google Scholar] [CrossRef]
- Dubey, P.; Srivastava, S.; Chandra, R.; Ramana, C.V. Toughness enhancement in zirconium-tungsten-nitride nanocrystalline hard coatings. AIP Adv. 2016, 6, 075211. [Google Scholar] [CrossRef]
- Kindlund, H.; Sangiovanni, D.G.; Martínez-de-Olcoz, L.; Lu, J.; Jensen, J.; Birch, J.; Petrov, I.; Greene, J.E.; Chirita, V.; Hultman, L. Toughness enhancement in hard ceramic thin films by alloy desing. APL Mater. 2013, 1, 042104. [Google Scholar] [CrossRef]
- Niyomsoan, S.; Grant, W.; Olson, D.L.; Mishra, B. Variation of color in titanium and zirconium nitride decorative thin films. Thin Solid Films 2002, 415, 187–194. [Google Scholar] [CrossRef]
- Zheng, Q.; Mei, A.B.; Tuteja, M.; Sangiovanni, D.G.; Hultman, L.; Petrov, I.; Greene, J.E.; Cahill, D.G. Phono and electron contributions to the thermal conductivity of v Nx epitaxial layers. Phys. Rev. Mater. 2017, 1, 065002. [Google Scholar] [CrossRef]
- Chun, J.-S.; Petrov, I.; Greene, J.E. Dense fully 111-textured TiN diffusion barriers: Enhanced lifetime through microstructure control during layer growth. J. Appl. Phys. 1999, 86, 3633. [Google Scholar] [CrossRef]
- Araujo, R.A.; Zhang, X.; Wang, H. Epitaxial cubic HfN diffusion barriers deposited on Si (001) by using a TiN buffer laeyer. J. Vac. Sci. Technol. B 2008, 26, 1871. [Google Scholar] [CrossRef]
- Min, K.-H.; Chun, K.-C.; Kim, K.-B. Comparative study of tantalum and tantalum nitrides (Ta2N and TaN) as a diffusion barrier for Cu. J. Vac. Sci. Technol. B 1996, 14, 3263. [Google Scholar] [CrossRef]
- Becker, J.S.; Gordon, R.G. Diffusion barrier properties of tungsten nitride films grown by atomic layer deposition from bis(tertbutylimido)bis(dimethylamido)tungsten and ammonia. Appl. Phys. Lett. 2003, 82, 2239. [Google Scholar] [CrossRef]
- Kim, H.; Detavenier, C.; van der Straten, O.; Rossnagel, S.M.; Kellock, A.J.; Park, D.-G. Robust TaNx diffusion barrier for Cu-interconnect technology with subnanometer thickness by metal-organic plasma-enhanced atomic layer deposition. J. Appl. Phys. 2005, 98, 014308. [Google Scholar] [CrossRef]
- Mühlbacher, M.; Greczynski, G.; Sartory, B.; Schalk, N.; Lu, J.; Petrov, I.; Greene, J.E.; Hultman, L.; Mitterer, C. Enhanced Ti0.84Ta0.16N diffusion barriers, grown by a hybrid sputtering technique with no substrate heating, between Si(001) wafers and Cu overlayers. Sci. Rep. 2018, 8, 5360. [Google Scholar] [CrossRef]
- McIntyre, D.; Greene, J.E.; Håkansson, G.; Sundgren, J.-E.; Münz, W.-D. Oxidation of metastable single-phase polycrystalline Ti0.5Al0.5N films: Kinetics and mechanisms. J. Appl. Phys. 1990, 67, 1542. [Google Scholar] [CrossRef]
- Greene, J.E. Review article: Tracing the recorded history of thin-film sputter deposition: From the 1800s to 2017. J.Vac. Sci. Technol. A 2017, 35, 05C204. [Google Scholar] [CrossRef]
- Lee, T.; Seo, H.; Hwang, H.; Howe, B.; Kodambaka, S.; Greene, J.E.; Petrov, I. Fully strained low-temperature epitaxy of TiN/MgO(001) layers using high-flux, low-energy ion irradiation during reactive magnetron sputter deposition. Thin Solid Films 2010, 518, 5169–5172. [Google Scholar] [CrossRef]
- Hakansson, G.; Sundgren, J.-E.; McIntyre, D.; Greene, J.E.; Munz, W.-D. Microstructure and physical properties of polycrystalline metastable Ti0.5Al0.5N alloys grown by magnetron sputter deposition. Thin Solid Films 1987, 153, 55–65. [Google Scholar] [CrossRef]
- Greene, J.E. Nucleation, Growth, and Microstructure Evolution in Films Grown by Physical Vapor Deposition. In Deposition Technologies for Films and Coatings; Bunshah, R.F., Ed.; Applied Science Publisher: Westwood, NJ, USA, 1994; p. 681. [Google Scholar]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, S117. [Google Scholar] [CrossRef]
- Greene, J.E. Thin Film Nucleation, Growth: Microstructural Evolution: An Atomic Scale View. In Handbook of Deposition Technologies for Thin Films and Coatings, 3rd ed.; Martin, P., Ed.; Applied Science Publisher: Burlington, MA, USA, 2010. [Google Scholar]
- Dubey, P.; Martinez, G.; Srivastava, S.; Chandra, R.; Ramana, C.V. Effect of bias induced microstructure on the mechanical properties of nanocrystalline zirconium tungsten nitride coatings. Surf. Coat. Technol. 2017, 313, 121–128. [Google Scholar] [CrossRef]
- Oettel, H.; Wiedemann, R.; Preisler, S. Residual stresses in nitride hard coatings prepared by magnetron sputtering and arc evaporation. Surf. Coat. Technol. 1995, 74, 273–278. [Google Scholar] [CrossRef]
- Falub, C.V.; Karimi, A.; Ante, M.; Kalss, W. Interdependence between stress and texture in arc evaporated Ti–Al–N. thin films. Surf. Coat. Technol. 2007, 201, 5891–5898. [Google Scholar] [CrossRef]
- Hörling, A.; Hultman, L.; Odén, M.; Sjölén, J.; Karlsson, L. Thermal stability of arc evaporated high aluminum-content Ti1−xAlxNTi1−xAlxN thin films. J. Vac. Sci. Technol. A 2002, 20, 1815. [Google Scholar] [CrossRef]
- Thornton, J.A. The microstructure of sputter-deposited coating. J. Vac. Sci. Technol. A 1986, 4, 3059. [Google Scholar] [CrossRef]
- Harper, J.M.E.; Cuomo, J.J.; Gambino, R.J. Nuclear Instruments and Methods in Physics Research. In Ion Bombardment Modification of Surfaces: Fundamentals and Applications; Auciello, O., Kelly, R., Eds.; Elsevier: Amsterdam, Switzerland, 1984. [Google Scholar]
- Winters, H.F.; Kay, E. Gas incorporation into sputtered films. J. Appl. Phys. 1967, 38, 3928. [Google Scholar] [CrossRef]
- Thornton, J.A.; Tabock, J.; Hoffman, D.W. Internal stresses in metallic films deposited by cylindrical magnetron sputtering. Thin Solid Films 1979, 64, 111. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Bolz, S.; Kölker, W.; Schiffers, C.; Lemmer, O.; Petrov, I.; Greene, J.E.; Hultman, L. Novel strategy for low-temperature, high-rate growth of dense, hard, and stress-free refractory ceramic thin films. J. Vac. Sci. Technol. A 2014, 32, 41515. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Johansson, M.P.; Jensen, J.; Petrov, I.; Greene, J.E.; Hultman, L. Role of Tin+ and Aln+ ion irradiation (n = 1, 2) during Ti1−xAlxN alloy film growth in a hybrid HIPIMS/magnetron mode. Surf. Coat. Technol. 2012, 206, 4202–4211. [Google Scholar] [CrossRef]
- Greczynski, G.; Petrov, I.; Greene, J.E.; Hultman, L. A paradigm shift in thin-film growth by magnetron sputtering: from gas-ion to metal-ion irradiation of the growing film. J. Vac. Sci. Technol. A 2019, 37, 060801. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Petrov, I.; Greene, J.E.; Bolz, S.; Kölker, W.; Schiffers, C.; Lemmer, O.; Hultman, L. Metal versus rare-gas ion irradiation during Ti1−xAlxN film growth by hybrid high power pulsed magnetron/dc magnetron co-sputtering using synchronized pulsed substrate bias. J. Vac. Sci. Technol. A 2012, 30, 061504. [Google Scholar] [CrossRef]
- Fager, H.; Tengstrand, O.; Lu, J.; Bolz, S.; Mesic, B.; Kölker, W.; Schiffers, C.; Lemmer, O.; Greene, J.E.; Hultman, L.; et al. Low-temperature growth of dense and hard Ti0.41Al0.51Ta0.08N films via hybrid HIPIMS/DC magnetron co-sputtering with synchronized metal-ion irradiation. J. Appl. Phys. 2017, 121, 171902. [Google Scholar] [CrossRef]
- Chang, C.L.; Shih, S.-G.; Chen, P.-H.; Chen, W.-C.; Ho, C.-T.; Wu, W.-Y. Effect of duty cycles on the deposition and characteristics of high power impulse magnetron sputtering deposited TiN thin films. Surf. Coat. Technol. 2014, 259, 232–237. [Google Scholar] [CrossRef]
- Elmkhah, H.; Attarzadeh, F.; Fattah-Alhosseini, A.; Kim, K.H. Microstructural and electrochemical comparison between TiN coatings deposited through HIPIMS and DCMS techniques. J. Alloys Compd. 2018, 735, 422–429. [Google Scholar] [CrossRef]
- Zhou, D.; Peng, H.; Zhu, L.; Guo, H.; Gong, S. Microstructure, hardness and corrosion behaviour of Ti/TiN multilayer coatings produced by plasma activated EB-PVD. Surf. Coat. Technol. 2014, 258, 102–107. [Google Scholar] [CrossRef]
- Ananthakumar, R.; Subramanian, B.; Kobayashi, A.; Jayachandran, M. Electrochemical corrosion and materials properties of reactively sputtered TiN/TiAlN multilayer coatings. Ceram. Int. 2012, 38, 477–485. [Google Scholar] [CrossRef]
- Jensen, J.; Martin, D.; Surpi, A.; Kubart, T. ERD analysis and modification of TiO2 thin films with heavy ions. Nucl. Instrum. Methods Phys. Res. Sect. B 2010, 268, 1893–1989. [Google Scholar] [CrossRef]
- Janson, M.S. CONTES: Conversion of Time-Energy Spectra—A Program. for ERDA Data Analysis; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Birkholz, M. Thin Film Analysis by X-ray Scattering; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2006. [Google Scholar]
- Tafel, J. Ueber die Polarisation bei Kathodischer Wasserstoffentwicklung. Z. Physik. Chem. 1905, 50, 6661. [Google Scholar]
- GAMRY Instruments, Echem Analyst TM Software. Available online: https://www.gamry.com/assets/Uploads/EchemAnalystSoftwareManual.pdf (accessed on 3 December 2019).
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Fenker, M.; Balzer, M.; Kapps, H. Corrosion protection with hard coatings on steel: Past approaches and current research efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part II.: EIS interpretation of corrosion behavior. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Brooks, A.R.; Clayton, C.R.; Doss, K.; Lu, Y.C. On the role of Cr in the passivity of stainless steel. J. Electrochem. Soc. 1986, 133, 12. [Google Scholar] [CrossRef]
- Cheng, L.; Da, Z.Y.; Guo, Q.L.; Min, Q. Corrosion resistance and hemocompatibility of multilayered Ti/TiN-coated surgical AISI 316L stainless steel. Mater. Lett. 2005, 59, 3813–3819. [Google Scholar] [CrossRef]
- Kirchheim, R.; Heine, B.; Fishmeister, H.; Hofmann, S.; Knote, H.; Stolz, U. The passivity of iron- chromium alloys. Corros. Sci. 1989, 29, 899–917. [Google Scholar] [CrossRef]
- Liu, C.; Leyand, A.; Bi, Q.; Matthews, A. Corrosion resistance of multi-layered plasma-assisted physical vapour deposition TiN and CrN coatings. Surf. Coat. Technol. 2001, 141, 164–173. [Google Scholar] [CrossRef]
- Jung, R.-H.; Tsuchiya, H.; Fujimoto, S. XPS characterization of passive films formed on Type 304 stainless steel in humid atmosphere. Corros. Sci. 2012, 58, 62–68. [Google Scholar] [CrossRef]
- Nie, X.; Meletis, E.I.; Jiang, J.C.; Leyland, A.; Yerokhin, A.L.; Matthews, A. Abrasive wear/corrosion properties and TEM analysis of Al2O3 coatings fabricated using plasma electrolysis. Surf. Coat. Technol. 2002, 149, 245–251. [Google Scholar] [CrossRef]
- Fasuba, O.A.; Yerokhin, A.; Matthews, A.; Leyland, A. Corrosion behaviour and galvanic coupling with steel of Al-based coating alternatives to electroplated cadmium. Mater. Chem. Phys. 2013, 141, 128–137. [Google Scholar] [CrossRef]
- Nam, N.D.; Vaka, M.; Hung, N.T. Corrosion behavior of TiN, TiAlN, TiAlSiN-coated 316L stainless steel in simulated proton exchange membrane fuel cell environment. J. Power Sources 2014, 268, 240–245. [Google Scholar] [CrossRef]
- Nam, N.D.; Jo, D.S.; Kim, J.G.; Yoon, D.H. Corrosion protection of CrN/TiN multi-coating for bipolar plate of polymer electrolyte membrane fuel cell. Thin Solid Films 2011, 519, 6787–6791. [Google Scholar] [CrossRef]
- Ghasemi, S.; Shanaghi, A.; Chu, P.K. Corrosion behavior of reactive sputtered Ti/TiN nanostructured coating and effects of intermediate titanium layer on self-healing properties. Surf. Coat. Technol. 2017, 326, 156–164. [Google Scholar] [CrossRef]
- Liu, C.; Leyand, A.; Lyon, S.; Matthews, A. Electrochemical impedance spectroscopy of PVD-TiN coatings on mild steel and AISI316 substrates. Surf. Coat. Technol. 1995, 76, 615–631. [Google Scholar] [CrossRef]
- Yi, P.; Zhu, L.; Dong, C.; Xiao, K. Corrosion and interfacial contact resistance of 316L stainless steel coated with magnetron sputtered ZrN and TiN in the simulated cathodic environment of a proton-exchange membrane fuel cell. Surf. Coat. Technol. 2019, 363, 198–202. [Google Scholar] [CrossRef]
- Er, D.; Azar, G.T.P.; Kazmanli, K.; Ürgen, M. The corrosion protection ability of TiAlN coatings produced with CA-PVD under superimposed pulse bias. Surf. Coat. Technol. 2018, 346, 1–8. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Cai, F.; Yang, Y.; Wang, Q.; Zhang, S. Characterization and corrosion behaviors of TiN/TiAlN multilayer coatings by ion source enhanced hybrid arc ion plating. Surf. Coat. Technol. 2019, 366, 355–365. [Google Scholar] [CrossRef]
- Kendig, M.; Mansfeld, F.; Tsai, S. Determination of the long term corrosion behavior of coated steel with A.C. impedance measurements. Corros. Sci. 1983, 23, 317–329. [Google Scholar] [CrossRef]
- Kendig, M.W.; Meyer, E.M.; Linberg, G.; Mansfeld, F. A computer analysis of electrochemical impedance data. Corrs. Sci. 1983, 23, 1007–1015. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. EIS comparison on corrosion performance of PVD TiN and CrN coated mild steel in 0.5 N NaCl aqueous solution. Corros. Sci. 2001, 43, 1953–1961. [Google Scholar] [CrossRef]
- Grips, V.K.W.; Barshilia, H.C.; Selvi, V.S.; Rajam, K.S. Effect of electroless nickel interlayer on the electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive dc magnetron sputtering. Electrochem. Act. 2006, 51, 3461–3468. [Google Scholar]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part, I.: EIS interpretation of corrosion behavior. Corros. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]


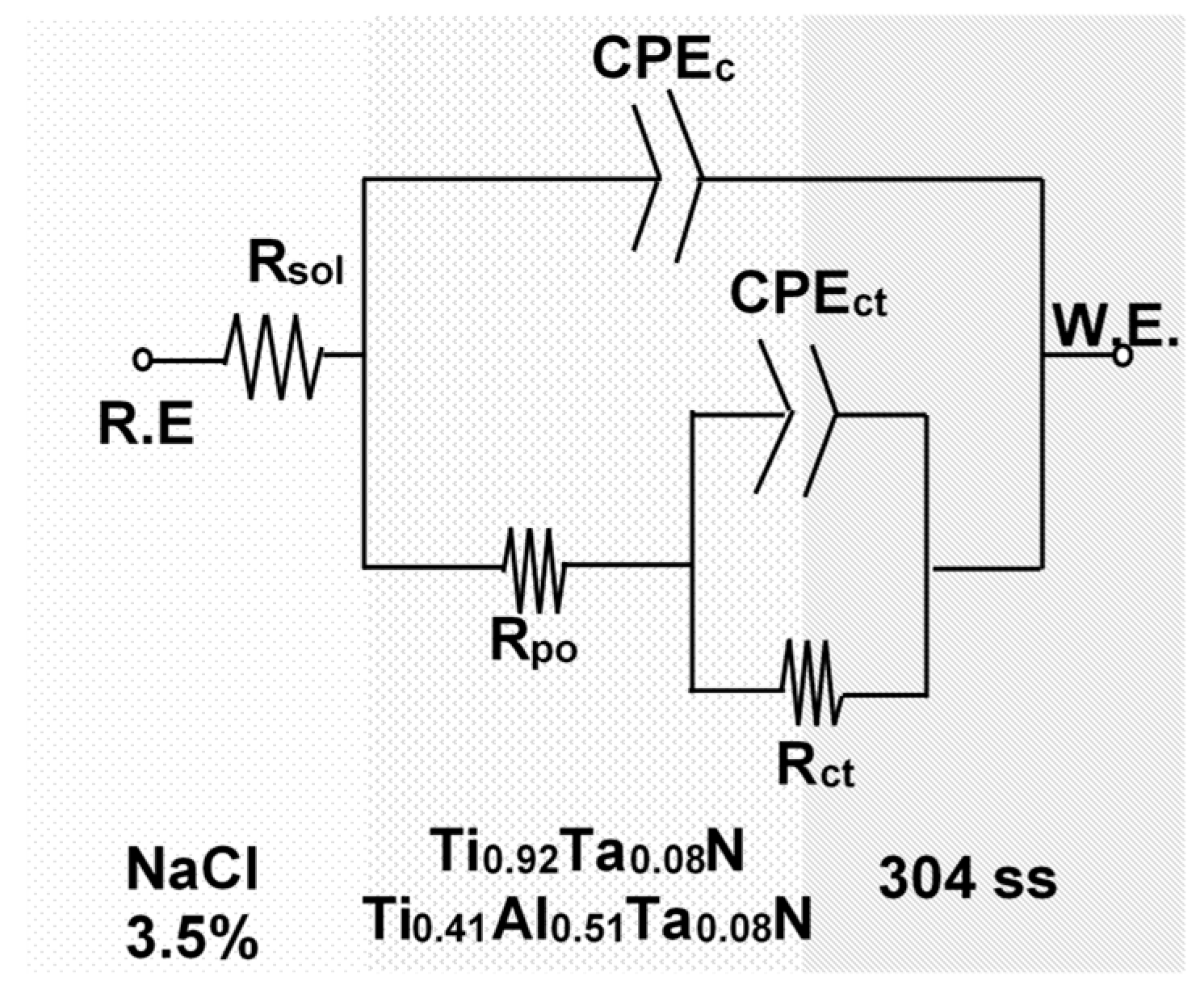

| Samples | jcorr [A·cm−2] | Ecorr [V] | ba [mV dec−1] | bc [mV dec−1] | Rp [kΩ cm−2] | Pe [%] |
|---|---|---|---|---|---|---|
| Ti0.92Ta0.08N | 1.88 × 10−8 | −1.70 × 10−1 | 0.647 | 0.262 | 2.28 × 104 | 99.95 |
| Ti0.41Al0.51Ta0.08N | 5.40 × 10−8 | −2.04 × 10−1 | 1.002 | 0.242 | 8.30 × 103 | 99.84 |
| 304 stainless steel | 3.60 × 10−5 | −4.50 × 10−1 | 0.061 | 0.112 | 2.47 | - |
| Ti0.92Ta0.08N/304 ss | |||||||
|---|---|---|---|---|---|---|---|
| Time [h] | Re [Ω·cm2] | Rct [Ω·cm2] | Rpo [Ω·cm2] | CPEc [S smc/cm2] | mc | CPEdl [S smdl/cm2] | mdl |
| 1 | 61.73 | 2.0 ± 0.31 × 1010 | 3.9 ± 0.8 × 104 | 6.6 ± 0.2 × 10−6 | 8.7 × 10−1 | 5.6 ± 0.2 × 10−6 | 6.1 × 10−1 |
| 24 | 60.68 | 4.3 ± 0.80 × 1010 | 1.9 ± 0.3 × 104 | 1.3 ± 0.05 × 10−5 | 8.2 × 10−1 | 5.2 ± 0.6 × 10−6 | 8.8 × 10−1 |
| 48 | 62.93 | 1.7 ± 0.0002 × 1011 | 1.1 ± 0.1 × 105 | 9.8 ± 0.2 × 10−6 | 8.3 × 10−1 | 7.4 ± 0.4 × 10−6 | 7.7 × 10−1 |
| 72 | 60.41 | 2.7 ± 0.03 × 1010 | 5.8 ± 1.0 × 104 | 7.6 ± 0.2 × 10−6 | 8.5 × 10−1 | 5.4 ± 0.2 × 10−6 | 6.9 × 10−1 |
| 168 | 58.43 | 1.0 ± 0.06 × 1010 | 6.3 ± 1.2 × 104 | 9.5 ± 0.3 × 10−6 | 8.4 × 10−1 | 4.7 ± 0.3 × 10−6 | 7.3 × 10−1 |
| Ti0.41Al0.51Ta0.08N/304 ss | |||||||
| 1 | 60.80 | 3.3 ±0.009 × 1010 | 2.0 ± 0.4 × 104 | 7.1 ± 0.3 × 10−7 | 9.3 × 10−1 | 5.0 ± 0.08 × 10−6 | 4.3 × 10−1 |
| 24 | 61.47 | 1.3 ± 0.08 × 1010 | 2.9 ± 0.3 × 104 | 8.1 ± 0.3 × 10−7 | 9.1 × 10−1 | 4.6 ± 0.07 × 10−6 | 5.3 × 10−1 |
| 48 | 62.29 | 4.0 ± 0.0008 × 1011 | 2.3 ± 0.2 × 104 | 7.8 ± 0.3 × 10−7 | 9.2 × 10−1 | 4.8 ± 0.06 × 10−6 | 5.4 × 10−1 |
| 72 | 64.99 | 9.2 ± 0.0005 × 1010 | 2.7 ± 0.3 × 104 | 9.5 ± 0.4 × 10−7 | 8.9 × 10−1 | 4.8 ± 0.06 × 10−6 | 5.2 × 10−1 |
| 168 | 61.85 | 9.6 ± 4.5 × 1012 | 2.8 ± 0.3 × 104 | 1.1 ± 3.7 × 10−8 | 8.8 × 10−1 | 5.0 ± 0.06 × 10−6 | 5.5 × 10−1 |
| 304 ss | |||||||
| 1 | 75.00 | 2.6 ± 0.01 × 10−1 | 1.0 ± 0.4 × 102 | 8.3 ± 1.3 × 10−5 | 9.3 × 10−1 | 2.8 ± 1.2 × 10−5 | 5.1 × 10−1 |
| 24 | 68.00 | 4.6 ± 0.3 × 100 | 2.2 ± 0.3 × 102 | 1.7 ± 0.3 × 10−4 | 5.1 × 10−1 | 1.7 ± 0.8 × 10−4 | 8.6 × 10−1 |
| 48 | 73.00 | 3.6 ± 0.02 × 10−1 | 1.7 ± 0.5 × 102 | 1.8 ± 0.4 × 10−4 | 5.1 × 10−1 | 1.8 ± 0.7 × 10−4 | 8.9 × 10−1 |
| 72 | 65.00 | 1.6 ± 1.1 × 10−1 | 3.7 ± 0.2 × 102 | 1.6 ± 0.2 × 10−4 | 8.1 × 10−1 | 1.3 ± 0.7 × 10−4 | 5.2 × 10−1 |
| 168 | 73.00 | 1.1 ± 9.2 × 10−1 | 1.7 ± 0.2 × 102 | 1.4 ± 0.4 × 10−4 | 7.0 × 10−1 | 9.0 ± 6.1 × 10−5 | 9.2 × 10−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chipatecua Godoy, Y.; Tengstrand, O.; Olaya Florez, J.; Petrov, I.; Bustos, E.; Hultman, L.; Herrera-Gomez, A.; Greene, J.E.; Greczynski, G. Corrosion Resistant TiTaN and TiTaAlN Thin Films Grown by Hybrid HiPIMS/DCMS Using Synchronized Pulsed Substrate Bias with No External Substrate Heating. Coatings 2019, 9, 841. https://doi.org/10.3390/coatings9120841
Chipatecua Godoy Y, Tengstrand O, Olaya Florez J, Petrov I, Bustos E, Hultman L, Herrera-Gomez A, Greene JE, Greczynski G. Corrosion Resistant TiTaN and TiTaAlN Thin Films Grown by Hybrid HiPIMS/DCMS Using Synchronized Pulsed Substrate Bias with No External Substrate Heating. Coatings. 2019; 9(12):841. https://doi.org/10.3390/coatings9120841
Chicago/Turabian StyleChipatecua Godoy, Yuri, Olof Tengstrand, Jairo Olaya Florez, Ivan Petrov, Erika Bustos, Lars Hultman, Alberto Herrera-Gomez, J.E. Greene, and Grzegorz Greczynski. 2019. "Corrosion Resistant TiTaN and TiTaAlN Thin Films Grown by Hybrid HiPIMS/DCMS Using Synchronized Pulsed Substrate Bias with No External Substrate Heating" Coatings 9, no. 12: 841. https://doi.org/10.3390/coatings9120841
APA StyleChipatecua Godoy, Y., Tengstrand, O., Olaya Florez, J., Petrov, I., Bustos, E., Hultman, L., Herrera-Gomez, A., Greene, J. E., & Greczynski, G. (2019). Corrosion Resistant TiTaN and TiTaAlN Thin Films Grown by Hybrid HiPIMS/DCMS Using Synchronized Pulsed Substrate Bias with No External Substrate Heating. Coatings, 9(12), 841. https://doi.org/10.3390/coatings9120841





