Fabrication of Fe3O4@SiO2 Nanofluids with High Breakdown Voltage and Low Dielectric Loss
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Nanofluids
- Fe3O4 nanoparticles: Fe3O4 nanoparticles were prepared by coprecipitation method. A total of 5.4 g of iron (III) chloride hexahydrate and 3.9 g of iron (II) chloride tetrahydrate was mixed in 100 mL of deionized water. The obtained solution was slowly added by 25 mL of ammonia with string at 60 °C for 12 h. The precipitated iron oxide was washed twice with deionized water. The black powder was subsequently dried in vacuum at 70 °C for 24 h.
- Fe3O4@SiO2 nanoparticles: A total of 3.0 g of iron oxide was dispersed into 500 mL of ethanol and ultrasonic treated for 1 h at room temperature, then 2, 4, and 8 mL of tetraethyl orthosilicate (TEOS) were added to the mixtures to obtain Fe3O4@SiO2 nanoparticles with different SiO2 shell thickness. The nanoparticles were subsequently centrifuged and washed several times.
- Modification of Fe3O4@SiO2 nanoparticles: A total of 2.0 g of Fe3O4@SiO2 nanoparticle was dissolved in 300 mL of ethanol, and 0.1 g of oleic acid was then added to the mixture under mechanical agitation for 1 h. After that, the product was washed by ethanol and dried at 60 °C for 24 h.
- Preparation of nanofluids: The three Fe3O4@SiO2 nanoparticles were dispersed into a type of insulating oil, the FR3 mechanized from Cargill, by ultrasonic treatment. They were tagged as sample A, B, and C, respectively. Before the electrical performance test, the three nanofluids and the FR3 were dried at 85 °C under 50 Pa for 72 h to reduce the water content of samples below 60 mg/kg.
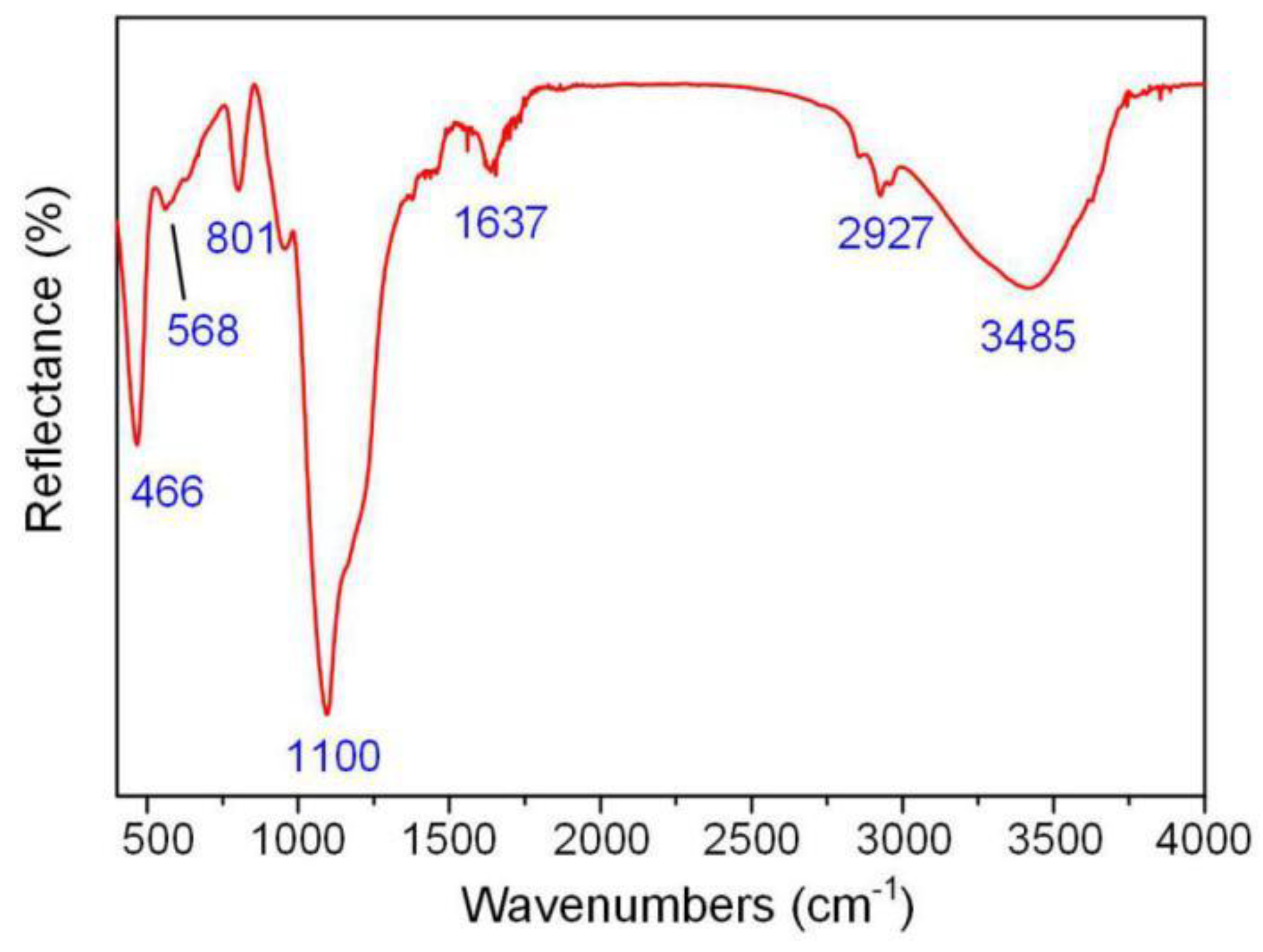
2.2. Nanoparticle Characterization
3. Results and Discussion
3.1. Dispersion Stability of Nanofluids
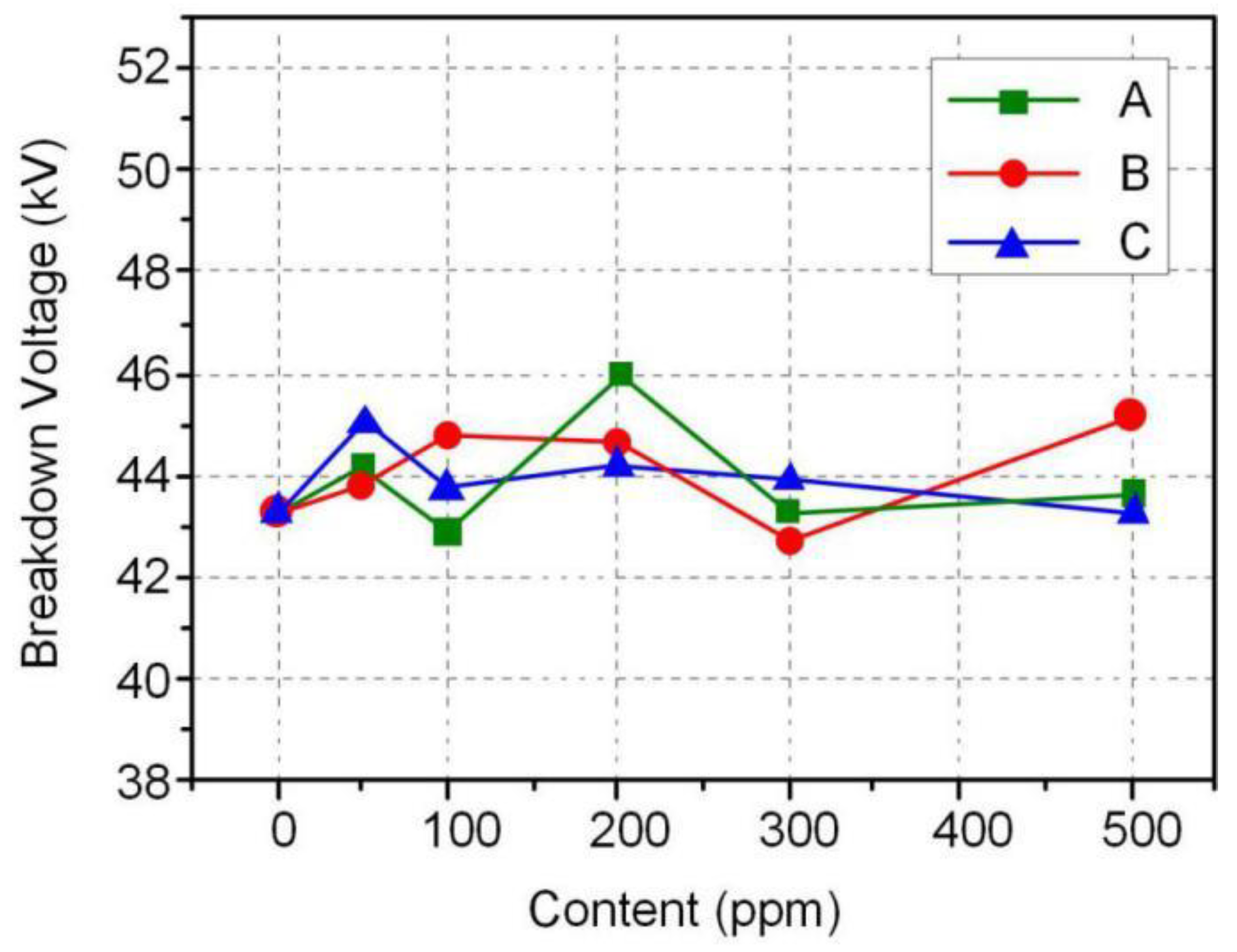
3.2. Breakdown Voltage of Nanofluids
3.3. Dielectric Properties of Nanofluids
4. Conclusions
- In this work, core-shell Fe3O4@SiO2 nanoparticles were synthesized. The TEM test showed the SiO2 shell thickness increased with the increase of TEOS concentration. Zeta potential size test showed the three Fe3O4@SiO2 were uniform nanoparticles.
- The nanofluids with high breakdown voltage and low dielectric loss were developed by dispersing Fe3O4@SiO2 nanoparticles with different thicknesses of SiO2 shell. The long-term dispersion stability of Fe3O4@SiO2 nanoparticles in insulating oil was characterized by natural deposition method. The AC and positive lightning breakdown voltage of nanofluids were significantly improved compared with that of FR3 oil, but the improvement is not obvious for the negative lightning breakdown voltage.
- As the thickness of the SiO2 shell increases, the relative dielectric constant and the dielectric loss factor decrease. When the SiO2 shell is 50 nm, the dielectric loss factor of nanofluids is basically the same as that of FR3 oil. This work will be beneficial to the application of nanofluids in transformers.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, C.; Yoo, H.S.; Oh, J.M. Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr. Appl. Phys. 2007, 8, 710–712. [Google Scholar] [CrossRef]
- Yao, W.; Huang, Z.; Li, J.; Wu, L.; Xiang, C. Enhanced electrical insulation and heat transfer performance of vegetable oil based nanofluids. J. Nanomater. 2018, 2018, 4504208. [Google Scholar] [CrossRef]
- Thabet, A.; Allam, M.; Shaaban, S.A. Investigation on enhancing breakdown voltages of transformer oil nanofluids using multi-nanoparticles technique. Inst. Eng. Technol. 2017, 12, 1171–1176. [Google Scholar]
- Li, J.; Du, B.; Wang, F.; Yao, W.; Yao, S. The effect of nanoparticle surfactant polarization on trapping depth of vegetable insulating oil-based nanofluids. Phys. Lett. A 2016, 380, 604–608. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Q.; Zhou, Y.; Li, C.; Ge, Y.; Qi, B. Effect of Fe3O4 nanoparticles on positive streamer propagation in transformer oil. AIP Adv. 2016, 6, 035110. [Google Scholar] [CrossRef]
- Takada, T.; Hayase, Y.; Tanaka, Y.; Okamoto, T. Space charge trapping in electrical potential well caused by permanent and induced dipoles. Annu. Rep. Conf. Electr. Insul. Dielectr. Phenom. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhong, Y.; Zhang, S.; Zhou, Y. Effect of nanoparticles on charge transport in nanofluid-impregnated pressboard. J. Appl. Phys. 2012, 111, 1243221. [Google Scholar] [CrossRef]
- Lanje, A.S.; Sharma, S.J.; Ningthoujam, R.S.; Ahn, J.S.; Pode, R.B. Low temperature dielectric studies of zinc oxide (ZnO) nanoparticles prepared by precipitation method. Adv. Powder Technol. 2013, 24, 331–335. [Google Scholar] [CrossRef]
- Hwang, J.G.; Zahn, M.; O’Sullivan, F.M.; Pettersson, L.A.; Hjortstam, O.; Liu, R. Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J. Appl. Phys. 2010, 107, 014310. [Google Scholar] [CrossRef]
- Mozaffari, S.; Li, W.; Dixit, M.; Seifert, S.; Lee, B.; Kovarik, L.; Mpourmpakis, G.; Karim, A.M. The role of nanoparticle size and ligand coverage in size focusing of colloidal metal nanoparticles. Nanoscale Adv. 2019, 1, 4052–4066. [Google Scholar] [CrossRef]
- Li, W.; Ivanov, S.; Mozaffari, S.; Shanaiah, N.; Karim, A.M. Palladium acetate trimer: Understanding its ligand-induced dissociation thermochemistry using isothermal titration calorimetry, X-ray absorption fine structure, and 31P nuclear magnetic resonance. Organometallics 2019, 38, 451–460. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Cheng, Y.; Zhang, X. The dielectric properties improvement of cable insulation layer by different morphology nanoparticles doping into LDPE. Coatings 2019, 9, 204. [Google Scholar] [CrossRef]
- Tkach, A.; Santos, A.; Zlotnik, S.; Serrazina, R.; Okhay, O.; Bdikin, I.; Costa, M.E.; Vilarinho, P. Strain-mediated substrate effect on the dielectric and ferroelectric response of potassium sodium niobate thin films. Coatings 2018, 8, 449. [Google Scholar] [CrossRef]
- Li, M.; Huang, X.; Wu, C.; Xu, H.; Jiang, P.; Tanaka, T. Fabrication of two-dimensional hybrid sheets by decorating insulating PANI on reduced graphene oxide for polymer nanocomposites with low dielectric loss and high dielectric constant. J. Mater. Chem. 2012, 22, 23477–23484. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Saviuc, C.; Chifiriuc, M.C.; Hristu, R.; Mihaiescu, D.E.; Balaure, P.; Stanciu, G.; Lazar, V. Inhibitory activity of Fe3O4/Oleic acid/usnic acid-core/shell/extra-shell nanofluid on S. aureus biofilm development. IEEE Trans. Nanobiosci. 2011, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- IEC 60156: 2018. Insulating Liquids—Determination of the Breakdown Voltage at Power Frequency; IEC: Geneva, Switzerland, 2018.
- IEC 60897: 2018. Methods for the Determination of the Lightning Breakdown Voltage of Insulating Liquids; IEC: Geneva, Switzerland, 2018.
- Li, J.; Zhang, Z.; Zou, P. Preparation of a vegetable oil-based nanofluid and investigation of its breakdown and dielectric properties. IEEE Electr. Insul. Mag. 2012, 28, 43–50. [Google Scholar] [CrossRef]
- Miao, J.; Dong, M.; Ren, M.; Wu, X.; Shen, L.; Wang, H. Effect of nanoparticle polarization on relative permittivity of transformer oil-based nanofluids. J. Appl. Phys. 2013, 113, 204103. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Shi, Y.; Liu, Q. Fabrication of Fe3O4@SiO2 Nanofluids with High Breakdown Voltage and Low Dielectric Loss. Coatings 2019, 9, 716. https://doi.org/10.3390/coatings9110716
Du B, Shi Y, Liu Q. Fabrication of Fe3O4@SiO2 Nanofluids with High Breakdown Voltage and Low Dielectric Loss. Coatings. 2019; 9(11):716. https://doi.org/10.3390/coatings9110716
Chicago/Turabian StyleDu, Bin, Yu Shi, and Qian Liu. 2019. "Fabrication of Fe3O4@SiO2 Nanofluids with High Breakdown Voltage and Low Dielectric Loss" Coatings 9, no. 11: 716. https://doi.org/10.3390/coatings9110716
APA StyleDu, B., Shi, Y., & Liu, Q. (2019). Fabrication of Fe3O4@SiO2 Nanofluids with High Breakdown Voltage and Low Dielectric Loss. Coatings, 9(11), 716. https://doi.org/10.3390/coatings9110716



