Nanotechnology to Improve the Performances of Hydrodynamic Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Paint Application
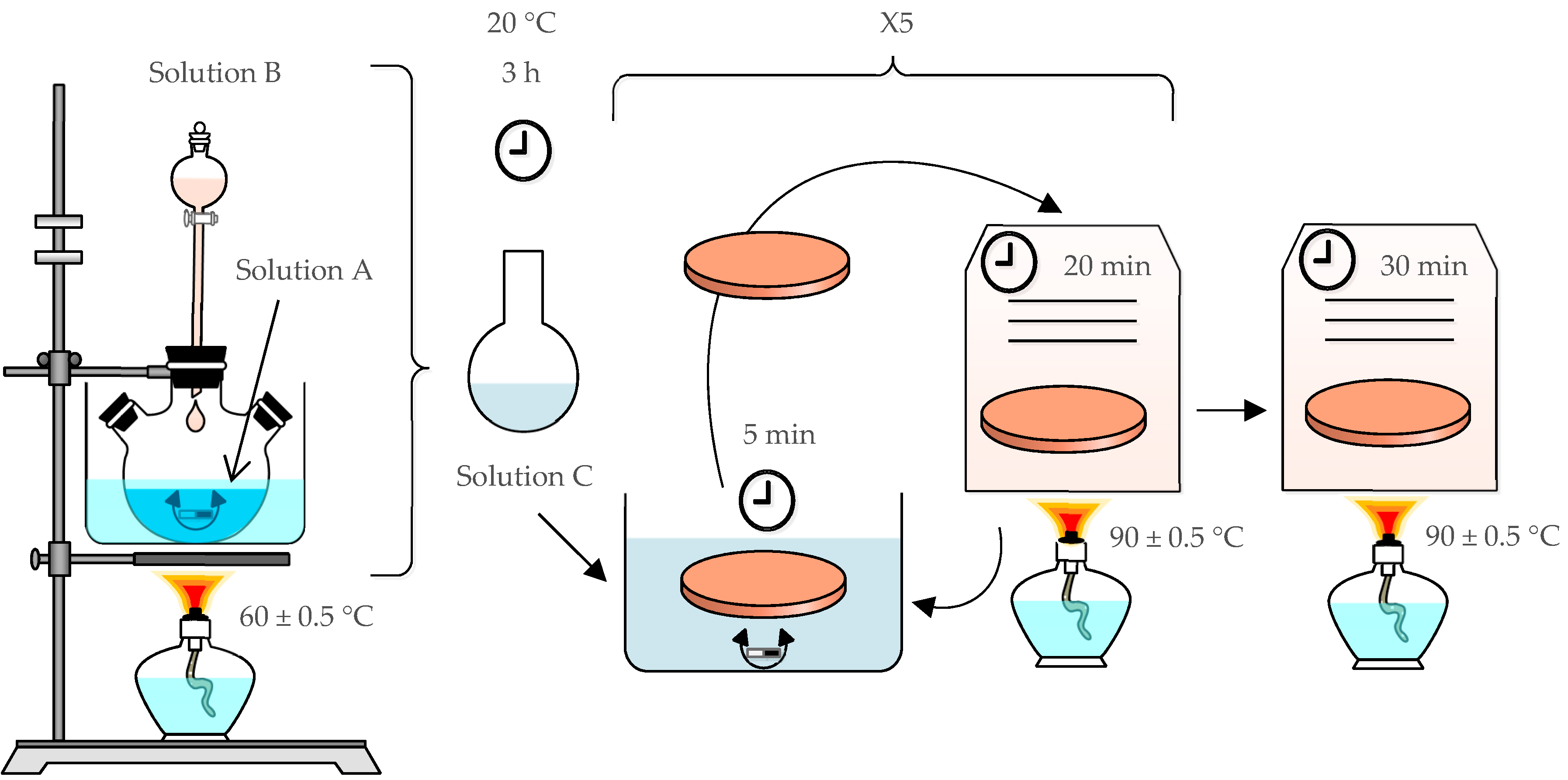
2.2. SuperHydrophobic Surface Creation
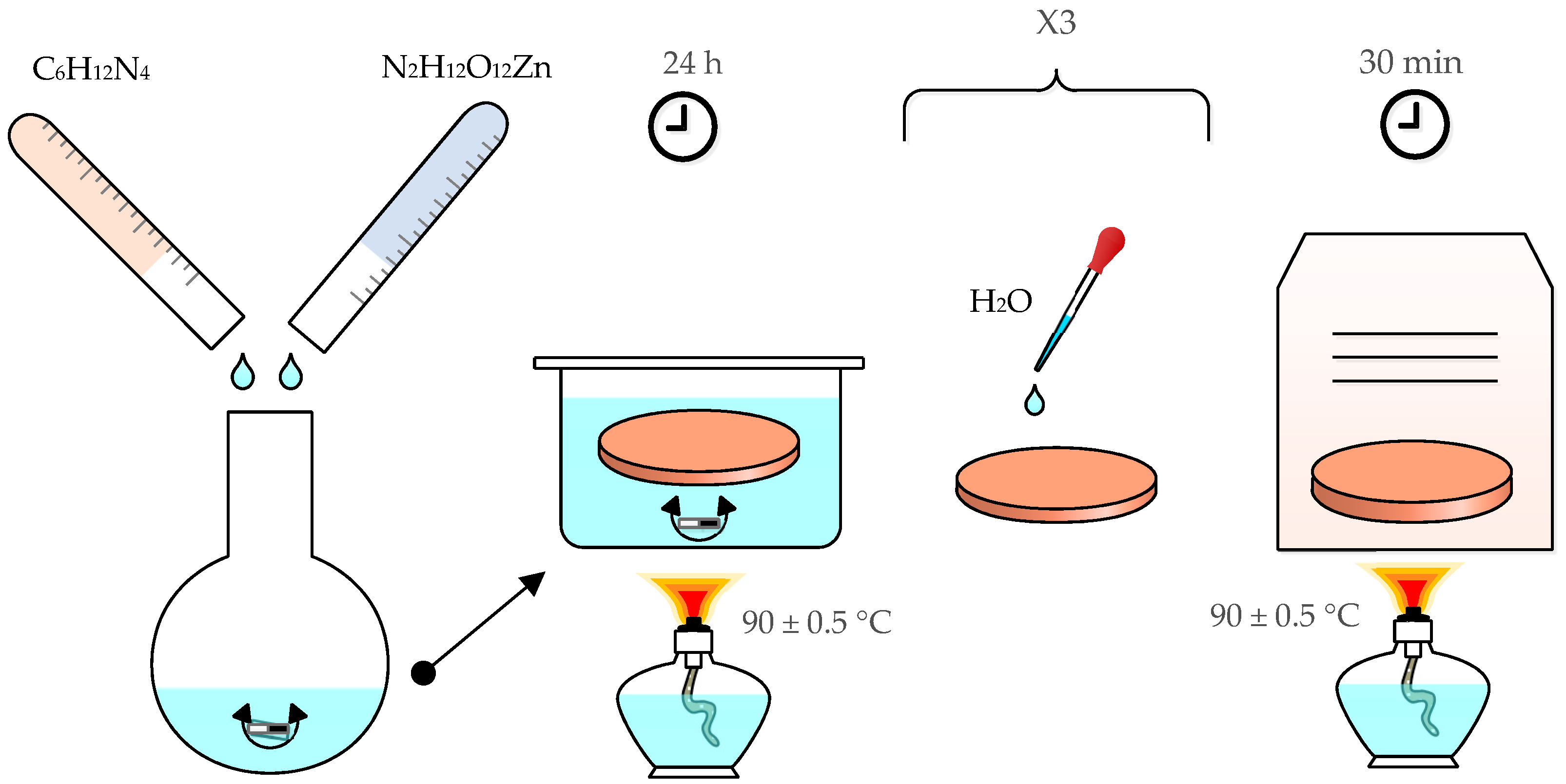
2.2.1. ZnO Seed Synthesis
- Solution A was obtained by dissolving 90 mmol/L of zinc acetate dihydrate in methanol.
- Solution B was obtained by dissolving 75 mmol/L of sodium hydroxide in methanol.
- Solution C was obtained by slowly adding solution B (1 drop/s) to the solution A while stirring at 60 °C. At the end, a transparent seed solution was obtained.
2.2.2. ZnO Nanorod Synthesis
2.2.3. Octadecyltrimethoxysilane (ODS) Deposition
3. Characterizations
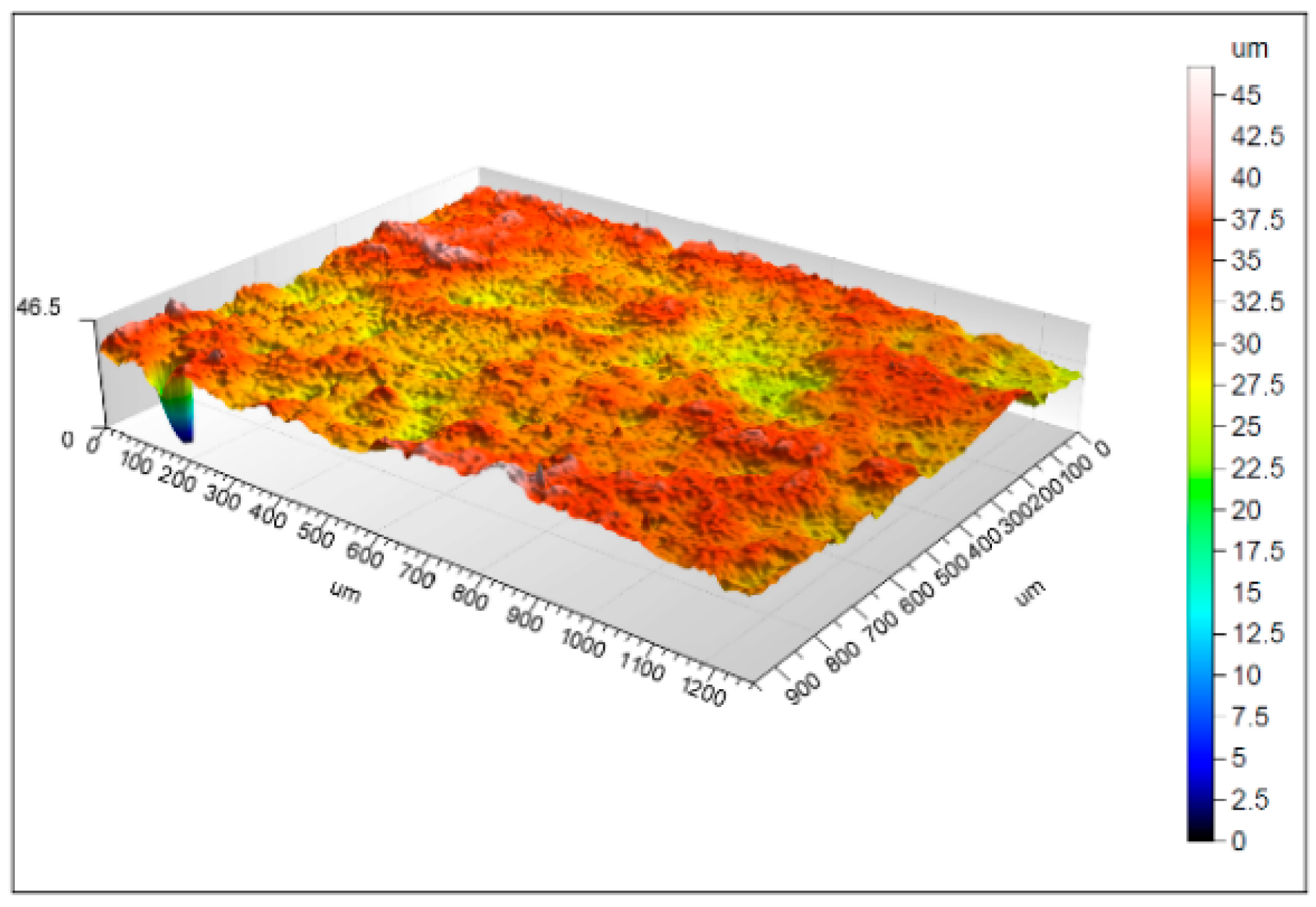
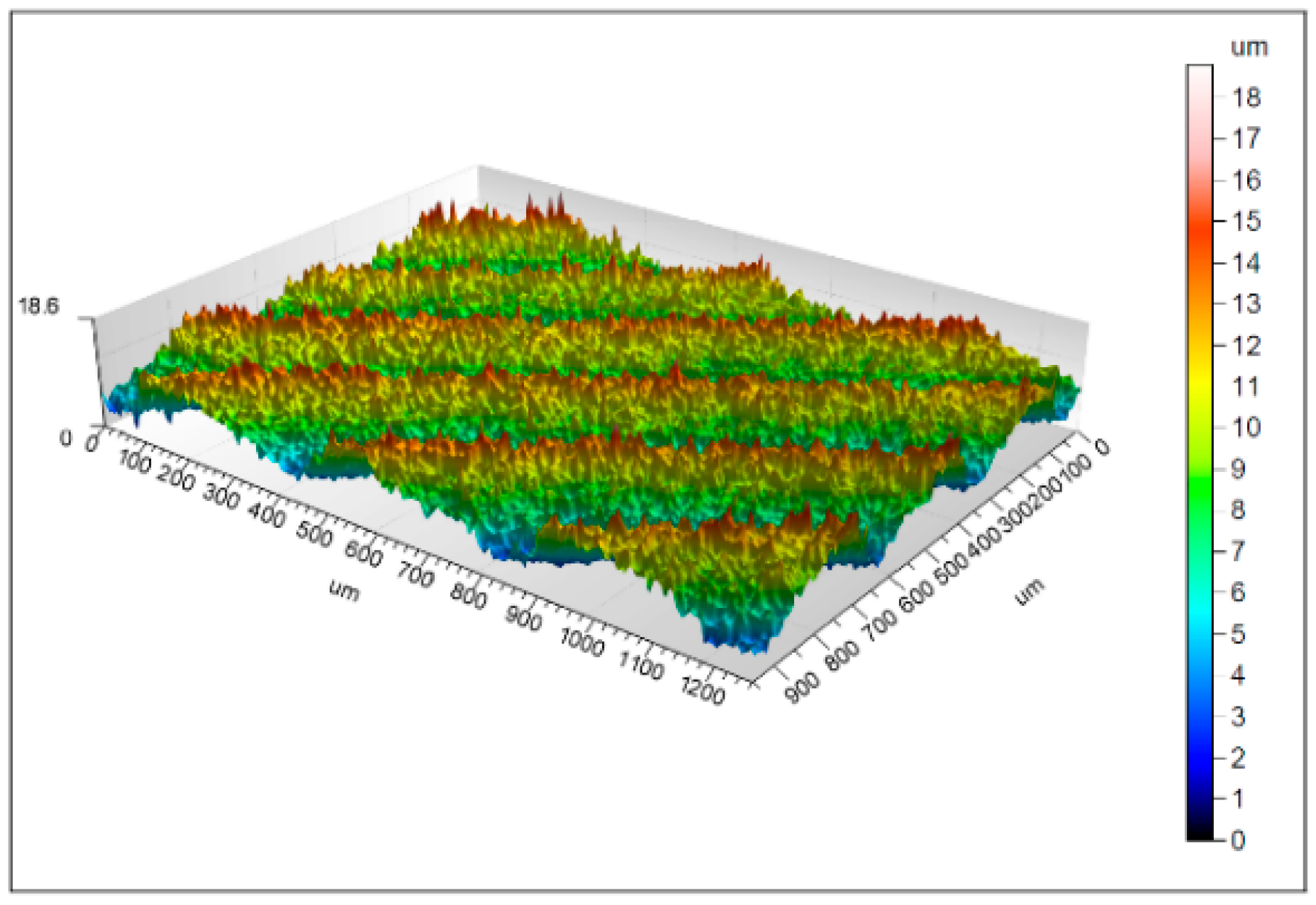
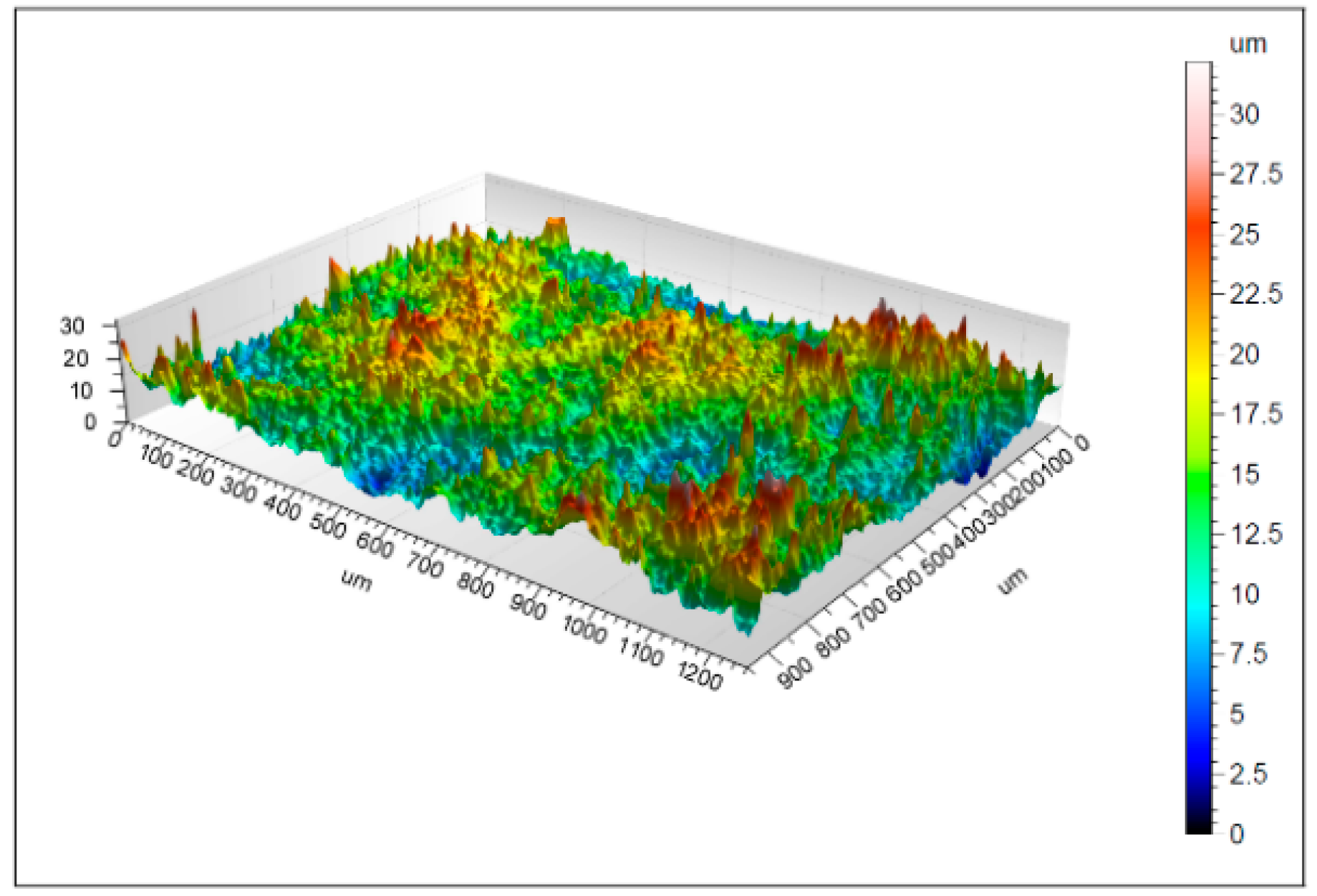
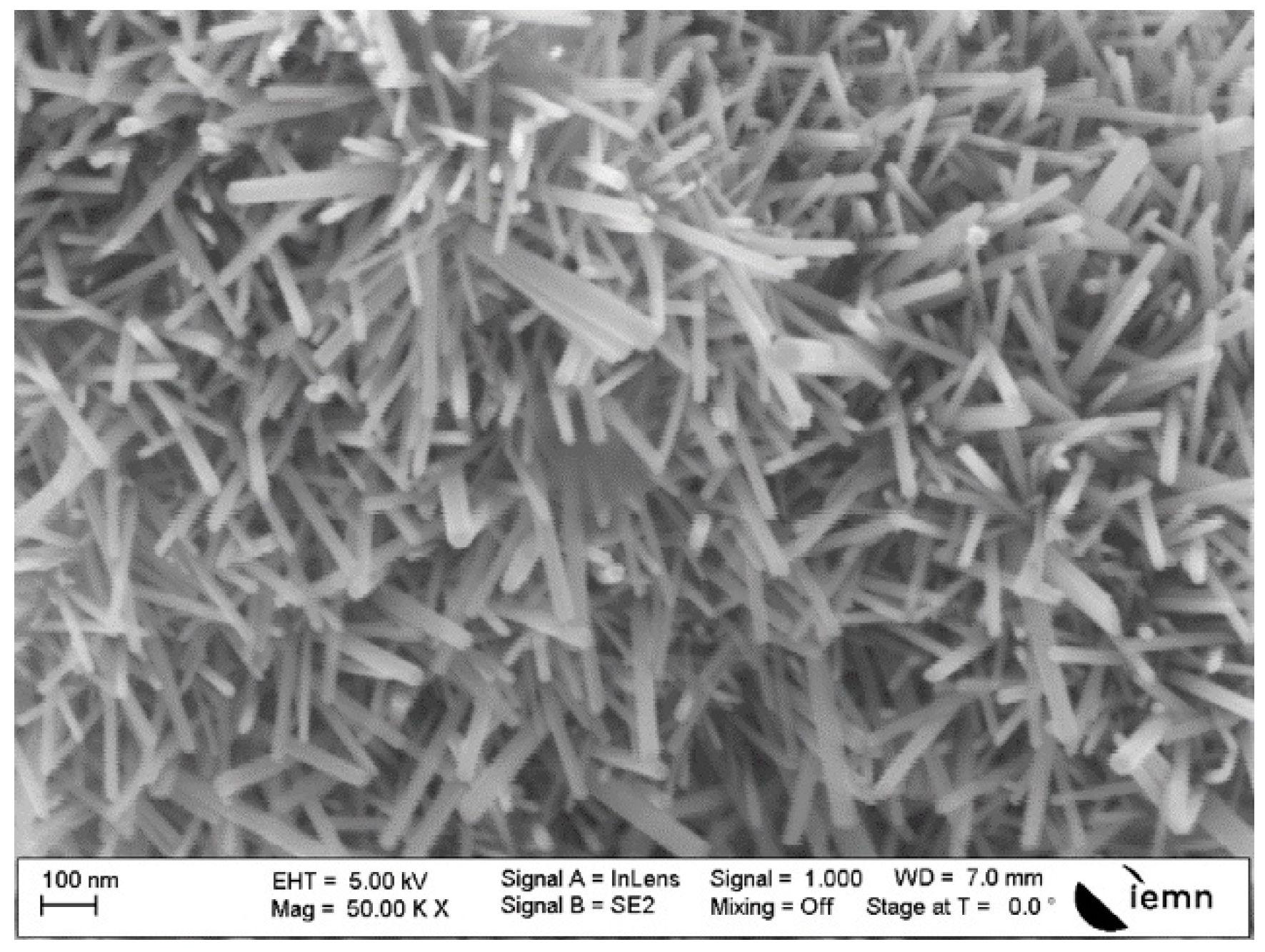
3.1. Morphology Properties
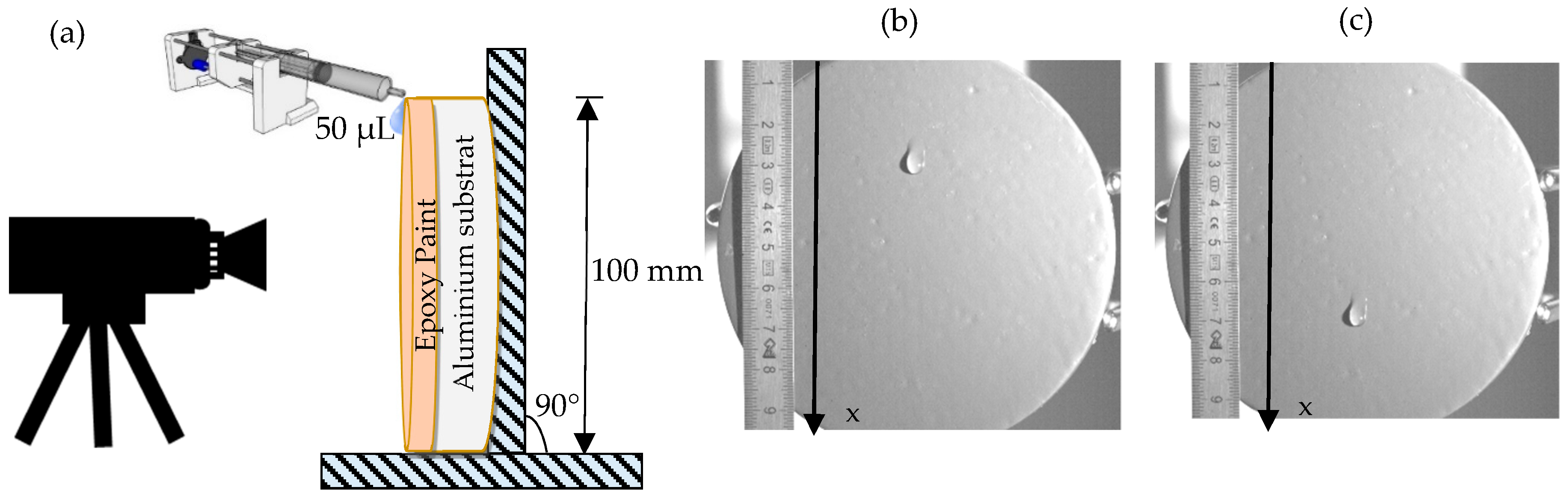
3.2. Hydrophobic Properties
- Aluminum substrate only;
- The substrate coated by the epoxy paint;
- The epoxy painted sample treated by ZnO + ODS.
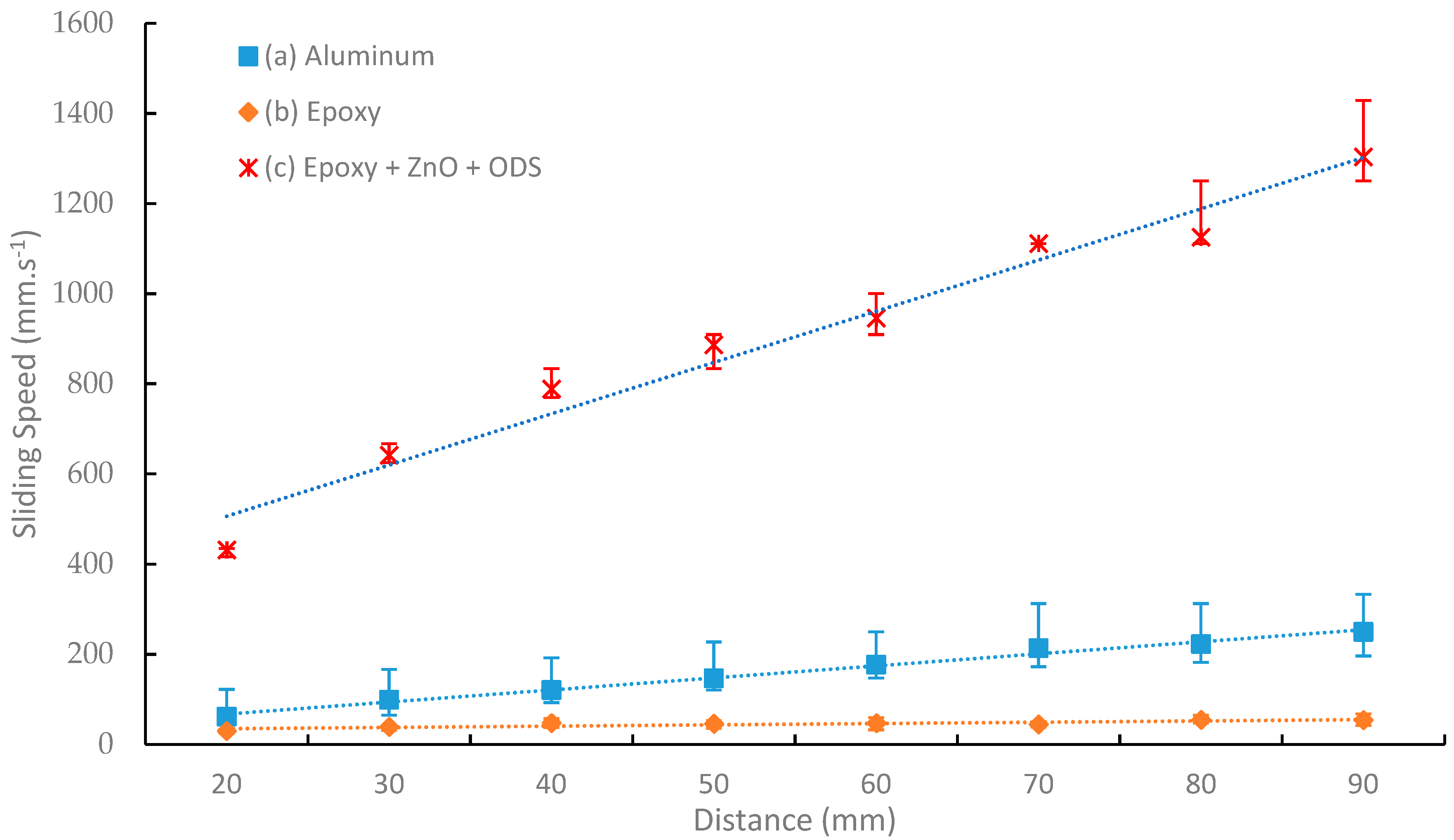
3.3. Hydrodynamic Properties
4. Results and Discussion
4.1. Surface Morphology
4.2. Nanostructural Results
4.3. Wetting Results
4.4. Hydrodynamic Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lai, D.L.; Kong, G.; Li, X.C.; Che, C.S. Corrosion resistance of ZnO nanorod superhydrophobic coatings with rose petal effect or lotus leaf effect. J. Nanosci. 2019, 19, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Kano, T.; Isobe, T.; Matsushita, S.; Nakajima, A. Hydrophobicity and Leidenfrost point of ZnO nanorod array combined with nanoscale roughness on the topmost surface. Mater. Chem. Phys. 2018, 217, 192–198. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, M.; Zhang, Y.; Wei, H.; Shi, F. Extraordinary drag-reducing effect of a superhydrophobic coating on a macroscopic model ship at high speed. J. Mater. Chem. A 2013, 1, 5886–5891. [Google Scholar] [CrossRef]
- Simpson, J.; Hunter, S.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Y.; Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. A 1805, 95, 65–87. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. J. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Hochmannova, L.; Vytrasova, J. Photocatalytic and antimicrobial effects of interior paints. Prog. Org. Coat. 2010, 67, 1–5. [Google Scholar] [CrossRef]
- Ashraf, M.; Dumont, F.; Campagne, C.; Champagne, P.; Perwuelz, A.; Leriche, A.; Chihib, N.E. Development of antibacterial polyester fabric by growth of ZnO nanorods. J. Eng. Fibers Fabr. 2014, 9, 15–22. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Amelioration of anticorrosion and hydrophobic properties of epoxy/PDMS composite coatings containing nano ZnO particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Epoxy/polyaniline-ZnO nanorods hybrid nanocomposite coatings: Synthesis, characterization and corrosion protection performance of conducting paints. Prog. Org. Coat. 2014, 77, 146–159. [Google Scholar] [CrossRef]
- Valença, D.P.; Alves, K.G.B.; de Melo, C.P.; Bouchonneau, N. Study of the efficiency of polypyrrole/ZnO nanocomposites as additives in anticorrosion coatings. Mater. Res. 2015, 18 (Suppl. 2), 273–278. [Google Scholar] [CrossRef]
- Shen, S.; Zuo, Y. The improved performance of Mg-rich epoxy primer on AZ91D magnesium alloy by addition of ZnO. Corros. Sci. 2014, 87, 167–178. [Google Scholar] [CrossRef]
- Lepore, E.; Pugno, N. Superhydrophobic polystyrene by direct copy of a lotus leaf. BioNanoScience 2011, 1, 136–143. [Google Scholar] [CrossRef]
- Xu, Q.F.; Mondal, B.; Lyons, A.M. Fabricating superhydrophobic polymer surfaces with excellent abrasion resistance by a simple lamination templating method. ACS Appl. Mater. Interfaces 2011, 3, 3508–3514. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Champagne, P.; Perwuelz, A.; Campagne, C.; Leriche, A. Photocatalytic solution discoloration and self-cleaning by polyester fabric functionalized with ZnO nanorods. J. Ind. Text. 2015, 44, 884–898. [Google Scholar] [CrossRef]
| Substrate | WCA (°) | SA (°) |
|---|---|---|
| (a) Aluminum | 93 | 22 |
| (b) Epoxy | 97 | 46 |
| (c) ZnO + ODS | 152 | 7 |
| (d) SHS | 150 | 10 |
| (e) LL | 153 | 26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, A.; Champagne, P.; Keirsbulck, L.; Dogheche, E.H. Nanotechnology to Improve the Performances of Hydrodynamic Surfaces. Coatings 2019, 9, 808. https://doi.org/10.3390/coatings9120808
Alshehri A, Champagne P, Keirsbulck L, Dogheche EH. Nanotechnology to Improve the Performances of Hydrodynamic Surfaces. Coatings. 2019; 9(12):808. https://doi.org/10.3390/coatings9120808
Chicago/Turabian StyleAlshehri, Ali, Philippe Champagne, Laurent Keirsbulck, and El Hadj Dogheche. 2019. "Nanotechnology to Improve the Performances of Hydrodynamic Surfaces" Coatings 9, no. 12: 808. https://doi.org/10.3390/coatings9120808
APA StyleAlshehri, A., Champagne, P., Keirsbulck, L., & Dogheche, E. H. (2019). Nanotechnology to Improve the Performances of Hydrodynamic Surfaces. Coatings, 9(12), 808. https://doi.org/10.3390/coatings9120808





