Surface Modification of Esophageal Stent Materials by a Drug-Eluting Layer for Better Anti-Restenosis Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immobilizing 5-Fu onto the PDA/PEI Layer
2.2. Characterization of PDA/PEI/5-Fu Layers
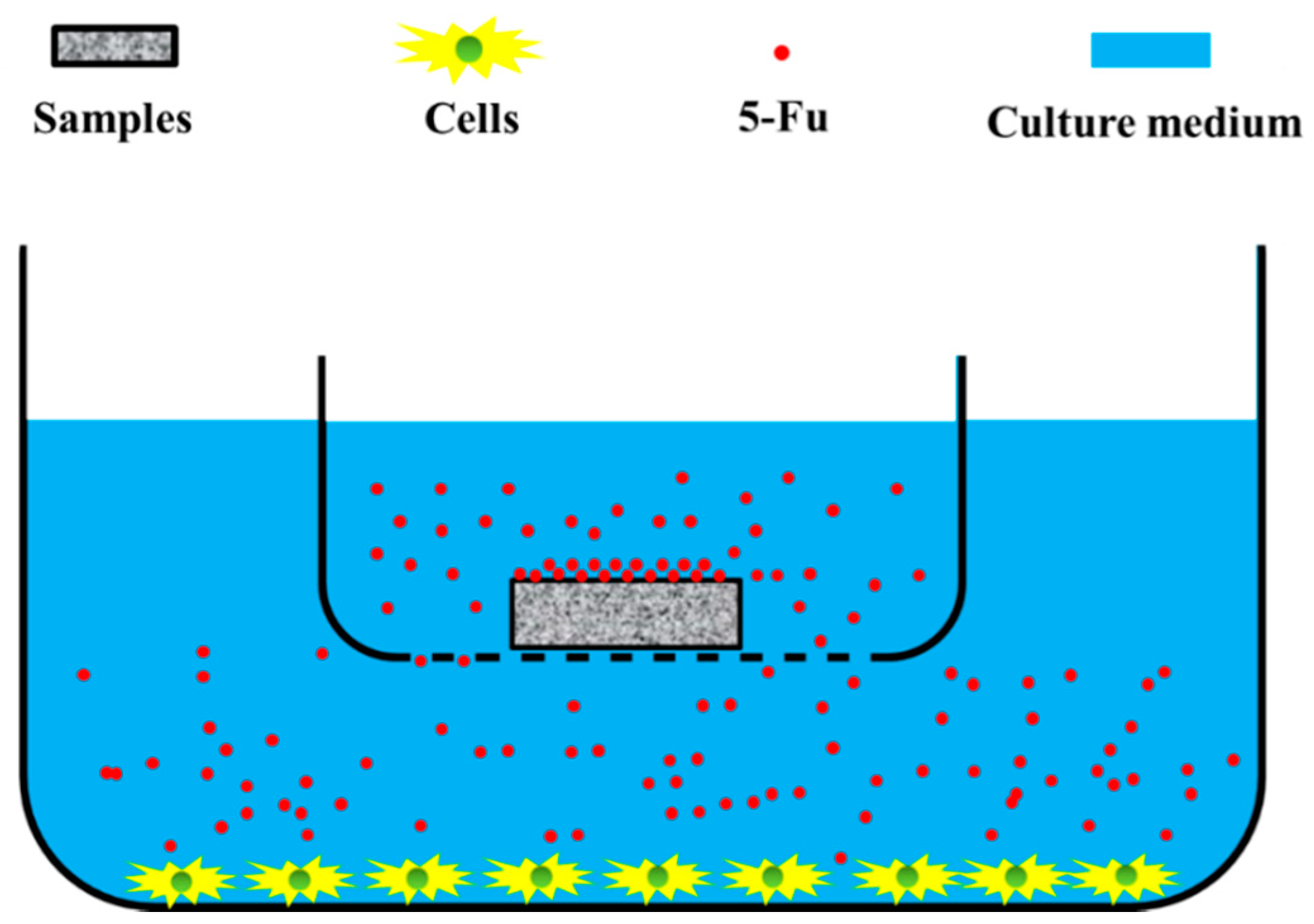
2.3. Anti-Restenosis Function of PDA/PEI/5-Fu Layers
2.4. Statistical Analysis
3. Results and Discussion
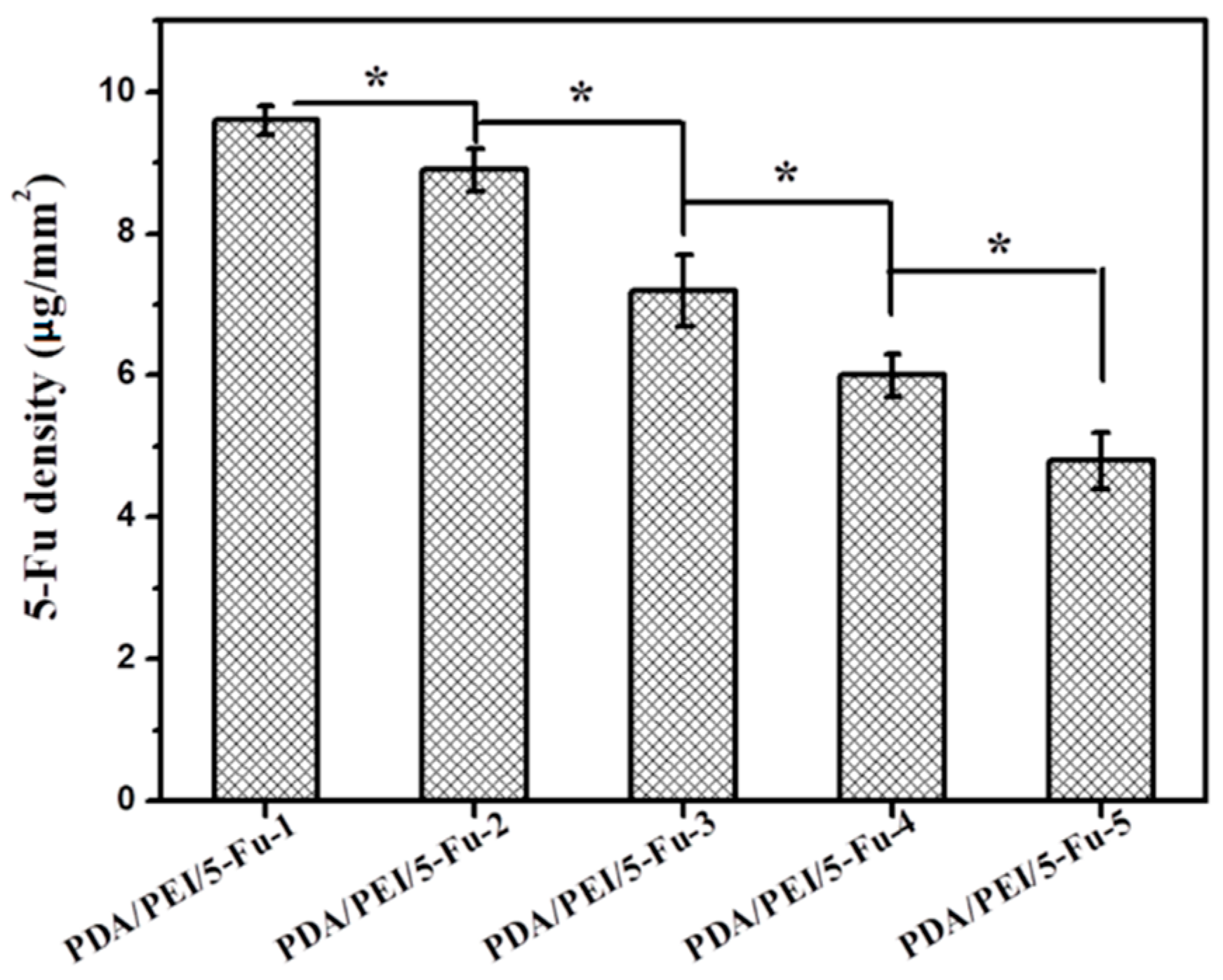
3.1. Quantity of Immobilized 5-Fu
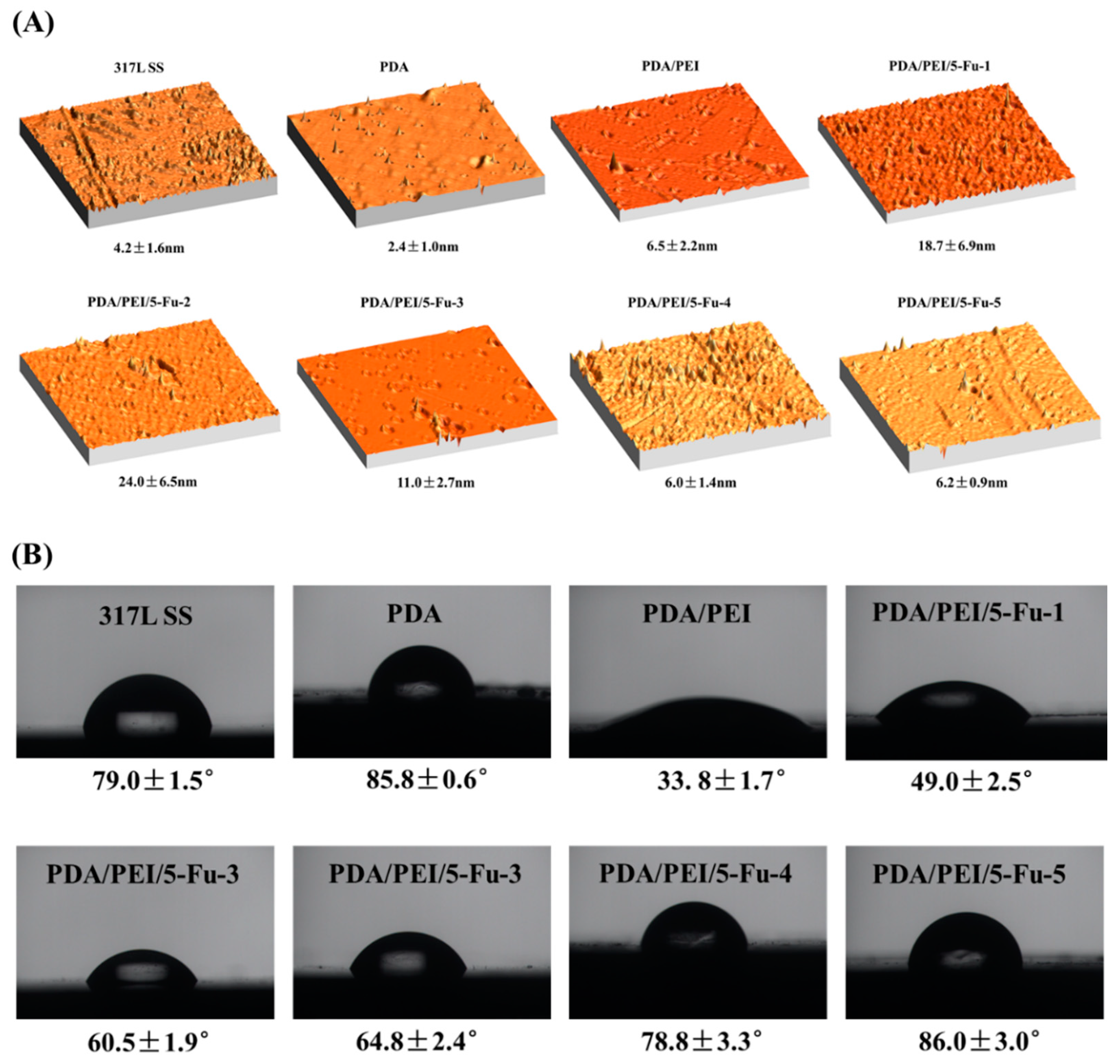
3.2. AFM and Wettability
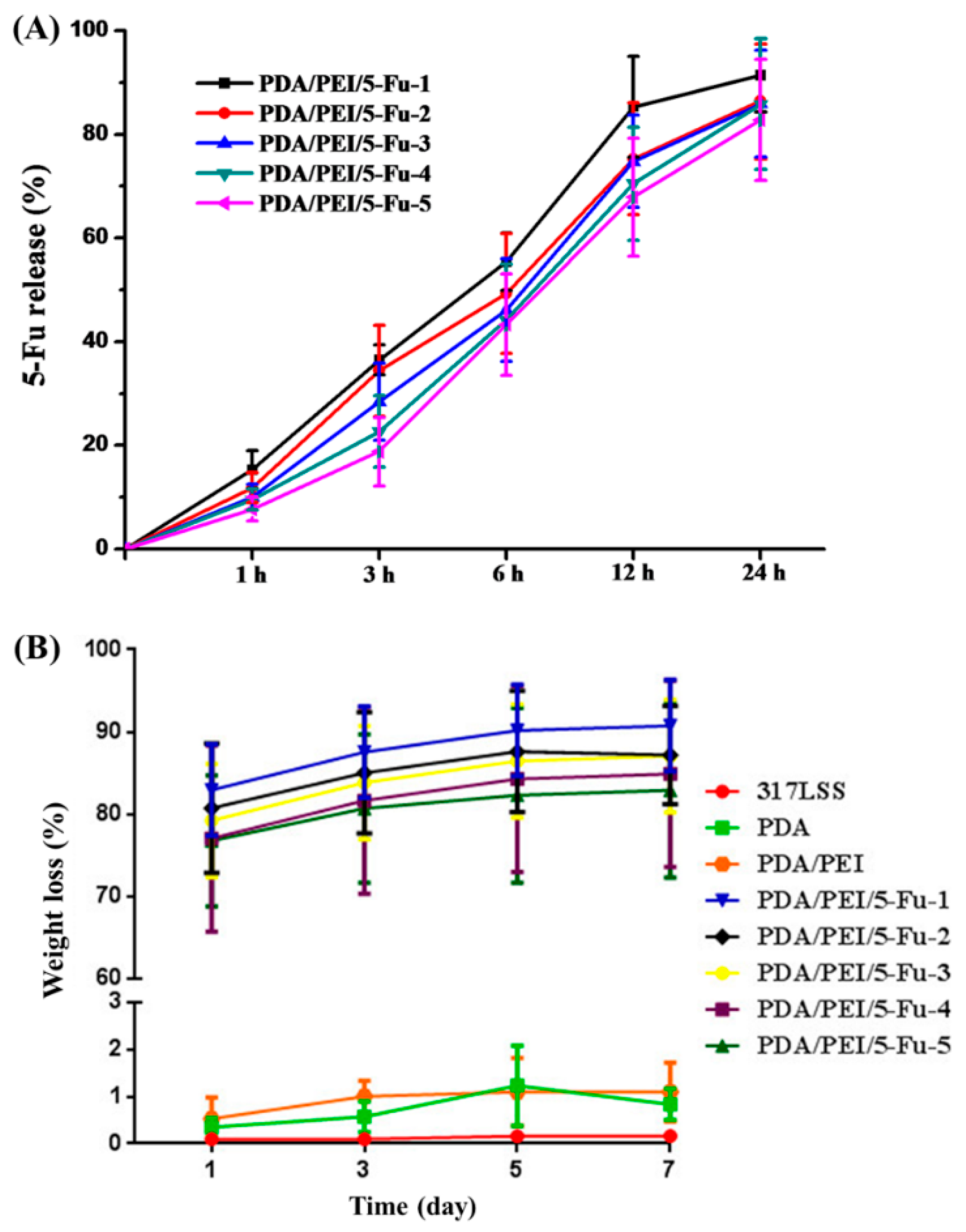
3.3. 5-Fu Release and the Layers’ Biodegradability
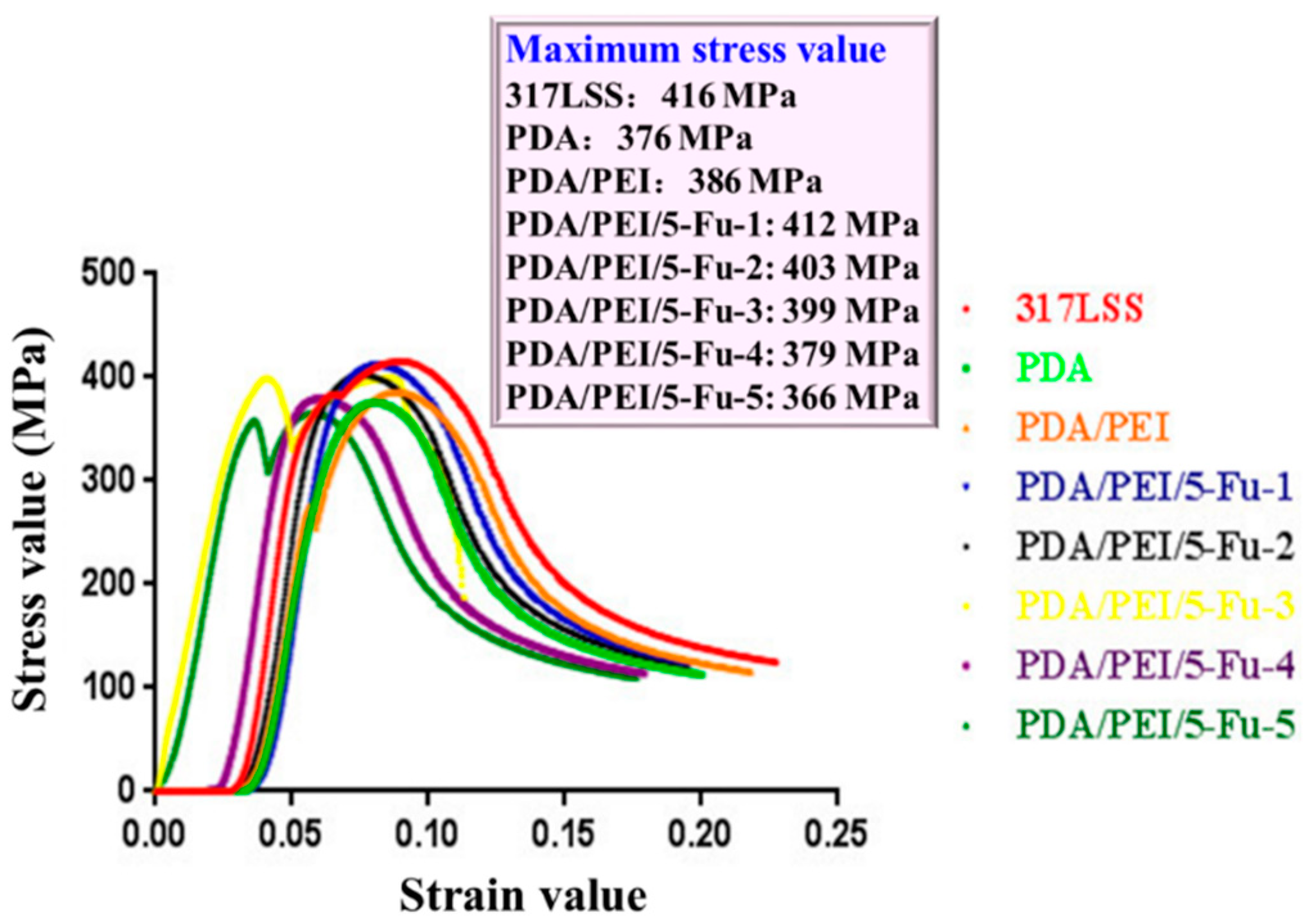
3.4. The Layers’ Biomechanical Properties
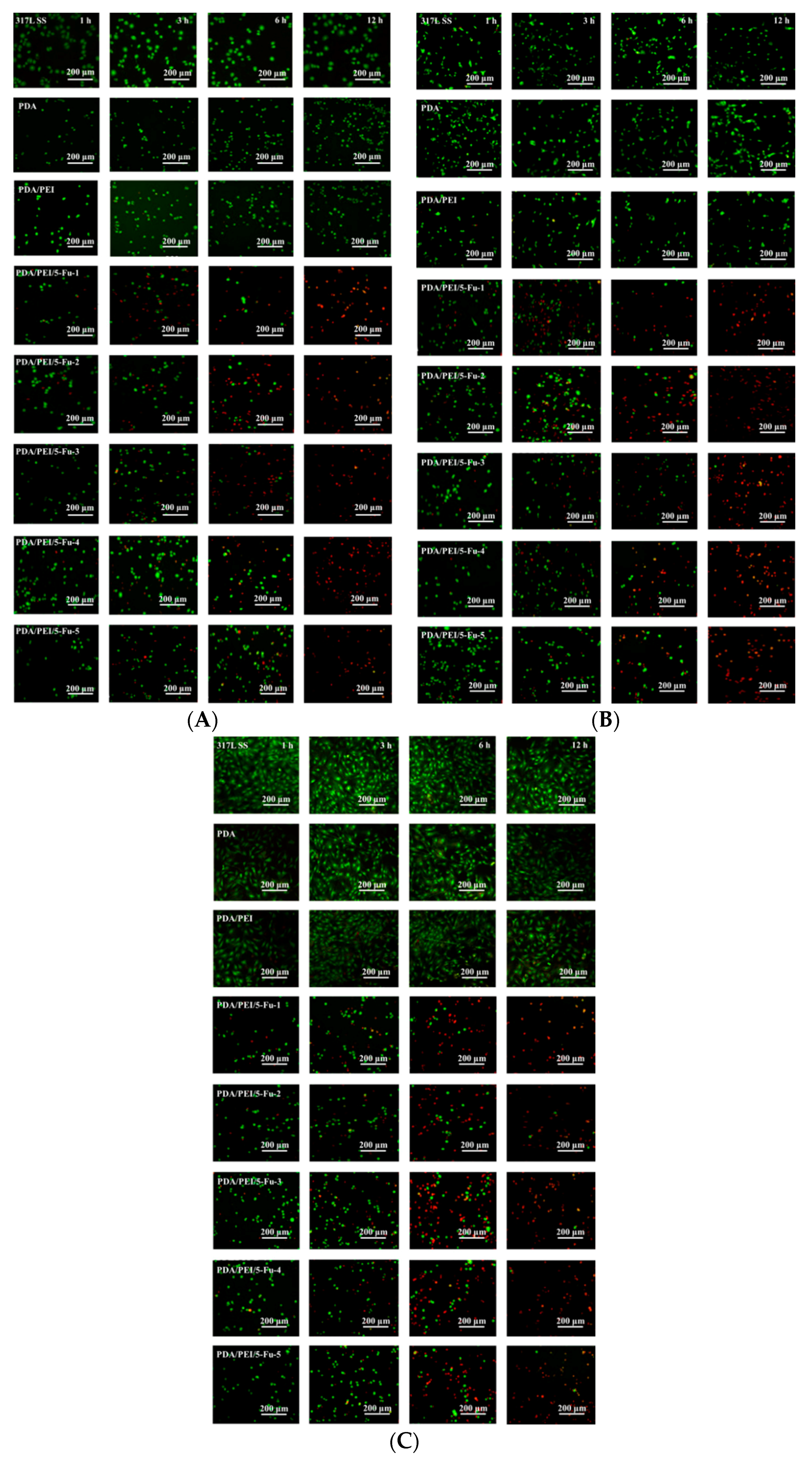
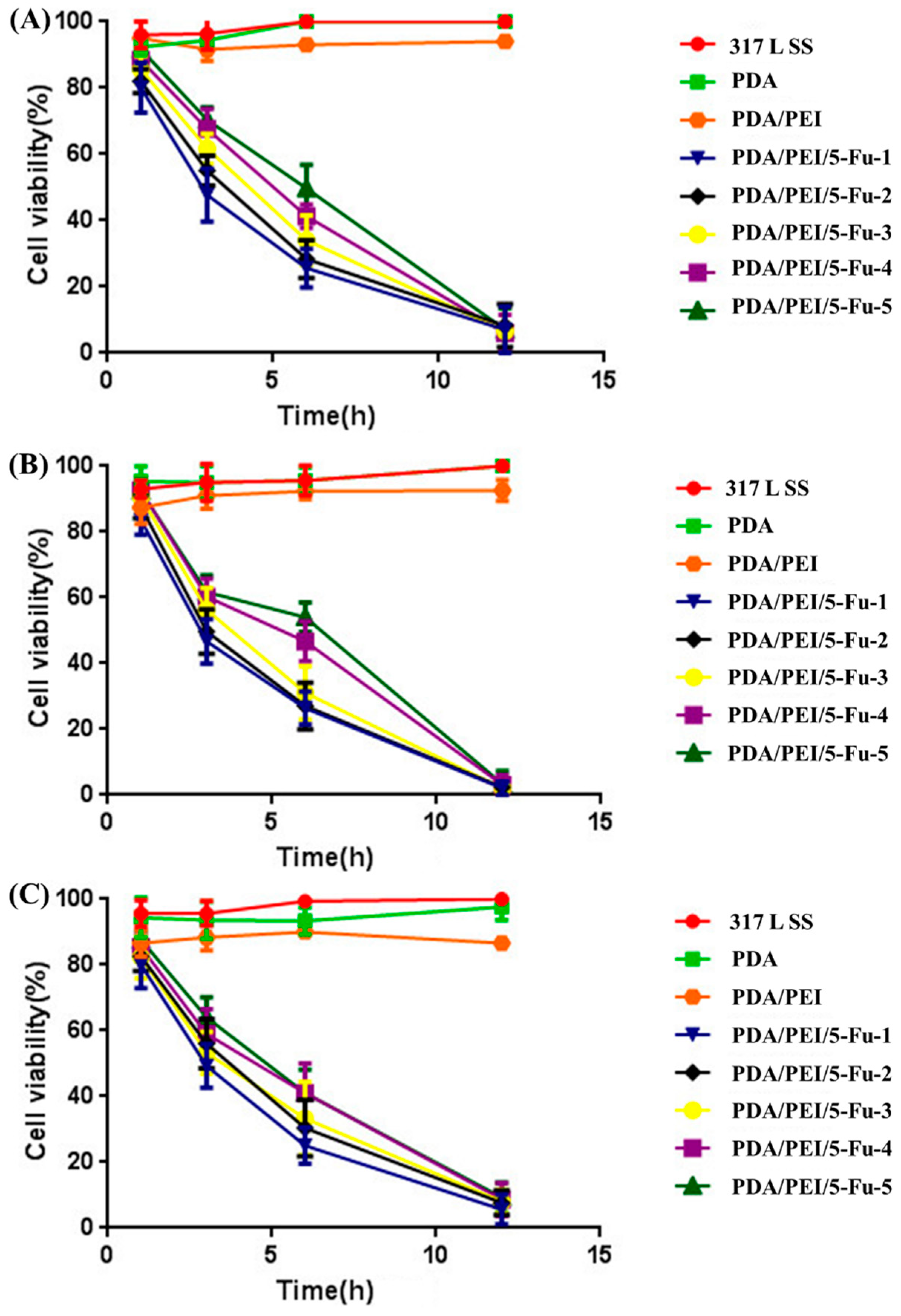
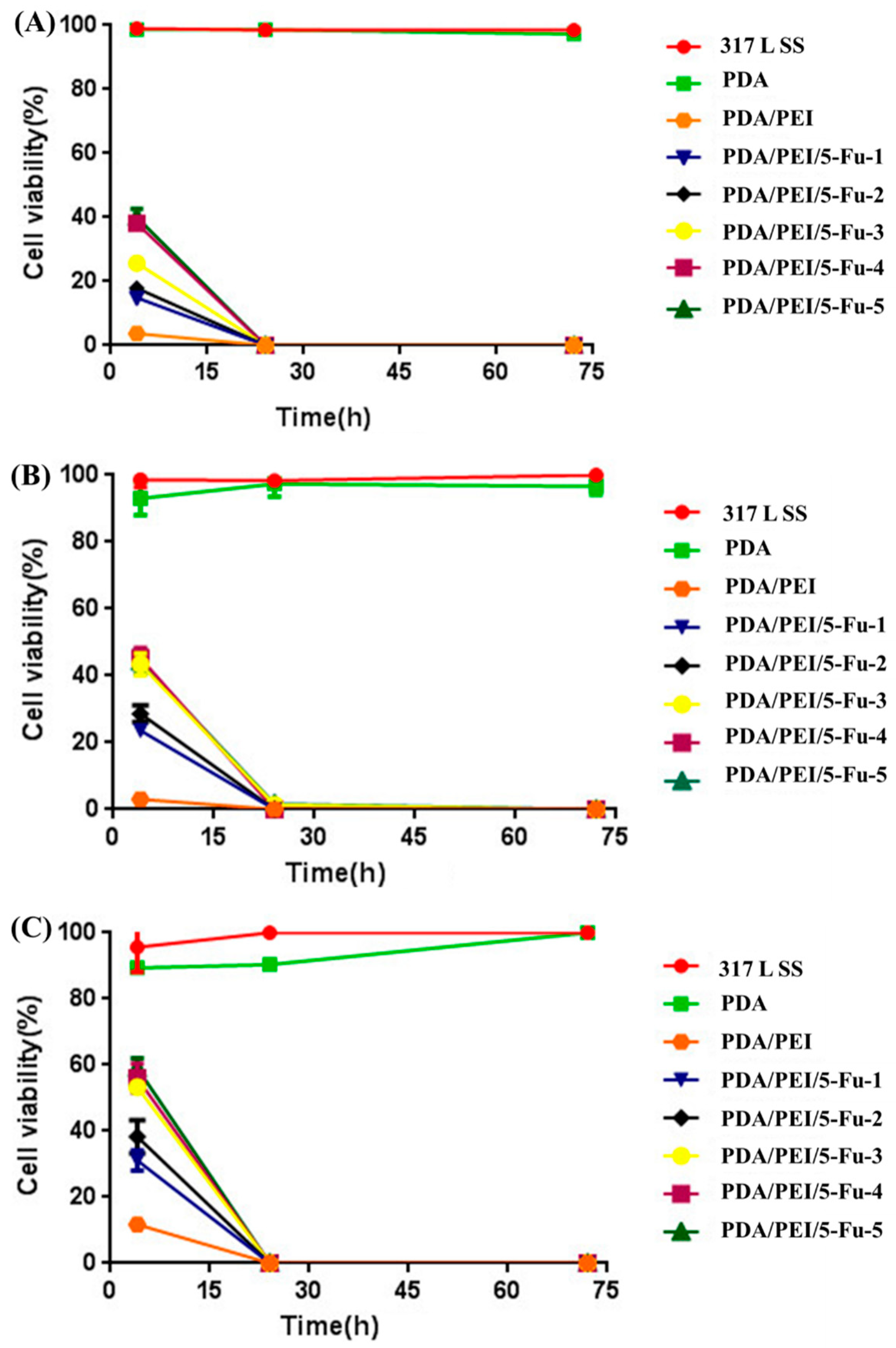
3.5. Effects of Released 5-Fu on Restenosis Related Cells’ Apoptosis and Necrosis
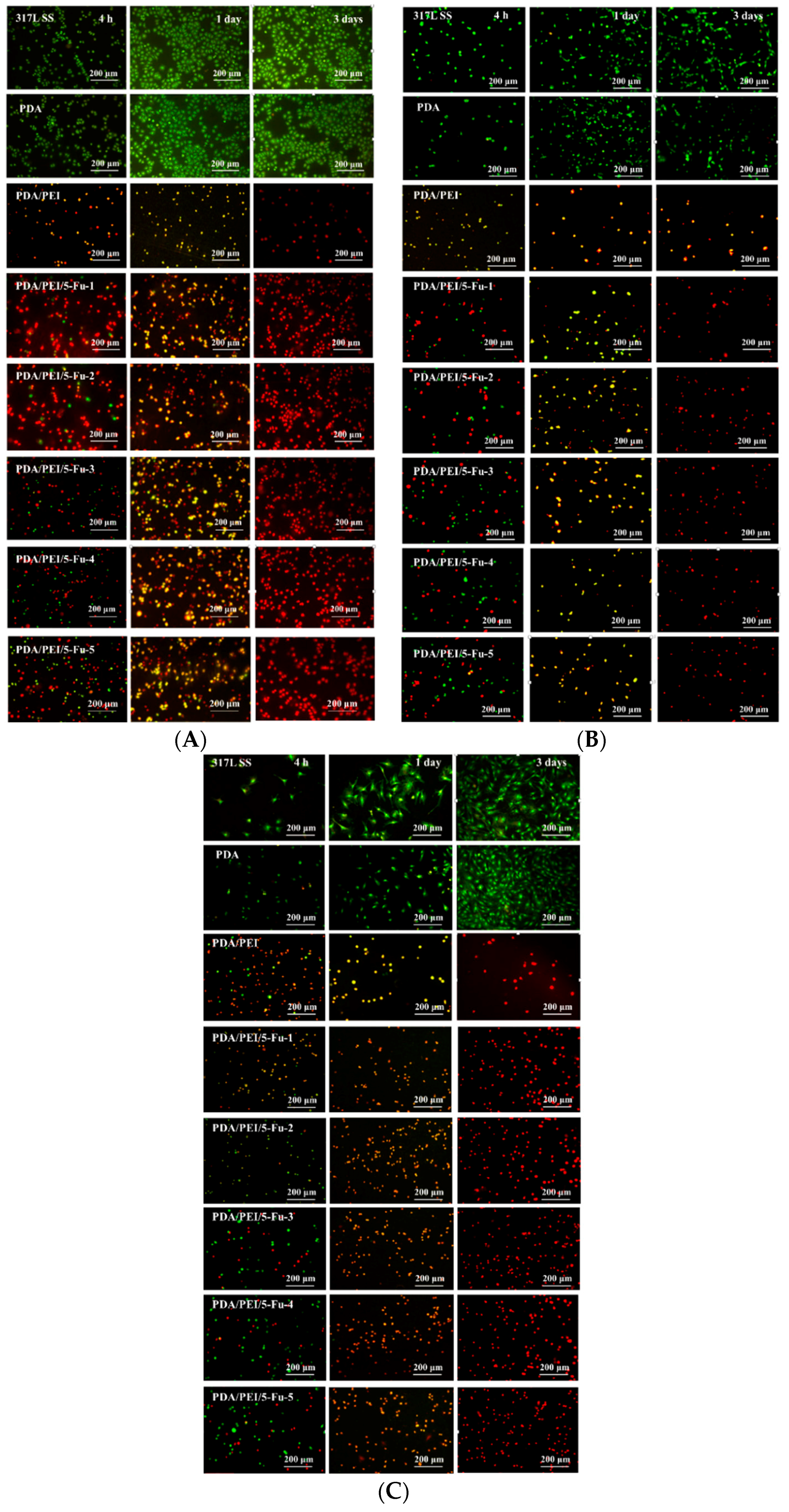
3.6. Effects of PDA/PEI/5-Fu Layers on Restenosis Related Cells’ Apoptosis and Necrosis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bjelovic, M.; Stojakov, D.; Spica, B.; Velicković, D.; Dragan, G.; Skrobić, O.; Djurasić, L.; Grujić, D.; Pesko, P. Minimally invasive esophagectomy in the treatment of esophageal cancer. Acta Chir. Iugosl. 2011, 58, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Yang, Y.H.; Lai, C.H.; Chen, W.C. Outcome of patients with esophageal cancer: A nationwide analysis. Ann. Surg. Oncol. 2013, 20, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.R., Jr. Multidisciplinary management of esophageal cancer. Surg. Oncol. Clin. N. Am. 2013, 22, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Vleggaar, F.P.; Siersema, P.D. Expandable stents for malignant esophageal disease. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, B.D.; Siersema, P.D. Esophageal stenting in clinical practice: An overview. Curr. Treat. Options Gastroenterol. 2018, 16, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Guo, S.; Wang, Z. A type of esophageal stent coating composed of one 5-fluorouracil-containing EVA layer and one drug-free protective layer: In vitro release, permeation and mechanical properties. J. Control. Release 2007, 118, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, C.; Li, B.; Yang, H.L.; Cheng, Y.S.; Cui, W.G. A highly flexible paclitaxel-loaded poly(ε-caprolactone) electrospun fibrous-membrane-covered stent for benign cardia stricture. Acta Biomater. 2013, 9, 8328–8336. [Google Scholar] [CrossRef] [PubMed]
- Tokar, J.L.; Banerjee, S.; Barth, B.A.; Desiletsi, D.J.; Kaul, V.; Kethi, S.R.; Pedrosa, M.C.; Pfau, P.R.; Pleskow, D.K.; Varadarajulu, S. Drug-eluting/biodegradable stents. Gastro Intest. Endosc. 2011, 74, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, Y.; Rong, H.; Lei, L.; Guo, S.R. Development and application of a validated gradient elution HPLC method for simultaneous determination of 5-fluorouracil and paclitaxel in dissolution samples of 5-fluorouracil/paclitaxel-co-eluting stents. J. Pharm. Biomed. Anal. 2012, 59, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Dua, K.S. Expandable stents for benign esophageal disease. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Wu, F.; Zhang, K.; He, Z.K.; Zou, D.; Luo, X.; Fan, Y.H.; Yang, P.; Zhao, A.S.; Huang, N. Controlling molecular weight of hyaluronic acid conjugated on amine-rich Surface: Towards better multifunctional biomaterials for cardiovascular implants. ACS Appl. Mater. Interfaces 2017, 9, 30343–30358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Bai, Y.X.; Wang, X.F.; Li, Q.; Guan, F.X.; Li, J.A. Surface modification of esophageal stent materials by a polyethylenimine layer aiming at anti-cancer function. J. Mater. Sci. Mater. Med. 2017, 28, 125. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, S.; Miyamoto, S.; Kikuchi, O.; Goto, T.; Amanuma, Y.; Muto, M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology 2015, 149, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, J.A.; Li, N.; Wang, K.B.; Li, X.; Wang, J. Surface modification of cardiovascular stent material-316L SS with estradiol loaded poly(trimethylene carbonate) film for better biocompatibility. Polymers 2017, 9, 598. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, J.A.; Zhang, K.; He, Z.K.; Yang, P.; Zou, D.; Huang, N. Multi-functional coating based on hyaluronic acid and dopamine conjugate for potential application on surface modification of cardiovascular implanted devices. ACS Appl. Mater. Interfaces 2016, 8, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, X.L.; Wong, Y.S.; Zheng, W.J.; Zhang, Y.B.; Cao, W.Q.; Chen, T.F. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS Nano 2012, 6, 6578–6591. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Zhang, K.; Xu, Y.; Chen, J.; Yang, P.; Zhao, Y.C.; Zhao, A.S.; Huang, N. A novel co-culture models of human vascular endothelial cells and smooth muscle cells by hyaluronic acid micro-pattern on titanium surface. J. Biomed. Mater. Res. Part A 2014, 102A, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Li, G.C.; Zhang, K.; Liao, Y.Z.; Yang, P.; Maitz, M.F.; Huang, N. Co-culture of vascular endothelial cells and smooth muscle cells by hyaluronic acid micro-pattern on titanium surface. Appl. Surf. Sci. 2013, 273, 24–31. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Z.Q.; Zhou, J.K.; Xing, Q.; Ma, S.S.; Li, Q.H.; Zhang, Y.T.; Yao, M.H.; Wang, X.F.; Li, Q.; et al. Potential application of an injectable hydrogel scaffold loaded with mesenchymal stem cells for treating traumatic brain injury. J. Mater. Chem. B 2018, 6, 2982–2992. [Google Scholar] [CrossRef]
- Li, J.A.; Qin, W.; Zhang, K.; Wu, F.; Yang, P.; He, Z.K.; Zhao, A.S.; Huang, N. Controlling mesenchymal stem cells differentiate into contractile smooth muscle cells on a TiO2 micro/nano interface: Towards benign pericytes environment for endothelialization. Colloids Surf. B Biointerfaces 2016, 145, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Zou, D.; Zhang, K.; Luo, X.; Yang, P.; Jing, Y.Y.; Zhang, Y.X.; Cui, G.L.; Huang, N. Strong Multi-functions based on conjugating chondroitin sulfate on amine-rich surface direct vascular cells fate for cardiovascular implanted devices. J. Mater. Chem. B 2017, 5, 8299–8313. [Google Scholar] [CrossRef]
- Li, J.A.; Yang, P.; Zhang, K.; Ren, H.L.; Huang, N. Preparation of SiO2/TiO2 and TiO2/TiO2 micropattern and their effects on platelet adhesion and endothelial cell regulation. Nucl. Instrum. Methods Phys. Res. B 2013, 307, 575–579. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, P.; Ding, Y.; Maitz, M.F.; Yang, Z.; Tu, Q.; Xiong, K.; Leng, Y.; Huang, N. A biocompatible and functional adhesive amine-rich coating based on dopamine polymerization. J. Mater. Chem. B 2015, 3, 72–81. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Statz, A.R.; Rho, J.; Park, T.G.; Messersmith, P.B. Substrate independent layer by layer assembly by using messel-adhesive-inspired polymers. Adv. Mater. 2008, 20, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Li, J.A.; Wu, F.; He, Z.K.; Yang, P.; Huang, N. Effect of micropatterned TiO2 nanotubes thin film on the deposition of endothelial extracellular matrix: For the purpose of enhancing surface biocompatibility. Biointerphases 2015, 10, 04A302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Zhang, K.; Wu, J.J.; Zhang, L.J.; Yang, P.; Tu, Q.F.; Huang, N. Tailoring of the titanium surface by preparing cardiovascular endothelial extracellular matrix layer on the hyaluronic acid micro-pattern for improving biocompatibility. Colloids Surf. B Biointerfaces 2015, 128, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Xue, G.N.; Ye, C.Y.; Wang, Y.; Zhao, A.S.; Huang, N.; Li, J.A. The effect of anti-CD133/fucoidan bio-coatings on hemocompatibility and EPC capture. J. Biomater. Sci. Polym. Ed. 2017, 28, 2066–2081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, X.F.; Guan, F.X.; Li, Q.; Li, J.A. Immobilization of Ophiopogonin D on stainless steel surfaces for improving surface endothelialization. RSC Adv. 2016, 6, 113893–113898. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Zhu, F.; Willette-Brown, J.; Song, N.Y.; Lomada, D.; Song, Y.; Xue, L.; Gray, Z.; Zhao, Z.; Davis, S.R.; Sun, Z.; et al. Autoreactive T cells and chronic fungal infection drive esophageal carcinogenesis. Cell Host Microbe 2017, 21, 478–493.e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Shen, W.Q.; Liu, K. Radioactive self-expanding stents for palliative management of unresectable esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2017, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ni, Z.; Jing, D.; He, L.; Qiao, L.; Liu, L.; Wei, X.; Jiang, M.; Tang, S.; Xu, H. Novel stent in the palliation of malignant esophageal strictures: A retrospective study. Dis. Esophagus 2017, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, M.Q.; Zhang, Y.L.; Guo, Y.C.; Zhao, Y.L.; Wu, Q.W.; Zhao, A.Y.; Zhang, X.Z. Application of esophageal irradiation stents coated with I-125 particles in advanced esophageal cancer. J. BUON 2017, 22, 265–269. [Google Scholar] [PubMed]
- Gan, Z.; Jing, J.; Zhu, G.Y.; Qin, Y.L.; Teng, G.J.; Guo, G.H. Preventive effects of I-125 seeds on benign restenosis following esophageal stent implantation in a dog model. Mol. Med. Rep. 2015, 11, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Zhang, K.; Xu, R.; Liu, H.; Guan, F.; Luo, H.; Chen, Y.; Li, J. Surface Modification of Esophageal Stent Materials by a Drug-Eluting Layer for Better Anti-Restenosis Function. Coatings 2018, 8, 215. https://doi.org/10.3390/coatings8060215
Bai Y, Zhang K, Xu R, Liu H, Guan F, Luo H, Chen Y, Li J. Surface Modification of Esophageal Stent Materials by a Drug-Eluting Layer for Better Anti-Restenosis Function. Coatings. 2018; 8(6):215. https://doi.org/10.3390/coatings8060215
Chicago/Turabian StyleBai, Yuxin, Kun Zhang, Ru Xu, Hongtao Liu, Fangxia Guan, Huiwen Luo, Ye Chen, and Jingan Li. 2018. "Surface Modification of Esophageal Stent Materials by a Drug-Eluting Layer for Better Anti-Restenosis Function" Coatings 8, no. 6: 215. https://doi.org/10.3390/coatings8060215
APA StyleBai, Y., Zhang, K., Xu, R., Liu, H., Guan, F., Luo, H., Chen, Y., & Li, J. (2018). Surface Modification of Esophageal Stent Materials by a Drug-Eluting Layer for Better Anti-Restenosis Function. Coatings, 8(6), 215. https://doi.org/10.3390/coatings8060215





