Effects of Chitosan Coating Structure and Changes during Storage on Their Egg Preservation Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
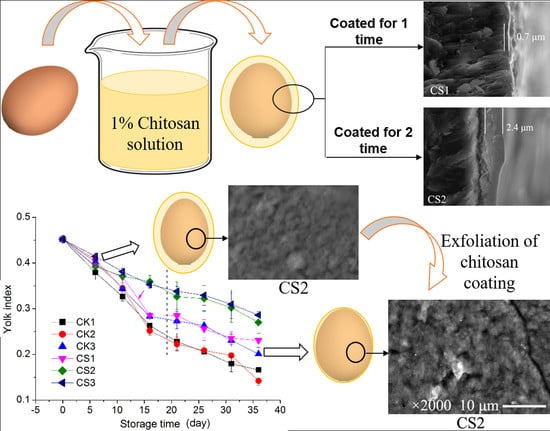
2.2. Treatment of Eggs
2.3. Characterizations of Eggs
2.3.1. Weight Loss
2.3.2. Haugh Unit
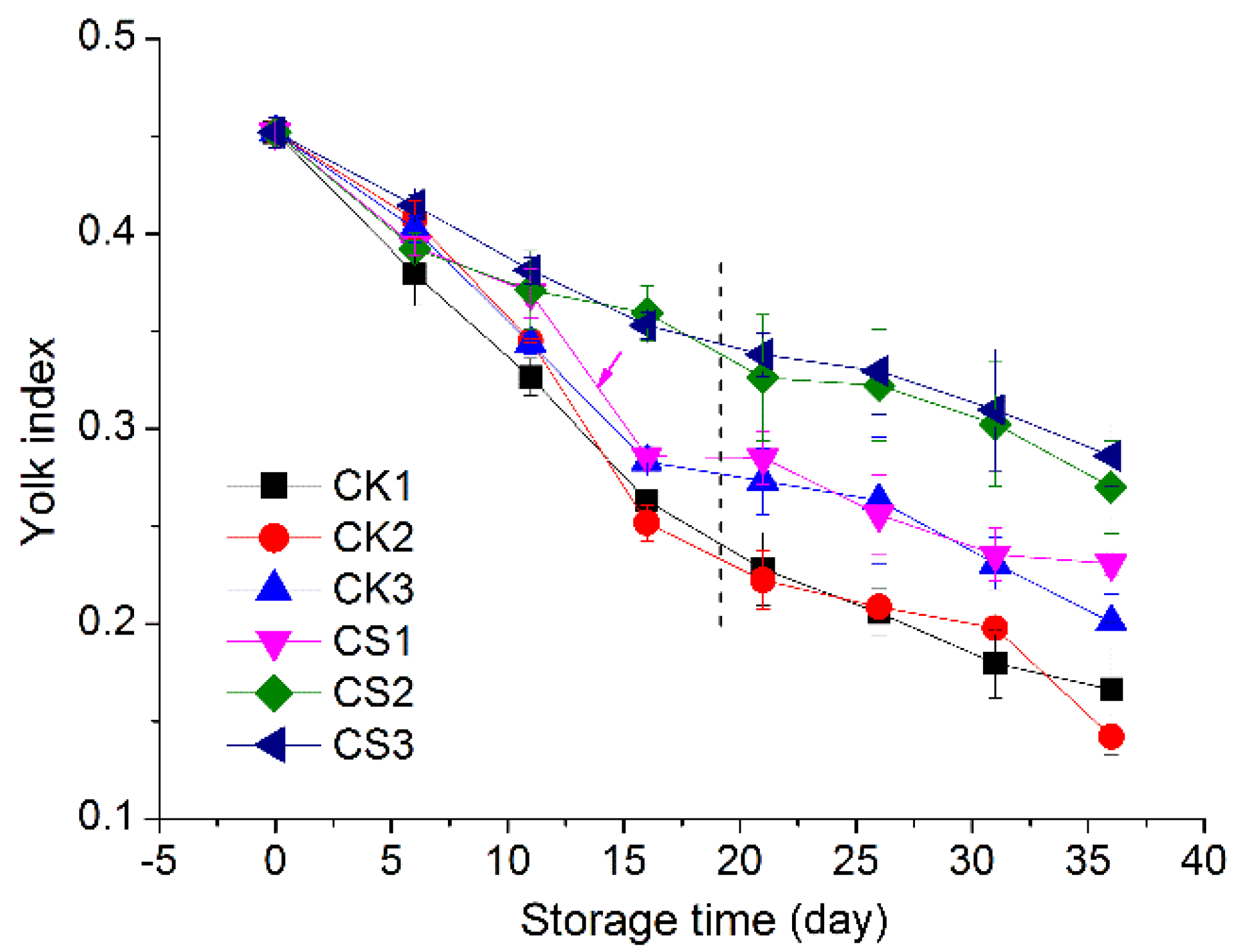
2.3.3. Yolk Index
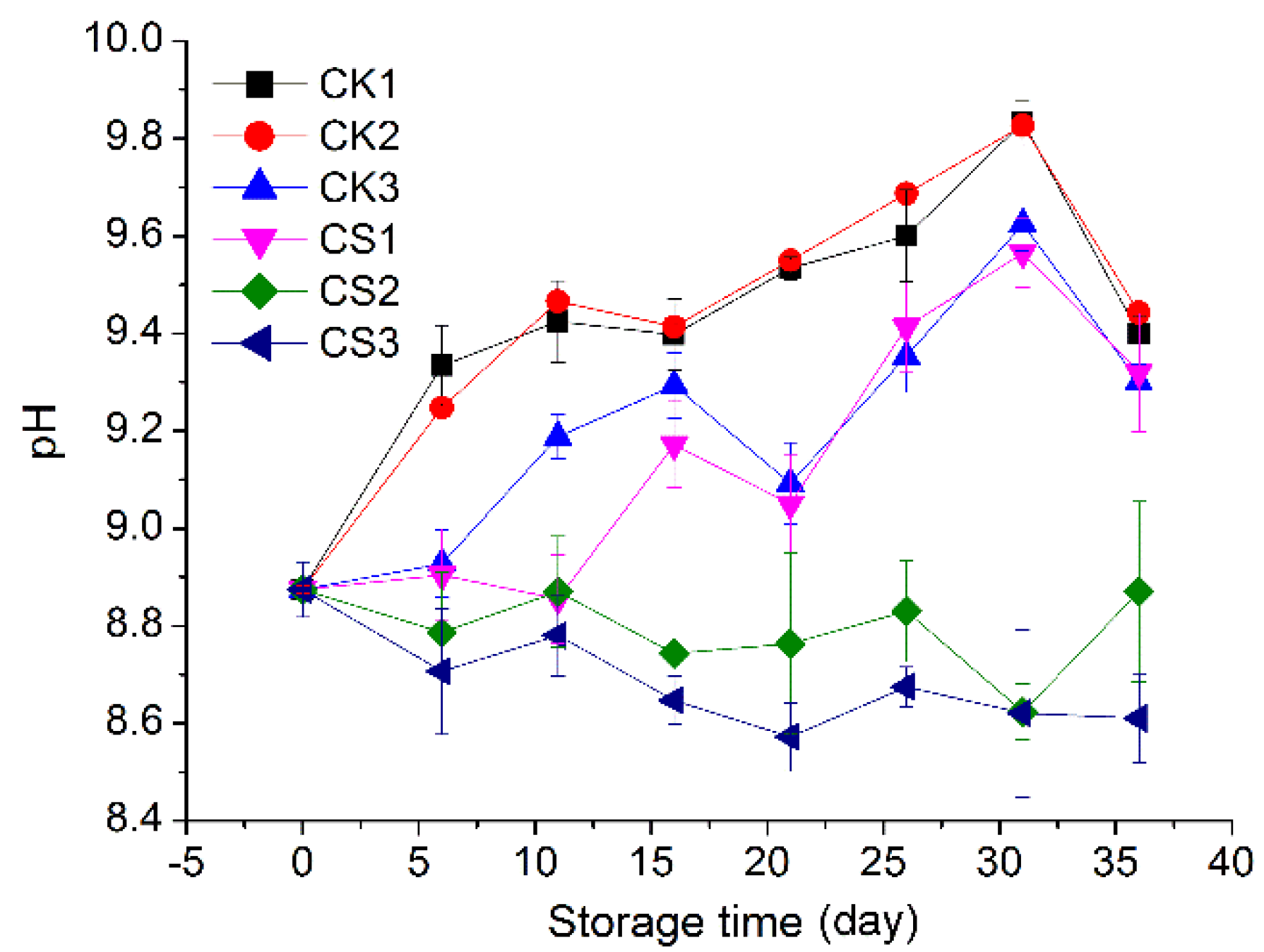
2.3.4. pH of Albumen
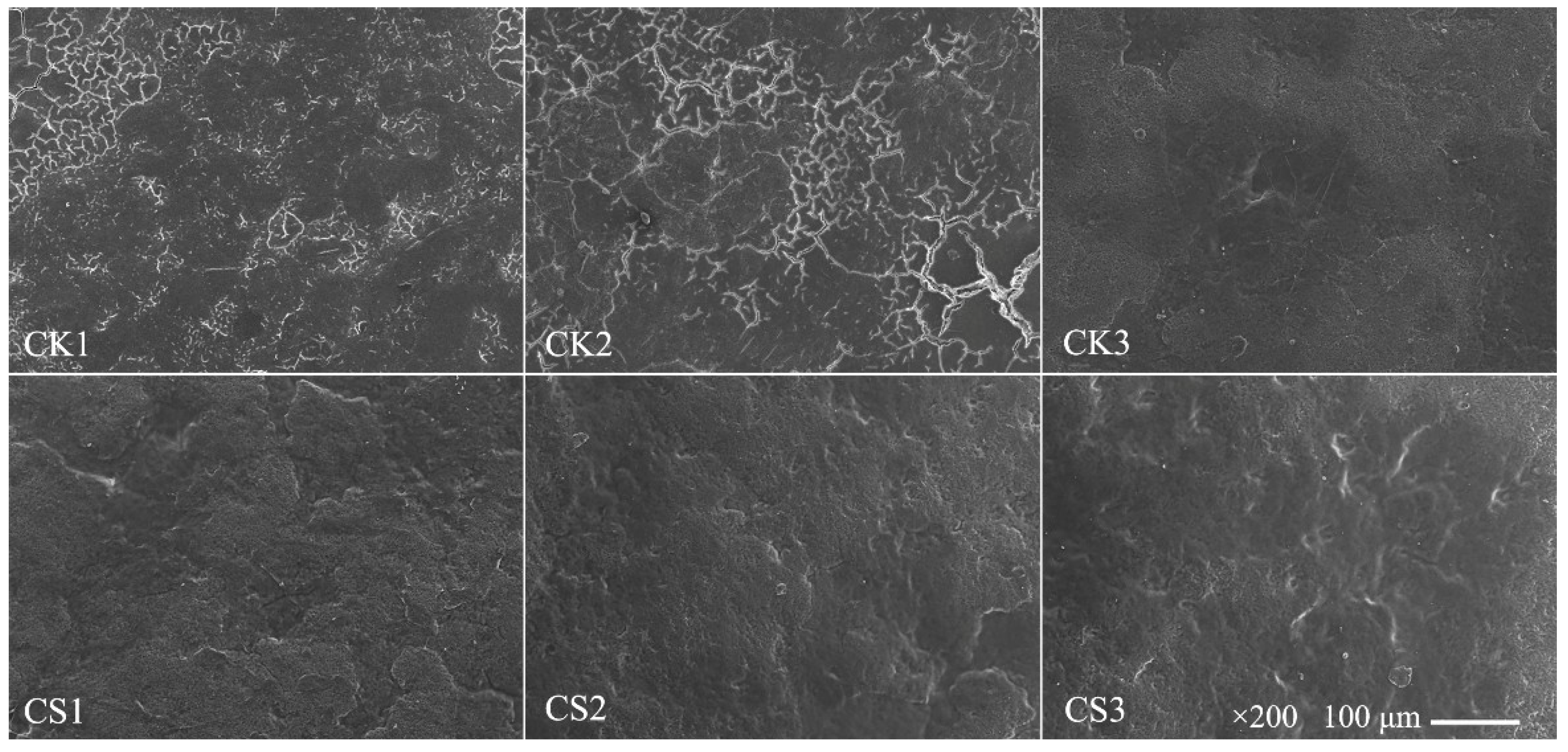
2.3.5. Scanning Electron Microscopy (SEM) of Eggshells
2.3.6. Fourier Transform Infrared (FTIR) Spectra of Eggshells
2.3.7. Statistical Analysis
3. Results
3.1. Weight Loss
3.2. Haugh Unit
3.3. Yolk Index
3.4. pH of Albumen
3.5. Morphology of Eggshells
3.6. FTIR Spectra of Eggshells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuroli, S.; Kanoo, T.; Itoh, H.; Ohkawa, Y. Nondestructive measurement of yolk viscosity in lightly heated chicken shell eggs. J. Food Eng. 2017, 205, 18–24. [Google Scholar] [CrossRef]
- Kostogrys, R.B.; Filipiak-Florkiewicz, A.; Dereń, K.; Drahun, A.; Czyzynska-Cichon, I.; Cieślik, E.; Szymczyk, B.; Franczyk-Zarow, M. Effect of dietary pomegranate seed oil on laying hen performance and physicochemical properties of eggs. Food Chem. 2017, 221, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Sharoba, A.M.; Khalaf, H.H.; El-Tanahy, H.H.; Cutter, C.N. Efficacy of antimicrobial pullulan-based coating to improve internal quality and shelf-life of chicken eggs during storage. J. Food Sci. 2015, 80, M1066–M1074. [Google Scholar] [CrossRef] [PubMed]
- Damaziak, K.; Marzec, A.; Riedel, J.; Szeliga, J.; Koczywas, E.; Cisneros, F.; Michalczul, M.; Łukasiewicz, M.; Gozdowski, D.; Siennicka, A. Effect of dietary canthaxanthin and iodine on the production performance and egg quality of laying hens. Poult. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sert, D.; Aygun, A.; Demir, M.K. Effects of ultrasonic treatment and storage temperature on egg quality. Poult. Sci. 2011, 90, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Yüceer, M.; Aday, M.S.; Caner, C. Ozone treatment of shell eggs to preserve functional quality and enhance shelf life during storage. J. Sci. Food Agric. 2016, 96, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Priyadharshini, M.L.M.; Nirmala, K.; Raman, M.; Raj, G.D. Bactericidal paper trays doped with silver nanoparticles for egg storing applications. Bull. Mater. Sci. 2016, 39, 819–826. [Google Scholar] [CrossRef]
- Aygun, A.; Sert, D. Effects of vacuum packing on eggshell microbial activity and egg quality in table eggs under different storage temperatures. J. Sci. Food Agric. 2013, 93, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.N.; No, H.K.; Prinyawiwatkul, W. Internal quality and shelf life of eggs coated with oils from different sources. J. Food Sci. 2011, 76, S325–S329. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.C.; Assis, D.C.S.; Menezes, L.D.M.; Oliveira, D.D.; Lima, A.L.; Souza, M.R.; Heneine, L.G.D.; Cançado, S.V. Effects of packaging, mineral oil coating, and storage time on biogenic amine levels and internal quality of eggs. Poult. Sci. 2014, 93, 3171–3178. [Google Scholar] [CrossRef] [PubMed]
- Caner, C.; Yüceer, M. Efficacy of various protein-based coating on enhancing the shelf life of fresh eggs during storage. Poult. Sci. 2015, 94, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, D.; Hooshmand, K.; Kemmer, G.C.; Kirkensgaard, J.J.K.; Mortensen, K.; Giosafatto, C.V.L.; Holse, M.; Hebelstrup, K.H.; Bao, J.; Stelte, W.; et al. Cross-linked amylose bio-plastic: A transgenic-based compostable plastic alternative. Int. J. Mol. Sci. 2017, 18, 2075. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of Citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kopacic, S.; Bauer, W.; Walzl, A.; Leitner, E.; Zankel, A. Alginate and chitosan as a functional barrier for paper-based packaging materials. Coatings 2018, 8, 235. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT—Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Bhale, S.; No, H.K.; Prinyawiwatkul, W.; Farr, A.J.; Nadarajah, K.; Meyers, S.P. Chitosan coating improves shelf life of eggs. J. Food Sci. 2003, 68, 2378–2383. [Google Scholar] [CrossRef]
- Kim, S.H.; No, H.K.; Prinyawiwatkul, W. Plasticizer types and coating methods affect quality and shelf life of eggs coated with chitosan. J. Food Sci. 2008, 73, S111–S117. [Google Scholar] [CrossRef] [PubMed]
- AMS-56 United States Standards, Grades, and Weight Classes for Shell Eggs; USDA: Washington, DC, USA, 2000.
- Pujols, K.D.; Osorio, L.; Carrillo, E.P.; Wardy, W.; Torrico, D.D.; No, H.K.; Corredor, J.A.H.; Prinyawiwatkul, W. Comparing effects of α- vs. β-chitosan coating and emulsion coatings on egg quality during room temperature storage. Int. J. Food Sci. Technol. 2014, 49, 1383–1390. [Google Scholar] [CrossRef]
- Legendre, G.; Vallée-Réhel, K.; Linossier, I.; Faÿ, F. Evaluation of ionically cross-linked chitosan coating aimed at eggs’ protection. Int. J. Food Sci. Technol. 2015, 50, 736–743. [Google Scholar] [CrossRef]
- Jin, T.Z.; Gurtler, J.B.; Li, S.-Q. Development of antimicrobial coatings for improving the microbiological safety and quality of shell eggs. J. Food Prot. 2013, 76, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Torrico, D.D.; Wardy, W.; Carabante, K.M.; Pujols, K.D.; Xu, Z.; No, H.K.; Prinyawiwatkul, W. Quality of eggs coated with oil-chitosan emulsion: Combined effects of emulsifier types, initial albumen quality, and storage. LWT—Food Sci. Technol. 2014, 57, 35–41. [Google Scholar] [CrossRef]
- Yi, S.-Z.; Liu, Q.; Xu, D.; Li, D. Preservation effects of chitosan/nano-montmorillonite coating on clean eggs. Packag. Eng. 2017, 7, 12. (In Chinese) [Google Scholar]
- Suresh, P.V.; Raj, K.R.; Nidheesh, T.; Pal, G.K.; Sakhare, P.Z. Application of chitosan for improvement of quality and shelf life of table eggs under tropical room conditions. J. Food Sci. Technol. 2015, 52, 6345–6354. [Google Scholar] [CrossRef] [PubMed]
- Jirangrat, W.; Torrico, D.D.; No, J.; No, H.K.; Prinyawiwatkul, W. Effects of mineral oil coating on internal quality of chicken eggs under refrigerated storage. Int. J. Food Sci. Technol. 2010, 45, 490–495. [Google Scholar] [CrossRef]
- Munoz, A.; Dominguez-Gasca, N.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.B. Importance of eggshell cuticle composition and maturity for avoiding trans-shell Salmonella contamination in chicken eggs. Food Control 2015, 55, 31–38. [Google Scholar] [CrossRef]
- Pan, C.; Zhao, Y.X.; Liao, S.F.; Chen, F.; Qin, S.Y.; Wu, X.S.; Zhou, H.; Huang, K.H. Effect of selenium-enriched probiotics on laying performance, egg quality, egg selenium content, and egg glutathione peroxidase activity. J. Agric. Food Chem. 2011, 59, 11424–11431. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, J.; Huang, M.J.; Xu, Q.; Ma, M.H. The changes of secondary structures and properties of lysozyme along with the egg storage. Int. J. Biol. Macromol. 2016, 92, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Huang, M.J.; Wang, J.; Xu, Q.; Hammad, H.H.M.; Ma, M.H. A study of storage impact on ovalbumin structure of chicken egg. J. Food Eng. 2018, 219, 1–7. [Google Scholar] [CrossRef]
- Omana, D.A.; Liang, Y.; Kav, N.N.; Wu, J. Proteomic analysis of egg white proteins during storage. Proteomics 2011, 11, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Nongtaodum, S.; Jangchud, A.; Jangchud, K.; Dhamvithee, P.; No, H.K.; Prinyawiwatkul, W. Oil coating affects internal quality and sensory acceptance of selected attributes of raw eggs during storage. J. Food. Sci. 2013, 78, S329–S335. [Google Scholar] [CrossRef] [PubMed]
- Markovski, J.S.; Markovic, D.D.; Dokic, V.R.; Mitric, M.; Ristic, M.D.; Onjia, A.E.; Marinkovic, A.D. Arsenate adsorption on waste eggshell modified by goethite, α-MnO2 and goethite/α-MnO2. Chem. Eng. J. 2014, 237, 430–442. [Google Scholar] [CrossRef]
- Pop, O.L.; Vodnar, D.V.; Suharoschi, R.; Mudura, E.; Socaciu, C. L. plantarum ATCC 8014 entrapment with prebiotics and lucerne green juice and their behavior in simulated gastrointestinal conditions. J. Food Process Eng. 2015, 39, 433–441. [Google Scholar] [CrossRef]
- Dominguez-Gasca, N.; Muñoz, A.; Rodriguez-Navarro, A.B. Quality assessment of chicken eggshell cuticle by infrared spectroscopy and staining techniques: A comparative study. Br. Poult. Sci. 2017, 58, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Galvis-Sánchez, A.C.; Castro, M.C.R.; Biernacki, K.; Gonçalves, M.P.; Souza, H.K.S. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar] [CrossRef]
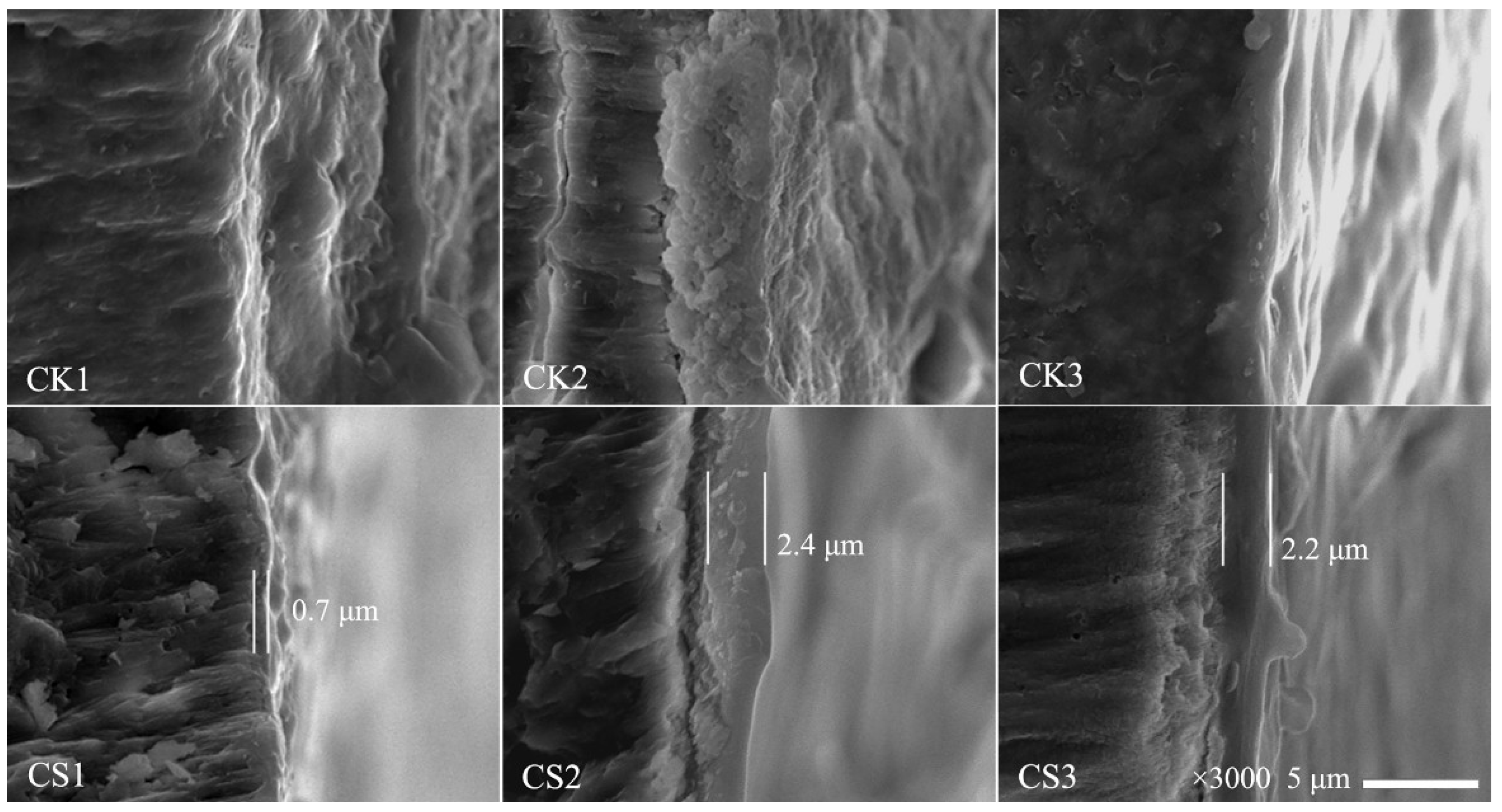
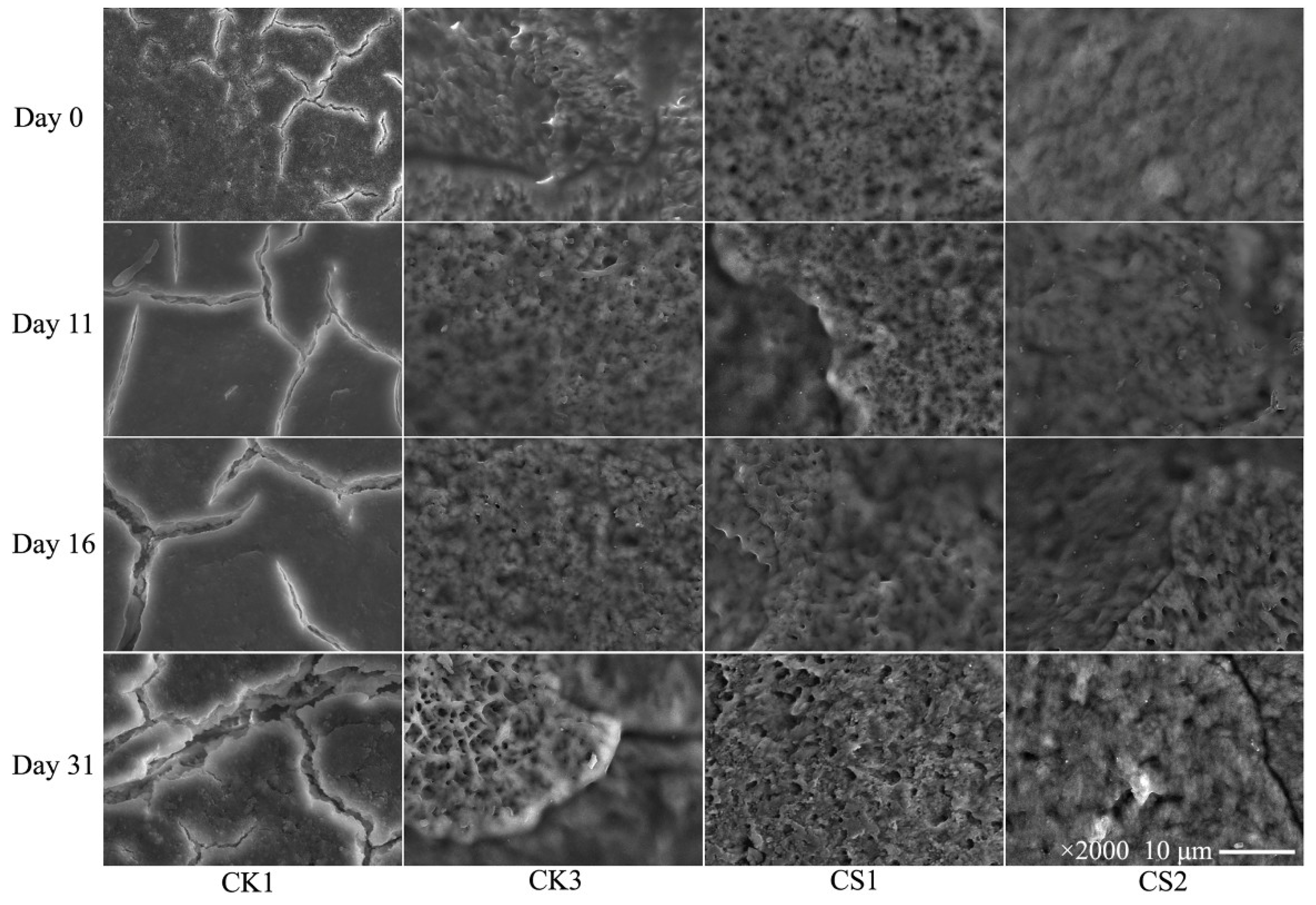

| Storage Time (day) | Weight Loss (%) | |||||
|---|---|---|---|---|---|---|
| CK1 | CK2 | CK3 | CS1 | CS2 | CS3 | |
| 6 | 0.90 ± 0.07 a | 1.01 ± 0.37 a | 0.88 ± 0.17 a | 0.78 ± 0.11 a | 0.92 ± 0.17 a | 0.80 ± 0.19 a |
| 11 | 1.90 ± 0.15 a | 2.12 ± 0.81 a | 1.72 ± 0.47 a | 1.66 ± 0.24 a | 1.67 ± 0.45 a | 1.68 ± 0.42 a |
| 16 | 2.65 ± 0.23 a | 2.76 ± 0.02 a | 2.46 ± 0.45 a,b | 2.31 ± 0.32 a,b | 1.91 ± 0.27 b | 2.01 ± 0.32 b |
| 21 | 3.22 ± 0.24 a,b | 3.27 ± 0.39 a | 3.18 ± 0.58 a,b | 2.95 ± 0.41 a,b | 2.46 ± 0.35 b | 2.61 ± 0.42 a,b |
| 26 | 3.93 ± 0.33 a,b | 4.21 ± 0.02 a | 3.87 ± 0.69 a,b,c | 3.58 ± 0.48 a,b,c | 2.99 ± 0.42 c | 3.19 ± 0.50 b,c |
| 31 | 4.72 ± 0.36 a | 4.74 ± 0.56 a | 4.19 ± 0.07 a,b | 3.96 ± 0.25 a,b | 3.61 ± 0.52 b | 3.84 ± 0.60 b |
| 36 | 5.92 ± 0.48 a,b | 6.18 ± 0.04 a | 5.09 ± 0.09 b,c | 5.02 ± 0.47 b,c | 4.38 ± 0.63 c | 4.64 ± 0.73 c |
| Storage Time (day) | Haugh Unit | |||||
|---|---|---|---|---|---|---|
| CK1 | CK2 | CK3 | CS1 | CS2 | CS3 | |
| 0 | 83.83 ± 0.81 | 83.83 ± 0.81 | 83.83 ± 0.81 | 83.83 ± 0.81 | 83.83 ± 0.81 | 83.83 ± 0.81 |
| 6 | 65.43 ± 2.85 c | 62.34 ± 0.55 c | 73.69 ± 4.56 b | 74.90 ± 1.56 a,b | 78.92 ± 3.98 a,b | 80.44 ± 4.70 a |
| 11 | 62.92 ± 1.67 c | 61.72 ± 1.27 c | 68.68 ± 1.89 b | 73.37 ± 4.43 a,b | 73.37 ± 4.43 a,b | 73.54 ± 3.50 a |
| 16 | 58.10 ± 2.14 c | 57.89 ± 1.40 c | 60.02 ± 3.46 b,c | 62.04 ± 3.42 b,c | 72.22 ± 4.58 a | 73.09 ± 1.75 a |
| 21 | 56.54 ± 3.93 b | 54.39 ± 2.31 b | 54.35 ± 2.69 b | 54.03 ± 0.96 b | 66.27 ± 1.41 a | 68.34 ± 5.01 a |
| 26 | 54.54 ± 5.25 c | 57.04 ± 2.33 b | 53.33 ± 1.79 c | 55.16 ± 1.16 b,c | 63.28 ± 3.81 a | 65.79 ± 3.76 a |
| 31 | 53.91 ± 2.89 b | 52.81 ± 2.98 b | 53.29 ± 3.89 b | 54.55 ± 7.41 b | 60.56 ± 5.26 a | 56.95 ± 1.65 a,b |
| 36 | 51.39 ± 2.74 a | 50.53 ± 3.20 a | 52.37 ± 1.08 a | 53.96 ± 0.91 a | 57.95 ± 8.11 a | 56.53 ± 2.89 a |
| Samples | 0–16 Days | 21–36 Days | ||
|---|---|---|---|---|
| Slope | R2 | Slope | R2 | |
| CK1 | −0.0117 | 0.9990 | −0.0042 | 0.9852 |
| CK2 | −0.0124 | 0.9585 | −0.0050 | 0.8514 |
| CK3 | −0.0106 | 0.9916 | −0.0050 | 0.9591 |
| CS1 | −0.0098 | 0.9536 | −0.0037 | 0.9162 |
| CS2 | −0.0057 | 0.9064 | −0.0038 | 0.8962 |
| CS3 | −0.0062 | 0.9991 | −0.0035 | 0.9625 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Wang, J.; Ren, D.; Wu, X. Effects of Chitosan Coating Structure and Changes during Storage on Their Egg Preservation Performance. Coatings 2018, 8, 317. https://doi.org/10.3390/coatings8090317
Xu D, Wang J, Ren D, Wu X. Effects of Chitosan Coating Structure and Changes during Storage on Their Egg Preservation Performance. Coatings. 2018; 8(9):317. https://doi.org/10.3390/coatings8090317
Chicago/Turabian StyleXu, Dan, Jing Wang, Dan Ren, and Xiyu Wu. 2018. "Effects of Chitosan Coating Structure and Changes during Storage on Their Egg Preservation Performance" Coatings 8, no. 9: 317. https://doi.org/10.3390/coatings8090317
APA StyleXu, D., Wang, J., Ren, D., & Wu, X. (2018). Effects of Chitosan Coating Structure and Changes during Storage on Their Egg Preservation Performance. Coatings, 8(9), 317. https://doi.org/10.3390/coatings8090317