Influence of Polymer Composition and Substrate on the Performance of Bioinspired Coatings with Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Formation and Adhesive Properties Tests
2.3. Characterization
2.4. Antimicrobial Test
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Waite, J.H.; Tanzer, M.L. Polyphenolic substance of mytilus edulis: Novel adhesive containing L-dopa and hydroxyproline. Science 1981, 212, 1038. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Payra, D.; Fujii, Y.; Das, S.; Takaishi, J.; Naito, M. Rational design of a biomimetic glue with tunable strength and ductility. Polym. Chem. 2017, 8, 1654–1663. [Google Scholar] [CrossRef]
- Wang, X.; Ye, Q.; Liu, J.; Liu, X.; Zhou, F. Low surface energy surfaces from self-assembly of perfluoropolymer with sticky functional groups. J. Colloid Interface Sci. 2010, 351, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Payra, D.; Naito, M.; Fujii, Y.; Yamada, N.L.; Hiromoto, S.; Singh, A. Bioinspired adhesive polymer coatings for efficient and versatile corrosion resistance. RSC Adv. 2015, 5, 15977–15984. [Google Scholar] [CrossRef]
- Ham, H.O.; Liu, Z.; Lau, K.H.; Lee, H.; Messersmith, P.B. Facile DNA immobilization on surfaces through a catecholamine polymer. Angew. Chem. Int. Ed. Engl. 2011, 50, 732–736. [Google Scholar] [CrossRef]
- Shen, D.; Xu, B.; Huang, X.; Zhuang, Q.; Lin, S. (PtBA-co-PPEGMEMA-co-PDOMA)-g-PPFA polymer brushes synthesized by sequential RAFT polymerization and ATRP. Polym. Chem. 2018, 9, 2821–2829. [Google Scholar] [CrossRef]
- Chiloeches, A.; Echeverría, C.; Cuervo-Rodríguez, R.; Plachà, D.; López-Fabal, F.; Fernández-García, M.; Muñoz-Bonilla, A. Adhesive antibacterial coatings based on copolymers bearing thiazolium cationic groups and catechol moieties as robust anchors. Prog. Org. Coat. 2019, 136, 105272. [Google Scholar] [CrossRef]
- Wang, X.; Jing, S.; Liu, Y.; Liu, S.; Tan, Y. Diblock copolymer containing bioinspired borneol and dopamine moieties: Synthesis and antibacterial coating applications. Polymer 2017, 116, 314–323. [Google Scholar] [CrossRef]
- ASTM International. ASTM E2149-13a, Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents under Dynamic Contact Conditions; ASTM International: West Conshohocken, PA, USA, 2013; Available online: www.astm.org (accessed on 8 October 2019).
- Zhang, H.; Zhao, T.; Newland, B.; Duffy, P.; Annaidh, A.N.; O’Cearbhaill, E.D.; Wang, W. On-demand and negative-thermo-swelling tissue adhesive based on highly branched ambivalent PEG–catechol copolymers. J. Mater. Chem. B 2015, 3, 6420–6428. [Google Scholar] [CrossRef]
- Matos-Perez, C.R.; White, J.D.; Wilker, J.J. Polymer composition and substrate influences on the adhesive bonding of a biomimetic, cross-linking polymer. J. Am. Chem. Soc. 2012, 134, 9498–9505. [Google Scholar] [CrossRef]
- Tejero, R.; Arbe, A.; Fernández-García, M.; López, D. Nanostructuration by self-assembly in N-alkyl thiazolium and triazolium side-chain polymethacrylates. Macromolecules 2015, 48, 7180–7193. [Google Scholar] [CrossRef]
- Munoz-Bonilla, A.; Lopez, D.; Fernandez-Garcia, M. Providing antibacterial activity to poly (2-hydroxy ethyl methacrylate) by copolymerization with a methacrylic thiazolium derivative. Int. J. Mol. Sci. 2018, 19, 4120. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Kumbharkar, S.C.; Rewar, A.S.; Kharul, U.K. Polybenzimidazole based film forming polymeric ionic liquids: Synthesis and effects of cation–anion variation on their physical properties. Polym. Chem. 2014, 5, 4083. [Google Scholar] [CrossRef]
- Chen, C.; Hess, A.R.; Jones, A.R.; Liu, X.; Barber, G.D.; Mallouk, T.E.; Allcock, H.R. Synthesis of new polyelectrolytes via backbone quaternization of poly (aryloxy- and alkoxyphosphazenes) and their small molecule counterparts. Macromolecules 2012, 45, 1182–1189. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Kharul, U.K. N-substitution of polybenzimidazoles: Synthesis and evaluation of physical properties. Eur. Polym. J. 2009, 45, 3363–3371. [Google Scholar] [CrossRef]
- Choi, K.-Y.; Yi, M.H. Synthesis and characterization of N-alkylated polyamide-imides. Die Angew. Makromol. Chem. 1994, 222, 103–109. [Google Scholar] [CrossRef]
- Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups. Polym. Chem. 2015, 6, 3449–3459. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
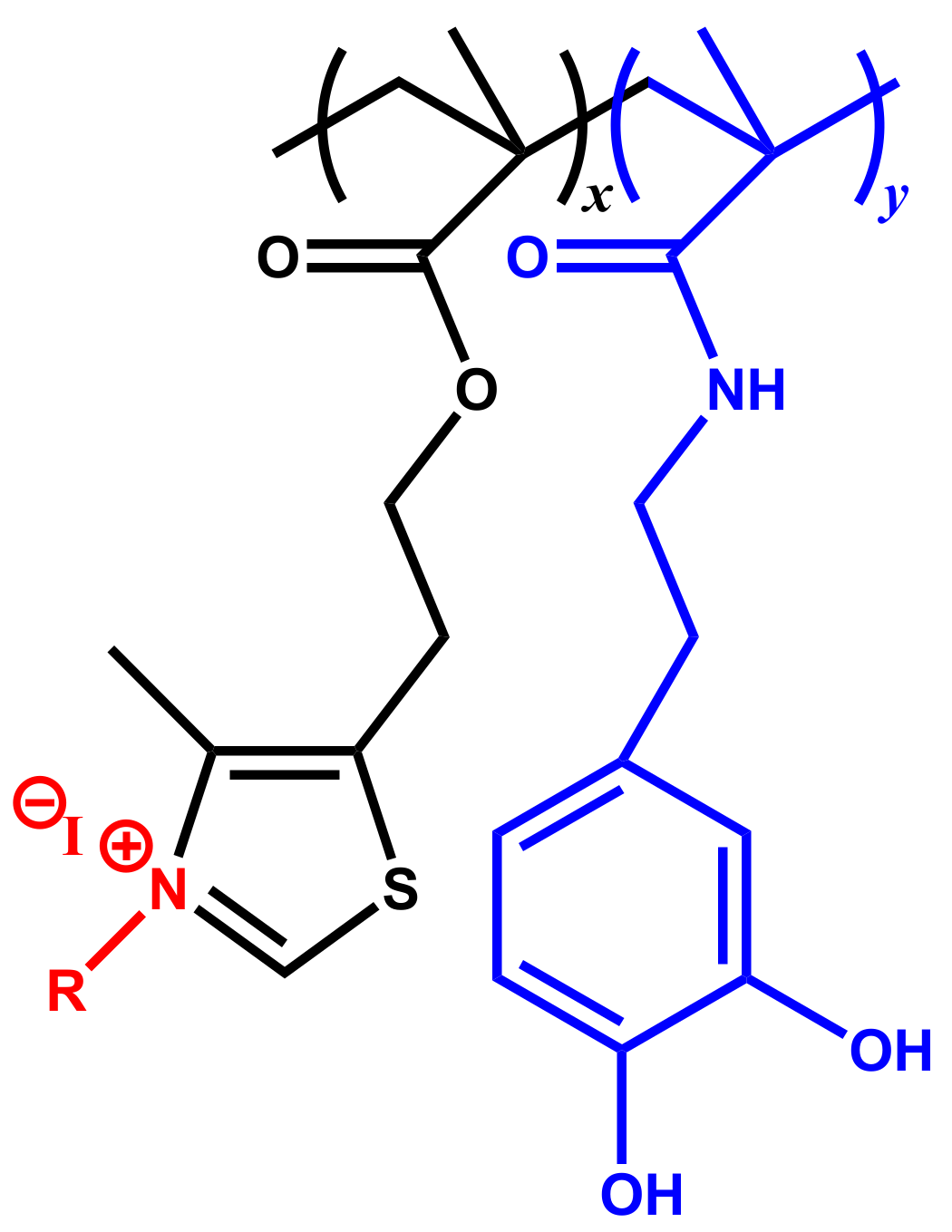
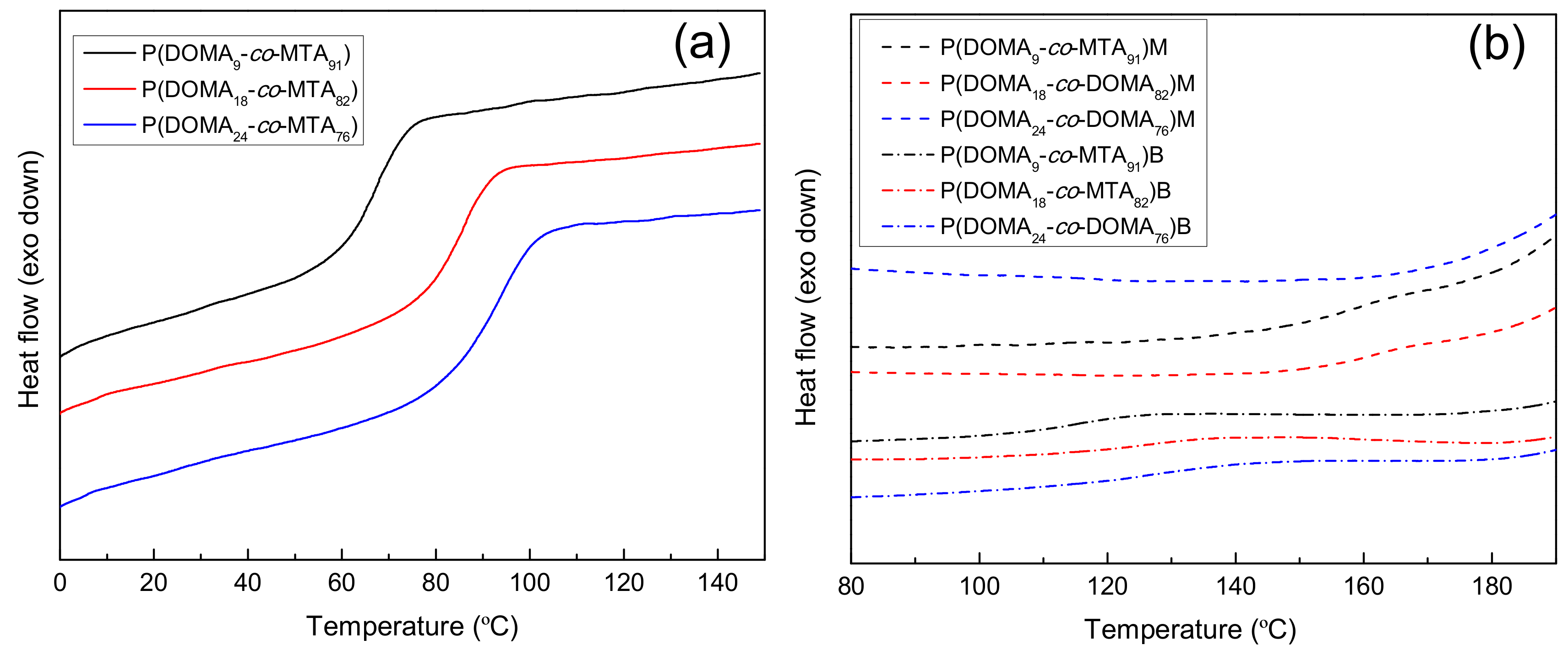
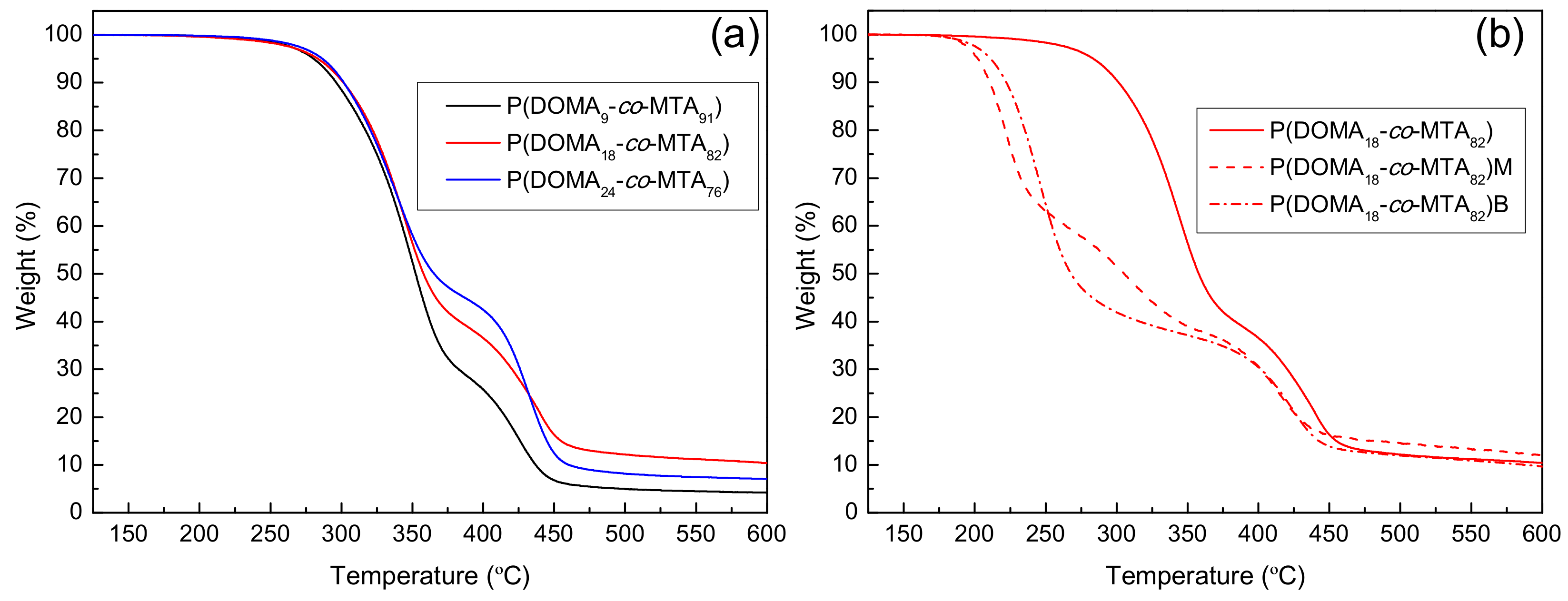
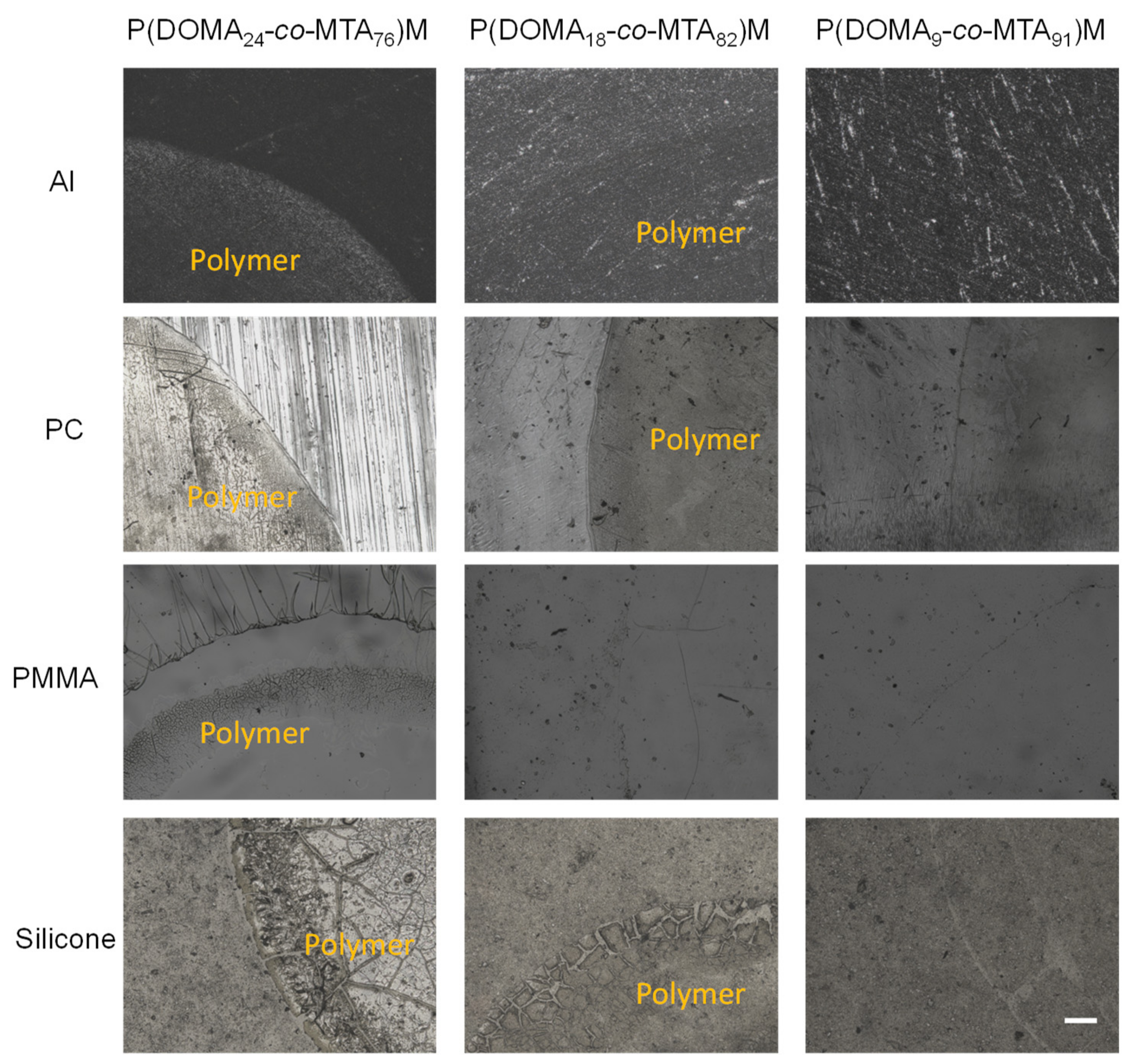
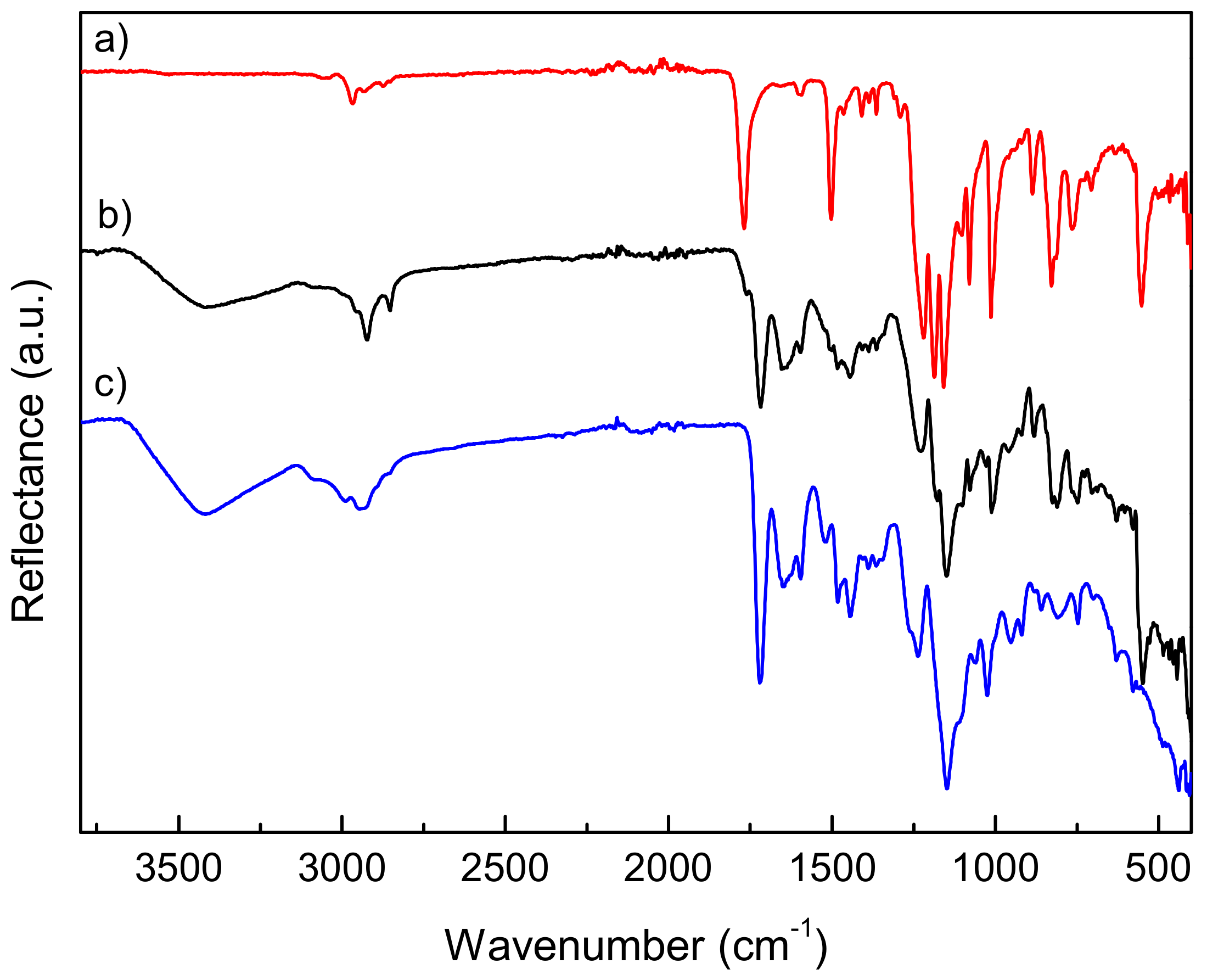
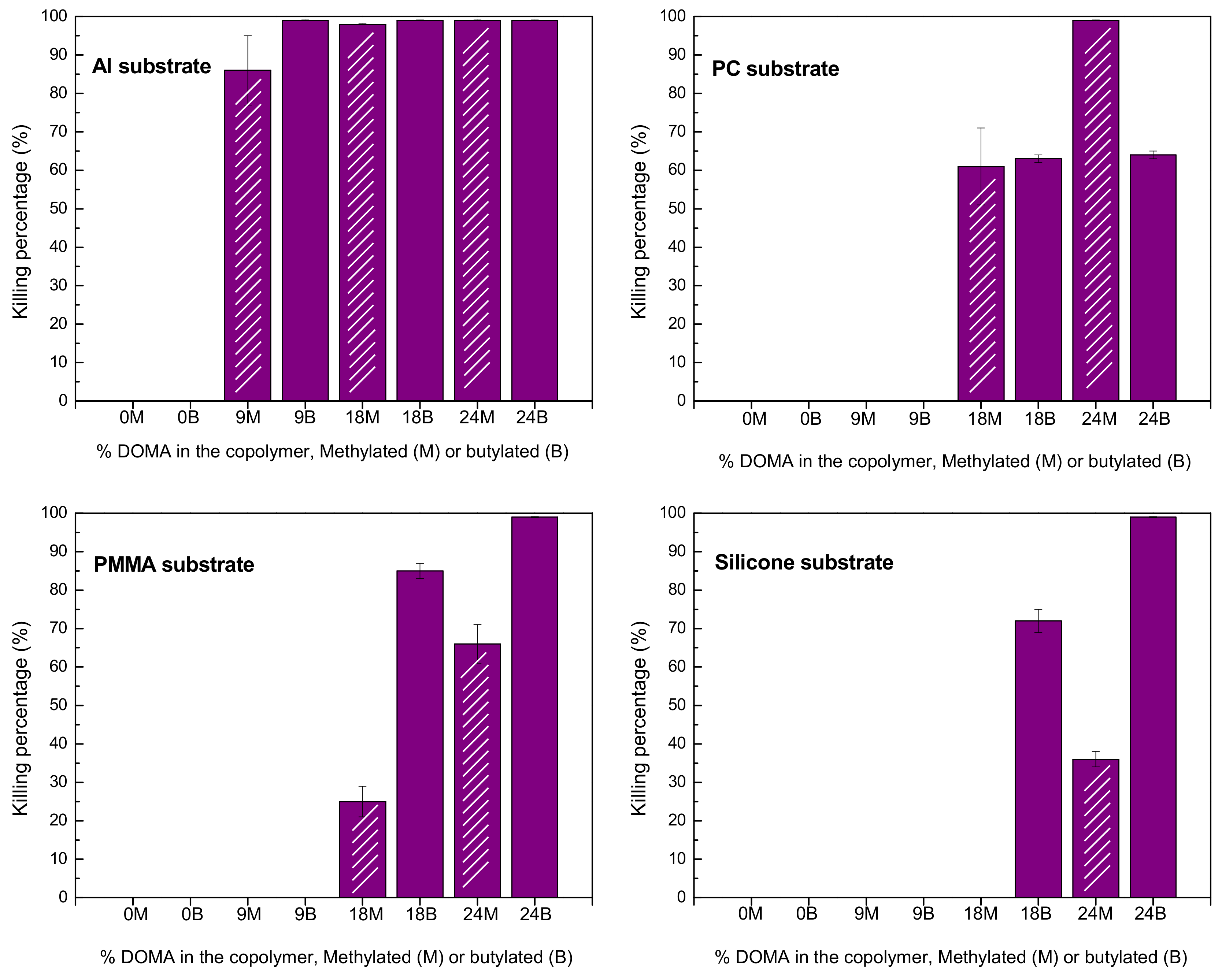
| Copolymers | Tg (°C) | Td10 (°C) | Tdmax1 (°C) | wt.%1 | Tdmax2 (°C) | wt.%2 | Tdmax3 (°C) | wt.%3 |
|---|---|---|---|---|---|---|---|---|
| P(DOMA9-co-MTA91) | 68.5 | 280 | 350 | 71 | – | – | 424 | 23 |
| P(DOMA18-co-MTA82) | 87.5 | 283 | 343 | 60 | – | – | 439 | 34 |
| P(DOMA24-co-MTA76) | 95.0 | 287 | 339 | 54 | – | – | 433 | 40 |
| P(DOMA9-co-MTA91)M | 160.0 | 198 | 221 | 42 | 318 | 26 | 408 | 21 |
| P(DOMA18-co-MTA82)M | 162.0 | 202 | 223 | 40 | 312 | 21 | 415 | 24 |
| P(DOMA24-co-MTA76)M | – | 200 | 226 | 40 | 318 | 22 | 416 | 21 |
| P(DOMA9-co-MTA91)B | 115.0 | 206 | 243 | 66 | – | – | 412 | 28 |
| P(DOMA18-co-MTA82)B | 125.0 | 211 | 245 | 61 | – | – | 424 | 29 |
| P(DOMA24-co-MTA76)B | 126.0 | 210 | 245 | 62 | – | – | 420 | 26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiloeches, A.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A. Influence of Polymer Composition and Substrate on the Performance of Bioinspired Coatings with Antibacterial Activity. Coatings 2019, 9, 733. https://doi.org/10.3390/coatings9110733
Chiloeches A, Echeverría C, Fernández-García M, Muñoz-Bonilla A. Influence of Polymer Composition and Substrate on the Performance of Bioinspired Coatings with Antibacterial Activity. Coatings. 2019; 9(11):733. https://doi.org/10.3390/coatings9110733
Chicago/Turabian StyleChiloeches, Alberto, Coro Echeverría, Marta Fernández-García, and Alexandra Muñoz-Bonilla. 2019. "Influence of Polymer Composition and Substrate on the Performance of Bioinspired Coatings with Antibacterial Activity" Coatings 9, no. 11: 733. https://doi.org/10.3390/coatings9110733
APA StyleChiloeches, A., Echeverría, C., Fernández-García, M., & Muñoz-Bonilla, A. (2019). Influence of Polymer Composition and Substrate on the Performance of Bioinspired Coatings with Antibacterial Activity. Coatings, 9(11), 733. https://doi.org/10.3390/coatings9110733








