1. Introduction
Within the past few years, the rates of periodontal and peri-implant disease have increased exponentially, boosted by population ageing, chronic diseases and people’s current lifestyle (stress, obesity, bad habits, etc.). Periodontal diseases include gingivitis and periodontitis which are infectious diseases in nature. In developed countries, about 60% of people over 30 suffer from this condition, which represents the main cause of tooth loss in adult patients. Furthermore, there is growing evidence of a possible association between periodontitis and various systemic diseases and conditions such as respiratory disease, chronic kidney disease, rheumatoid arthritis, cognitive impairment, adverse pregnancy outcomes, obesity, metabolic syndrome and cancer [
1]. Preventing the progression of these serious health concerns is thus of critical importance.
It may be difficult to comprehend how to prevent and treat periodontal diseases without knowledge of the key-stone role of bacterial plaque. Since Silness et al. [
2] proved the relationship between oral bacteria and gingivitis, the concept of the role of bacteria in periodontal disease etiology has evolved from the “specific and nonspecific plaque hypothesis” [
3] to the concept of the opportunistic or ecological nature of periodontal microbiota [
4]. Today we know that many of the microorganisms involved in periodontal diseases are uncultivable but identifiable through advanced nucleic acid probe technologies. They make up the periodontitis-associated microbiota and are organized in microbial clusters which have the potential to disrupt host defenses and induce an immune reaction that ultimately leads to soft and hard periodontal tissue breakdown.
Traditional methods of periodontal prevention and treatment are based on bacterial control by mechanical removal. Dental scaling and root planning aim to remove supragingival and subgingival calcified deposits, to reduce subgingival biofilm and also to detach and eliminate loosely bound endotoxin from root surfaces, thus restoring sufficient biocompatibility to facilitate healing by the formation of a long junctional epithelium [
5]. However, the mechanical treatment of periodontal disease, whether surgical or non-surgical, is limited principally by its inability to completely eliminate pathogenic bacteria from the periodontal tissues, thereby affecting a sustained shift towards a nonpathogenic subgingival microflora. As a consequence, recolonization of treated periodontal sites by pathogenic bacteria following root-surface debridement is associated with poorer therapeutic outcomes. The principal sources of re-infection are endogenous and include the sequestration of organisms within the residual biofilm at poorly accessible sites, such as furcation and within root grooves, invasion of epithelial cells by certain pathogens and recolonization of periodontal sites from other oral niches [
6]. As a matter of fact, there is insufficient evidence to determine the effects of routine scale and polish treatments [
7].
The aforementioned limitations prompted the use of antimicrobial drugs as adjunctive chemical methods for eliminating periodontal bacteria. However, most local antimicrobial delivery systems have had limited success because of the lack of substantivity from sustained-release devices and their tendency to facilitate bacterial resistance. On the other hand, the use of systemic antibiotics remains highly controversial, except in the management of aggressive periodontitis, as a result of issues related to its ecological impact on microbiome [
8,
9]. However, due to the importance of bacterial control in periodontal disease treatment, novel adjunctive antimicrobial approaches have emerged within the scientific and clinical literature in recent years. These include photodynamic therapy, prebiotics and probiotics, and the “one-stage full-mouth disinfection” clinical approach [
10,
11]. However, the long term efficacy of these approaches remains unclear. In this regard and attending to the emerging importance of bioactive glasses as antimicrobials, we have explored the role of new bioactive glasses in the prevention of periodontal disease in an in vivo experimental study.
For research purposes, the ligature model has been extensively used in preclinical studies on experimental periodontitis to promote tissue breakdown in a short period of time [
12]. The placement of a ligature in a subgingival position disrupts the soft tissue seal around teeth and implants and opens the pocket for biofilm formation. While a ligature made of cotton or silk may not induce bone loss by itself, the developing inflammatory process in the connective tissue that results from biofilm formation mediates tissue destruction during the experiment [
13].
A new generation of inorganic biocides based on CaO-enriched soda-lime glasses and ZnO-rich glasses has been recently developed [
14,
15]. These glasses are suitable for application in several strategic fields in which the control of an unwanted heterogeneous microbial population represents a serious threat to human health. The efficacy of a coating made of this kind of glasses to prevent peri-implant diseases was recently pointed out in a previous in vivo research study [
16].
Furthermore, in our previous works [
17,
18,
19], a new glass-ceramic composed of combeite and nepheline in a residual soda-lime glassy matrix was fabricated by sintering at 750 °C for 1 h. The antibacterial activity of this glass-ceramic, free of P
2O
5, effectively diminishes the growth (logarithm of reduction >3) of bacteria (such as
S. aureus, E. coli,
S. epidermidis,
M. lutea, and
P. aeruginosa) [
17] as well as yeast (
C. krusei) [
18]. It has also been shown that this glass-ceramic inhibits adhesion and biofilm formation [
17]. Furthermore, in vitro biocompatibility assays with human stem cells demonstrated that the new glass-ceramic also shows excellent biocompatibility [
17].
The main objective of the present work is to study the influence of an antimicrobial glass/glass-ceramic addition, similar to the ones mentioned before, to a flowable resin composite. The addition of a glass-ceramic or a particulate glass containing a high ZnO fraction to a conventional dental polymer composite can play and important role in preventing periodontitis. To this end, in vivo experiments have been carried out on Beagle dog fangs subjected to a ligature-induced periodontitis model.
2. Materials and Methods
2.1. Glass/Glass-Ceramic Synthesis and Characterization
A glass and glass-ceramic were prepared in a similar way to that previously described by the authors [
14,
15,
17]. Briefly, a glass powder from the SiO
2–Na
2O–Al
2O
3–CaO–B
2O
3 system with the following chemical composition was used (wt.%): 41.6 SiO
2, 20.0 Na
2O, 19.5 CaO, 10.1 Al
2O
3, 6.4 B
2O
3, 0.21 MgO, and 0.61 K
2O (Nanoker Research, Oviedo, Spain). The precursor powder was thermally treated in a conventional furnace in air at 750 °C for 1 h at a heating rate of 5 °C/min leading to glass-ceramic material. After thermal treatment, the powders were ground in an alumina mortar and subsequently sieved through a <40 µm mesh. The average particle size obtained, as measured using a laser technique (LS Particle Size Analyzer 13 320, Beckman Coulter, Indianapolis, IN, USA), was found to be
d50 = 10.30 ± 0.55 µm (
Table 1). This glass-ceramic was labelled as G3-GC.
A zinc-containing glass, belonging to the B
2O
3–SiO
2–Na
2O–Al
2O
3–ZnO system, and with the following chemical composition (wt.%): 19.39 SiO
2; 34.24 B
2O
3; 5.55 Na
2O; 5.13 Al
2O
3 and 34.73 ZnO was prepared by fusion of mixtures of Na
2CO
3, SiO
2, B
2O
3, ZnO and Al
2O
3 in Pt crucibles at 1250 °C for 1 h in an electric furnace. After melting, it was quenched by dipping the crucible into water, dried, grinded in an alumina mortar and sieved down to 40 µm. The average particle size (Beckman Coulter LS Particle Size Analyzer 13 320) was found to be
d50 = 7.53 ± 0.1 µm (
Table 1). This glass powder was labelled as 35ZnO-G.
2.2. Composite Synthesis and Characterization
A commercially available flowable dental composite FiltekTM Supreme XTE Flowable Restorative (3M ESPE, St. Paul, MN, USA) was reinforced with varying amounts of the antimicrobial glass/glass-ceramic (0, 13, 26 wt.%). This resin, according to its manufacturer, contains: bis-GMA, TEGDMA and Procrylat resins and the inorganic filler loading is approximately 65% by weight (46% by volume).
Plates of dimensions 1.5 cm × 1.5 cm were prepared by homogeneously mixing the resin composite with 13, 26 wt.% of glass/glass-ceramic powders. Light curing was performed according to the manufacturers’ instructions. Photopolymerization was performed using a curing lamp (BA optimal 10, B.A International, Kingsthorpe, UK). The glass-ceramic or glass composites were labelled as G3-GC13, G3-GC26, 35ZnO-G13, 35ZnO-G26.
The obtained composite was analyzed by means of FESEM and EDS analysis to evaluate the homogeneity of particle dispersion. The analyses were performed both on sample surfaces and cross-sections. For cross-section evaluation, the composites were cut with a cut-off machine (TM3-A Boccadoro, Losone, Switzerland) and subsequently polished down to 1 µm.
2.3. In Vitro Antibacterial Test
Previous to the in vivo test, the bactericidal efficacy of the resin composites was evaluated according to ISO 22196:2011 [
20]. This standard specifies a method of evaluating the antibacterial activity of antibacterial treated plastics and other non-porous product surfaces. ISO 22196:2011 recommends using
Staphylococcus aureus (ATCC 6538P) and
Escherichia coli (ATCC 8739) bacterial species for the bactericidal capability test. The analysis was performed in triplicate. For each microorganism, 3 specimens of the untreated material (control) were used to measure viable cells immediately after inoculation and another 3 specimens of the untreated material (control) were used to measure viable cells after 24 h of incubation. 3 specimens of each treated test material were used to measure viable cells after 24 h of incubation.
The square test composites (1.5 cm × 1.5 cm): 6 untreated resins and 3 filled with glass/glass-ceramic composites were placed in petri dishes and inoculated with 50 µL of the test inoculum (containing 2.7 × 10
5 CFU/mL of
Escherichia coli or 4.5 × 10
5 CFU/mL of
Staphylococcus aureus). The inoculum was covered with a sterile polyethylene coverslip (1.4 cm × 1.4 cm), which allows the inoculum to spread well, prevents it from drying and ensures good contact with the antimicrobial surface. Immediately after inoculation, half of the untreated composites were supplied with 5 mL of phosphate-buffer saline to recover bacteria from the surface of the specimens. The recovered bacteria cell suspension was then serially diluted in physiological saline. Petri dishes containing plate count agar were inoculated with these recovered bacterial dilutions in duplicate. These plates were incubated for 24–48 h at 37 °C, after which CFU were determined. The remaining 3 untreated and glass/glass-ceramic composites were incubated for 24 h at 37 °C and 95% humidity. After this time, the bacterial cells were recovered from the surface and the number of microorganisms present on the surfaces was determined in the way previously described. The reduction in microorganisms in relation to the initial concentrations and the control surface was calculated. Antibacterial activity, delineated as
R, was calculated following Equation:
where
R is antibacterial activity;
U0 is the average logarithm of the number of viable bacteria, in cells/cm
2, recovered from untreated test specimens immediately after inoculation;
Ut is the average logarithm of the number of viable bacteria, in cells/cm
2, recovered from untreated test specimens after 24 h; and
At is the average logarithm of the number of viable bacteria, in cell/cm
2, recovered from treated test specimens after 24 h.
Converting logarithmic (base 10) reduction R to percentage reduction (P) is possible applying the following formula: P = 100 × (1 − 10−R). This means that the R-values (log10 scale) of 1, 2, 3, 4, 5 and 6 correspond to microbial load reductions of 90%, 99%, 99.9%, 99.99%, 99.999% and 99.9999%, respectively.
2.4. In Vivo Test
2.4.1. Animals
This study was carried out in strict accordance with Directive 2010/63/EU on the protection of animals used for scientific purposes. The protocol was approved by the Ethics Committee for Animal Research Welfare of the Minimally Invasive Surgery Center (Cáceres, Spain). Five Beagle dogs were used in this experiment to meet the goal of reduction, the second of the 3R’s (Replacement, Reduction and Refinement), a widely accepted ethical framework for conducting scientific experiments. In addition, it is common practice to employ this number of animals for such experiments and thus satisfy statistical power requirements. At the initiation of the experiment, the dogs were 5 years old and weighed 19 kg on average.
During all procedures, veterinary assistance was used continuously and all efforts were made to minimize suffering. General anaesthesia was induced with intravenous injected propofol 10 mg/kg (Propofol Hospira, Hospira Productos Farmacéuticos y Hospitalarios, Madrid, Spain). One no. 7 endotracheal tube with a balloon cuff was placed and connected to a circular anesthesia circuit (Leon Plus, Heinen & Löwenstein, Bad Ems, Germany). The anesthesia was sustained with sevofluorane (Sevorane, Abbott Laboratories, Madrid, Spain). Multimodal analgesia was employed in the perioperatory [ketorolac 1 mg/kg (Toradol 30 mg, Roche, Basel, Seitzerland), tramadol 1.7 mg/kg (Adolonta inyec., Grünenthal, Aachen, Germany) and buprenorfine 0.01 mg/kg (Buprex, Reckitt Benckiser Pharmaceuticals Limited, Berkshire, UK)].
2.4.2. Dental Preparations
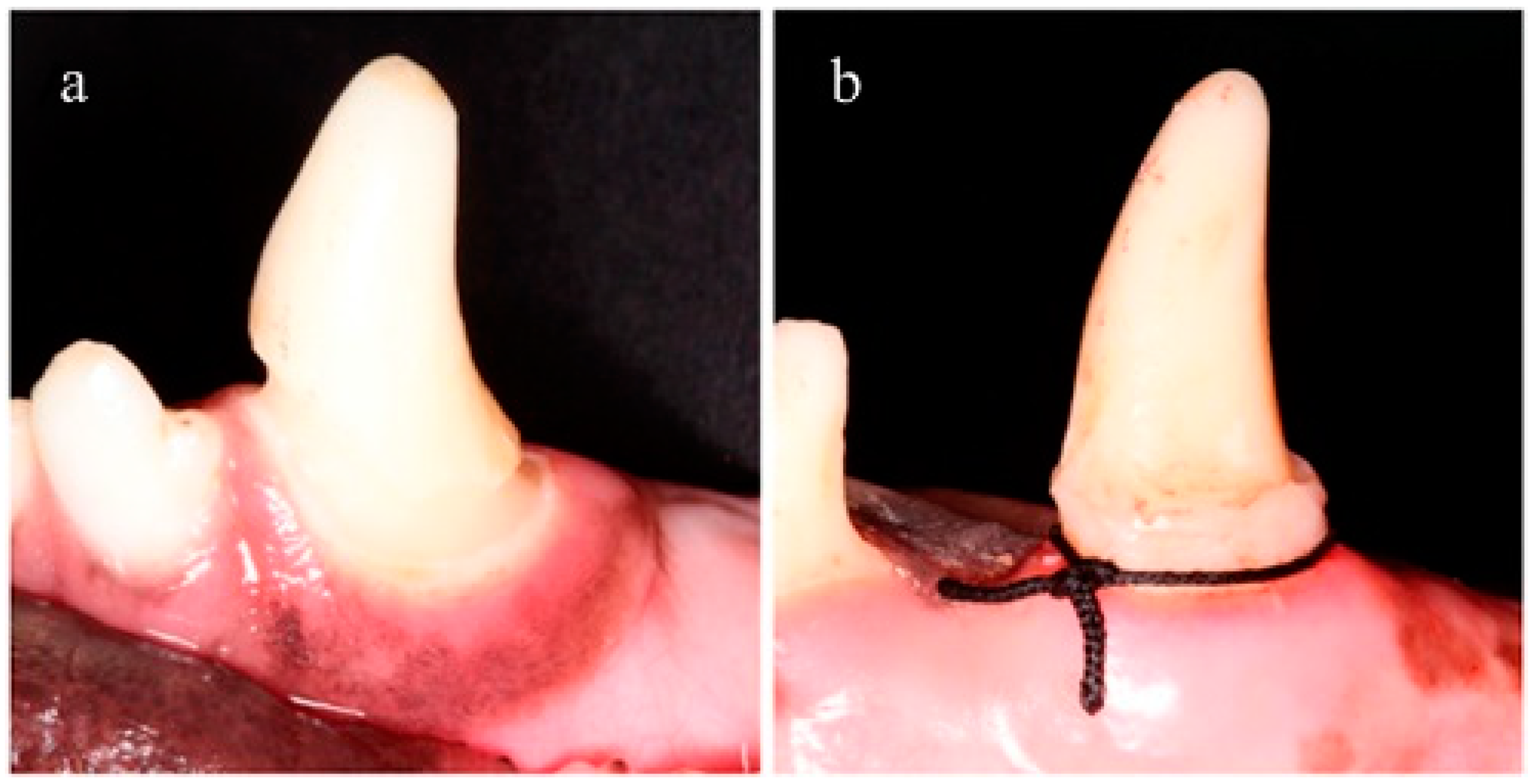
Sample size was 20 canines (5 dogs) but the study unit was each aspect (medial and distal) of each fang. Negative controls were used to exclude spontaneous bone loss or recession due to any etiology unrelated with the experiments. Negative controls consisted of 2 canines (4 aspects) without intervention (no class V obturation nor periodontitis ligature induction). Class V dental preparations (named CV) (Black’s classification) were performed in 18 canines (36 aspects) using a 0.5 mm diameter drill bur (Komet Dental, Lemgo, Germany) and affecting the enamel next to the supra gingival margin all the way around the neck of the crown (
Figure 1a). Dental preparations were conditioned by a self-etching one-step primer and bonding solution (3M ESPE ScotchBond Universal Adhesive, St. Paul, MN, USA) that was light-cured for 20 s (BA optimal 10, B.A International, Kingsthorpe, UK). Consecutively, these preparations were filled with different restorative materials. A universal restorative dental composite (3M ESPE Filtek Supreme XTE, St. Paul, MN, USA) was used as the control material (
n = 6, 12 aspects). In the test branches (for each group
n = 6, 12 aspects), G3-GC or 35ZnO-G powders were mixed (26 wt.%) with the commercial dental composite to provide them with antimicrobial properties, as previously described. The mixing was performed by hand with a stainless steel dental cement spatula on a glass slab. Dental materials were randomly allocated. All preparations were filled via incremental addition using a dental spatula (Dental Spatula XTS Composite No 03 Flexi Thin, Hu-Friedy Mfg Co., Chicago, IL, USA) and light-cured.
2.4.3. Experimental Periodontitis
The traditional ligature model was used to induce acute breakdown of periodontal tissues as described by Lindhe et al. in 1973 [
12]. Dental preparations were overfilled to provide retention for ligatures since canines have a conical shape and ligatures tend to slip off and to be loose. Ligatures were placed in a yuxtagingival position and no plaque control procedures were performed (
Figure 1b).
2.4.4. Bone Loss Measurements
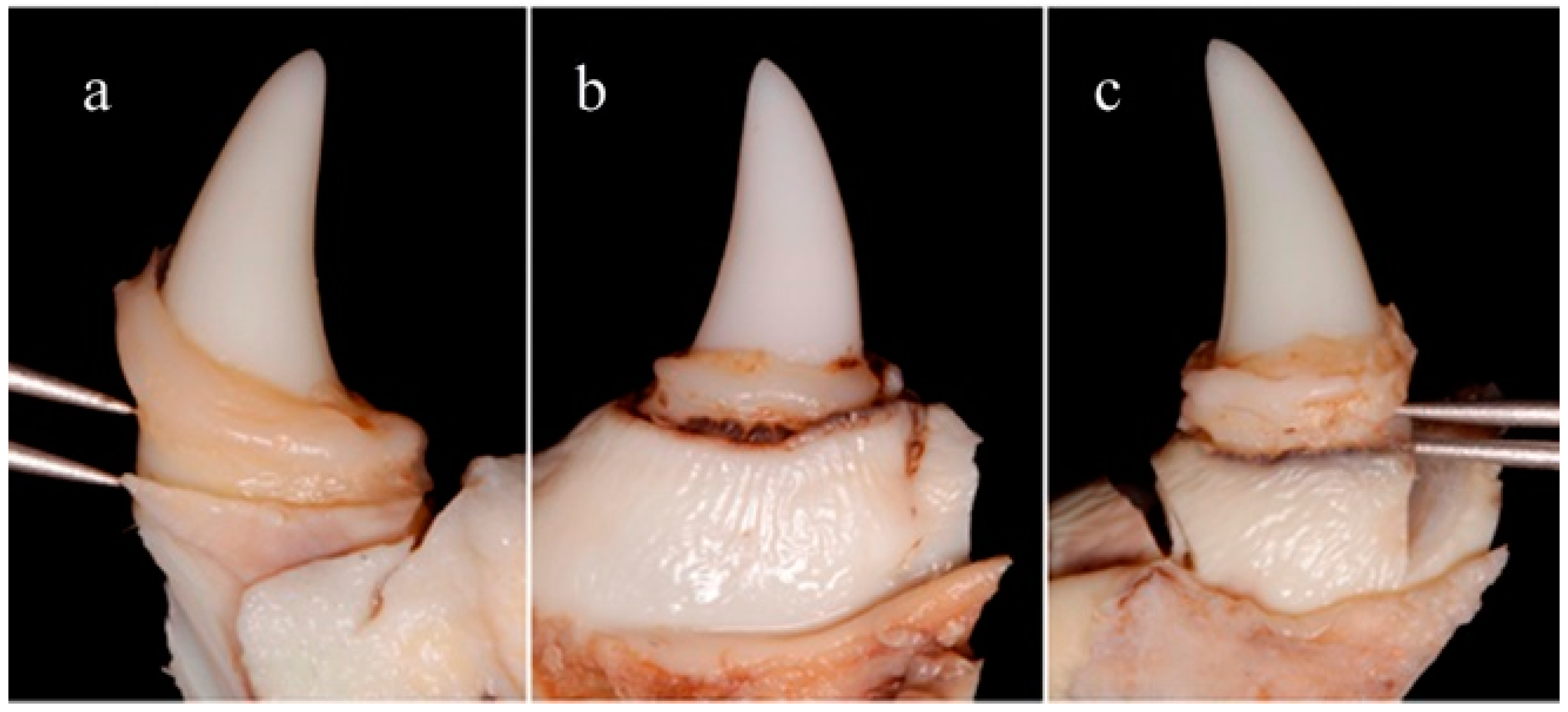
After 8 weeks of ligature periodontitis induction the animals were euthanized with a lethal dose of Sodium-Pentothal. Blocks containing the canines were retrieved from the jaw bone using an oscillating autopsy saw (Exakt, Kulzer, Germany). The dissected specimens were sent to two blinded appraisers who measured the medial and distal aspects of the canines, to assess the distance between the apical portion of the dental restoration and the residual marginal bone level. Distance was assessed using a double-pointed compass (EMI Deluxe EKG Caliper, Fullerton, CA, USA) as can be observed in
Figure 2. A trained program was implemented to increase inter- and intra-examiner reliability. Mean values for all variables were calculated for the mesial and distal aspect of each tooth. Comparisons were made using absolute values (final bone loss in millimeters). Differences were analyzed using a non-parametric (Mann-Whitney) method. The null hypothesis was rejected at
p ≤ 0.05.
The cross-sections of the dissected specimens, after polishing (<1 µm), and their corresponding external surfaces were also characterized by Field Emission Scanning Electron Microscopy (FESEM) (FEI: Quanta FEG 650, Hillsboro, OR, USA) and Energy Dispersive Spectroscopy (EDS) equipment from EDAX-AMETEK GmbH (Meerbusch, Germany).
4. Discussion
Diverse research with different additives has been carried out to develop effective antibacterial resin-based composites. Most of these additives are organic bactericides, such as: quaternary ammonium dimethacrylate (QADM) [
21], dimethylaminohexadecyl methacrylates (DMAHDM) [
22], dimethyl-hexadecylmethacryloxyethyl-ammonium iodide (DHMAI) [
23], quaternary ammonium polyethylenimine (PEI) nanoparticles [
24], chlorhexidine [
25], triclosan [
26] or chitosan [
27]. Inorganic coatings have emerged as an alternative approach since they present several advantages compared to traditional organic agents, which include chemical stability, thermal resistance and protracted action, and they have not yet been associated with resistant organisms [
28]. Silver is the most studied antibacterial metal ion for the treatment of periodontitis and perimplantitis [
29]. The use of different experimental fillers is another important strategy. In this sense, multifunctional bioactive glasses and glass-ceramics could be an alternative antimicrobial system. The bioactivity of the glass/glass-ceramic has made the current composite an interesting candidate for potential applications in the dental field. Tauböck et al. functionalized a dentin bonding resin with 20 wt.% ultrafine SiO
2-Na
2O-CaO-P
2O
5-Bi
2O
3 particles. This bioactive glass loading does not influence microhardness, and the viscosity of the composite remained below that of a flowable dental composite [
30]. However, it did not evaluate the possibility of conferring antimicrobial properties to the dental resin, at least to the author’s knowledge. Khvostenko et al. investigated the effect of adding 15 wt.% bioglass (65% SiO
2-31% CaO-4% P
2O
5 (mol.%)) to a resin composite on bacterial biofilms penetrating into marginal gaps of simulated tooth fillings in vitro during cyclic mechanical loading. A significantly lower degree of bacterial biofilm penetration into the gap was found [
31]. The results obtained in the present in vivo experiment, although limited by sample size, due to the ethical issues involved in the use of Beagle dogs in in vivo experiments, demonstrate the antimicrobial performance of a dental resin matrix containing different bioglass fillers and can thus expand the clinical applications of these materials in the treatment of dental diseases in the near future.
It has been proven that the concentration required to obtain the desired antimicrobial properties (26 wt.%) does not compromise tooth-bonding efficacy nor workability. The dental adhesive remained on the tooth until the end of the in vivo test (
Figure 2). Further studies are ongoing in order to complete the mechanical characterization.
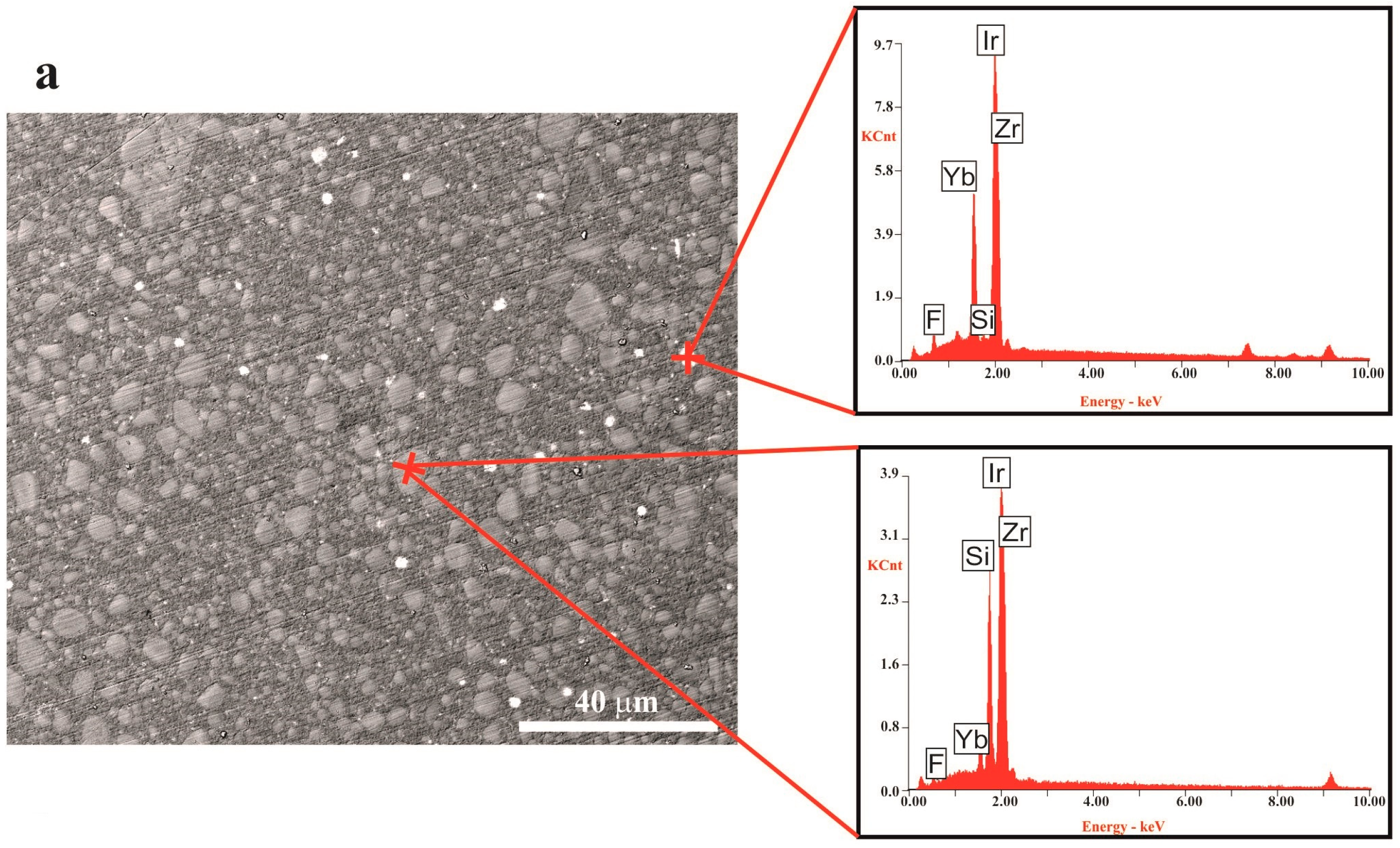
It is important to point out that both powders display a different particle size distribution as is made clear in
Table 1. The G3-GC26 powder has a larger average grain size (
d50 = 10.3 µm) and course fraction than the Zn-containing glass (
d50 = 7.5 µm). Particle distribution was found to be very homogeneous in both cases and no aggregates are observed in the polymer matrix (
Figure 3).
Although the bactericidal properties of these glasses have been extensively proved [
14,
17], in vitro tests were carried out before the in vivo test to ensure that the composites preserve their efficacy. In agreement with the results obtained in vitro
, the in vivo tests were performed using the composites containing 26 wt.% glass or glass-ceramic particulate filler.
At 24 h, time at which the in vitro tests were carried out according to the standard, the composite reinforced with 35ZnO-G26 looks more active than the one containing G3-GC26. This trend can be explained taking into account the different dissolution behavior of the glasses. However, after 72 h, both materials show the same antimicrobial performance [
14,
17]. Due to differences in microbiological test protocols, previous results obtained by other authors cannot be directly compared to one another or to the results of the present study.
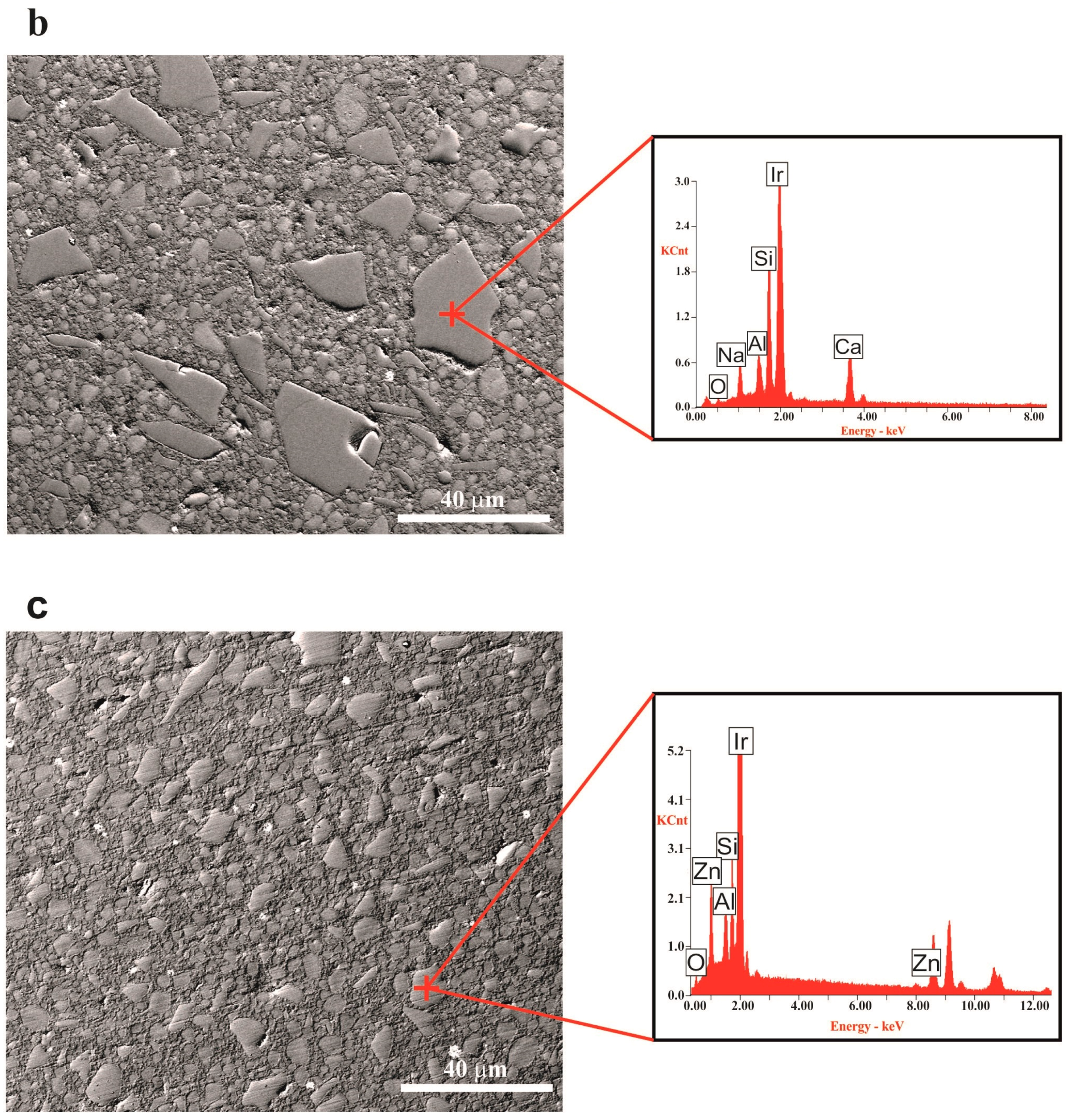
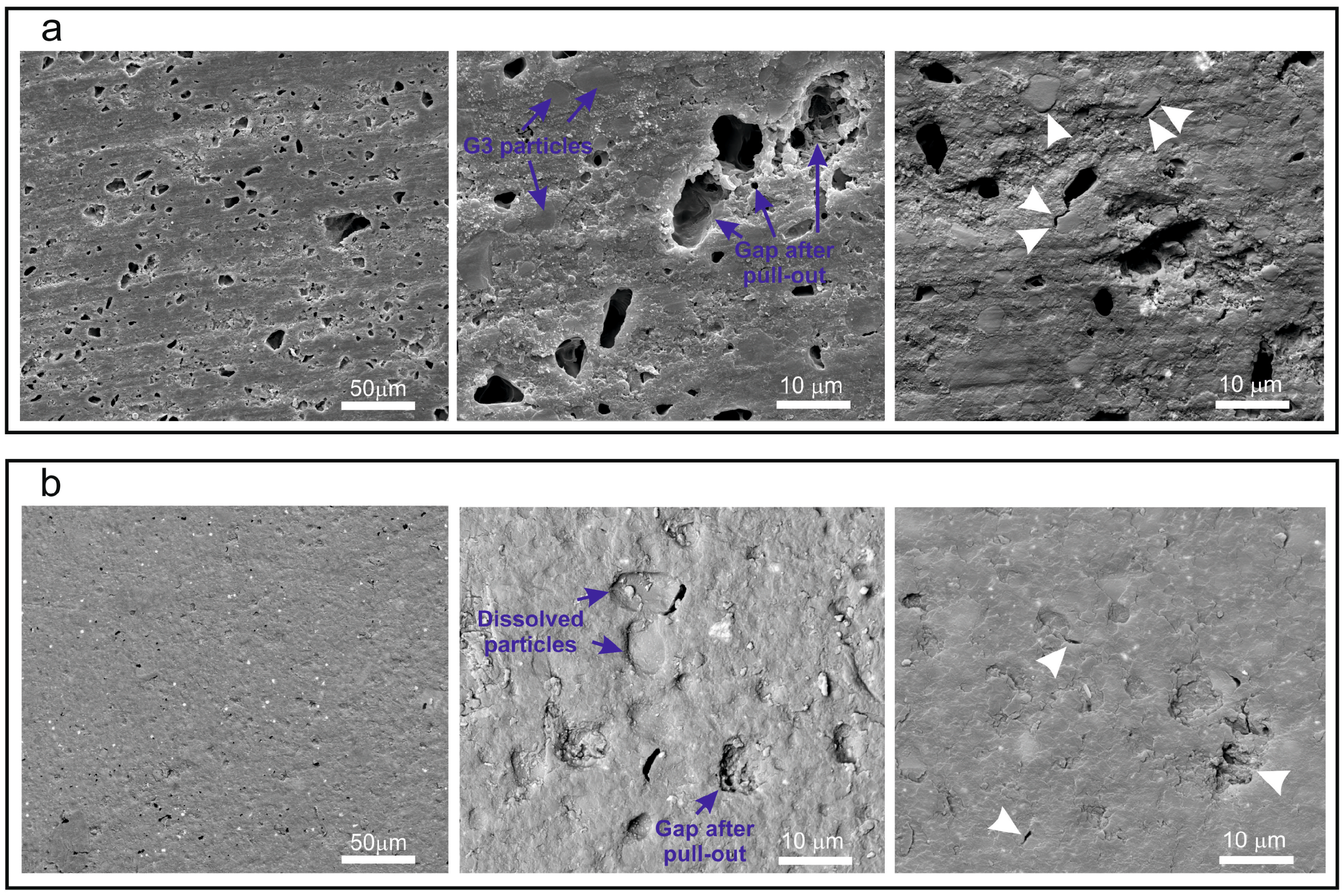
In the FESEM micrographs corresponding to the external surface of the composite toroid (
Figure 4), after the in vivo test, we can observe that, in both cases (G3-GC26 and 35ZnO-G26 glass composites), grains >10 µm have been pulled-out leaving a gap. In the particular case of the G3-GC26 glass-ceramic, almost all grains <10 µm (~50% of the volume fraction) have remained, resisting the wear of the dog’s tongue without an appreciable dissolution effect (
Figure 4a). As observed in
Figure 3, two crystalline phases precipitate during G3-GC powder annealing at 750 °C: nepheline (Na
6.65Al
6.24Si
9.76O
32) and combeite (Na
2Ca
2Si
3O
9). In a previous study [
17], nepheline crystals were described as elongated (>10 µm) while combeite crystals were found to be equiaxed and much smaller (<50 nm). The presence of nepheline needle-like crystals drastically improves the wear resistance of this composite. This fact can explain its good behavior in the prevention of the induced periodontitis (
Table 3). Therefore, its antimicrobial activity is not significantly affected during the 4 months of testing in the dog’s mouth.
Conversely, in the case of the Zn-containing glass composite, 35ZnO-G shows the typical XRD pattern of a perfect glass [
14]. Taking into account that its granulometric distribution is noticeably narrower than that corresponding to G3-GC and that it is more soluble, the majority of the grains <5 µm (~25% of the volume fraction) were dissolved as a consequence of the continuous abrasion to which it is subjected inside the dog’s mouth. It is, therefore, logical to think that the biocidal activity of this composite has been significantly reduced and was most probably limited to the first weeks of implantation. Nevertheless, statistical results (
p = 0.217) tend to be significant and suggest that with a larger number of subjects more relevant differences might be detected in terms of bone loss between 35ZnO-G and positive control groups.
Since negative controls did not experience any bone loss during the experiment, it can be assumed that the bone loss achieved around positive controls and cases is totally due to experimental ligature-induced periodontitis.