Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
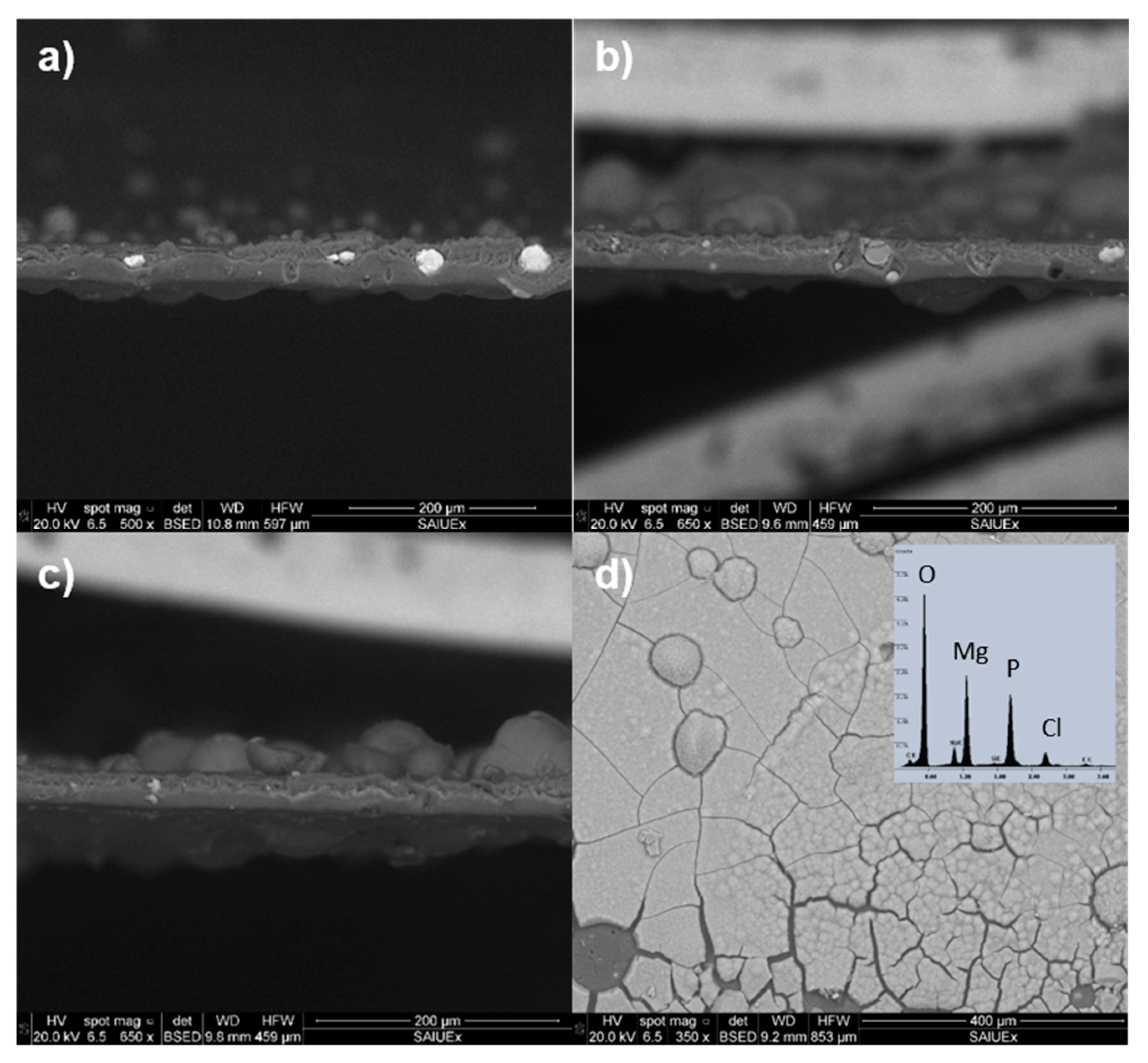
2.2. Surface Structure Characterization
2.3. X-Ray Diffraction
2.4. Surface Tension
2.5. Surface Charge
2.6. In Vitro Degradation Evaluation
2.7. Bacterial Adhesion Assays
2.8. Statistical Analyses
3. Results and Discussion
3.1. Surface of Films Characterization
3.2. Bacterial Adhesion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wan, P.; Yuan, C.; Tan, L.L.; Li, Q.; Yang, K. Fabrication and evaluation of bioresorbable PLLA/magnesium and PLLA/magnesium fluoride hybrid composites for orthopedic implants. Compos. Sci. Technol. 2014, 98, 36–43. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, H.; Ying, J.; Qiao, F.; Peng, M. Preparation and mechanical properties of carbon fiber reinforced hydroxyapatite/polylactide biocomposites. J. Mater. Sci. Mater. Med. 2009, 20, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Kandadai, M.A.; Cech, J.; Roth, S.; Curran, S.A. Poly(L-lactide) (PLLA)/Multiwalled Carbon Nanotube (MWCNT) Composite: Characterization and Biocompatibility Evaluation. J. Phys. Chem. B 2006, 110, 12910–12915. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Fujitani, W.; Kawaguchi, N.; Daito, K.; Niido, T.; Uchinaka, A.; Mori, S.; Kojima, Y.; Manabe, M.; Nishida, K.; et al. The preparation of PLLA/calcium phosphate hybrid composite and its evaluation of biocompatibility. Dent. Mater. J. 2012, 31, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Felfel, R.M.; Hossain, K.M.Z.; Parsons, A.J.; Rudd, C.D.; Ahmed, I. Accelerated in vitro degradation properties of polylactic acid/phosphate glass fibre composites. J. Mater. Sci. 2015, 50, 3942–3955. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Dewidar, M.; Lim, J.; Lim, J.K. Enhanced biocorrosion resistance of surface modified magnesium alloys using inorganic/organic composite layer for biomedical applications. Ceram. Int. 2014, 40, 2237–2247. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Gavilán, R.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L. In vitro degradation of biodegradable polylactic acid/magnesium composites: Relevance of Mg particle shape. Acta Biomater. 2016, 32, 348–357. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Frutos, E.; González-Carrasco, J.L.; Muñoz, M.; Multigner, M.; Chao, J.; Benavente, R.; Lieblich, M. Novel PLLA/magnesium composite for orthopedic applications: A proof of concept. Mater. Lett. 2012, 74, 239–242. [Google Scholar] [CrossRef]
- Wu, Y.H.; Li, N.; Cheng, Y.; Zheng, Y.F.; Han, Y. In vitro Study on Biodegradable AZ31 Magnesium Alloy Fibers Reinforced PLGA Composite. J. Mater. Sci. Technol. 2013, 29, 545–550. [Google Scholar] [CrossRef]
- Sawai, J.; Kojima, H.; Igarashi, H.; Hashimoto, A.; Shoji, S.; Sawaki, T.; Hakoda, A.; Kawada, E.; Kokugan, T.; Shimizu, M. Antibacterial characteristics of magnesium oxide powder. World J. Microbiol. Biotechnol. 2000, 16, 187–194. [Google Scholar] [CrossRef]
- Coelho, C.C.; Araújo, R.; Quadros, P.A.; Sousa, S.R.; Monteiro, F.J. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Mater. Sci. Eng. C 2019, 97, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, C.; Zhou, L.; Bai, J.; Guo, C.; Xue, F.; Lin, P.; Chu, P.K. Fully degradable PLA-based composite reinforced with 2D-braided Mg wires for orthopedic implants. Compos. Sci. Technol. 2017, 142, 180–188. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H.; Ni, J.; Zhang, S.; Zhang, X. Development of PLA/Mg composite for orthopedic implant: Tunable degradation and enhanced mineralization. Compos. Sci. Technol. 2017, 147, 8–15. [Google Scholar] [CrossRef]
- Li, X.; Guo, C.; Liu, X.; Liu, L.; Bai, J.; Xue, F.; Lin, P.; Chu, C. Impact behaviors of poly-lactic acid based biocomposite reinforced with unidirectional high-strength magnesium alloy wires. Prog. Nat. Sci. Mater. Int. 2014, 24, 472–478. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Zhai, Z.; Liu, L.; Li, H.; Yang, K.; Tan, L.; Wan, P.; Liu, X.; Ouyang, Z.; et al. Antibacterial properties of magnesium in vitro and in an in vivo model of implant-associated methicillin-resistant Staphylococcus aureus infection. Antimicrob. Agents Chemother. 2014, 58, 7586–7591. [Google Scholar] [CrossRef]
- Robinson, D.A.; Griffith, R.W.; Shechtman, D.; Evans, R.B.; Conzemius, M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010, 6, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Eifler, R.; Rais, B.; Mueller, P.P. Alkalization is responsible for antibacterial effects of corroding magnesium. J. Biomed. Mater. Res. Part A 2015, 103, 3526–3532. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhao, Y.; Cheng, M.; Wang, Q.; Wang, Q.; Wang, J.; Jiang, Y.; An, Z.; Zhang, X. Anti-biofilm properties of magnesium metal via alkaline pH. RSC Adv. 2015, 5, 21434–21444. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.; Pacha-Olivenza, M.Á.; González-Martín, M.L. Bactericidal effect of magnesium ions over planktonic and sessile Staphylococcus epidermidis and Escherichia coli. Mater. Chem. Phys. 2019, 221, 342–348. [Google Scholar] [CrossRef]
- Hickey, D.J.; Muthusamy, D.; Webster, T.J. Electrophoretic deposition of MgO nanoparticles imparts antibacterial properties to poly-L-lactic acid for orthopedic applications. J. Biomed. Mater. Res. Part A 2017, 105, 3136–3147. [Google Scholar] [CrossRef]
- Ma, R.; Lai, Y.X.; Li, L.; Tan, H.L.; Wang, J.L.; Li, Y.; Tang, T.T.; Qin, L. Bacterial inhibition potential of 3D rapid-prototyped magnesium-based porous composite scaffolds—An in vitro efficacy study. Sci. Rep. 2015, 5, 13775. [Google Scholar] [CrossRef]
- Fernández-Calderón, M.C.; Cifuentes, S.C.; Pacha-Olivenza, M.A.; Gallardo-Moreno, A.M.; Saldaña, L.; González-Carrasco, J.L.; Blanco, M.T.; Vilaboa, N.; González-Martín, M.L.; Pérez-Giraldo, C. Antibacterial effect of novel biodegradable and bioresorbable PLDA/Mg composites. Biomed. Mater. 2017, 12, 015025. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Lieblich, M.; González-Carrasco, J.L.; Benavente, R.; Lorenzo, V.; Detsch, R.; Boccaccini, A.R.; Ferrari, B. Development of biocompatible and fully bioabsorbable PLA/Mg films for tissue regeneration applications. Acta Biomater. 2019. [Google Scholar] [CrossRef]
- Harris, L.G.; Richards, R.G. Staphylococci and implant surfaces: A review. Injury 2006, 37, S3–S14. [Google Scholar] [CrossRef]
- Pacha-Olivenza, M.A.; Gallardo-Moreno, A.M.; Méndez-Vilas, A.; Bruque, J.M.; González-Carrasco, J.L.; González-Martín, M.L. Effect of UV irradiation on the surface Gibbs energy of Ti6Al4V and thermally oxidized Ti6Al4V. J. Colloid Interface Sci. 2008, 320, 117–124. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Ju, L.; Chaudhury, M.K.; Good, R.J. Estimation of the polar parameters of the surface tension of liquids by contact angle measurements on gels. J. Colloid Interface Sci. 1989, 128, 313–319. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, M.; Simões, L.C. The effects of sodium hypochlorite against selected drinking water-isolated bacteria in planktonic and sessile states. Sci. Total Environ. 2016, 565, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Moreno, A.M.; Vadillo-Rodríguez, V.; Perera-Núñez, J.; Bruque, J.M.; González-Martín, M.L. The zeta potential of extended dielectrics and conductors in terms of streaming potential and streaming current measurements. Phys. Chem. Chem. Phys. 2012, 14, 9758–9767. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bei, J.; Wang, S. Enhanced cell affinity of poly (D,L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 2002, 23, 2607–2614. [Google Scholar] [CrossRef]
- Cai, K.; Yao, K.; Cui, Y.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Gao, L. Influence of different surface modification treatments on poly(D,L-lactic acid) with silk fibroin and their effects on the culture of osteoblast in vitro. Biomaterials 2002, 23, 1603–1611. [Google Scholar] [CrossRef]
- Onder, O.C.; Nazeer, M.A.; Yilgör, E.; Yilgör, I. Spontaneous formation of microporous poly(lactic acid) coatings. Prog. Org. Coat. 2018, 125, 249–256. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Ye, L.; Zhao, X.; Coates, P.; Caton-Rose, F. Structure and biocompatibility improvement mechanism of highly oriented poly(lactic acid) produced by solid die drawing. Eur. Polym. J. 2017, 97, 68–76. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Ye, L.; Coates, P.; Caton-Rose, F.; Martyn, M. Fibrillation of chain branched poly (lactic acid) with improved blood compatibility and bionic structure. Chem. Eng. J. 2015, 279, 767–776. [Google Scholar] [CrossRef]
- Paragkumar, N.T.; Edith, D.; Six, J.L. Surface characteristics of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar] [CrossRef]
- Van Oss, C.J. Interfacial Forces in Aqueous Media, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kolská, Z.; Kasálková, N.S.; Siegel, J.; Švorčík, V. Electrokinetic Potential for Characterization of Nanosctructured Solid Flat Surfaces. J. Nano Res. 2013, 25, 31–39. [Google Scholar] [CrossRef]
- Bastekova, K.; Guselnikova, O.; Postnikov, P.; Elashnikov, R.; Kunes, M.; Kolska, Z.; Švorčík, V.; Lyutakov, O. Spatially selective modification of PLLA surface: From hydrophobic to hydrophilic or to repellent. Appl. Surf. Sci. 2017, 397, 226–234. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Porous poly(L-lactic acid)/apatite composites created by biomimetic process. J. Biomed. Mater. Res. 1999, 45, 285–293. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Meng, Z.X.; Li, H.F.; Sun, Z.Z.; Zheng, W.; Zheng, Y.F. Fabrication of mineralized electrospun PLGA and PLGA/gelatin nanofibers and their potential in bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 699–706. [Google Scholar] [CrossRef]
- Babaie, E.; Ren, Y.; Bhaduri, S.B. Microwave sintering of fine grained MgP and Mg substitutes with amorphous tricalcium phosphate: Structural, and mechanical characterization. J. Mater. Res. 2016, 31, 995–1003. [Google Scholar] [CrossRef]
- Babaie, E.; Lin, B.; Goel, V.K.; Bhaduri, S.B. Evaluation of amorphous magnesium phosphate (AMP) based non-exothermic orthopedic cements. Biomed. Mater. 2016, 11, 055010. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Tamimi, F.; Le Nihouannen, D.; Bassett, D.C.; Ibasco, S.; Gbureck, U.; Knowles, J.; Wright, A.; Flynn, A.; Komarova, S.V.; Barralet, J.E. Biocompatibility of magnesium phosphate minerals and their stability under physiological conditions. Acta Biomater. 2011, 7, 2678–2685. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F.; Harris, L.; Douglas, J. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Moreno, A.M.; Navarro-Pérez, M.L.; Vadillo-Rodríguez, V.; Bruque, J.M.; González-Martín, M.L. Insights into bacterial contact angles: Difficulties in defining hydrophobicity and surface Gibbs energy. Colloids Surf. B Biointerfaces 2011, 88, 373–380. [Google Scholar] [CrossRef] [PubMed]
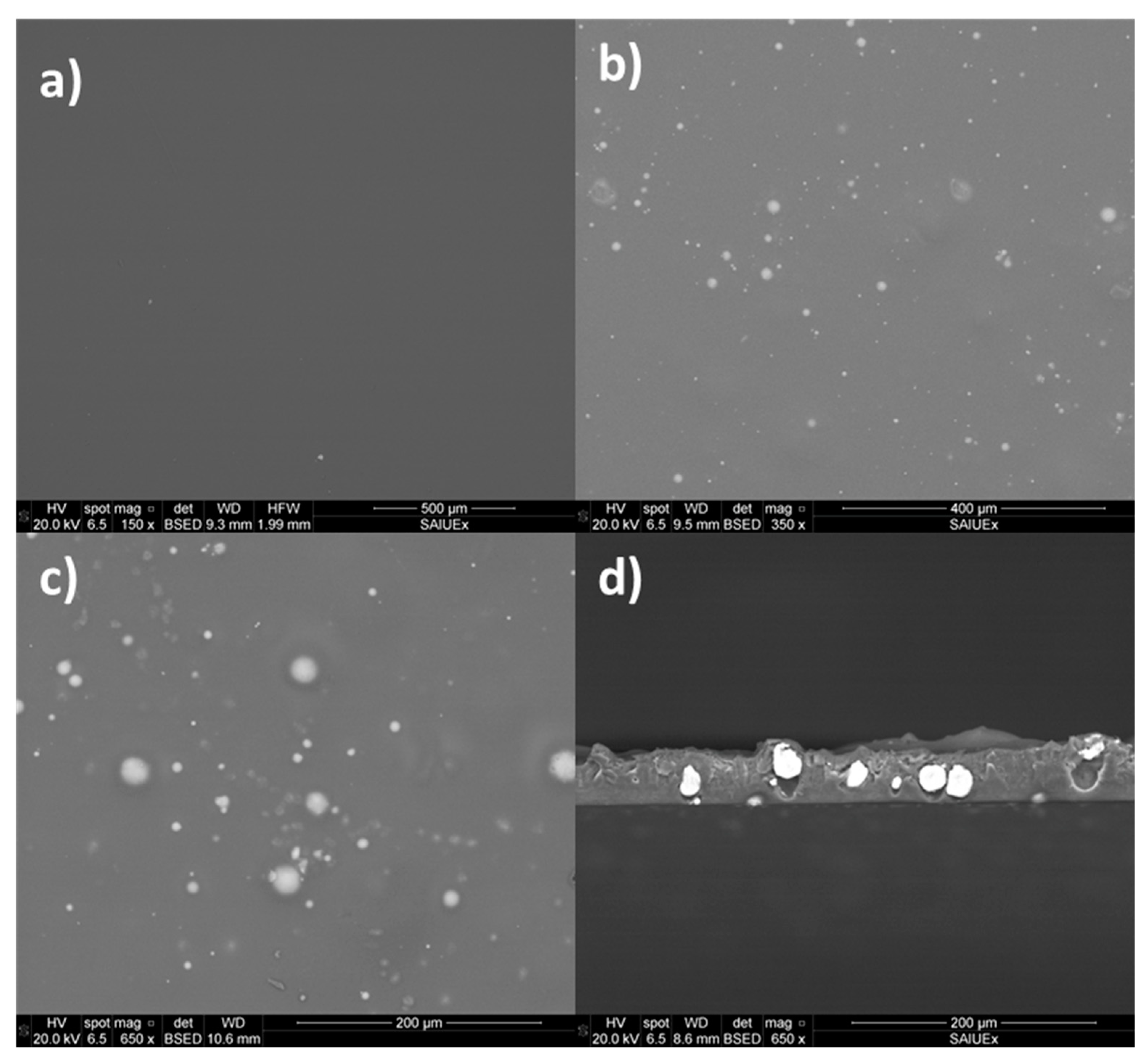
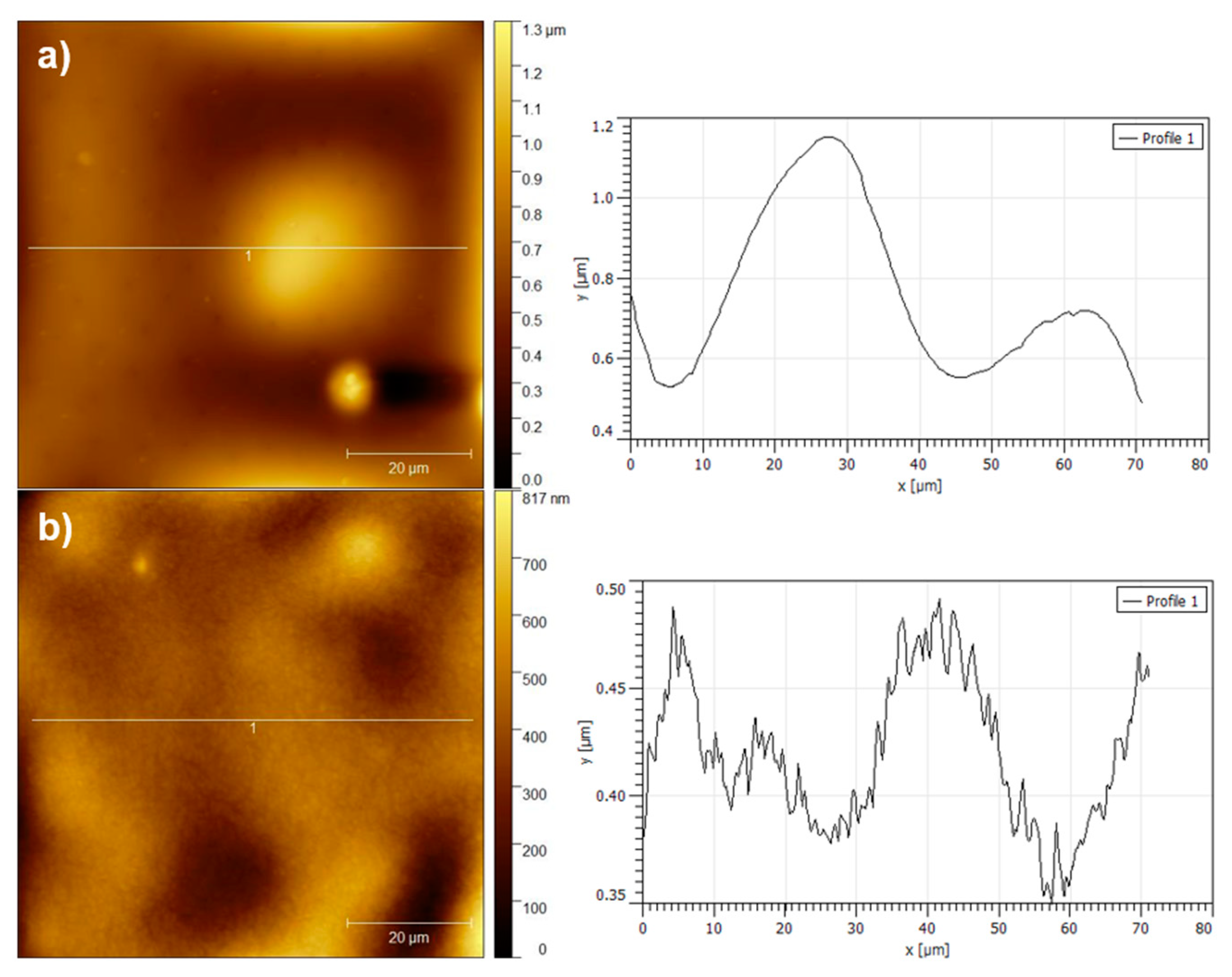
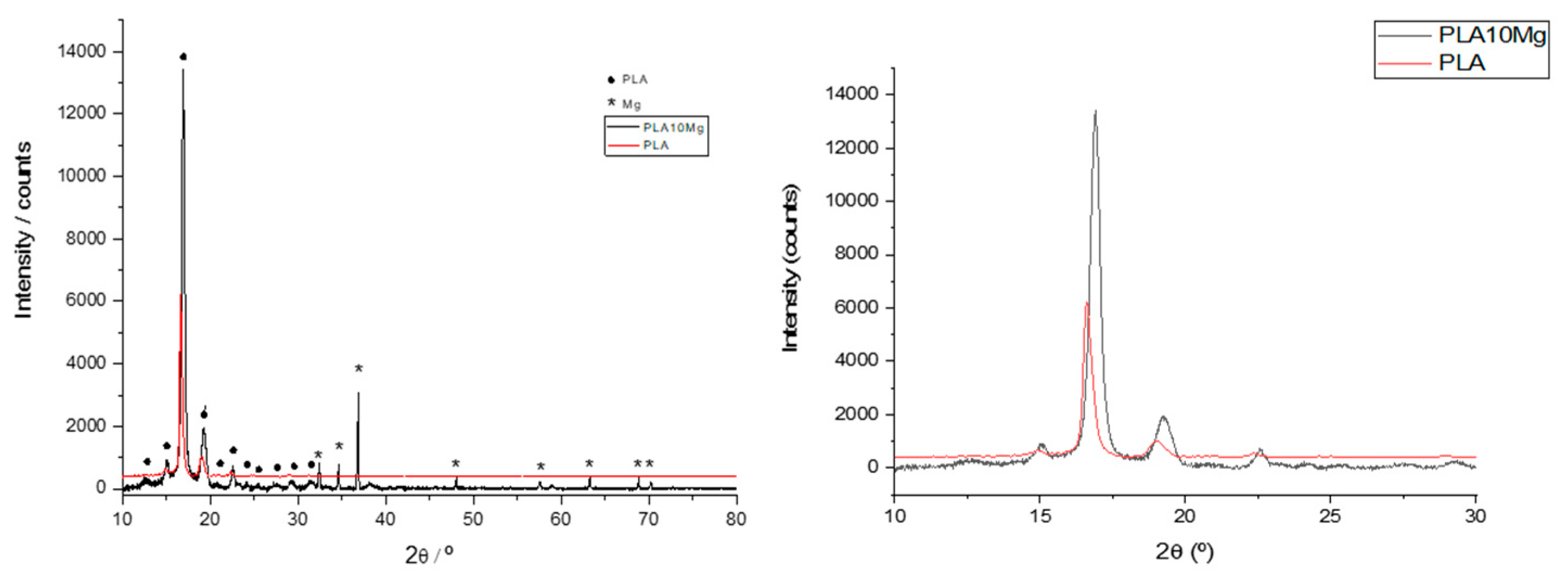
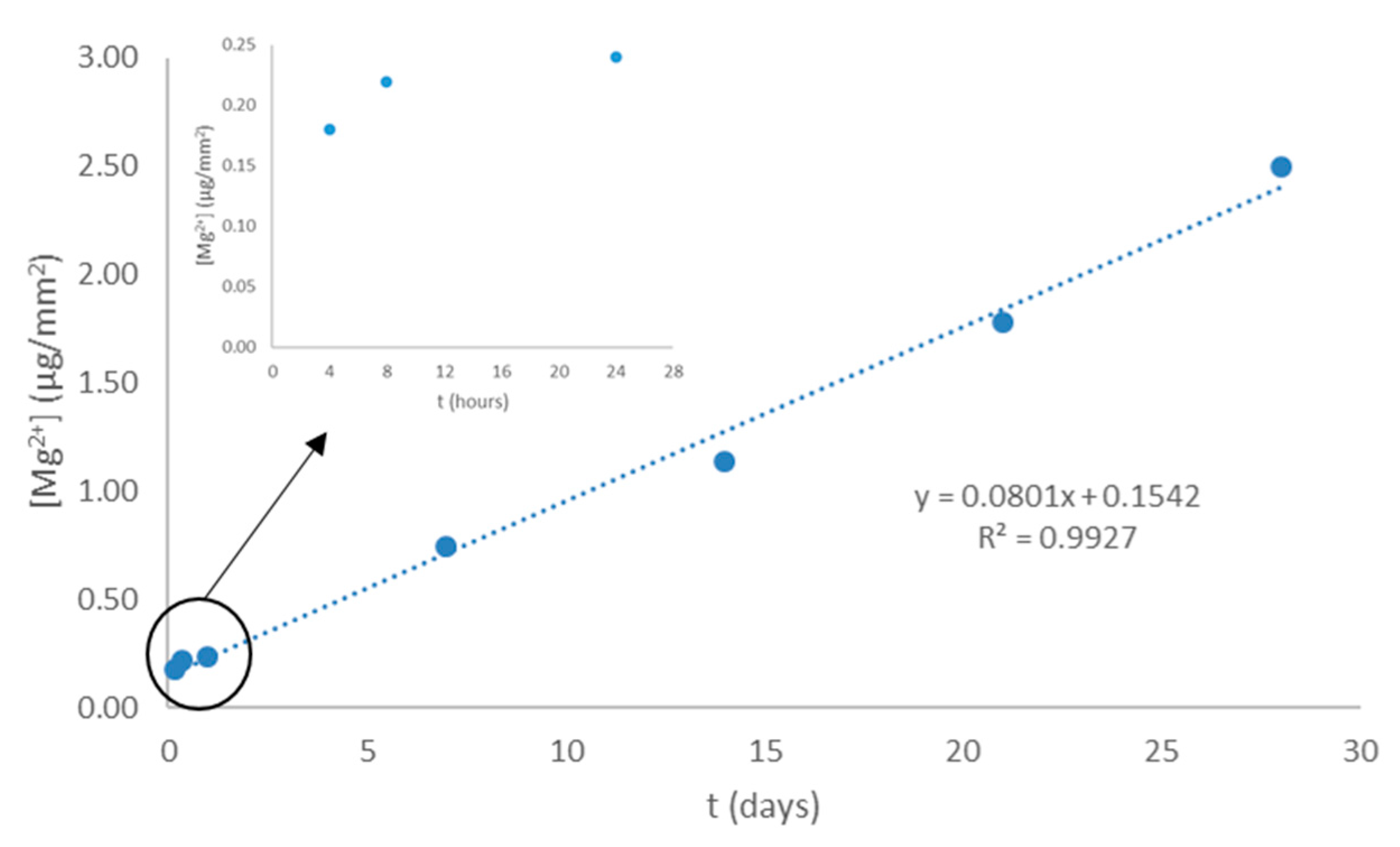
| Sample | θW [°] | θF [°] | θD [°] | γT (mJ·m−2) | γLW (mJ·m−2) | γAB (mJ·m−2) | ΔGSWS (mJ·m−2) | |
|---|---|---|---|---|---|---|---|---|
| New film | PLA10Mg | 106 ± 1 | 88 ± 1 | 58 ± 3 | 30 ± 3 | 28 ± 2 | 1.9 ± 0.6 | −67 ± 5 |
| PLA | 106 ± 2 | 90 ± 4 | 66 ± 2 | 25 ± 2 | 24 ± 1 | 1.2 ± 1.0 | −70 ± 10 | |
| Aged film | PLA10Mg | 97 ± 4 | 86 ± 4 | 63 ± 7 | 28 ± 4 | 25 ± 3 | 2.9 ± 2.5 | −48 ± 14 |
| PLA | 98 ± 4 | 89 ± 4 | 64 ± 2 | 27 ± 3 | 24 ± 2 | 3.4 ± 2.3 | −46 ± 15 | |
| Adhesion of S. Epidermidis (Bacteria·104 per cm2) | Viability (%) | [Mg2+] (ppm) | pH | ||
|---|---|---|---|---|---|
| New Film | PLA10Mg | 110 ± 2 | 0 | 14.33 ± 0.92 | 6.77 ± 0.02 |
| PLA | 129 ± 1 | 100 | - | 6.61 ± 0.01 | |
| Aged Film | PLA10Mg | 138 ± 2 | 0 | 14.79 ± 0.54 | 6.77 ± 0.01 |
| PLA | 120 ± 5 | 100 | - | 6.60 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque-Agudo, V.; Romero-Guzmán, D.; Fernández-Grajera, M.; González-Martín, M.L.; Gallardo-Moreno, A.M. Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability. Coatings 2019, 9, 814. https://doi.org/10.3390/coatings9120814
Luque-Agudo V, Romero-Guzmán D, Fernández-Grajera M, González-Martín ML, Gallardo-Moreno AM. Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability. Coatings. 2019; 9(12):814. https://doi.org/10.3390/coatings9120814
Chicago/Turabian StyleLuque-Agudo, Verónica, Daniel Romero-Guzmán, María Fernández-Grajera, M. Luisa González-Martín, and Amparo M. Gallardo-Moreno. 2019. "Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability" Coatings 9, no. 12: 814. https://doi.org/10.3390/coatings9120814
APA StyleLuque-Agudo, V., Romero-Guzmán, D., Fernández-Grajera, M., González-Martín, M. L., & Gallardo-Moreno, A. M. (2019). Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability. Coatings, 9(12), 814. https://doi.org/10.3390/coatings9120814




