Microstructure and Tribocorrosion Properties of Ni-Based Composite Coatings in Artificial Seawater
Abstract
1. Introduction
2. Materials and Methods
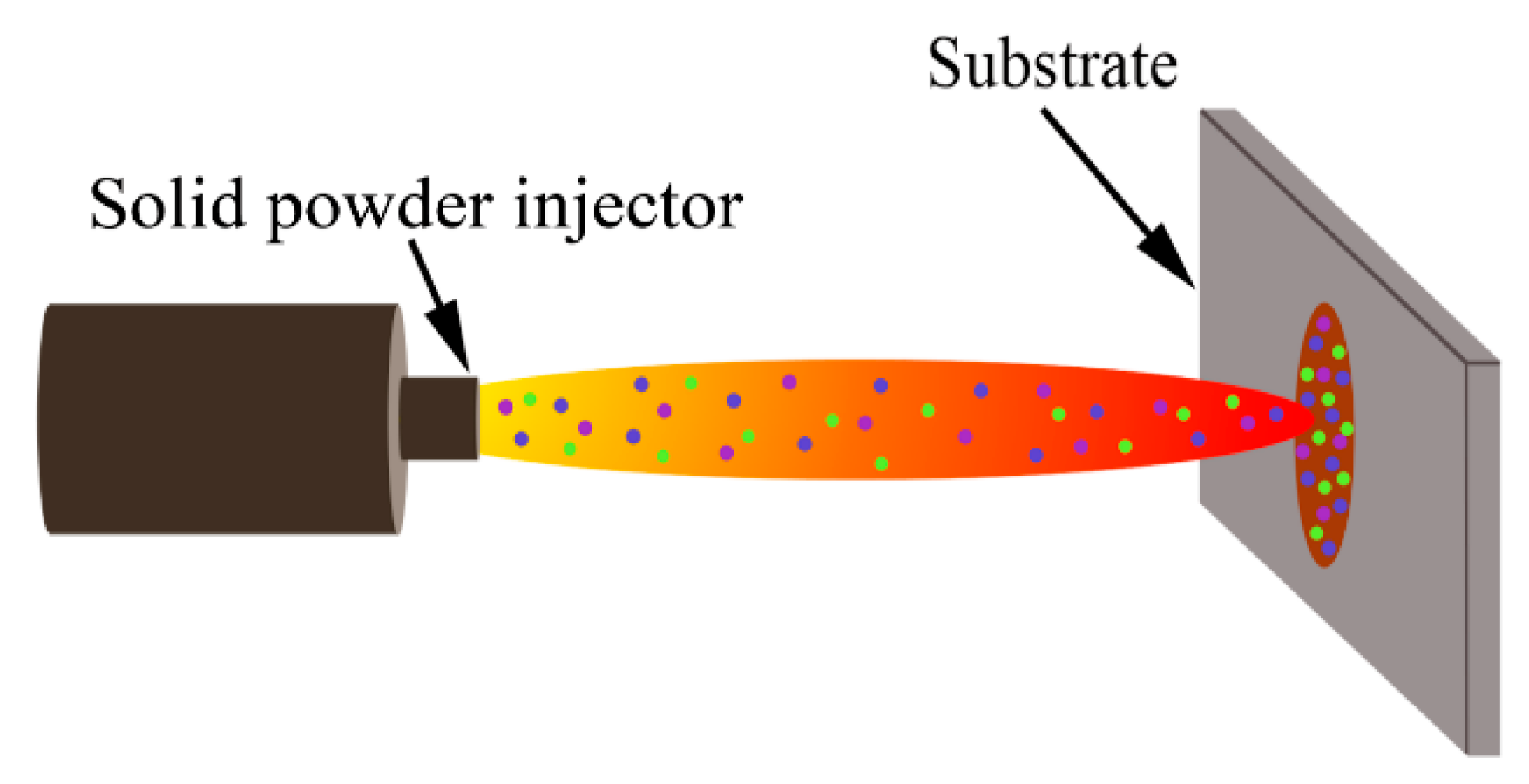
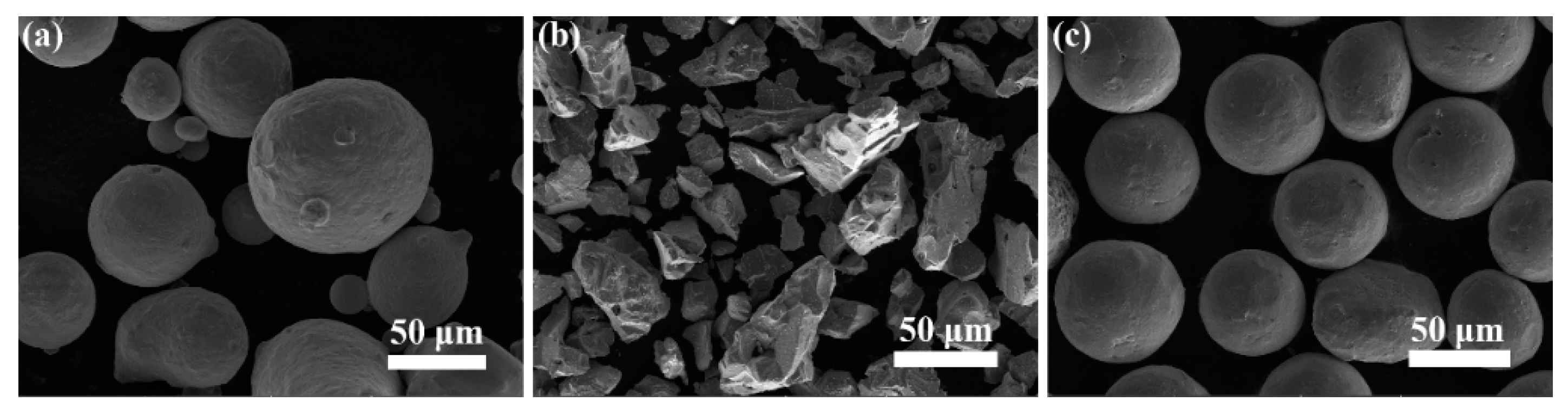
2.1. Material Preparation
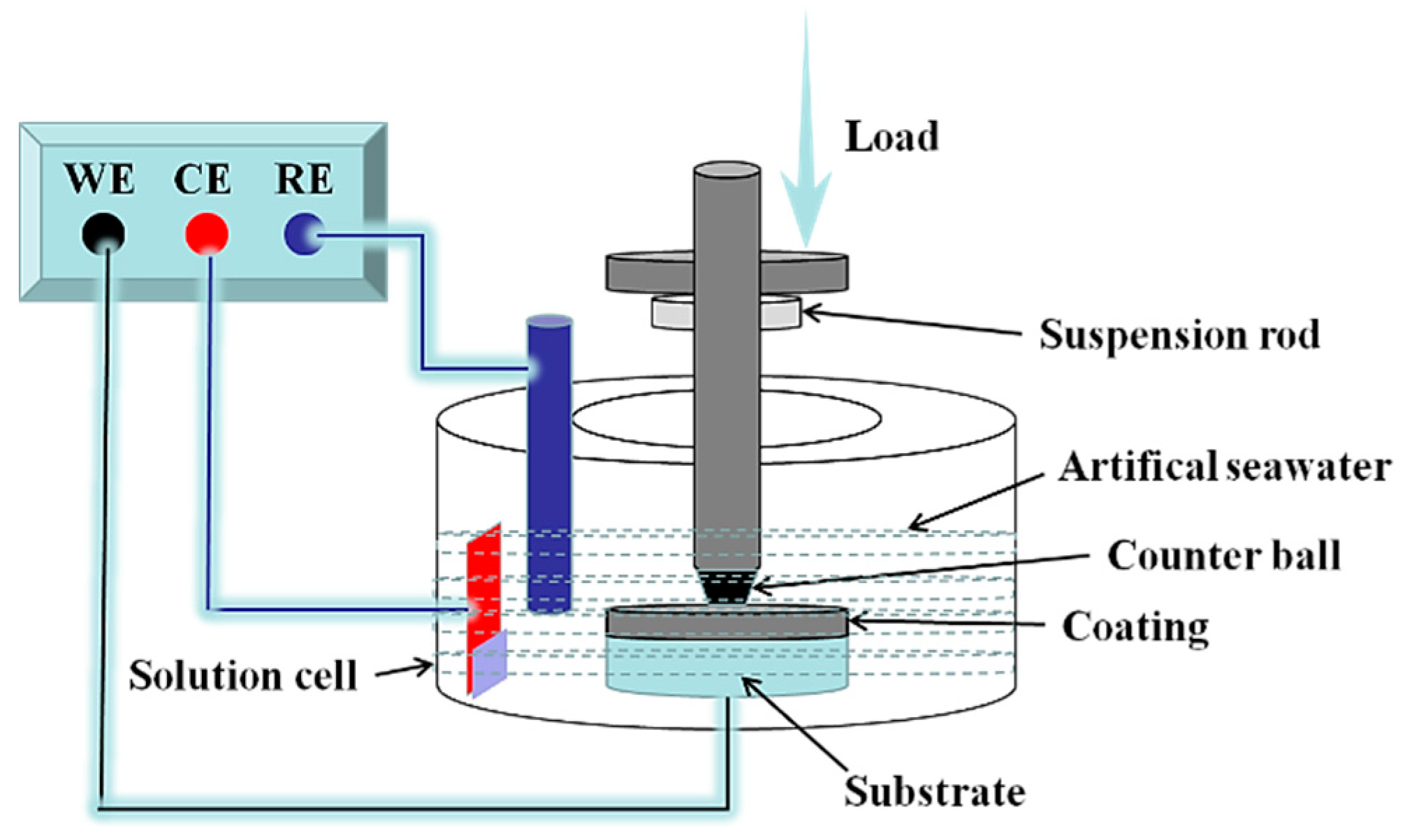
2.2. Tribocorrosion Tests
3. Results and Discussion
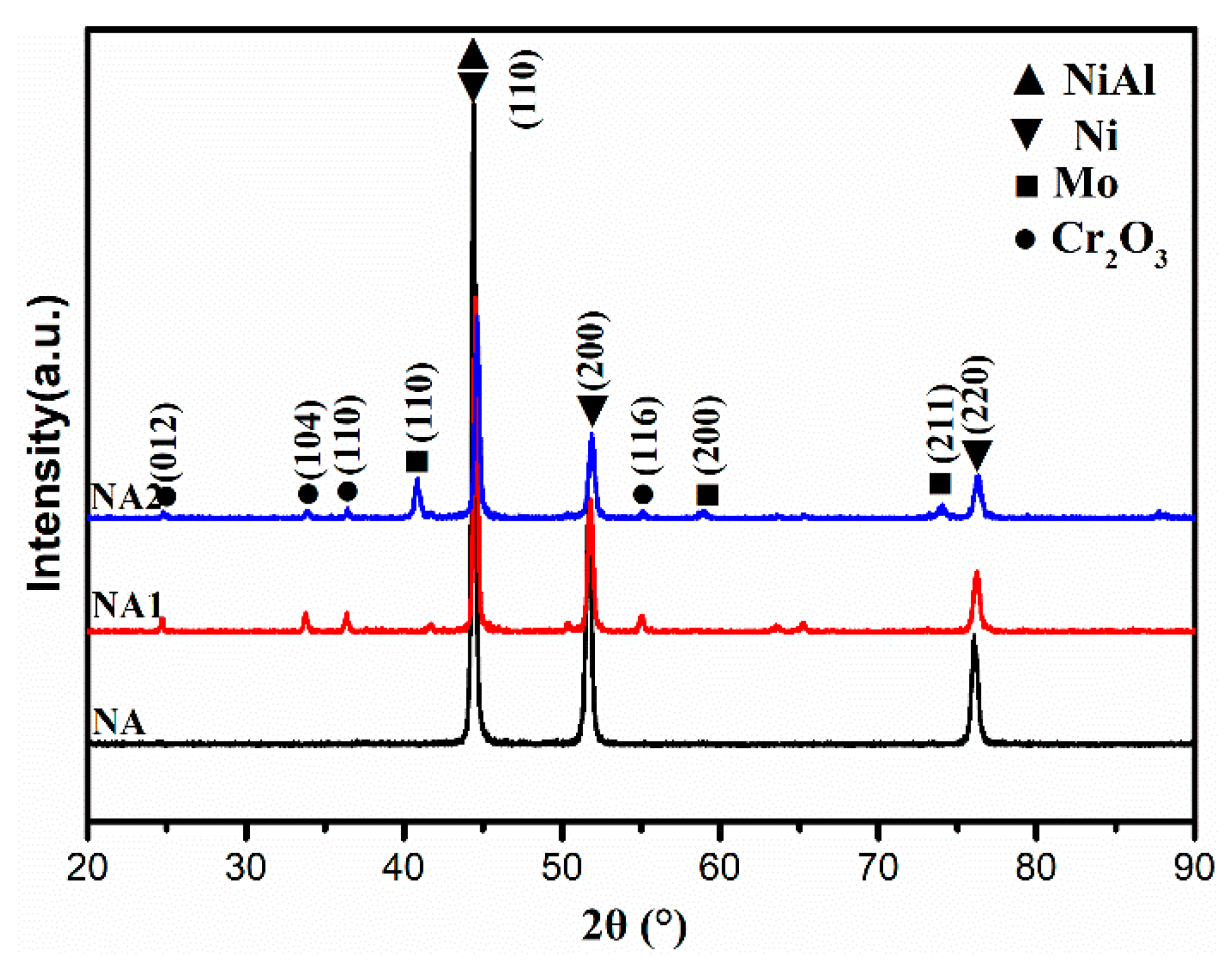
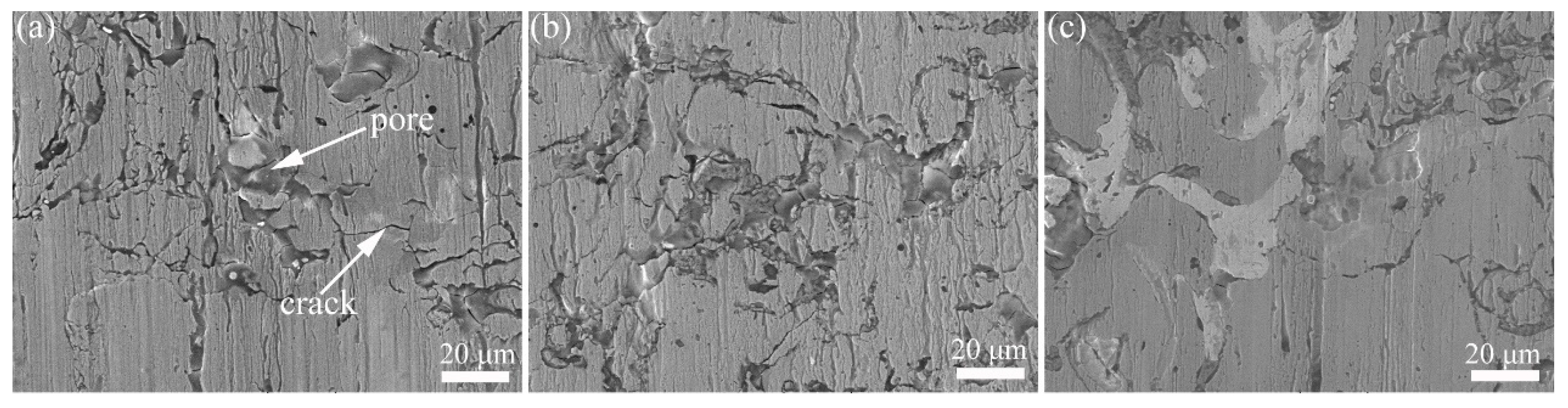
3.1. Microstructure and Microhardness of Composite Coatings
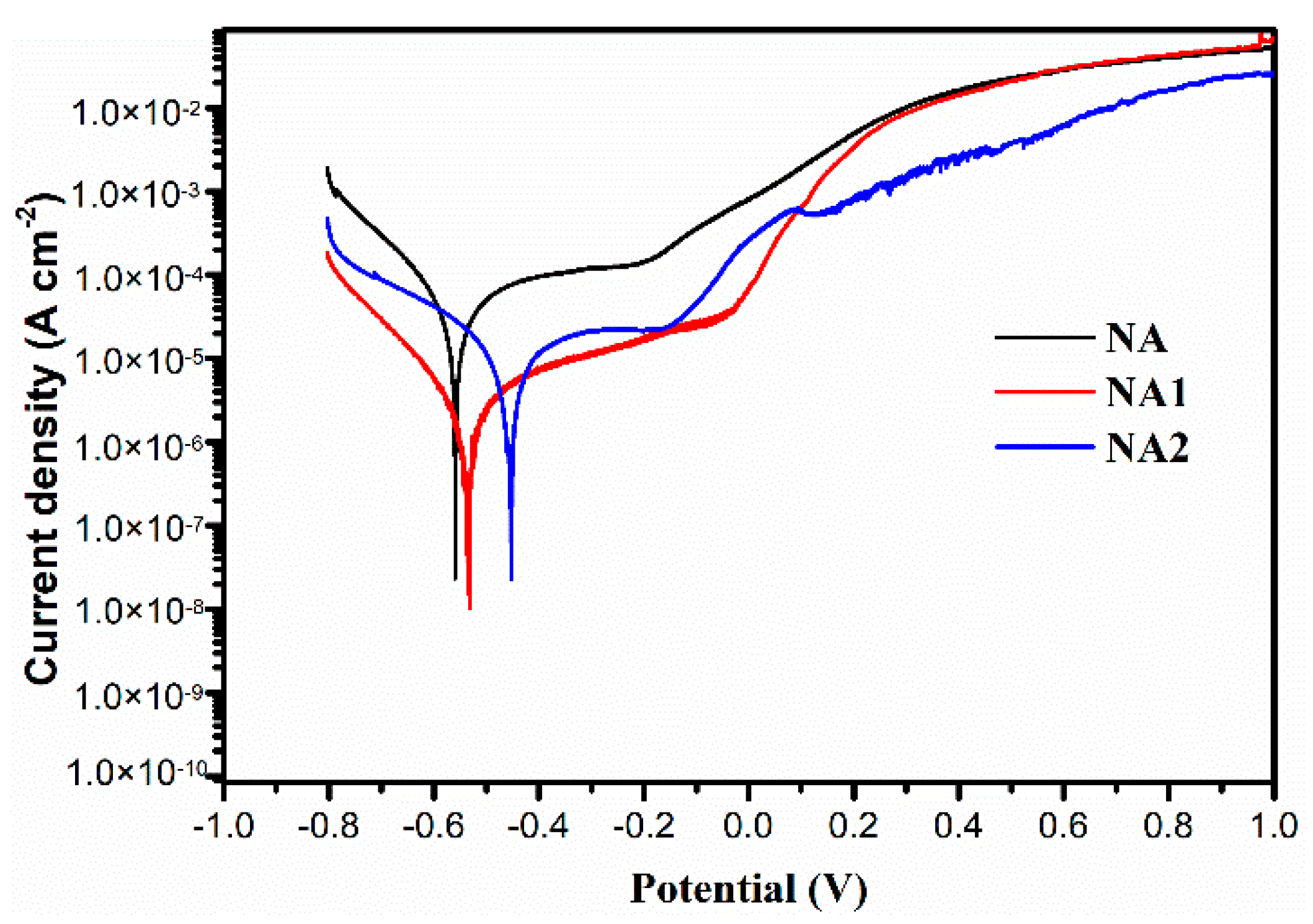
3.2. Electrochemical Behavior of Composite Coatings
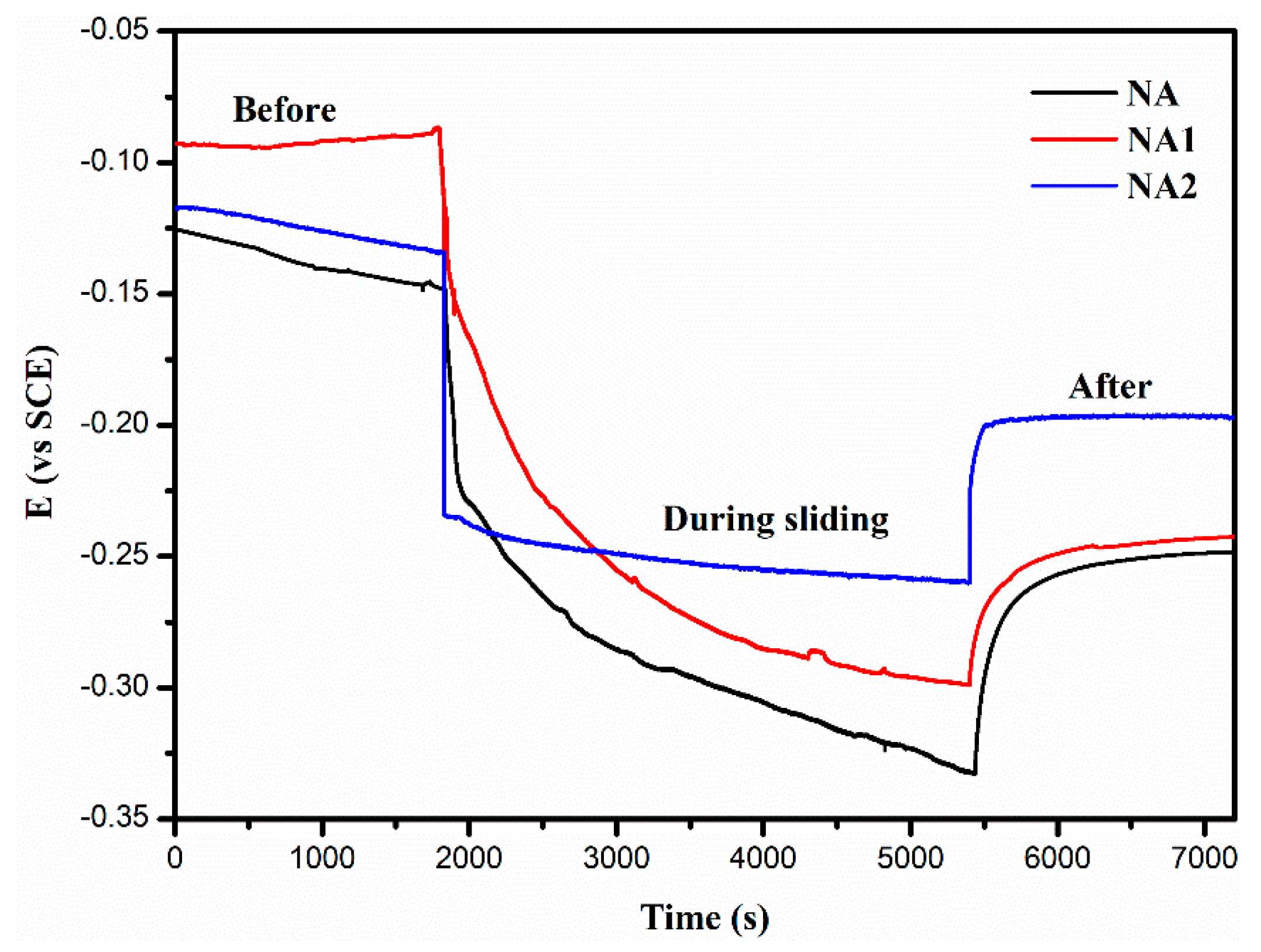
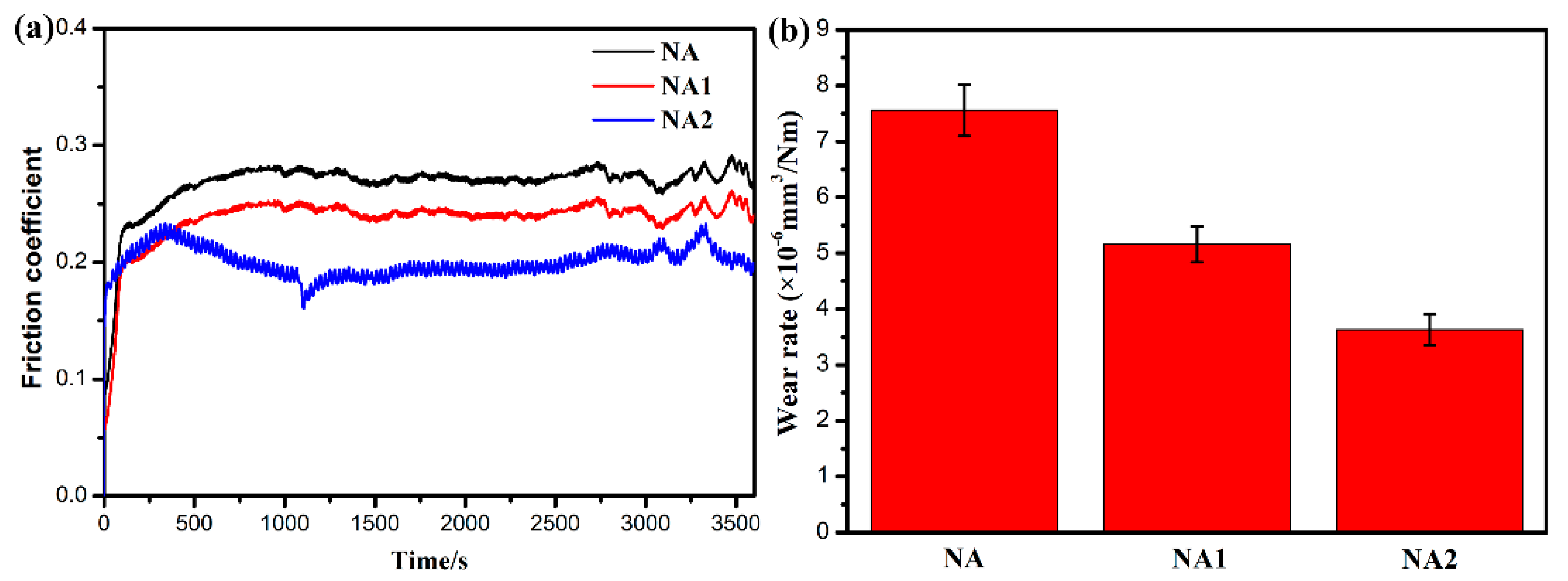
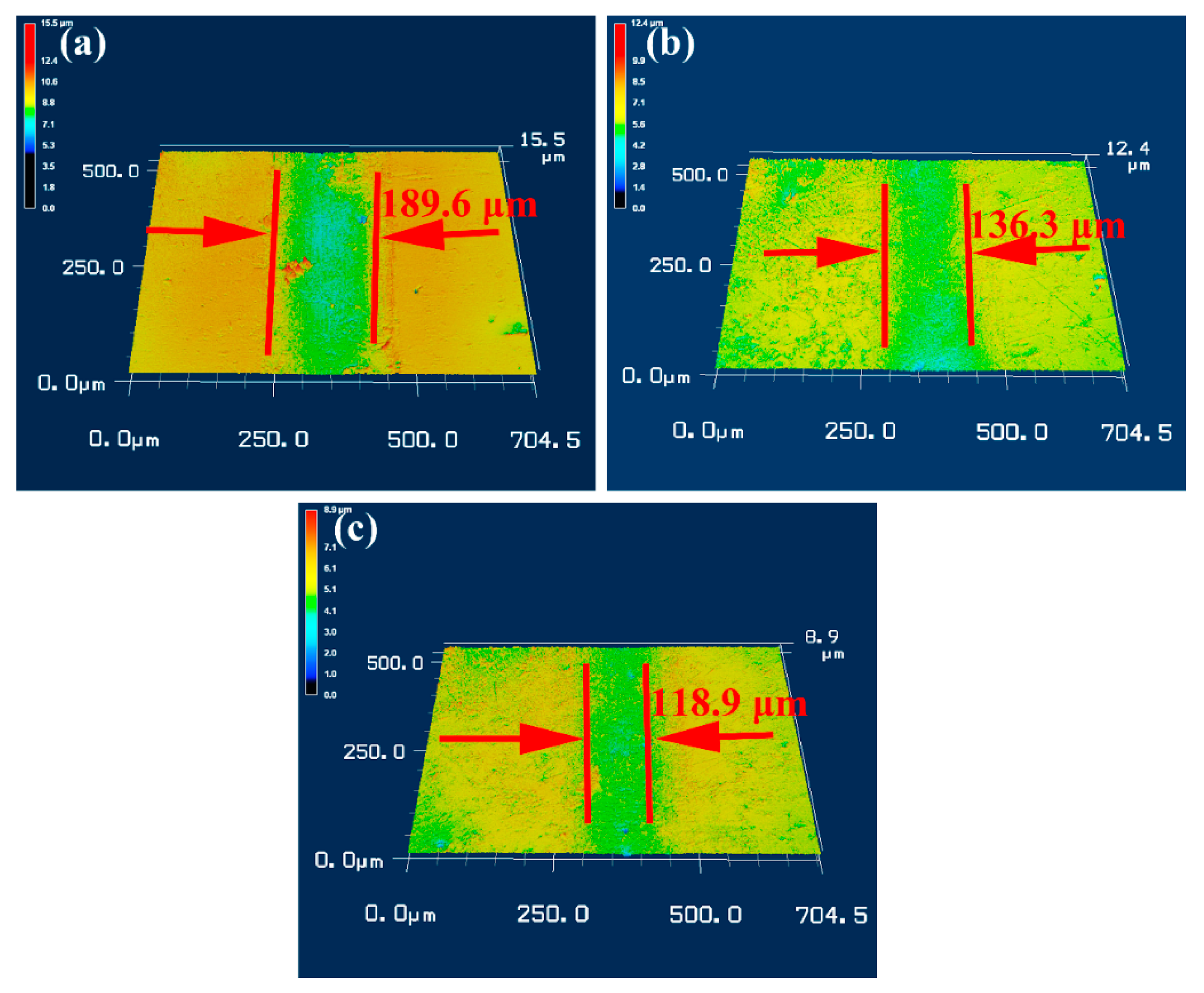
3.3. Tribological Behavior of NiAl Matrix Composite Coatings
4. Conclusions
- Upon adding Cr2O3 and Mo, the microhardness of the composite coatings improves from 195.1 to 362.2 HV. Meanwhile, the Cr2O3 and Mo phases distribute uniformly and the interface of these two phases has no evident cracks.
- The Ni-based solid solution diffraction peaks slightly shift to the right after the addition of Mo, which is maybe due to the partial solid solution of the element Mo into the matrix.
- The NiAl–Cr2O3–Mo composite coating has the lowest corrosion current density, friction coefficient and wear rate of 9.487 × 10−6 A/cm2, 0.18 and 3.63 × 10−6 mm3/Nm in all composite coatings.
- The NiAl–Cr2O3–Mo composite coating shows excellent tribocorrosion properties in artificial seawater.
Author Contributions
Funding
Conflicts of Interest
References
- Aribo, S.; Fakorede, A.; Ige, O.; Olubambi, P. Erosion-corrosion behaviour of aluminum alloy 6063 hybrid composite. Wear 2017, 376–377, 608–614. [Google Scholar] [CrossRef]
- Babu, P.; Sen, D.; Jyothirmayi, A.; Krishna, L.; Rao, D. Influence of microstructure on the wear and corrosion behavior of detonation sprayed Cr2O3–Al2O3 and plasma sprayed Cr2O3 coatings. Ceram. Int. 2018, 44, 2351–2357. [Google Scholar] [CrossRef]
- Bai, W.; Xie, Y.; Li, L.; Wang, X.; Gu, C.; Tu, J. Tribological and corrosion behaviors of Zr-doped graphite-like carbon nanostructured coatings on Ti6Al4V alloy. Surf. Coat. Technol. 2017, 320, 235–239. [Google Scholar] [CrossRef]
- Castillejo, F.; Marulanda, D.; Olaya, J.; Alfonso, J. Wear and corrosion resistance of niobium-chromium carbide coatings on AISI D2 produced through TRD. Surf. Coat. Technol. 2014, 254, 104–111. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel-titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Dalibón, E.; Escalada, L.; Simison, S.; Forsich, C.; Heim, D.; Bruhl, S. Mechanical and corrosion behavior of thick and soft DLC coatings. Surf. Coat. Technol. 2017, 312, 101–109. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; An, Y.; Hou, G.; Li, S.; Deng, W.; Zhou, H.; Chen, J. Effects of loads on corrosion-wear synergism of NiCoCrAlYTa coating in artificial seawater. Tribol. Int. 2018, 118, 421–431. [Google Scholar] [CrossRef]
- Dun, Y.; Zhao, X.; Tang, Y.; Dino, S.; Zuo, Y. Microstructure and corrosion resistance of a fluorosilane modified silane-graphene film on 2024 aluminum alloy. Appl. Surf. Sci. 2018, 437, 152–160. [Google Scholar] [CrossRef]
- Silva, R.; Nogueira, R.; Bastos, I. Tribocorrosion of UNS S32750 in chloride medium: Effect of the load level. Electrochim. Acta 2011, 56, 8839–8845. [Google Scholar] [CrossRef]
- Liu, X.; An, Y.; Li, S.; Zhao, X.; Hou, G.; Zhou, H.; Chen, J. An assessment of tribological performance on NiCoCrAlYTa coating under corrosive environments. Tribol. Int. 2017, 115, 35–44. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Structural, mechanical and tribocorrosion behaviour in artificial seawater of CrN/AlN nano-multilayer coatings on F690 steel substrates. Appl. Surf. Sci. 2018, 428, 404–414. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Bagheri, A.; Souza, J.; Silva, F.; Henriques, B. Tribocorrosion behavior of hot pressed CoCrMo alloys in artificial saliva. Tribol. Int. 2016, 97, 423–430. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, Z.; Chen, T.; Gong, F. Tribocorrosion failure mechanism of TiN/SiOx duplex coating deposited on AISI304 stainless steel. Materials 2016, 9, 963. [Google Scholar] [CrossRef]
- Adabi, M.; Amadeh, A. Formation mechanisms of Ni–Al intermetallics during heat treatment of Ni coating on 6061 Al substrate. Trans. Nonferrous Met. Soc. China 2015, 25, 3959–3966. [Google Scholar] [CrossRef]
- Ameri, S.; Sadeghian, Z.; Kazeminezhad, I. Effect of CNT addition approach on the microstructure and properties of NiAl-CNT nanocomposites produced by mechanical alloying and spark plasma sintering. Intermetallics 2016, 76, 41–48. [Google Scholar] [CrossRef]
- Beyhaghi, M.; Kashefi, M.; Kiani-Rashid, A.; Khaki, J.; Jonsson, S. In-situ synthesis of nanostructured NiAl-Al2O3 composite coatings on cast iron substrates by spark plasma sintering of mechanically activated powders. Surf. Coat. Technol. 2015, 272, 254–267. [Google Scholar] [CrossRef]
- Li, B.; Jia, J.; Gao, Y.; Han, M.; Wang, W. Microstructural and tribological characterization of NiAl matrix self-lubricating composite coatings by atmospheric plasma spraying. Tribol. Int. 2017, 109, 563–570. [Google Scholar] [CrossRef]
- Liu, E.; Bai, Y.; Gao, Y.; Yi, G.; Jia, J. Tribological properties of NiAl-based composites containing Ag3VO4 nanoparticles at elevated temperatures. Tribol. Int. 2014, 80, 25–33. [Google Scholar] [CrossRef]
- Geist, D.; Gammer, C.; Rentenberger, C.; Karnthaler, H. Sessile dislocations by reactions in NiAl severely deformed at room temperature. J. Alloy. Compd. 2015, 621, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.; Su, C.; Huang, T.; Liao, W. The microstructural characteristics and mechanical properties of Ni–Al/h-BN coatings deposited using plasma spraying. J. Alloy. Compd. 2011, 509, 8239–8245. [Google Scholar] [CrossRef]
- Liu, E.; Jia, J.; Bai, Y.; Wang, W.; Gao, Y. Study on preparation and mechanical property of nanocrystalline NiAl intermetallic. Mater. Des. 2014, 53, 596–601. [Google Scholar] [CrossRef]
- Guo, X.; Niu, Y.; Huang, L.; Ji, H.; Zheng, X. Microstructure and tribological property of TiC–Mo composite coating prepared by vacuum plasma spraying. J. Therm. Spray Technol. 2012, 21, 1083–1090. [Google Scholar] [CrossRef]
- Klimashin, F.; Euchner, H.; Mayrhofer, P. Computational and experimental studies on structure and mechanical properties of Mo–Al–N. Acta Mater. 2016, 107, 273–278. [Google Scholar] [CrossRef]
- Kong, L.; Bi, Q.; Zhu, S.; Qiao, Z.; Yang, J.; Liu, W. Effect of CuO on self-lubricating properties of ZrO2(Y2O3)–Mo composites at high temperatures. J. Eur. Ceram. Soc. 2014, 34, 1289–1296. [Google Scholar] [CrossRef]
- Liu, E.; Gao, Y.; Bai, Y.; Yi, G.; Wang, W.; Zeng, Z.; Jia, J. Tribological properties of self-lubricating NiAl/Mo-based composites containing AgVO3 nanowires. Mater. Charact. 2014, 97, 116–124. [Google Scholar] [CrossRef]
- Liu, E.; Gao, Y.; Jia, J.; Bai, Y.; Wang, W. Microstructure and mechanical properties of in situ NiAl–Mo2C nanocomposites prepared by hot-pressing sintering. Mater. Sci. Eng. A 2014, 592, 201–206. [Google Scholar] [CrossRef]
- Zhu, S.; Bi, Q.; Niu, M.; Yang, J.; Liu, W. Tribological behavior of NiAl matrix composites with addition of oxides at high temperatures. Wear 2012, 274, 423–434. [Google Scholar] [CrossRef]
- Navas, C.; Cadenas, M.; Cuetos, J.; Damborenea, J. Microstructure and sliding wear behaviour of Tribaloy T-800 coatings deposited by laser cladding. Wear 2006, 260, 838–846. [Google Scholar] [CrossRef]
- Du, L.; Zhang, W.; Zhang, W.; Zhang, T.; Lan, H.; Huang, C. Tribological and oxidation behaviors of the plasma sprayed NiCoCrAlY–Cr2O3–AgVO3 coating. Surf. Coat. Technol. 2016, 298, 7–14. [Google Scholar] [CrossRef]
- Kim, G.; Choi, H.; Han, C.; Uhm, S.; Lee, C. Characterization of atmospheric plasma spray NiCr–Cr2O3–Ag–CaF2/BaF2 coatings. Surf. Coat. Technol. 2005, 195, 107–115. [Google Scholar] [CrossRef]
- Ramazani, M.; Ashrafizadeh, F.; Mozaffarinia, R. The influence of temperature on frictional behavior of plasma-sprayed NiAl–Cr2O3 based self-adaptive nanocomposite coatings. J. Therm. Spray Technol. 2013, 22, 1120–1132. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Gao, Y.; Guo, H.; Kang, Y.; Zhao, S. Tribological performance of a Ni-based composite coating in artificial seawater. Coatings 2019, 7, 747. [Google Scholar] [CrossRef]
- Li, B.; Gao, Y.; Jia, J.; Han, M.; Guo, H.; Wang, W. Influence of heat treatments on the microstructure as well as mechanical and tribological properties of NiCrAlY–Mo–Ag coatings. J. Alloy. Compd. 2016, 686, 503–510. [Google Scholar] [CrossRef]
- Li, B.; Jia, J.; Han, M.; Gao, Y.; Wang, W.; Li, C. Microstructure, mechanical and tribological properties of plasma-sprayed NiCrAlY–Mo–Ag coatings from conventional and nanostructured powders. Surf. Coat. Technol. 2017, 324, 552–559. [Google Scholar] [CrossRef]
- Zhang, T.; Lan, H.; Huang, C.; Du, L.; Zhang, W. Formation mechanism of the lubrication film on the plasma sprayed NiCoCrAlY–Cr2O3–AgMo coating at high temperatures. Surf. Coat. Technol. 2017, 319, 47–54. [Google Scholar] [CrossRef]
- Yang, H.; Lee, C.; Hwang, S. The effect of nano-sized Cr2O3 addition on the characteristics of NiCr–Cr2O3–Ag–BaF2/CaF2 coating. Surf. Coat. Technol. 2006, 201, 38–44. [Google Scholar] [CrossRef]
- Tian, J.; Yao, S.; Luo, X.; Li, C.; Li, C. An effective approach for creating metallurgical self-bonding in plasma-spraying of NiCr–Mo coating by designing shell-core-structured powders. Acta Mater. 2016, 110, 19–30. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Xing, X.; Yuan, Z.; Yang, S.; Han, Z.; Yuan, G. Effects of Ni addition on tribocorrosion property of TiCu alloy. Tribol. Int. 2017, 107, 39–47. [Google Scholar] [CrossRef]
- Kannan, A.; Muralidharan, S.; Sarangapani, K.; Balaramachandran, V.; Kapali, V. Corrosion and anodic behaviour of zinc and its ternary alloys in alkaline battery electrolytes. J. Powder Sources 1995, 57, 93–98. [Google Scholar] [CrossRef]

| Composite Coating | NiAl (wt.%) | Cr2O3 (wt.%) | Mo (wt.%) |
|---|---|---|---|
| NA | 100 | 0 | 0 |
| NA1 | 80 | 20 | 0 |
| NA2 | 70 | 20 | 10 |
| Parameter | Value |
|---|---|
| Plasma gas flow Ar, L/min | 40 |
| Secondary gas flow H2, L/min | 5 |
| Spraying angle | 90° |
| Powder feed rate, g/min | 40 |
| Current, A | 500 |
| Voltage, V | 60 |
| Spray distance, mm | 110 |
| Constituent | Concentration (g/L) |
|---|---|
| NaCl | 24.530 |
| Na2SO4 | 4.090 |
| CaCl2 | 1.160 |
| MgCl2·6H2O | 11.110 |
| KCl | 0.695 |
| NaHCO3 | 0.201 |
| KBr | 0.100 |
| H3BO3 | 0.027 |
| SrCl2·6H2O | 0.042 |
| NaF | 0.003 |
| Coatings | NA | NA1 | NA2 |
|---|---|---|---|
| Vickers hardness (HV) | 195.1 ± 13.6 | 290.5 ± 15.8 | 362.2 ± 16.9 |
| Coatings | Ecorr (V, vs. SCE) | icorr (A/cm2) | βa (V/dec) | −βc (V/dec) | Rp (Ω) |
|---|---|---|---|---|---|
| NA | −0.583 | 8.896 × 10−5 | 0.059 | 0.046 | 1.262 × 102 |
| NA1 | −0.552 | 4.624 × 10−5 | 0.050 | 0.042 | 2.143 × 102 |
| NA2 | −0.490 | 9.487 × 10−6 | 0.042 | 0.037 | 9.003 × 102 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Li, C.; Gao, Y.; Guo, H.; Kang, Y.; Zhao, S. Microstructure and Tribocorrosion Properties of Ni-Based Composite Coatings in Artificial Seawater. Coatings 2019, 9, 822. https://doi.org/10.3390/coatings9120822
Li B, Li C, Gao Y, Guo H, Kang Y, Zhao S. Microstructure and Tribocorrosion Properties of Ni-Based Composite Coatings in Artificial Seawater. Coatings. 2019; 9(12):822. https://doi.org/10.3390/coatings9120822
Chicago/Turabian StyleLi, Bo, Cong Li, Yimin Gao, Hongjian Guo, Yunchuan Kang, and Siyong Zhao. 2019. "Microstructure and Tribocorrosion Properties of Ni-Based Composite Coatings in Artificial Seawater" Coatings 9, no. 12: 822. https://doi.org/10.3390/coatings9120822
APA StyleLi, B., Li, C., Gao, Y., Guo, H., Kang, Y., & Zhao, S. (2019). Microstructure and Tribocorrosion Properties of Ni-Based Composite Coatings in Artificial Seawater. Coatings, 9(12), 822. https://doi.org/10.3390/coatings9120822





