Hemin-Modified SnO2/Metglas Electrodes for the Simultaneous Electrochemical and Magnetoelastic Sensing of H2O2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hemin/SnO2–Metglas Electrodes
2.3. Characterization of the Hemin/SnO2-Metglas Film Electrodes
2.4. Experimental Setup for MR and CV Measurements
2.5. Experimental Preparation and Procedure
3. Results and Discussion
3.1. X-ray Diffraction
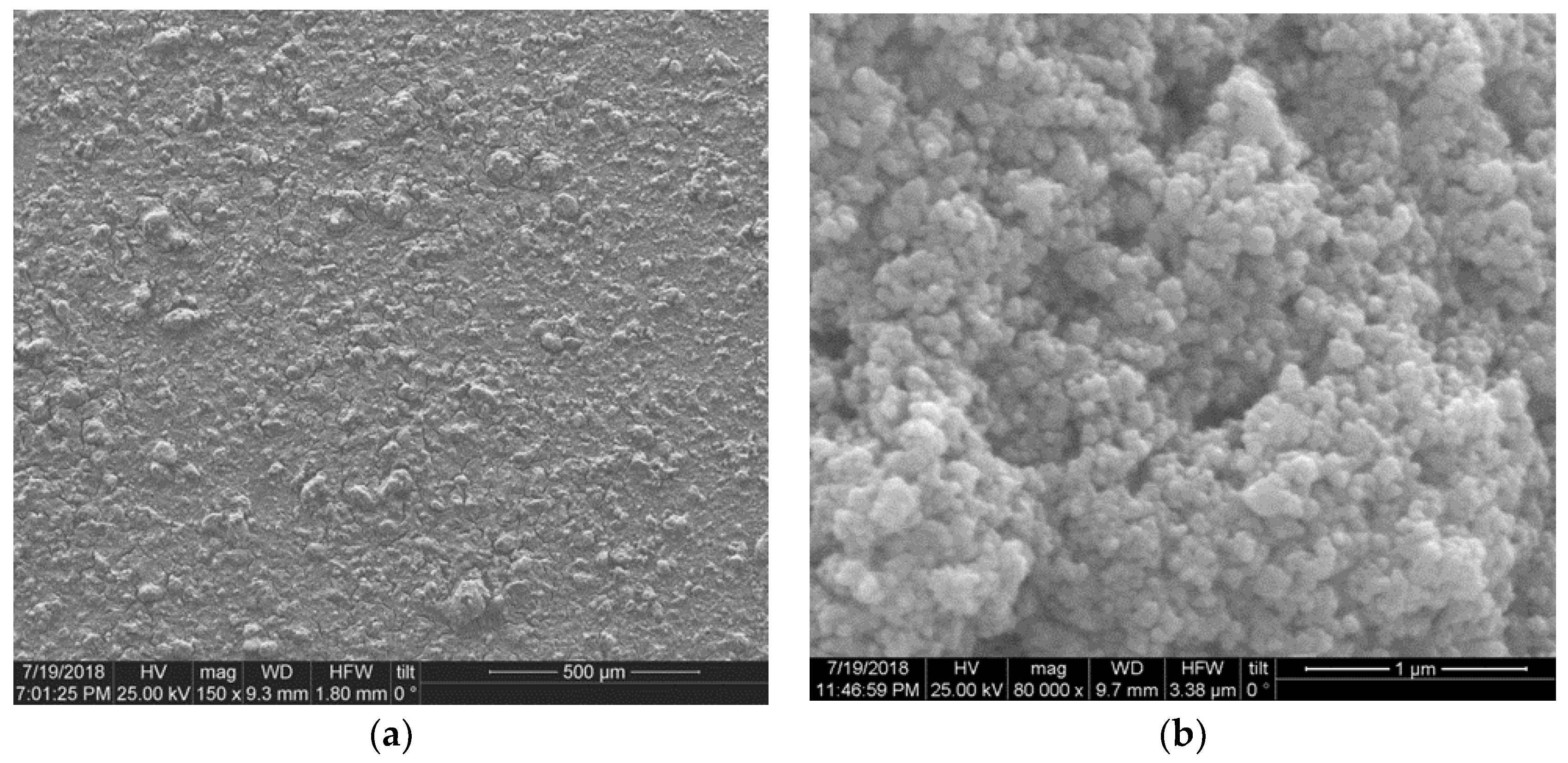
3.2. FE-SEM Imaging of Surface Topography
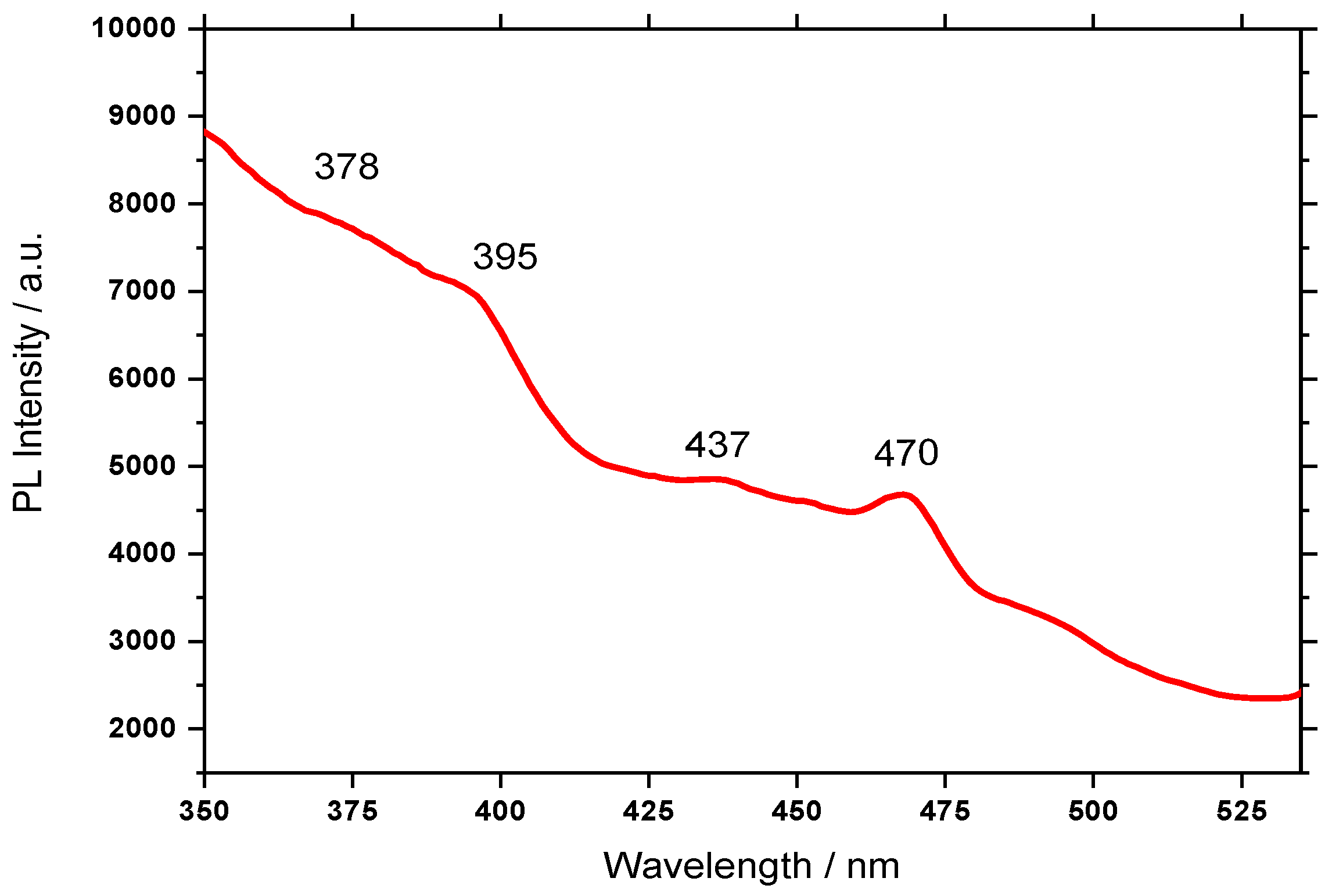
3.3. Photoluminescence Properties
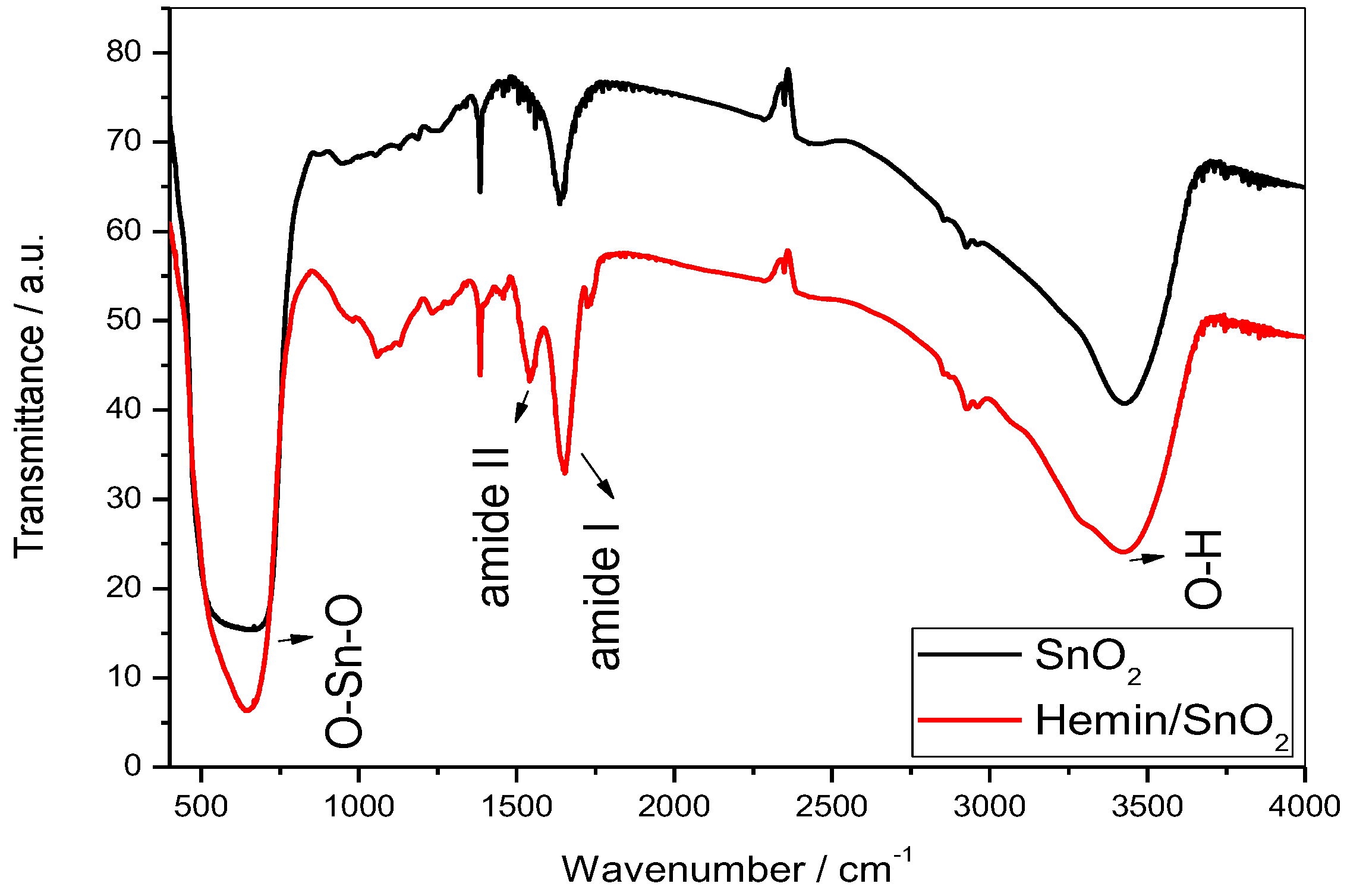
3.4. Fourier Transform Infrared Analysis
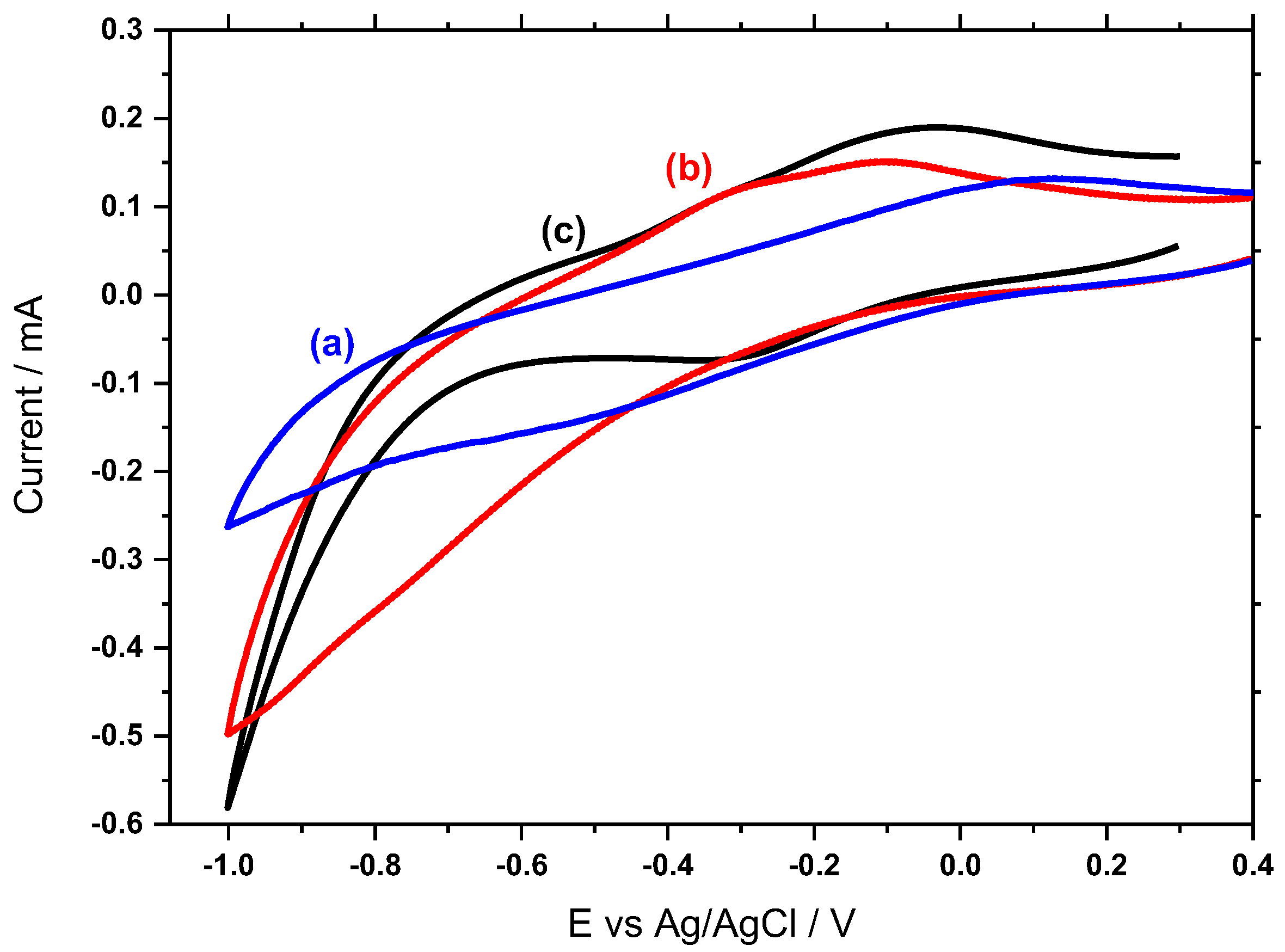
3.5. Electrochemical Behavior of Hemin/SnO2-Metglas and SnO2-Metglas Electrodes
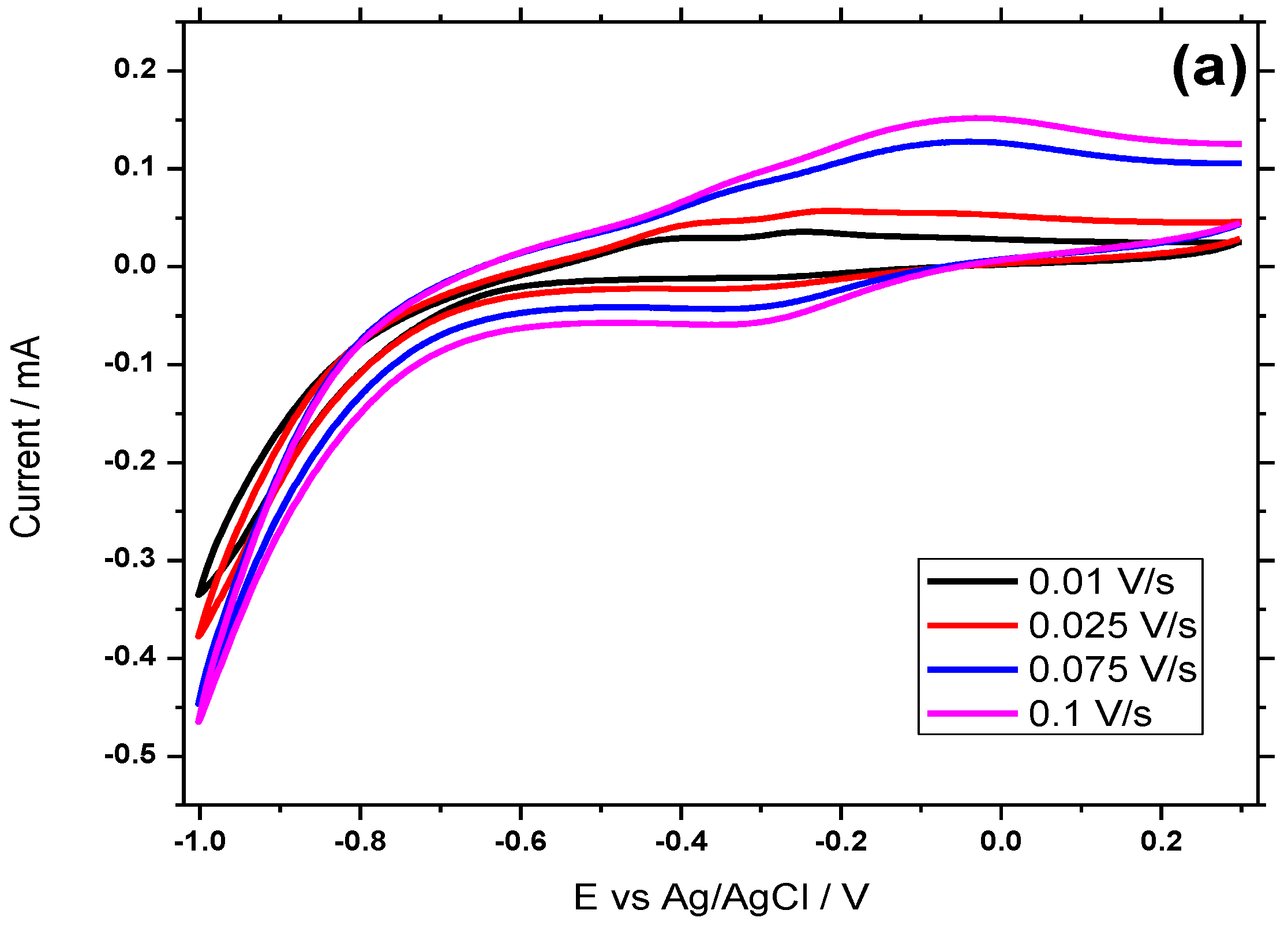
3.6. Electrocatalytic Behavior of H2O2 at the Hemin/SnO2-Metglas Electrode
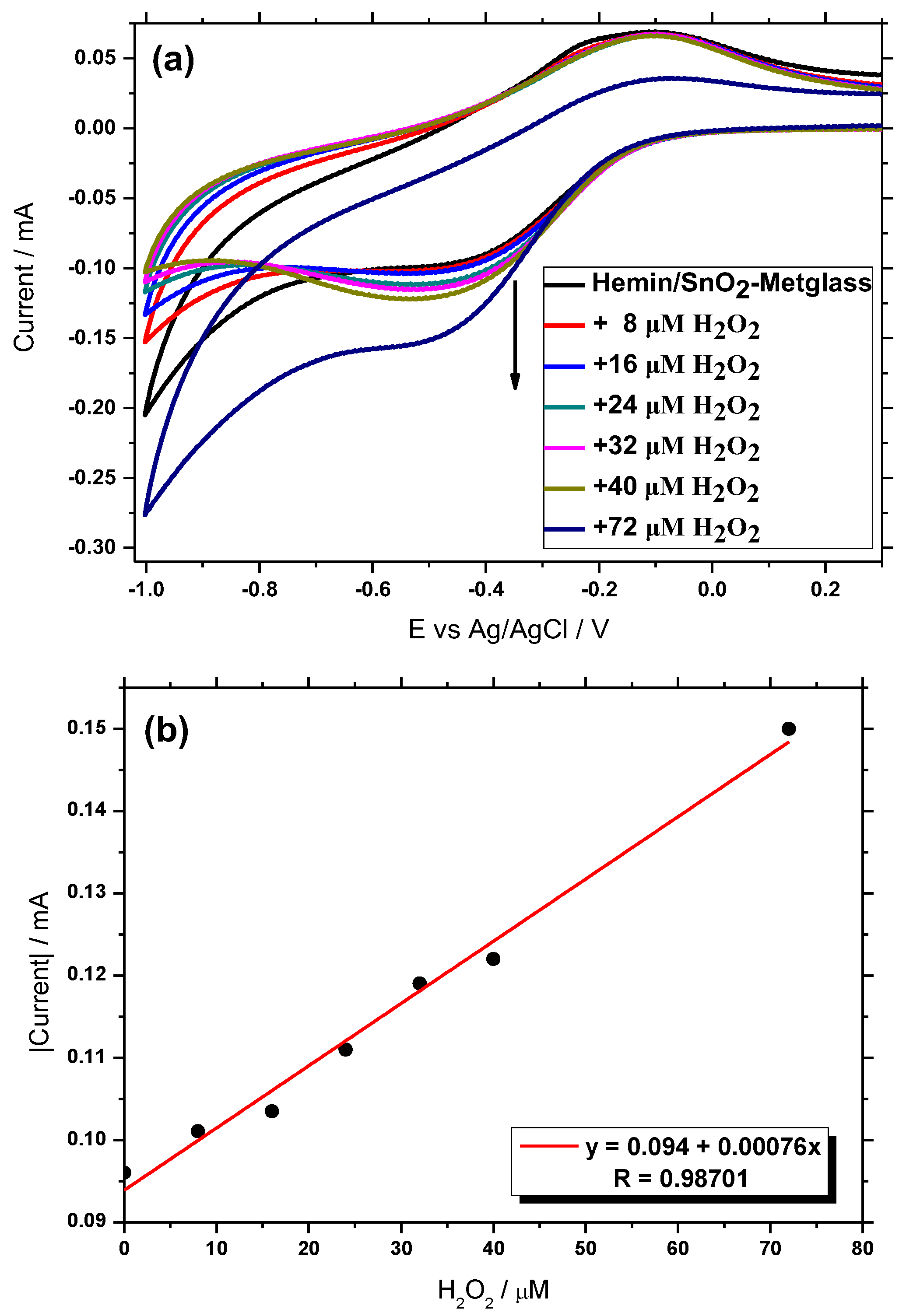
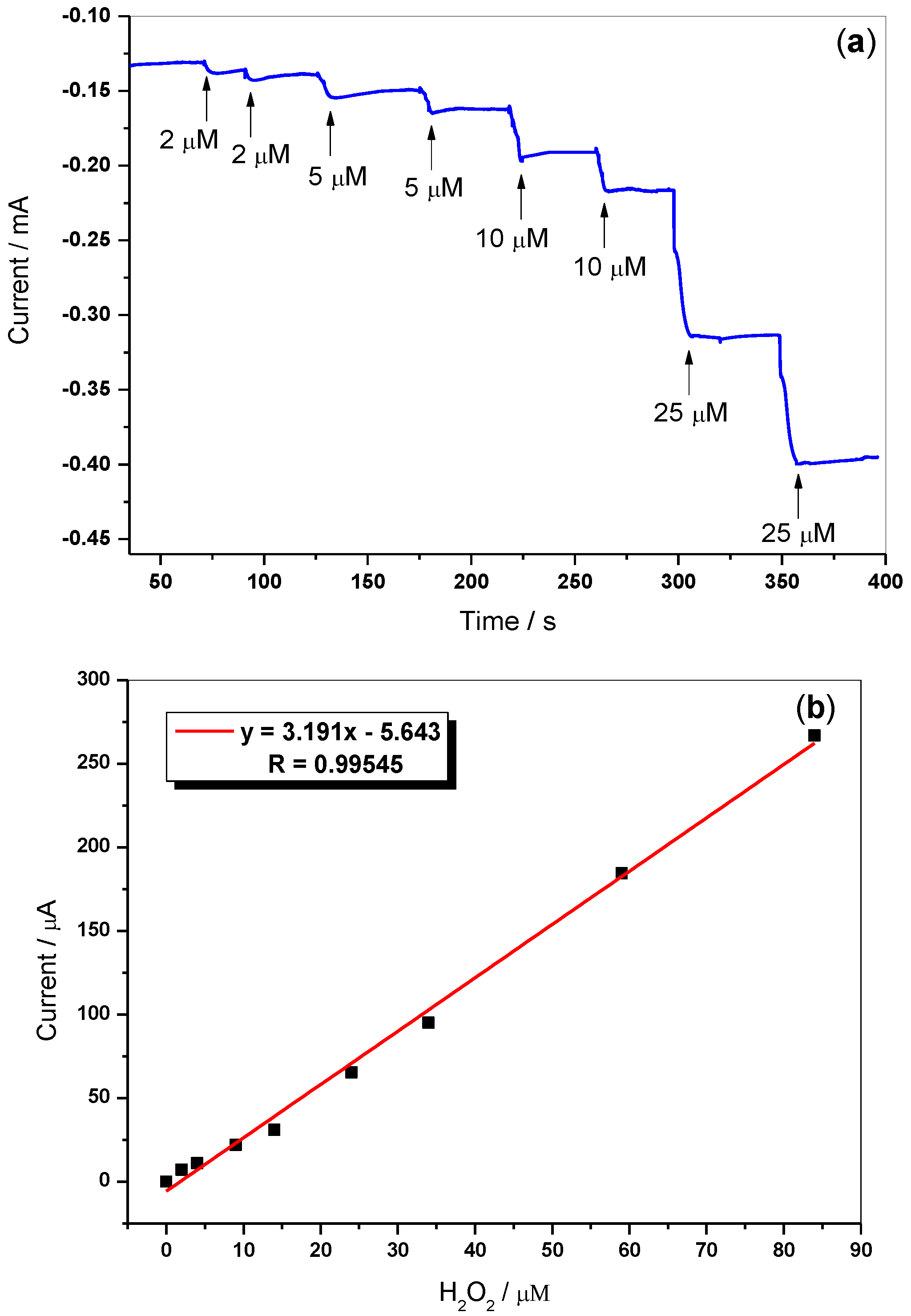
3.7. Amperometric Sensing of H2O2
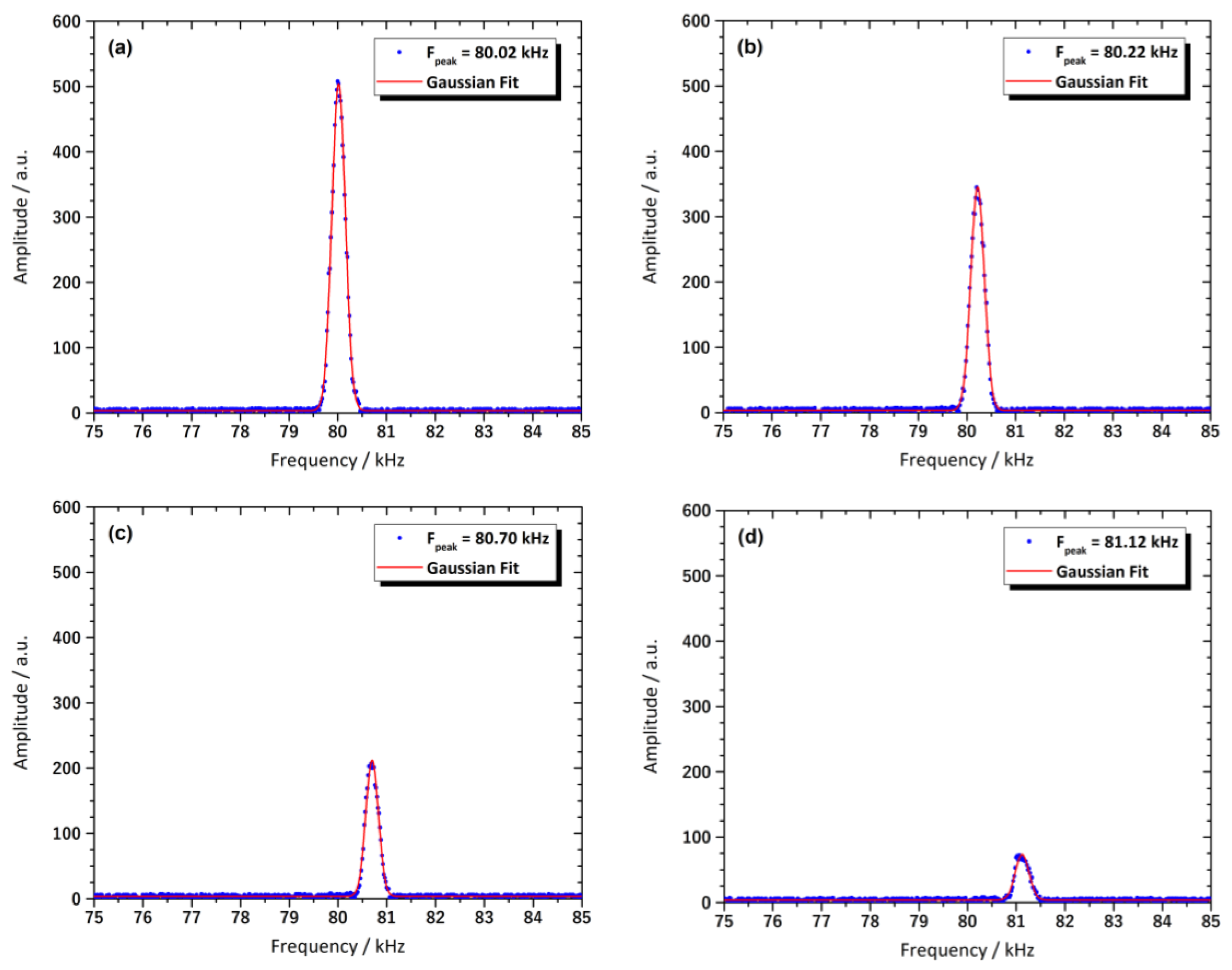
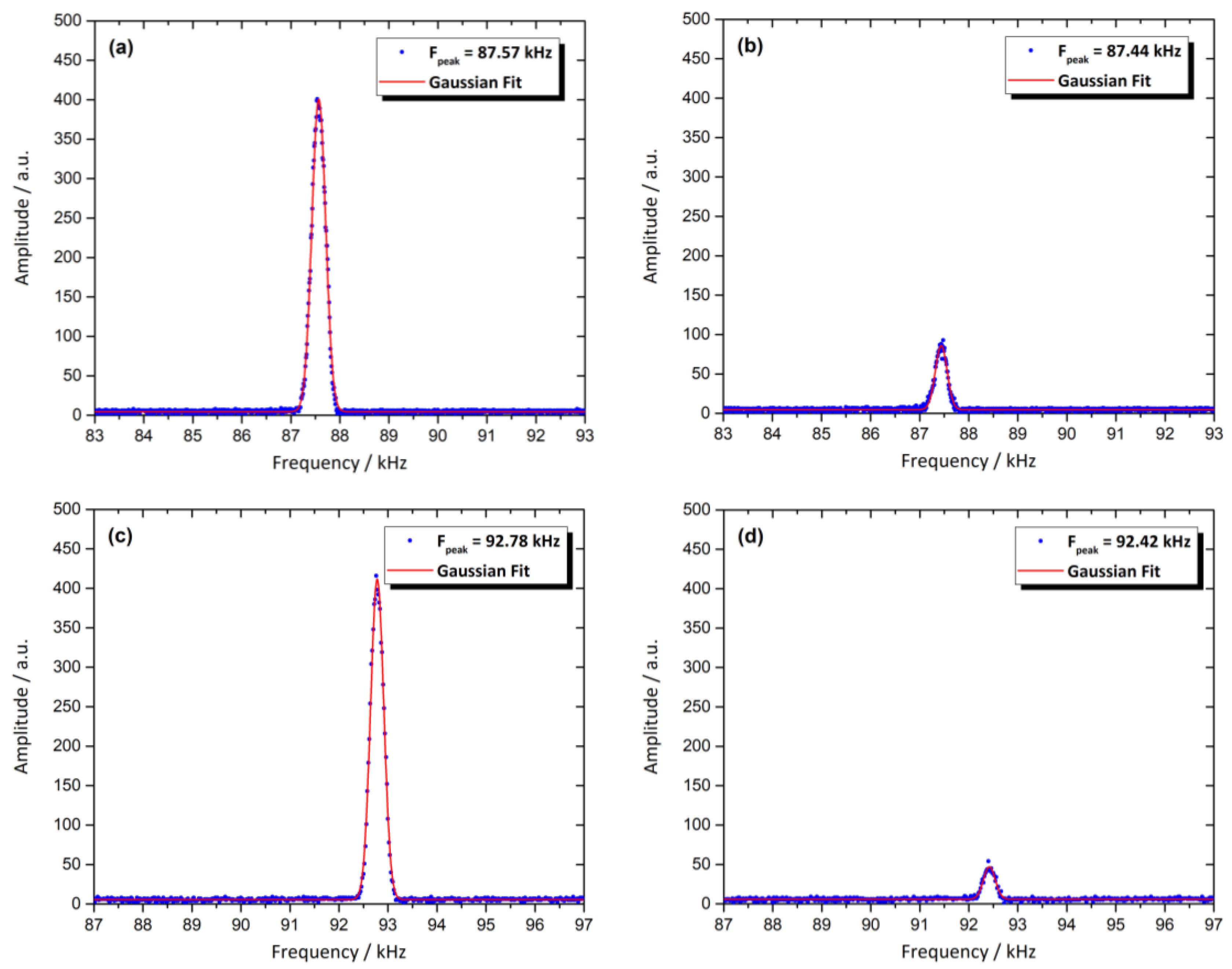
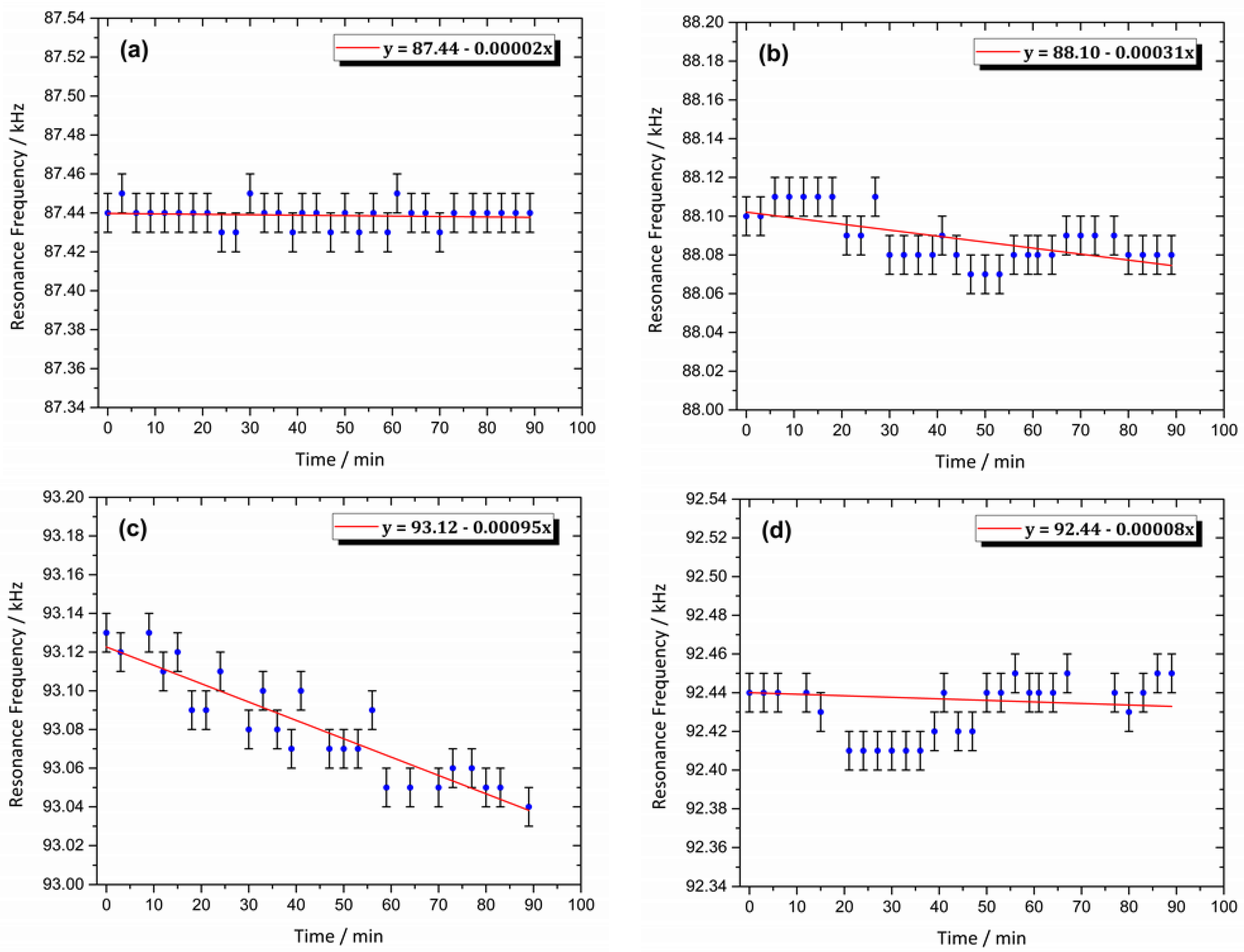
3.8. Magnetic Resonance Behavior of the Sensor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Woo, Y.A.; Lim, H.R.; Kim, H.J.; Chung, H. Determination of hydrogen peroxide concentration in antiseptic solutions using portable near-infrared system. J. Pharm. Biomed. Anal. 2003, 33, 1049–1057. [Google Scholar] [CrossRef]
- Wang, T.Y.; Zhu, H.C.; Zhuo, J.Q.; Zhu, Z.W.; Papakonstantinou, P.; Lubarsky, G.; Lin, J.; Li, M.X. Biosensor Based on Ultrasmall MoS2 Nanoparticles for Electrochemical Detection of H2O2 Released by Cells at the Nanomolar Level. Anal. Chem. 2013, 85, 10289–10295. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Jiang, X. A Facile One-Pot Synthesis of Copper Sulfide-Decorated Reduced Graphene Oxide Composites for Enhanced Detecting of H2O2 in Biological Environments. Anal. Chem. 2013, 85, 8095–8101. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.B.; Montes, R.H.O.; Richter, E.M.; Munoz, R.A.A. Rapid and selective determination of hydrogen peroxide residues in milk by batch injection analysis with amperometric detection. Food Chem. 2012, 133, 200–204. [Google Scholar] [CrossRef]
- Seders, L.A.; Shea, C.A.; Lemmon, M.D.; Maurice, P.A.; Talley, J.W. LakeNet: An Integrated Sensor Network for Environmental Sensing in Lakes. Environ. Eng. Sci. 2007, 24, 183–191. [Google Scholar] [CrossRef]
- Takahashi, A.; Hashimoto, K.; Kumazawa, S.; Nakayama, T. Determination of hydrogen peroxide by high-performance liquid chromatography with a cation-exchange resin gel column and electrochemical detector. Anal. Sci. 1999, 15, 481–483. [Google Scholar] [CrossRef]
- Matsubara, C.; Kawamoto, N.; Takamura, K. Oxo [5, 10, 15, 20-tetra(4-pyridyl) porphyrinato] titanium (IV): An ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide. Analyst 1992, 117, 1781–1784. [Google Scholar] [CrossRef]
- Zscharnack, K.; Kreisig, T.; Prasse, A.A.; Zuchner, T. A luminescence-based probe for sensitive detection of hydrogen peroxide in seconds. Anal. Chim. Acta 2014, 834, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ren, J.; Feng, L.; Qu, X. H2O2 triggered sol–gel transition used for visual detection of glucose. Chem. Commun. 2012, 48, 3739–3741. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, C.; Qi, Y.; Guo, S.; Liang, X. Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose. Sensors 2016, 16, 584. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Patra, C.R.; Arkalgud, J.R.; Boghossian, A.A.; Zhang, J.H.; Han, J.; Reuel, N.F.; Ahn, J.H.; Mukhopadhyay, D.; Strano, M.S. Single-Molecule Detection of H2O2 Mediating Angiogenic Redox Signaling on Fluorescent Single-Walled Carbon Nanotube Array. ACS Nano 2011, 5, 7848–7857. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Pashabadi, A.; Molaabasi, F. A novel electrochemical hydrogen peroxide biosensor based on hemoglobin capped gold nanoclusters-chitosan composite. RSC Adv. 2015, 5, 61725–61734. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, C.; Hu, S. A hydrogen peroxide biosensor based on direct electrochemistry of hemoglobin in Hb-Ag sol films. Sens. Actuators B 2008, 130, 816–822. [Google Scholar] [CrossRef]
- Duan, G.; Li, Y.; Wen, Y.; Ma, X.; Wang, Y.; Ji, J.; Wu, P.; Zhang, Z.; Yang, H. Direct electrochemistry and electrocatalysis of Hemoglobin/ZnO-Chitosan/nano-Au modified glassy carbon electrode. Electroanalysis 2008, 20, 2454–2459. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 85, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 1702168. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured material for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hayasaaka, T.; Liu, Y.; Liu, H.; Oliveira Jr, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Bai, X.Y.; Wang, X.M.; Shiu, K.K.; Zhu, Y.L.; Jiang, H. Highly Sensitive Graphene-Pt Nanocomposites Amperometric Biosensor and Its Application in Living Cell H2O2 Detection. Anal. Chem. 2014, 86, 9459–9465. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Jing, P.; Xu, W. Hemin on graphene nanosheets functionalized with flower-like MnO2 and hollow AuPd for the electrochemical sensing lead ion based on the specific DNAzyme. Biosens. Bioelectron. 2016, 86, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.; He, J.; Zhang, Y.; Yu, J.; Song, Y. Cu-Hemin Metal Organic Frameworks Chitosan Reduced Graphene Oxide Nanocomposites with Peroxidase-Like Bioactivity for Electrochemical Sensing. Electrochim. Acta 2016, 213, 691–697. [Google Scholar] [CrossRef]
- Yang, X.; Xiao, F.B.; Lin, H.W.; Wu, F.; Chen, D.Z.; Wu, Z.Y. A novel H2O2 biosensor based on Fe3O4–Au magnetic nanoparticles coated horseradish peroxidase and graphene sheets–Nafion film modified screen-printed carbon electrode. Electrochim. Acta 2013, 109, 750–755. [Google Scholar]
- Lv, X.C.; Weng, J. Ternary Composite of Hemin, Gold Nanoparticles and Graphene for Highly Efficient Decomposition of Hydrogen Peroxide. Sci. Rep. 2013, 3, 3285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Liao, K.; Wang, G.; Han, M. Pd nanoparticle-modified electrodes for nonenzymatic hydrogen peroxide detection. Nanoscale Res. Lett. 2015, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.H.; Luo, Y.L.; Lu, W.B.; Qin, X.Y.; Sun, X.P. Carbon nanoparticles-induced formation of polyaniline nanofibers and their subsequent decoration with Ag nanoparticles for nonenzymatic H2O2 detection. Russ. J. Electrochem. 2014, 50, 95–99. [Google Scholar] [CrossRef]
- Gu, T.T.; Wu, X.M.; Dong, Y.M.; Wang, G.L. Novel photoelectrochemical hydrogen peroxide sensor based on hemin sensitized nanoporous NiO based photocathode. J. Electoanal. Chem. 2015, 759, 27–31. [Google Scholar] [CrossRef]
- Huang, J.F.; Zhu, Y.H.; Zhong, H.; Yang, X.; Li, C.Z. Dispersed CuO nanoparticles on a silicon nanowire for improved performance of nanoenzymatic H2O2 detection. ACS Appl. Mater. Inter. 2014, 6, 7055–7062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yan, K.; Xu, Z.; Zhang, J. Hemin Modified TiO2 Nanoparticles with Enhanced Photoelectrocatalytic Activity for Electrochemical and Photoelectrochemical Sensing. J. Electrochem. Soc. 2016, 163, B526–B532. [Google Scholar] [CrossRef]
- Huan, Y.F.; Fei, Q.; Shan, H.Y.; Wang, B.J.; Xu, H.; Feng, G.D. A novel water-soluble sulfonated porphyrin fluorescence sensor for sensitive assays of H2O2 and glucose. Analyst 2015, 140, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wei, T.; Li, X.; Yang, J.; Zhang, J.; Fan, S.; Zhang, H. Synergistic-Effect-Controlled Tetraoctylammonium Bromide/Multi-Walled Carbon Nanotube@Hemin Hybrid Material for Construction of Electrochemical Sensor. J. Electrochem. Soc. 2017, 164, B147–B151. [Google Scholar] [CrossRef]
- Cao, Y.; Si, W.; Hao, Q.; Li, Z.; Lei, W.; Xia, X.; Li, J.; Wang, F.; Liu, Y. One–pot fabrication of Hemin-N-C composite with enhanced electrocatalysis and application of H2O2 sensing. Electrochim. Acta 2018, 261, 206–213. [Google Scholar] [CrossRef]
- Panagiotopoulos, A.; Gkouma, A.; Vassi, A.; Johnson, C.J.; Cass, A.E.G.; Topoglidis, E. Hemin modified SnO2 films on ITO-PET with enhanced activity for electrochemical sensing. Electroanalysis 2018. in Press. [Google Scholar] [CrossRef]
- Wang, Y.; Hosono, T.; Hasebe, Y. Hemin-adsorbed carbon felt for sensitive and rapid flow-amperometric detection of dissolved oxygen. Microchim. Acta 2013, 180, 1295–1302. [Google Scholar] [CrossRef]
- Santos, R.M.; Rodrigues, M.S.; Laranjinha, J.; Barbosa, R.M. Biomimetic sensor based on hemin/carbon nanotubes/chitosan modified microelectrode for nitric oxide measurement in the brain. Biosens. Bioelectron. 2013, 44, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavithra, L.; Devasena, T.; Pandian, K.; Gopinath, S.C.B. Amperometric determination of nitrite using natural fibers as template for titanium dioxide nanotubes with immobilized hemin as electron transfer mediator. Microchim. Acta 2018, 185, 194. [Google Scholar]
- Garcia de la Rosa, A.; Castro-Quezada, E.; Gutierrez-Granados, S.; Bedioui, F.; Alatorre-Ordaz, A. Stable hemin embedded in Nafion films for the catalytic reduction of trichloroacetic acid under hydrodynamic conditions. Electrochem. Commun. 2005, 7, 853–856. [Google Scholar]
- Obare, S.O.; Ito, T.; Balfour, M.H.; Meyer, G.J. Ferrous hemin oxidation by organic halides at nanocrystalline TiO2 interfaces. Nano Lett. 2003, 3, 1151–1153. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Gao, C.; Zhao, C.; Wang, Y.; Wang, J. Hemin immobilized into metal-organic frameworks as electrochemical biosensor for 2,4,6-trichlorophenol. Nanotechnology 2018, 29, 074003. [Google Scholar] [CrossRef] [PubMed]
- Reys, J.R.M.; Lima, P.R.; Cioletti, A.G.; Ribeiro, A.S.; Abreu, F.C.D.; Goulart, M.O.F.; Kubota, L.T. An amperometric sensor based on hemin adsorbed on silica gel modified with titanium oxide for electrocatalytic reduction and quantification of artemisinin. Talanta 2008, 77, 909–914. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Dong, S.J. Hemin functionalized graphene nanosheets-based dual biosensor platforms for hydrogen peroxide and glucose. Sens. Actuators B 2011, 160, 295–300. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.G.; Ren, J.S.; Qu, X.G. Polypyrrole nanoparticles as promising enzyme mimics for sensitive hydrogen peroxide detection. Chem. Commun. 2014, 50, 3030–3032. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Han, D.X.; Wang, X.H.; Qi, B.; Zhao, M.S. Polyoxometalates as peroxidase mimetics and their applications in H2O2 and glucose detection. Biosens. Bioelectron. 2012, 36, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Sagasti, A.; Bouropoulos, N.; Kouzoudis, D.; Panagiotopoulos, A.; Topoglidis, E.; Gutierrez, J. Nanostructured ZnO in a Metglas/ZnO/Hemoglobin modified electrode to detect the oxidation of Hemoglobin simultaneously by cyclic voltammetry and magnetoelastic resonance. Materials 2017, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Topoglidis, E.; Cass, A.E.G.; O’Regan, B.; Durrant, J.R. Immobilization and bioelectrochemistry of proteins on nanoporous TiO2 and ZnO films. J. Electroanal. Chem. 2001, 517, 20–27. [Google Scholar] [CrossRef]
- Willit, J.L.; Bowden, E.F. Adsorption and redox thermodynamics of strongly adsorbed cytochrome c on tin oxide electrodes. J. Phys. Chem. 1990, 94, 8241–8246. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Chai, Y. Mass load distribution dependence of mass sensitivity of magnetoelastic sensors under different resonance modes. Sensors 2015, 15, 20267–20278. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, Z.Y. Nonuniform mass detection using magnetostrictive biosensors operating under multiple harmonic resonance modes. J. Appl. Phys. 2010, 107, 114514. [Google Scholar] [CrossRef]
- Hernando, A.; Vazquez, M.; Barandiaran, J. Metallic glasses and sensing applications. J. Phys. E Sci. Instrum. 1988, 21, 1129. [Google Scholar] [CrossRef]
- Modzelewski, C.; Savage, H.; Kabacoff, L.; Clark, A. Magnetomechanical coupling and permeability in transversely annealed Metglas 2605 alloys. IEEE Trans. Magn. 1981, 17, 2837–2839. [Google Scholar] [CrossRef]
- Topoglidis, E.; Astuti, Y.; Duriaux, F.; Gratzel, M.; Durrant, J.R. Direct Electrochemistry and Nitric Oxide Interaction of Heme Proteins Adsorbed on Nanocrystalline Tin Oxide Electrodes. Langmuir 2003, 19, 6894–6900. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 41-1445 for SnO2; International Center for Diffraction Data: Newtown Township, PA, USA.
- Sivashankaran, N.S.L.; Vijayam, S.N.A.; PuthenKadathil, V.T.; Kunjkunju, J. Magnetic properties of Mn-doped SnO2 thin films prepared by the Sol–Gel dip coating method for dilute magnetic semiconductors. J. Am. Ceram. Soc. 2014, 97, 3184–3191. [Google Scholar] [CrossRef]
- Shikha, B.; Pandya, D.K.; Kashyap, S.C. Photoluminescence and Defects in Ultrathin SnO2 Films. AIP Conf. Proc. 2013, 1512, 1042–1043. [Google Scholar] [CrossRef]
- Seema, H.; Kemp, K.C.; Chandra, V.; Kin, K.S. Graphene–SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology 2012, 23, 355705. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.R.; Shukoor, I.; Tremel, W.; Wolfrum, U.; Kolb, U.; Naworth, T.; Langguth, P. Hemin-coupled iron (III)-hydroxide nanoparticles show increased uptake in Caco-2 cells. J. Pharm. Pharmacol. 2011, 63, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Altube, A.; Pierna, A.R. Thermal and electrochemical properties of cobalt containing Finemet type alloys. Electrochem. Acta 2004, 49, 303–311. [Google Scholar] [CrossRef]
- Laviron, E.J. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Song, H.; Ni, Y.; Kokot, S. A novel electrochemical biosensor based on the hemin-graphene nano-sheets and gold nano-particles hybrid film for the analysis of hydrogen peroxide. Anal. Chim. Acta 2013, 788, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Deac, A.R.; Morar, C.; Turdean, G.L.; Darabentu, M.; Gal, E.; Bende, A.; Muresan, L.M. Glassy carbon electrode modified with hemin and new melamine compounds for H2O2 amperometric detection. J. Solid State Electrochem. 2016, 20, 3071–3081. [Google Scholar] [CrossRef]






| Electrode | Preparation | Particle Size (nm) | Thickness (μm) | Insulating Region | Ref. |
|---|---|---|---|---|---|
| Hemin/SnO2-ITO/PET | Low temperature Hydrothermal method | 20–70 | 4 | At +0.2 V and more positive biases | [32] |
| Hemin-ZnO-Metglas | Hydrothermal method/sintering | 11–32 | 1 | none | [43] |
| Hemin/TiO2-FTO 1 | Hydrolysis/sol-gel/sintering | 10–15 | 2 | At −0.3 V and more positive biases | [37] |
| Hemin/TiO2-GCE 2 electrode | Flame synthesis technique | 10–50 | 10 | none | [28] |
| Cyt-c 3/SnO2/FTO Hb 4/SnO2/FTO | Sol-gel/sintering | 15–20 | 4 | At +0.2 V and more positive biases | [50] |
| Cyt-c/TiO2/FTO Cyt-c/ZnO/FTO | Sol-gel/sintering | 10–20 | 4 | At −0.15 V and more positive biases | [44] |
| Hemin/SnO2-Metglas | Low temperature Hydrothermal method | 20–70 | 11 | none | This work |
| Electrode | Method | Linear Range (M) | LOD (M) | Ref. |
|---|---|---|---|---|
| Hemin/SnO2-ITO/PET | CV | 1.5 × 10−6–90 × 10−6 | 1.5 × 10−6 | [32] |
| Hb/ZnO-Metglas | CV & MR | 25 × 10−6–350 × 10−6 | 25 × 10−6–50 × 10−6 | [43] |
| Hemin-graphene nano-sheets (H-GNs)/gold nano-particles (AuNPs) | CV & Amperometry | 0.3 × 10−6–1.8 × 10−3 | 0.11 × 10−6 | [58] |
| Hemin-TiO2 modified electrode | CV & Amperometry | 3.0 × 10−7–4.7 × 10−4 | 7.2 × 10−8 | [28] |
| Hemin-GCE | CV | 0–170 × 10−6 | 31.6 × 10−6 | [59] |
| ITO/NiO/Hemin | CV | 0.5 × 10−6–500 × 10−6 | 10−7 | [26] |
| Hemin/SnO2-Metglas | CV & MR | 2 × 10−6–90 × 10−6 | 1.6 × 10−7 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samourgkanidis, G.; Nikolaou, P.; Gkovosdis-Louvaris, A.; Sakellis, E.; Blana, I.M.; Topoglidis, E. Hemin-Modified SnO2/Metglas Electrodes for the Simultaneous Electrochemical and Magnetoelastic Sensing of H2O2. Coatings 2018, 8, 284. https://doi.org/10.3390/coatings8080284
Samourgkanidis G, Nikolaou P, Gkovosdis-Louvaris A, Sakellis E, Blana IM, Topoglidis E. Hemin-Modified SnO2/Metglas Electrodes for the Simultaneous Electrochemical and Magnetoelastic Sensing of H2O2. Coatings. 2018; 8(8):284. https://doi.org/10.3390/coatings8080284
Chicago/Turabian StyleSamourgkanidis, Georgios, Pavlos Nikolaou, Andreas Gkovosdis-Louvaris, Elias Sakellis, Ioanna Maria Blana, and Emmanuel Topoglidis. 2018. "Hemin-Modified SnO2/Metglas Electrodes for the Simultaneous Electrochemical and Magnetoelastic Sensing of H2O2" Coatings 8, no. 8: 284. https://doi.org/10.3390/coatings8080284
APA StyleSamourgkanidis, G., Nikolaou, P., Gkovosdis-Louvaris, A., Sakellis, E., Blana, I. M., & Topoglidis, E. (2018). Hemin-Modified SnO2/Metglas Electrodes for the Simultaneous Electrochemical and Magnetoelastic Sensing of H2O2. Coatings, 8(8), 284. https://doi.org/10.3390/coatings8080284






