Abstract
Water tightness of a concrete cover layer is important, as it is typically used as a protective coating of the steel reinforcement. Water tightness can be impaired by crack formation or by permeability. A bacteria-based lactate-derived healing agent (HA) can be added to concrete to enhance the potential for restoration of water tightness. Bacterial conversion of the included carbon source results in CO2 production and subsequent CaCO3 precipitation, similar to the mechanism of concrete carbonation. Carbonation is known to densify concrete, particularly when using ordinary Portland cement (OPC), but to a much lower extend in slag-based concrete (CEM III/B). To identify the effect of HA addition on concrete properties, this study focusses on the ingress of moisture in non-cracked concrete surfaces by assessing capillary water absorption. Surface properties were determined for sealed and unsealed surfaces of concrete—either based on OPC or CEM III/B—before and after curing under three different conditions: Dry, wet, or humid. HA addition to concrete containing slag cement generated a surface less prone to continued drying, but resulted in higher water absorption. In contrast, surface water absorption significantly decreased upon HA addition to OPC-based samples, independent of the curing regime. It is therefore concluded that HA in its current form is suitable for application in OPC, but less in CEM III/B-based mixtures.
1. Introduction
Concrete structures typically have steel reinforcement embedded to take over tensile stresses. In order to activate the steel under tension, the concrete surface layer cracks. When intact, the concrete cover delays the onset of steel corrosion by creating an alkaline environment [1] and preventing ingress of deleterious materials [2,3]. Ingress is typically limited by surface densification [4]. Surface density is enhanced by curing [5]. Carbon dioxide is a typical agent that can affect the steel through de-alkalinisation of the surrounding concrete matrix, called carbonation [6]. Carbonation can make the surface less susceptible to the ingress of water, particularly when using ordinary Portland cement (OPC) [7]. However, upon using high slag containing cement (CEM III/B), the surface becomes more open of structure [8] due to limited alkaline buffer in the slag-based matrix and carbonation of calcium silicate hydrate (CSH). A direct relation was found between carbonation depth and capillary absorption [9]. Further related to the reduction of water absorption is the limitation of deterioration mechanisms (e.g., freeze–thaw damage and transportation properties such as water permeability) [10]. The main material formed in the cementitious matrix upon carbonation are carbonates, and further enhancement of the sealing function should occur through promotion of additional carbonate depositions [11].
Enhanced carbonate formation potential can be built in by application of a bacteria-based agent to mortar or concrete [12]. Carbonates can be formed from the microbial metabolic conversion of an organic carbon source [12,13]. Limitation of freeze–thaw salt scaling damage was found for surface application of a bacteria-based agent in liquid form to concrete surfaces [14]. Regain of water-tightness of cracked mortar was found from incorporation of a solid bacteria-based additive or healing agent (HA) at the mixing stage [15]. Where negligible influence was found for mortar strength development and high sealing capacity of cracks upon immersion in water, the effect on the water transport properties of intact mortar or concrete was not determined.
It is interesting to assess whether inclusion of the healing agent as an additive to concrete readily affects the surface transport properties, also depending on the type of curing regime applied. Therefore, this study investigates whether the capillary absorption of a sealed or unsealed concrete surface differ before and after specific curing regimes (dry, humid, or submerged condition). As the bacterial metabolic conversion of organic compounds may be regarded as a source of carbonation (and therefore exert similar effect as the carbonation mechanism), a more pronounced densifying effect is expected for high clinker-containing cements (CEM I) in comparison to lower alkaline slag-containing cements (CEM III). Therefore, for this work, two types of cements widely used in The Netherlands—CEM I and CEM III/B [16]—were chosen to cover two extreme cases. The first, being a near full clinker cement (ordinary Portland cement, OPC, CEM I), and the second, a cement with approximately 70% replacement of clinker by slag (blast furnace slag cement, BFSC, CEM III/B). In addition to permeability aspects of the concrete cover, the effect of healing agent on cement hydration and concrete compressive strength development was also investigated. This should allow an assessment of whether the change in surface absorption properties is caused by chemical interaction of the HA with cement. It is expected that HA can be added to cementitious material featuring more alkalinity buffer, such as OPC, as the surface properties are not negatively affected and the surface is densified when immersed in water.
2. Materials and Methods
2.1. HA, Cement Paste and Concrete Material
Healing agent (HA) particles (2 mm diameter × 2–3 mm long cylinders) composed of lactate derivatives, calcium source, bacterial spores (of Bacillus cohnii-related strains), and activation nutrients were obtained from Corbion (Gorinchem, The Netherlands). Cement paste was made by mixing ordinary Portland cement (OPC, CEM I 42.5 N, ENCI, Rotterdam, The Netherlands) or blast furnace slag cement (CEM III/A 52.5 R, CEMEX or CEM III/B 42.5 N LH HS, ENCI, IJmuiden, The Netherlands) with water to obtain a water to cement ratio (w/c) of 0.50, following the mixing procedure as for standard mortar (NEN-EN 196-1 [17]). HA particles were added to the paste specimen at 3%, 4%, or 5% by mass of cement. Additional concrete specimens were prepared in accordance with standards (NEN-EN 206 [18], NEN 8005 [19]) with a designed strength class of C30/37, a slump of 120 mm, and a w/c of 0.50 or 0.55 as specified in Table 1. Constituents used were OPC and CEM III/B, tap water, river sand, and gravel of 0–4 mm and 4–16 mm, respectively. HA particles were added to the concrete specimens in a quantity of 15 kg·m−3, leading to an HA-to-cement ratio of 4% by mass and an HA-to-paste ratio of 3% by mass.

Table 1.
Concrete mixture proportions using a water to cement ratio of 0.55 or 0.50.
2.2. Isothermal Calorimetry
In order to determine the effect of HA addition on cement hydration, the released heat during cement hydration was monitored using an eight-channel isothermal calorimeter (TAM Air 3114/3236, Thermometric AB, Sollentuna, Sweden), operating at 600 mW and 20 ± 0.02 °C, positioned in a room conditioned at 20 ± 2 °C and 50% relative humidity. Heat of hydration was recorded on a computer for 3 days at 1 min intervals. In each 20 mL calorimeter glass ampoule, a paste mass of 10 g plus additional HA was added (see Table 2 for specific sample composition). Mass of samples increased due to the addition of healing agent. Final weight of samples was: 10.00 g, 10.20 g, 10.27 g, and 10.33 g for the samples containing 0%, 3%, 4% and 5% HA, respectively. Each composition was tested twofold. Sample heat capacity was balanced by water. Heat flow measured during the first hour was caused by the temperature difference of the placed sample and the calorimeter bath and therefore subtracted from the heat curve.

Table 2.
Sample types prepared for isothermal calorimetry determination with amount of healing agent (HA) addition expressed as percentage of cement mass. A = analysed, N/A = not analysed.
2.3. Cube Compressive Strength
Concrete cubes of 15 × 15 × 15 cm3 were produced in duplicate (see Table 1 for composition) using a vibrating table and cube compressive strength was determined according to guidelines (NEN-EN 12390-3 [20]) using a compressive bench (Matest ServoTronic, Macben, Treviolo, Italy). Samples were kept in foil after demoulding until testing at 1, 7, 14, or 28 days.
2.4. Capillary Absorption
Concrete cubes of 10 × 10 × 10 cm3 were produced according to guidelines (NEN–EN 12390–2 [21]) (see Table 1 for composition) using a vibrating table. Three cubes were produced for each concrete composition. In order to generate a porous surface on the top side of the specimen, curing was prevented by keeping the top surface exposed to air (laboratory condition: 20 ± 2 °C, 50% r.h.) from the moment of casting until testing at 28 days (“unsealed specimen”). All other sides were covered in foil from the moment of demoulding after 1 day until 28 days. Afterwards, the hardened concrete cubes were cut in half horizontally by a rotating diamond blade to obtain two samples of 5 cm thickness, corresponding to the maximum required concrete cover to reinforcing steel (NEN-EN 1992-1-1 [22]). The top half cube contained the porous non-cured surface (“uncured” U), while the bottom half cube contained the cured (“sealed” S) surface. Initial capillary suction was determined by immersing the expected porous top surface and non-porous bottom surface in 2 mm water and weighing the specimens at regular time intervals in accordance with the porosity test described in guideline NEN-EN 13057 [23]. Following the porosity test, specimens were further cured under either laboratory conditions (20 ± 2 °C, 50% r.h.), in a humidity room (20 ± 2 °C, ≥95% r.h.), or in a water bath (20 ± 2 °C) for 100 days. Subsequently, specimens were rinsed three times with tap water to remove surface deposits and dried in laboratory air for 7 days to reach a stable dry condition (<0.2% mass change in 24 h) before retesting capillary absorption.
3. Results
3.1. Isothermal Calorimetry
Figure 1 shows heat flow and cumulative heat development for OPC, CEM III/A, and CEM III/B samples without and with HA. For slag cement, mainly three parts can be distinguished in the graph. First a minimum during the dormant or induction phase, then a first maximum (clinker peak), followed—particularly in CEMIII-based samples—by a second maximum (slag peak). No slag peak thus occurs for OPC-based paste. Influence of HA addition was apparent for all cement types. During the induction or dormant phase (between one and 3 h), more energy evolved upon HA addition for all cement types. Afterwards, during the acceleration period, heat flow and accumulated heat was reduced upon the addition of HA. During the slow continued reaction, after the peaks the heat flow was higher for HA-containing paste than for control. When compared to the control cement paste, addition of healing agent reduced the first peak at around 12 h, and contributed to the clinker component more significantly for CEM I than for the slag-containing cement (Figure 2b). Interestingly, the peak reduction was similar for CEM III/A and CEM III/B. The second peak, attributed to the slag reaction occurring around 24 (CEM III/A) or 36 h (CEM III/B) is more significantly affected, especially for the cement with higher clinker replacement. Upon reduction of healing agent content, the main effect was a partial recovery of the dormant phase and slag reaction. Furthermore, the effect on total heat development at 3 days became limited for CEM III/B (Figure 2a).
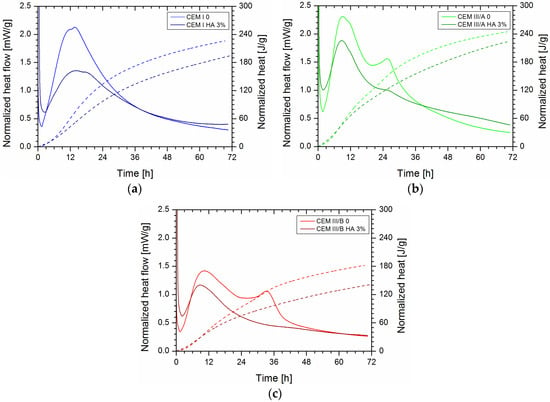
Figure 1.
Normalized heat flow (solid line) and accumulated heat (dashed line) for cement paste with or without 3% HA using (a) CEM I, (b) CEM III/A, and (c) CEM III/B. Top, lighter lines in all graphs represent samples without HA (control samples), and the darker bottom lines represent samples with HA.
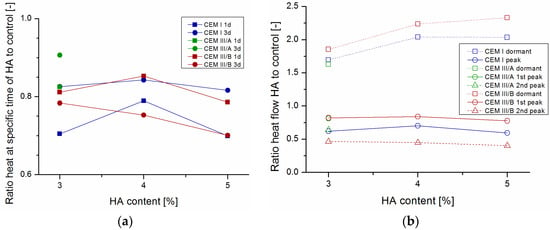
Figure 2.
Ratio of HA-containing cement paste to control for (a) cumulative heat at 1 and 3 days and (b) heat flow at minimum of dormant period and maximum of first peak (clinker) and second peak (slag). HA was added 3%, 4% and 5% by mass of cement (CEM I, CEM III/A, and CEM III/B).
3.2. Cube Compressive Strength
Table 3 shows cube compressive strength values and ratio of strength upon HA addition to control. As designed, similar strength development occurred for control samples with either OPC or CEM III/B. The strength ratio of HA to control indicates that negligible effect on compressive strength occurred upon HA addition to concrete with OPC. For concrete based on CEM III/B, however, the strength development of specimens amended with HA was substantially lower (around 50%) in comparison to reference samples without added HA. Furthermore, this ratio did not change significantly between days 7 and 28, indicating a more permanent effect.

Table 3.
Compressive strength (MPa) of concrete specimens in time without (0) or amended with 4% HA by mass of cement (HA), and ratio of compressive strength over reference concrete specimen (HA/0).
3.3. Capillary Absorption
Sorption coefficients of samples based on either OPC or CEM III/B, with and without healing agent addition, sealed or unsealed, and upon a 100-day curing condition were measured. The sorption coefficient was determined as the linear relationship between 0.5 and 4 h of capillary suction (g; mL) per surface area (dm−2) (water absorption) with the square root of time (h−0.5). The reason for choosing this time window is that partial capillary saturation was reached between 4 and 8 h (example in Figure 3), and a biased result at 12 min occurred due to the difference in measuring time between the first and last sample. Examples of water absorption in time are given in Figure 3a, where the partial saturation is indicated by non-linear behaviour and verified by visual observations of wetting of the non-immersed surface in Figure 3b. From Figure 3a it can further be clearly seen that initially unsealed surfaces absorb more water and that a higher sorption coefficient occurs, given the steeper slope. Shown in Figure 4 are the sorption coefficients (Figure 4a) and the changes in capillary absorption. Changes were determined between samples before and after 100 days of dry, humid, or wet curing (Figure 4b), sealed or unsealed (Figure 4c) and with and without healing agent (Figure 4d—all expressed relative (ratio) to control specimen. Lower values in the comparison thus indicate enhanced reduction in water absorption. Lower values for ratio comparisons indicate further enhancement of water absorption, as the sorption is relatively more reduced when compared to the initial values.
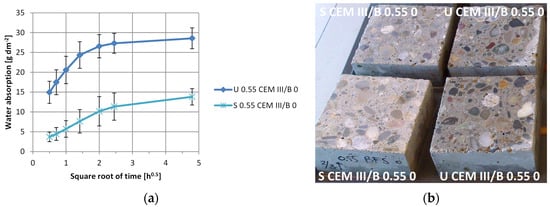
Figure 3.
Water absorption for unsealed (U) and sealed (S) surface of CEM III/B concrete with w/c 0.55 without HA. (a) Water absorption in the square root of time; (b) The partial saturation of specimens with initially unsealed surfaces 6 h into capillary absorption test (√time = 2.45). Error bars indicate minimum and maximum absorption values.
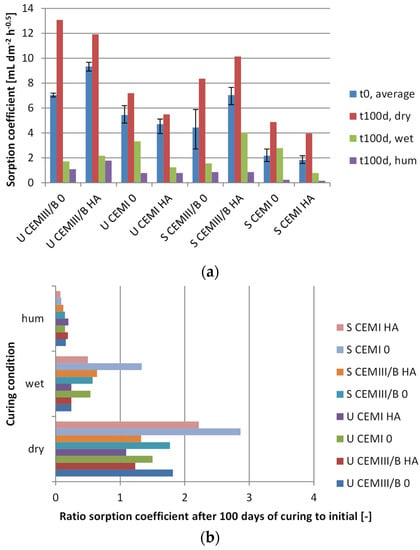
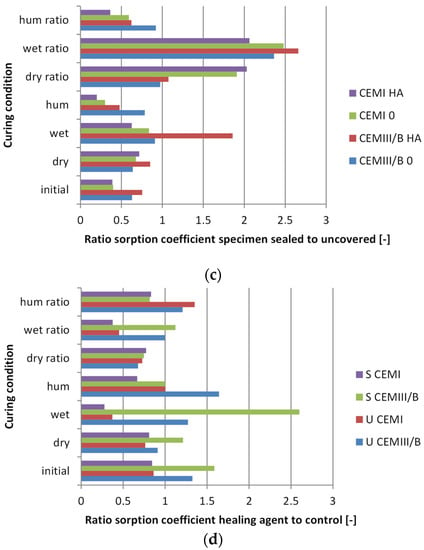
Figure 4.
Water absorption of concrete with or without healing agent (HA). (a) The measured sorption coefficient; (b) The ratio between sorption coefficient after 100 days of curing and 28 days of hardening; (c) The ratio of specimens initially sealed (S) and unsealed (U); (d) The ratio of healing agent-containing samples (HA) to control (0). Curing conditions were either in air (dry), a humid room (hum), or a water bath (wet). Curing condition ratios indicate the relative change in absorption when compared to the control.
Results showed that first day sealing (early age curing) of the concrete surface led (as expected) to a less-absorbing surface (Figure 4a). In case early age curing (first day sealing) was not applied, it was not possible to fully restore surface density by subsequent curing when compared to initially cured (sealed) specimens (Figure 4c). Even for initially cured (28 days sealed) specimens, subsequent placement (100 days) in dry conditions further increased porosity, as this treatment resulted in a higher water absorption rate. This effect appeared more pronounced for Portland- than for slag cement-based specimen (Figure 4b). Specimens cured under humid conditions (20 ± 2 °C, ≥95% r.h.) performed better than those cured under water, as water absorption of the former were substantially lower. The addition of healing agent to the concrete mix appeared to be particularly positive for OPC-based specimens, as substantial reduction of water absorption was observed in those specimens. Curing under water resulted in a significant decrease of water absorption for HA-containing specimens with OPC (Figure 4d).
4. Discussion
It was expected that the surface would become more dense for OPC-based concrete and more absorbing for CEM III/B-based concrete upon HA addition, due to an effect similar to carbonation. Surface water absorption upon the addition of healing agent was indeed lower when using CEM I and higher when using CEM III/B. In the case of CEM I, addition of HA led to densification for all situations (i.e., sealed and unsealed surfaces and before as well as after a curing regime). The significantly enhanced reduction in water absorption of specimen cured for 100 days under water for samples with added HA when compared to control may be due to the effect of carbonate deposition due to bacterial metabolic activity. Future work comparing the behaviour and properties of concrete surfaces under carbonation may indicate whether the system works similarly. Further densification of Portland cement concrete surfaces upon moisture curing occurred for both control and HA-containing concrete, and was indeed expected because of ongoing cement hydration. However, reduced initial absorption and less proneness to drying upon HA addition readily occurred under dry conditions, and can therefore not be explained by bacterial metabolism, nor by ongoing hydration. Instead, this indicates a change in material properties during hardening, supported by the fact that cement heat of hydration was affected. Whether this is caused by chemical interaction of HA with the cement matrix [24] is interesting to explore in future work. Further interesting is to assess whether the densification also has a reducing effect on durability-related mechanisms such as freeze–thaw damage with salt scaling or chloride ingress, as is expected for cement containing a minimum of 50% of clinker [25]. What effect the initial and further surface densification has on the reduction of steel reinforcement corrosion should also be determined [7]. Additionally, assessing changes in the pH of the concrete pore solution could indicate steel passivation potential.
Even though concrete compressive strength was similar for CEM I and CEM III/B specimens, the initial surface water absorption was higher for CEM III/B than for CEM I specimen. This observed phenomenon may correspond to a less dense surface for CEM III/B concrete, as found by other researchers [5]. Even though OPC was found to be more sensitive to early age curing (by sealing), curing is even more important for concrete using CEM III/B due to the inherently more absorbing surface. For the non-sealed specimens of both OPC and CEM III/B, saturation readily occurred within 24 h over a thickness of 50 mm during the water absorption test, which is the maximum cover thickness for protection of the steel reinforcement according to the European standard (NEN-EN 1992-1-1). Greater potential for water absorption reduction was found for initially non-sealed specimens when compared to sealed specimens, which corresponds to literature [26]. However, compared to initially cured (sealed) specimens, the surface water absorption still appeared to increase. Immediate curing is therefore recommended over curing at a later stage.
For concrete with CEM III/B, addition of HA caused a significant reduction in compressive strength, which remained similar from 7 days onward. Whether this strength reduction is permanent may become apparent when strength differences are determined beyond 28 days. Where the total heat of hydration remained around 80% of the control, the third hydration peak was reduced to approximately 40%, which is associated with the slag reaction [27]. This limitation in slag reaction could be the cause of the significant strength reduction. As slag is activated by calcium hydroxide (CH), the potential for competition over CH with HA addition as the cause of reduced slag reaction may be investigated in future work. The reduced slag reaction may also explain the more absorbing surface. It would be interesting to compare the results of HA addition to CEM III/B with properties of CEM III/C paste and concrete, as it contains a higher slag-to-clinker ratio and may mimic a reduced CH content.
As for CEM III/A, a limited effect on strength [15], hydration energy, and slag reaction occurred for HA addition; it would be interesting to see whether similar results can be found for the absorption rate of concrete surfaces with or without HA, as was found for OPC.
5. Conclusion
Applicability of healing agent addition to concrete was attributed to limited effect on general properties and enhanced reduction of water absorption by the concrete surface. Surface densification occurred upon healing agent addition to concrete containing clinker cement (OPC). However, when cement was used containing a high slag content as clinker replacement (CEM III/B), the surface had an increased water absorption. Furthermore, HA addition to CEM III/B based material showed significant reduction of the slag reaction and concrete strength development. In case of CEM I and CEM III/A however, the effect was limited. It is therefore recommended to modify the HA or to reduce the concentration for the application of HA in concrete using cement with clinker content below 50%. HA in the current from is suitable for addition to OPC-based concrete.
Acknowledgments
This research was financially supported by Agentschap NL under grant IOP-SHM01018 and BE Basic under grant F04.001. Input from Corbion Purac in healing agent development was greatly appreciated.
Author Contributions
Renee Mors and Henk Jonkers conceived and designed the experiments; Renee Mors performed the experiments; Renee Mors and Henk Jonkers analyzed the data; Renee Mors wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Poursaee, A.; Hansson, C.M. Reinforcing steel passivation in mortar and pore solution. Cem. Concr. Res. 2007, 37, 1127–1133. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction—A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Pease, B.J. Influence of Concrete Cracking on Ingress and Reinforcement Corrosion. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, September 2010. [Google Scholar]
- Song, H.W.; Lee, C.H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Bouwmeester, W.J.; Schlangen, E. Influence of curing on the pore structure of concrete. In Tailor Made Concrete Structures; Walraven, J.C., Stoelhorst, D., Eds.; Taylor & Francis: London, UK, 2008; pp. 65–70. [Google Scholar]
- Parrott, L.J. Variations of water absorption rate and porosity with depth from an exposed concrete surface: Effects of exposure conditions and cement type. Cem. Concr. Res. 1992, 22, 1077–1088. [Google Scholar] [CrossRef]
- Chi, J.M.; Huang, R.; Yang, C.C. Effects of carbonation on mechanical properties and durability of concrete using accelerated testing method. J. Mar. Sci. Technol. 2002, 10, 14–20. [Google Scholar]
- De Ceukelaire, L.; Van Nieuwenburg, D. Accelerated carbonation of a blast-furnace cement concrete. Cem. Concr. Res. 1993, 23, 442–452. [Google Scholar] [CrossRef]
- Carvajal, A.M.; Maturana, P.; Pino, C.; Poblete, J. Analysis of the relation between accelerated carbonation, porosity, compressive strength and capillary absorption in concrete, in the search of a new control method by durability. J. Rev. Constr. 2009, 8, 129–135. [Google Scholar]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]
- De Rooij, M.; Van Tittelboom, K.; De Belie, N.; Schlangen, E. State-of-the-Art Report of RILEM Technical Committee 221-SHC: Self-Healing Phenomena in Cement-Based Materials, 1st ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 1–18. [Google Scholar]
- Jonkers, H.M. Self healing concrete: A biological approach. In Self Healing Materials. An Alternative Approach to 20 Centuries of Materials Science; Van der Zwaag, S., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 195–204. [Google Scholar]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Field performance of bacteria-based repair system: Pilot study in a parking garage. Case Stud. Constr. Mater. 2015, 2, 11–17. [Google Scholar] [CrossRef]
- Mors, R.M.; Jonkers, H.M. Feasibility of lactate derivative based agent as additive for concrete for regain of crack water tightness by bacterial metabolism. Ind. Crops Prod. 2016. [Google Scholar] [CrossRef]
- Lanser, P.A.; Burger, A.M. Kooldioxide als een Stimulans voor Levenscyclusdenken in de Cement—en Betonindustrie; Cement & Beton Centrum: Hertogenbosch, The Netherlands, 2008. [Google Scholar]
- NEN-EN 196-1: 2005 Methods of Testing Cement–Part 1: Determination of Strength; Nederlands Normalisatie-instituut: Delft, The Netherlands, 2005.
- NEN-EN 206: 2014 Concrete–Specification, Performance, Production and Conformity; Nederlands Normalisatie-instituut: Delft, The Netherlands, 2014.
- NEN 8005 NL–Dutch Supplement to NEN-EN 206; Nederlands Normalisatie-instituut: Delft, The Netherlands, 2014. (In Dutch)
- NEN-EN 12390-3: 2009 Testing Hardened Concrete–Part 3: Compressive Strength of Test Specimens; Nederlands Normalisatie-instituut: Delft, The Netherlands, 2009.
- NEN-EN 12390-2: 2009 Testing Hardened Concrete–Part 2: Making and Curing Specimens for Strength Tests; Nederlands Normalisatie-instituut: Delft, The Netherlands, 2009.
- NEN-EN 1992-1-1: 2005 Eurocode 2: Design of Concrete Structures–Part 1–1: General Rules and Rules for Buildings; Nederlands Normalisatie-instituut: Delft, The Netherlands, 2005.
- NEN-EN 13057: 2002 Products and Systems for the Protection and Repair of Concrete Structures–Test Methods –Determination of Resistance of Capillary Absorption; Nederlands Normalisatie-instituut: Delft, The Netherlands, 2002.
- Singh, N.B.; Prabha, S.; Singh, A.K. Effect of lactic acid on the hydration of Portland cement. Cem. Concr. Res. 1986, 16, 545–553. [Google Scholar] [CrossRef]
- Holthuizen, P.E. Chloride Ingress of Carbonated Blast Furnace Slag Cement Mortars. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, June 2016. [Google Scholar]
- Dias, W.P.S. Reduction of concrete sorptivity with age through carbonation. Cem. Concr. Res. 2000, 30, 1255–1261. [Google Scholar] [CrossRef]
- Gruyaert, E.; Robeyst, N.; De Belie, N. Study of the hydration of Portland cement blended with blast-furnace slag by calorimetry and thermogravimetry. J. Therm. Anal. Calorim. 2010, 102, 941–951. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).