Preparation and Isothermal Oxidation Behavior of Zr-Doped, Pt-Modified Aluminide Coating Prepared by a Hybrid Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
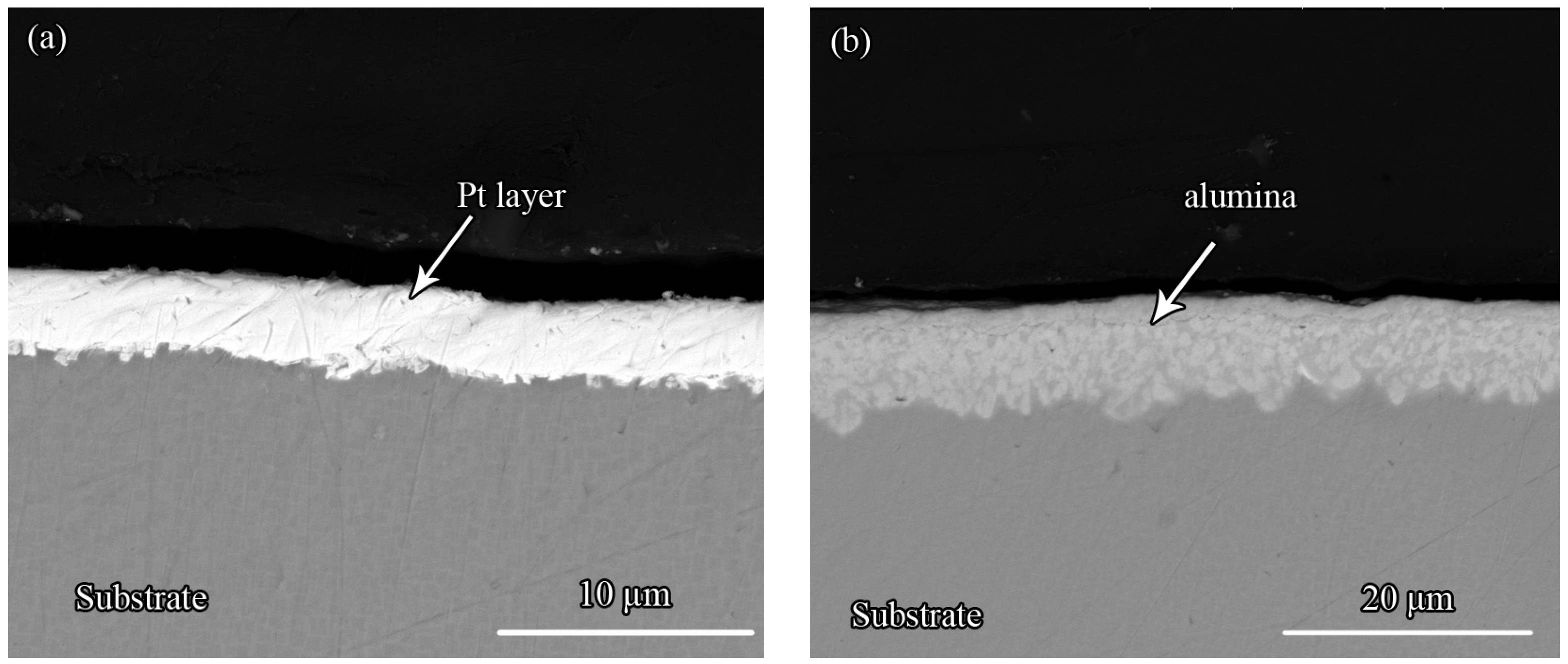
3.1. The Microstructure of the Pt Coating
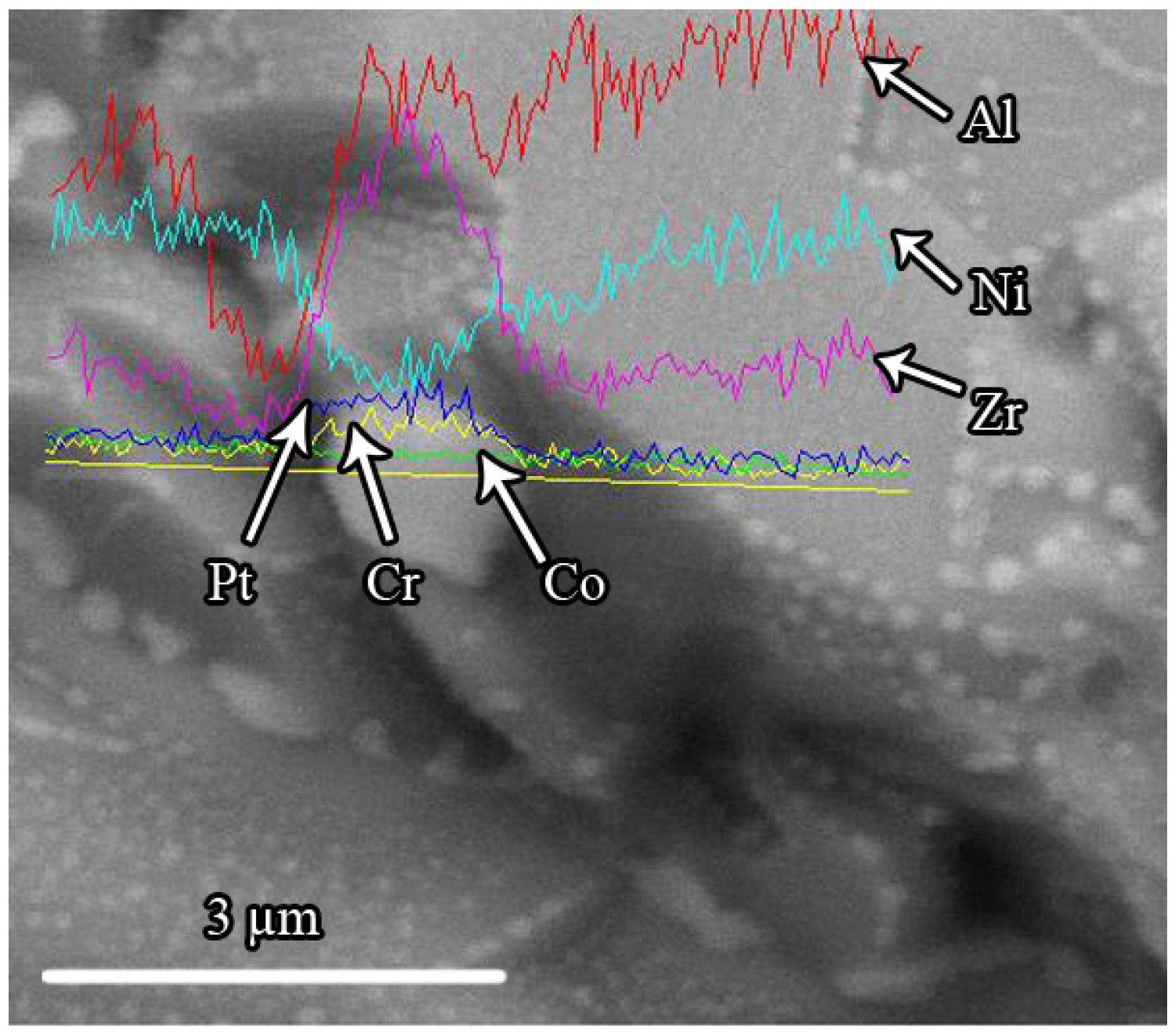
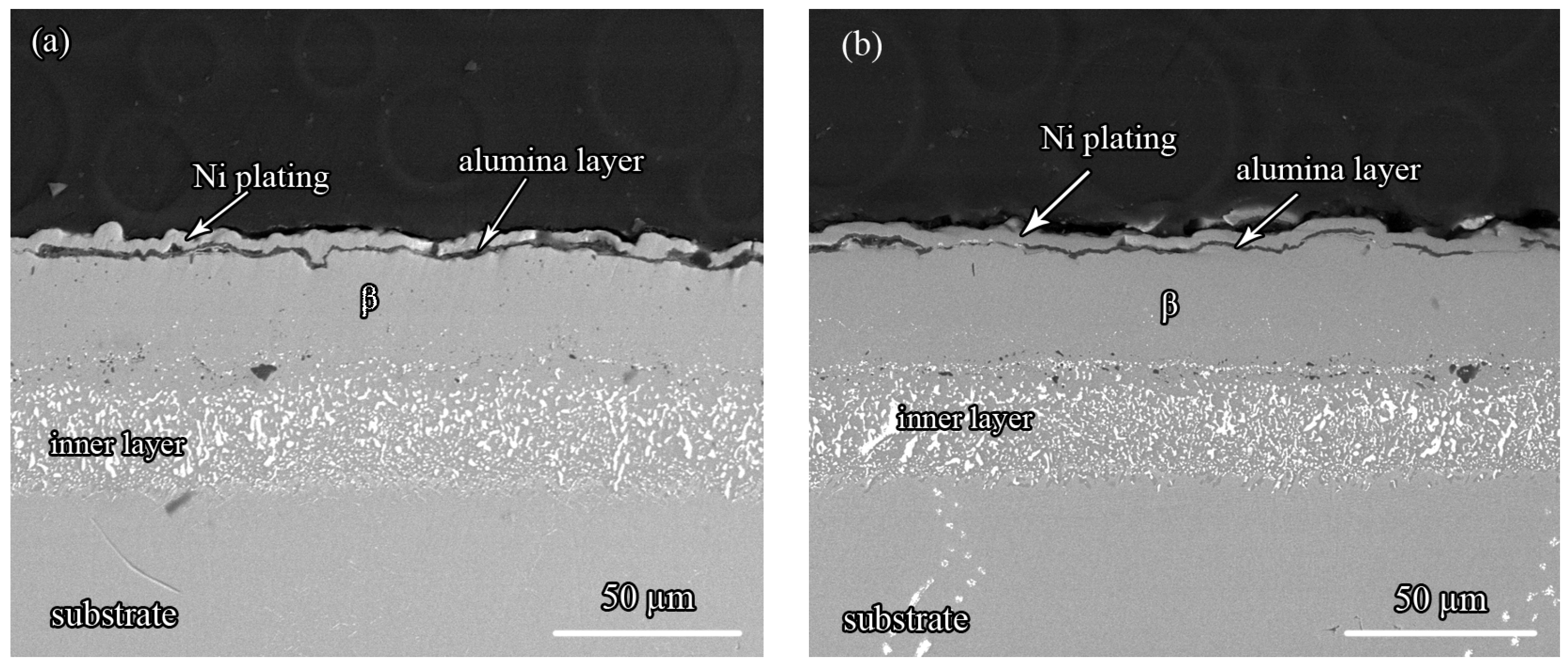
3.2. The Microstructure of the Zr-Doped, Pt-Modified Aluminide Coating
3.3. Oxidation Kinetic Curve
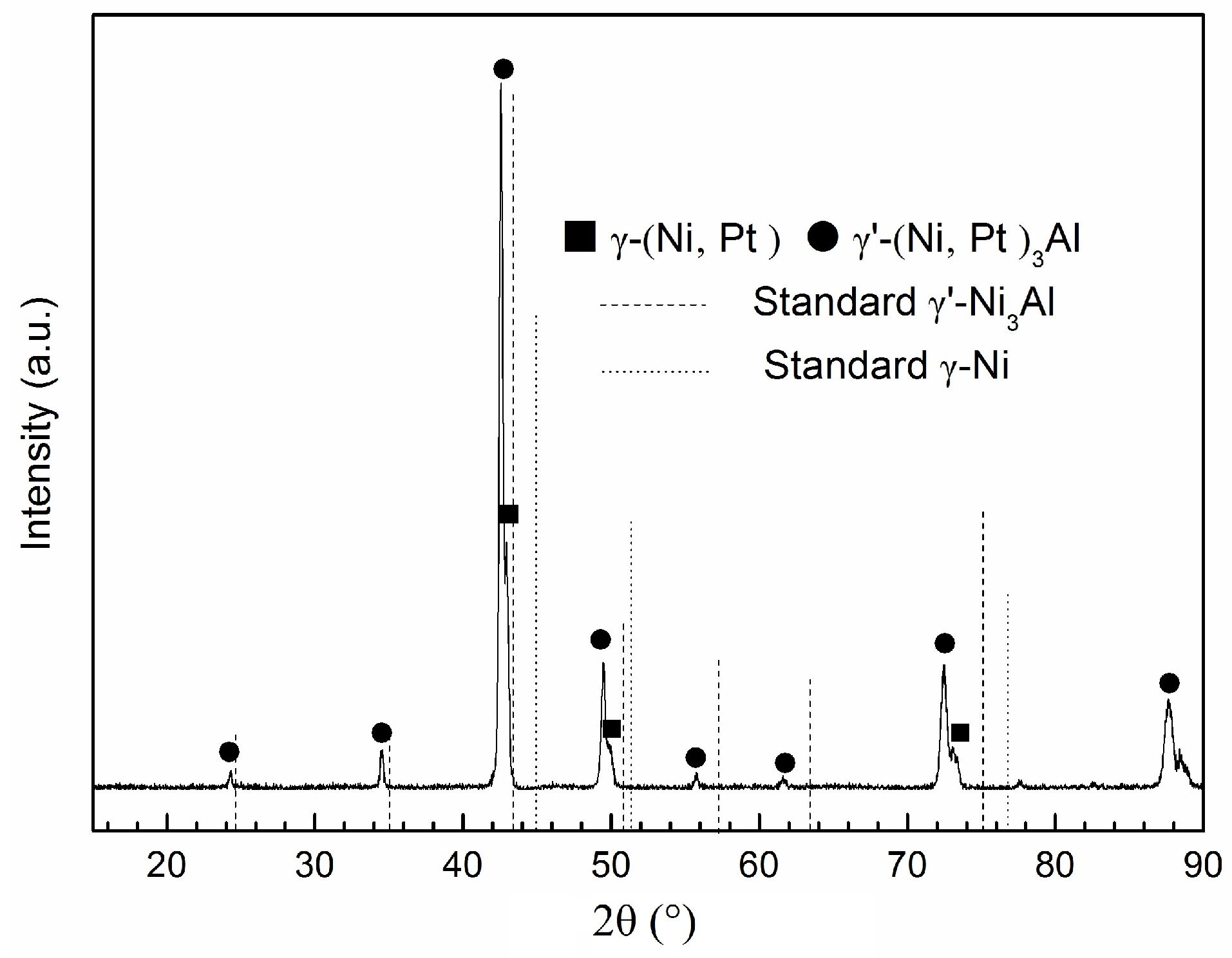
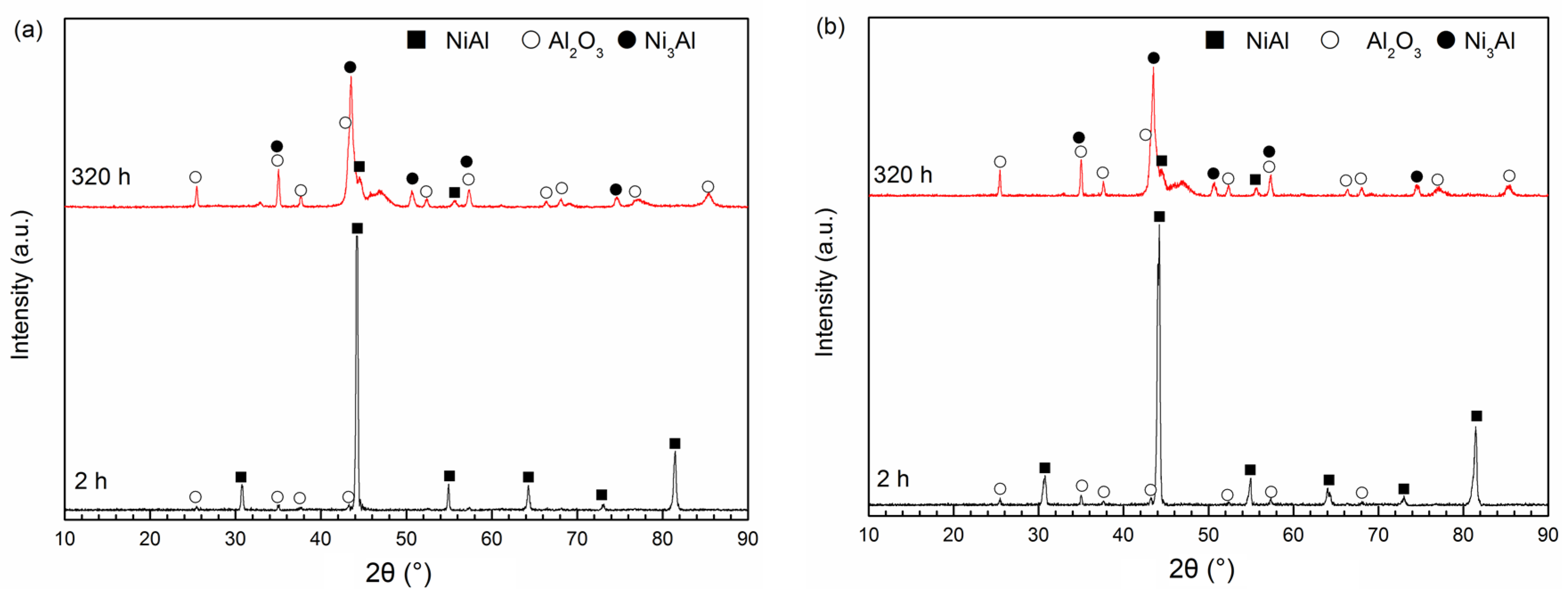
3.4. Oxidation Product
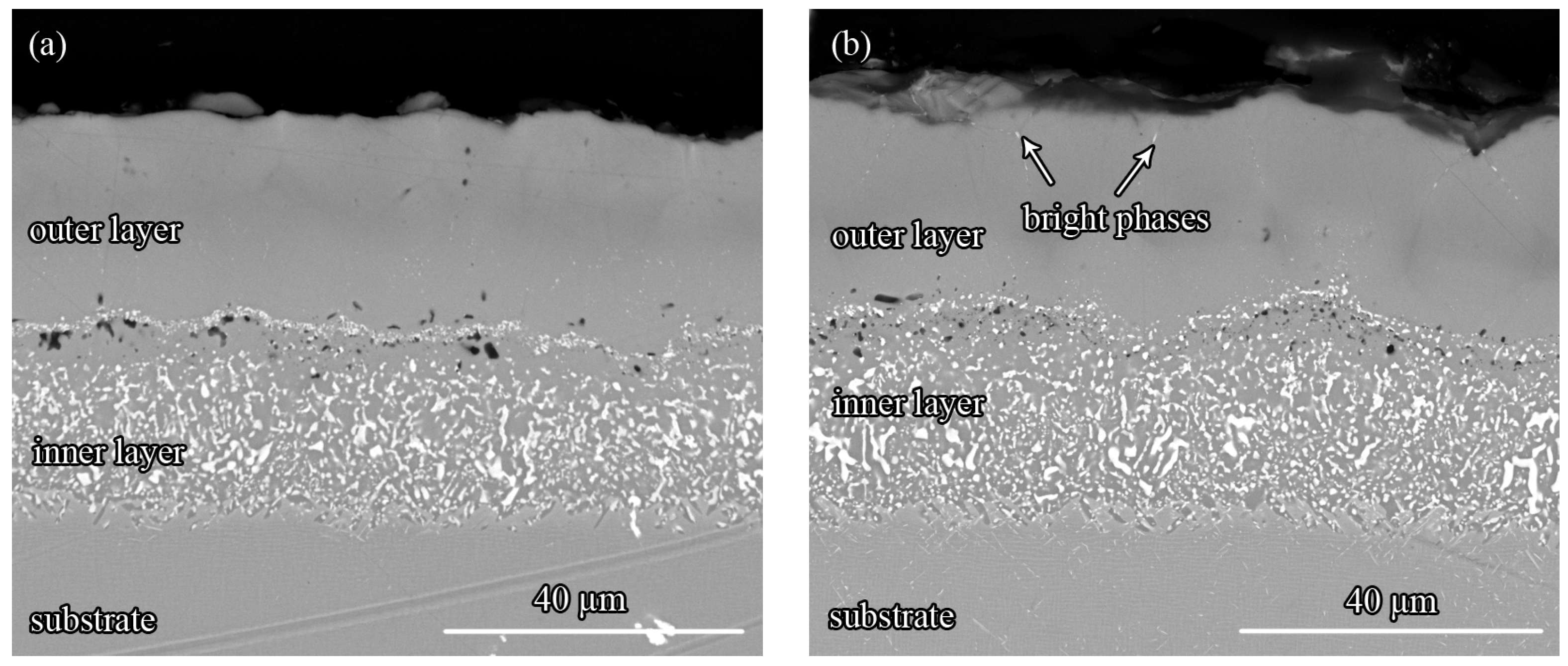
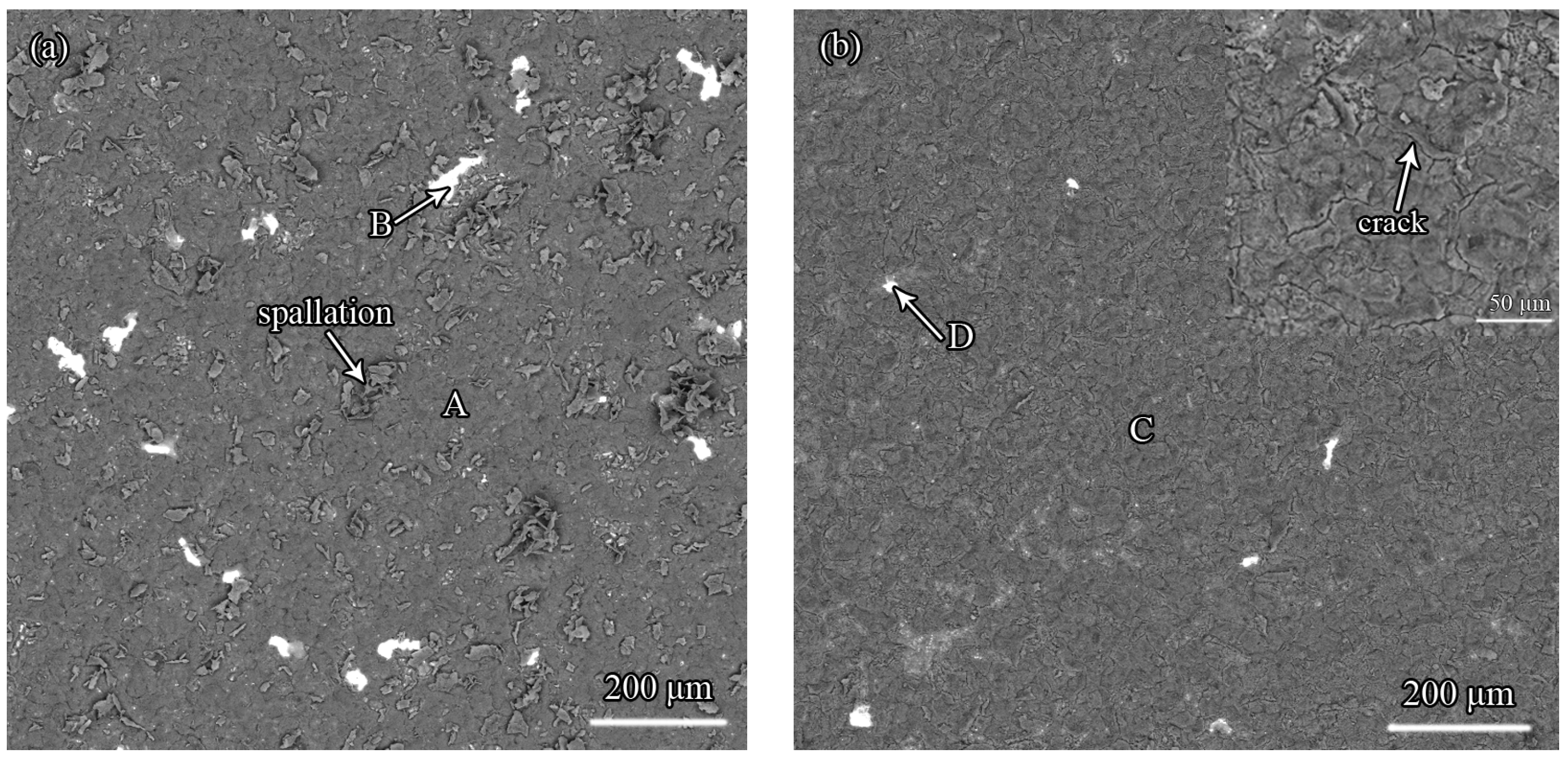
3.5. The Effect of Zr on the Oxidation Resistance of the Pt-Modified Aluminide Coating
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, Y.; Murakami, H.; Zhou, C. Cyclic-oxidation behavior of multilayered Pt/Ru-modified aluminide coating. J. Mater. Sci. Technol. 2011, 27, 280–288. [Google Scholar] [CrossRef]
- Wu, D.; Jiang, S.; Fan, Q.; Gong, J.; Sun, C. Hot corrosion behavior of a Cr-modified aluminide coating on a Ni-based superalloy. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 627–634. [Google Scholar] [CrossRef]
- Fan, Q.X.; Jiang, S.M.; Wu, D.L.; Gong, J.; Sun, C. Microstructure and hot corrosion behaviors of two Co modified aluminide coatings on a Ni-based superalloy at 700 °C. Appl. Surf. Sci. 2014, 311, 214–223. [Google Scholar] [CrossRef]
- Dai, P.; Wu, Q.; Ma, Y.; Li, S.; Gong, S. The effect of silicon on the oxidation behavior of NiAlHf coating system. Appl. Surf. Sci. 2013, 271, 311–316. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, S.; Yu, H.; Sun, C. Preparation and hot corrosion behaviour of Pt modified AlSiY coating on a Ni-based superalloy. Corros. Sci. 2016, 104, 162–172. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Morgiel, J.; Romanowska, J.; Sieniawski, J. Nanoparticles in zirconium-doped aluminide coatings. Mater. Lett. 2015, 139, 50–54. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, C.; Yao, H.; Bao, Z.; Zhu, S.; Wang, F. Preparation and enhanced oxidation performance of a Hf-doped single-phase Pt-modified aluminide coating. Corros. Sci. 2016, 113, 17–25. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, H.; Zhang, L.; Guo, H.; Gong, S. Microstructure and cyclic oxidation behaviour of low-Pt/Dy co-doped β-NiAl coatings on single crystal (SC) superalloy. Surf. Coat. Technol. 2016, 304, 108–116. [Google Scholar] [CrossRef]
- Khakpour, I.; Soltani, R.; Sohi, M.H. Microstructure and high temperature oxidation behaviour of Zr-doped aluminide coatings fabricated on nickel-based super alloy. Procedia Mater. Sci. 2015, 11, 515–521. [Google Scholar] [CrossRef]
- Pint, B.A.; Wright, I.G.; Lee, W.Y.; Zhang, Y.; Prüßner, K.; Alexander, K.B. Substrate and bond coat compositions: Factors affecting alumina scale adhesion. Mater. Sci. Eng. A 1998, 245, 201–211. [Google Scholar] [CrossRef]
- Hamadi, S.; Bacos, M.P.; Poulain, M.; Seyeux, A.; Maurice, V.; Marcus, P. Oxidation resistance of a Zr-doped NiAl coating thermochemically deposited on a nickel-based superalloy. Surf. Coat. Technol. 2009, 204, 756–760. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Pytel, M.; Romanowska, J.; Sieniawski, J. The Effect of Zirconium Addition on the Oxidation Resistance of Aluminide Coatings. J. Mater. Eng. Perform. 2015, 24, 1614–1625. [Google Scholar] [CrossRef]
- Wei, L.; Peng, H.; Jia, F.; Zheng, L.; Gong, S.; Guo, H. Cyclic oxidation behavior of Hf/Zr co-doped EB-PVD β-NiAl coatings at 1200 °C. Surf. Coat. Technol. 2015, 276, 721–725. [Google Scholar] [CrossRef]
- Sitek, R.; Kwaśniak, P.; Sopicka-Lizer, M.; Borysiuk, J.; Kamiński, J.; Mizera, J.; Kurzydłowski, K.J. Experimental and ab-initio study of the Zr- and Cr-enriched aluminide layer produced on an IN 713C Inconel substrate by CVD; investigations of the layer morphology, structural stability, mechanical properties, and corrosion resistance. Intermetallics 2016, 74, 15–24. [Google Scholar] [CrossRef]
- Cho, J.; Wang, C.M.; Chan, H.M.; Rickman, J.M.; Harmer, M.P. Role of segregating dopants on the improved creep resistance of aluminum oxide. Acta Mater. 1999, 47, 4197–4207. [Google Scholar] [CrossRef]
- Hong, S.J.; Hwang, G.H.; Han, W.K.; Lee, K.S.; Kang, S.G. Effect of zirconium addition on cyclic oxidation behavior of platinum-modified aluminide coating on nickel-based superalloy. Intermetallics 2010, 18, 864–870. [Google Scholar] [CrossRef]
- Skinner, P.E. Improvements in platinum plating: A new generation of electroplating baths. Platin. Met. Rev. 1989, 33, 102–105. [Google Scholar]
- Liu, R.; Jiang, S.; Guo, C.; Gong, J.; Sun, C. The alumina scale growth and interdiffusion behaviour of Pt modified AlSiY coating during cyclic oxidation. Corros. Sci. 2017, 120, 121–129. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Sayre, G. Factors affecting the microstructure of platinum-modified aluminide coatings during a vapor phase aluminizing process. Surf. Coat. Technol. 2009, 203, 1264–1272. [Google Scholar] [CrossRef]
- Mollard, M.; Pedraza, F.; Bouchaud, B.; Montero, X.; Galetz, M.C.; Schutze, M. Influence of the superalloy substrate in the synthesis of the Pt-modified aluminide bond coat made by slurry. Surf. Coat. Technol. 2015, 270, 102–108. [Google Scholar] [CrossRef]
- Jackson, M.R.; Rairden, J.R. The aluminization of platinum and platinum-coated IN-738. Metall. Trans. A 1977, 8, 1697–1707. [Google Scholar] [CrossRef]
- Hong, S.J.; Hwang, G.H.; Han, W.K.; Kang, S.G. The effect of Pt contents on the surface morphologies of Pt-modified aluminide coating. Surf. Coat. Technol. 2009, 203, 3066–3071. [Google Scholar] [CrossRef]
- Pedraza, F.; Kennedy, A.D.; Kopecek, J.; Moretto, P. Investigation of the microstructure of platinum-modified aluminide coatings. Surf. Coat. Technol. 2006, 200, 4032–4039. [Google Scholar] [CrossRef]
- Krishna, G.R.; Dasa, D.K.; Singhb, V.; Joshia, S.V. Role of Pt content in the microstructural development and oxidation performance of Pt–aluminide coatings produced using a high-activity aluminizing process. Mater. Sci. Eng. A 1998, 251, 40–47. [Google Scholar] [CrossRef]
- Benoist, J.; Badawi, K.F.; Malie, A.; Ramade, C. Microstructure of Pt modified aluminide coatings on Ni-based superalloys without prior Pt diffusion. Surf. Coat. Technol. 2005, 194, 48–57. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Zhao, C.; Hao, W.; Wang, X.; Xiao, P. The oxidation performance for Zr-doped nickel aluminide coating by composite electrodepositing and pack cementation. Corros. Sci. 2017, 123, 103–115. [Google Scholar] [CrossRef]
- Qian, L.; Fang, X.; Voisey, K.T.; Nekouie, V.; Zhou, Z. Incorporation and evolution of ZrO2 nano-particles in Pt-modified aluminide coating for high temperature applications. Surf. Coat. Technol. 2017, 311, 238–247. [Google Scholar] [CrossRef]
- Fan, Q.X.; Peng, X.; Yu, H.J.; Jiang, S.M.; Gong, J.; Sun, C. The isothermal and cyclic oxidation behaviour of two Co modified aluminide coatings at high temperature. Corros. Sci. 2014, 84, 42–53. [Google Scholar] [CrossRef]
- Prescott, R.; Mitchell, D.F.; Graham, M.J.; Doychak, J. Oxidation mechanisms of β-NiAl + Zr determined by SIMS. Corros. Sci. 1995, 37, 1341–1364. [Google Scholar] [CrossRef]
- Wang, C.M.; Cargill, G.S., III; Harmer, M.P.; Chan, H.M.; Cho, J. Atomic structural environment of grain boundary segregated Y and Zr in creep resistant alumina from EXAFS. Acta Mater. 1999, 47, 3411–3422. [Google Scholar] [CrossRef]
- Guo, H.; Li, D.; Zheng, L.; Gong, S.; Xu, H. Effect of co-doping of two reactive elements on alumina scale growth of β-NiAl at 1200 °C. Corros. Sci. 2014, 88, 197–208. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Z.; Kou, Z.; Chen, Y.; Zhao, G.; Zhao, X.; Guo, F.; Xiao, P. High temperature stress and its influence on surface rumpling in NiCoCrAlY bond coat. Acta Mater. 2017, 139, 122–137. [Google Scholar] [CrossRef]
- Bouchaud, B.; Balmain, J.; Pedraza, F. Cyclic and isothermal oxidation at 1100 °C of a CVD aluminised directionally solidified Ni superalloy. Oxid. Met. 2008, 69, 193–210. [Google Scholar] [CrossRef]
- Ke, P.; Wang, Q.; Hua, W.; Gong, J.; Sun, C.; Zhou, Y. Stresses and microstructural development of thermal barrier coatings using AIP/D-gun two-step processing. J. Am. Ceram. Soc. 2007, 90, 936–941. [Google Scholar] [CrossRef]



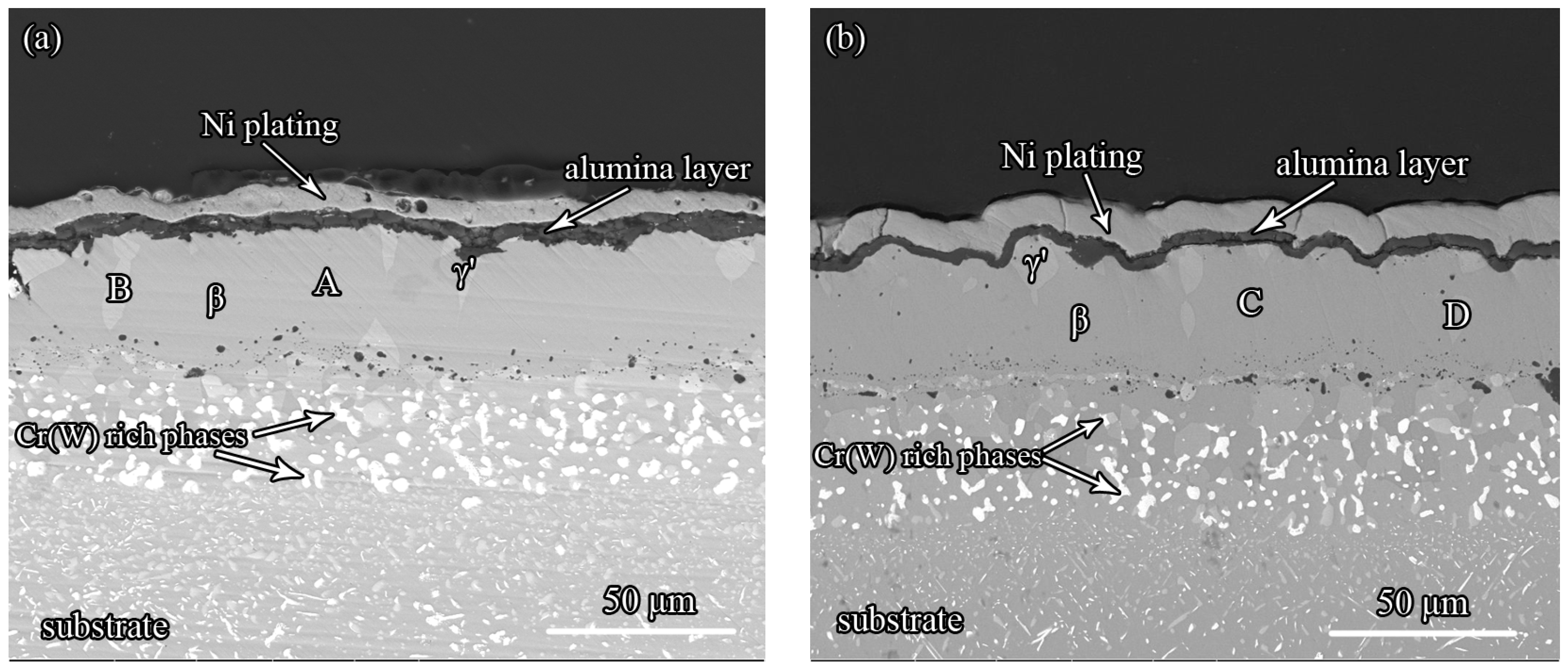
| Spot | Al (at.%) | O (at.%) | Ni (at.%) | W (at.%) | Cr (at.%) | Co (at.%) | Pt (at.%) | Hf (at.%) |
|---|---|---|---|---|---|---|---|---|
| A | 46.4 | 50.2 | 1.6 | 1.9 | – | – | – | – |
| B | 18.2 | 19.3 | 48.7 | 3.0 | 3.0 | 5.3 | 2.4 | 0.2 |
| C | 37.3 | 59.3 | 1.0 | 2.4 | – | – | – | – |
| D | 13.8 | 30.6 | 41.8 | 4.8 | 2.4 | 4.7 | 2.0 | – |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Yu, H.; Wang, T.-G.; Wu, Z.; Liu, Y. Preparation and Isothermal Oxidation Behavior of Zr-Doped, Pt-Modified Aluminide Coating Prepared by a Hybrid Process. Coatings 2018, 8, 1. https://doi.org/10.3390/coatings8010001
Fan Q, Yu H, Wang T-G, Wu Z, Liu Y. Preparation and Isothermal Oxidation Behavior of Zr-Doped, Pt-Modified Aluminide Coating Prepared by a Hybrid Process. Coatings. 2018; 8(1):1. https://doi.org/10.3390/coatings8010001
Chicago/Turabian StyleFan, Qixiang, Haojun Yu, Tie-Gang Wang, Zhenghuan Wu, and Yanmei Liu. 2018. "Preparation and Isothermal Oxidation Behavior of Zr-Doped, Pt-Modified Aluminide Coating Prepared by a Hybrid Process" Coatings 8, no. 1: 1. https://doi.org/10.3390/coatings8010001
APA StyleFan, Q., Yu, H., Wang, T.-G., Wu, Z., & Liu, Y. (2018). Preparation and Isothermal Oxidation Behavior of Zr-Doped, Pt-Modified Aluminide Coating Prepared by a Hybrid Process. Coatings, 8(1), 1. https://doi.org/10.3390/coatings8010001







