Electrochemical Behavior of Bilayer Thermal-Spray Coatings in Low-Temperature Corrosion Protection
Abstract
1. Introduction
2. Experimental Details
2.1. Materials and Methods
2.2. Thermal-Spray Experiments
2.3. Salt-Spray, OCP and Potentiodynamic Polarization Tests
2.4. Characterization of the As-Sprayed and Corroded Coatings
3. Results & Discussion
3.1. Microstructure Analysis of Coatings
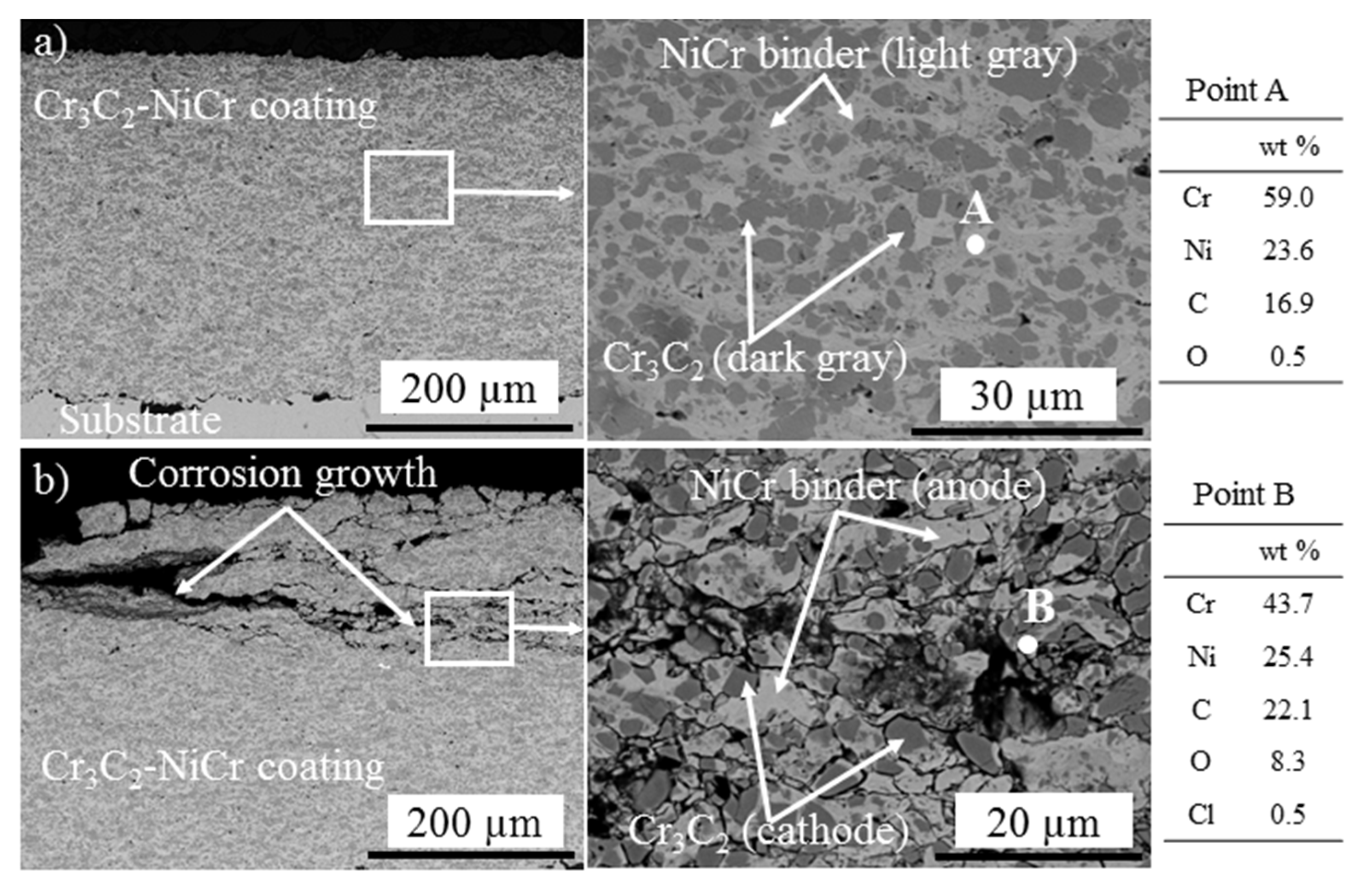
3.1.1. As-Sprayed and Corroded Cr3C2-NiCr
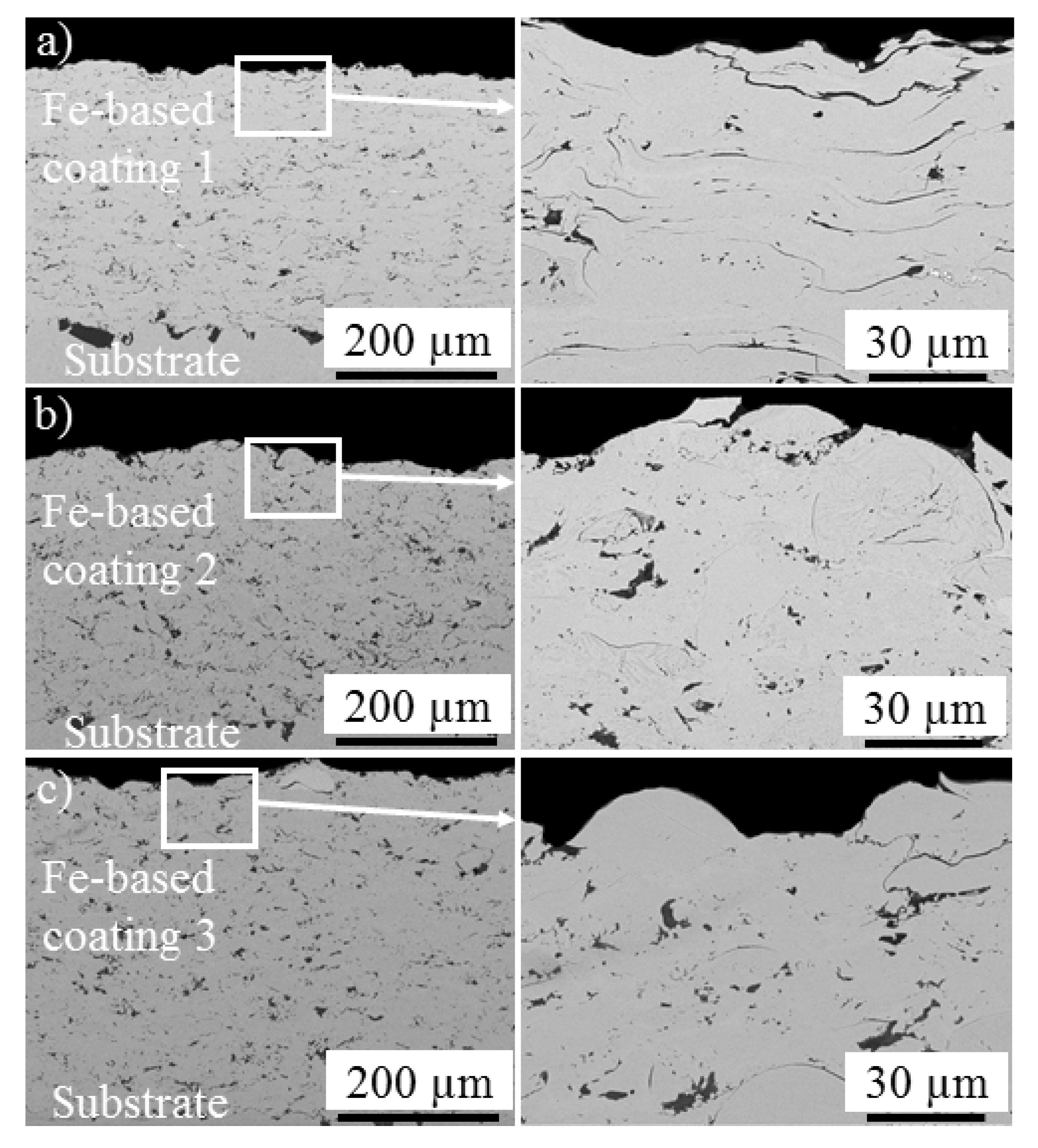
3.1.2. Single-Layer and Bilayer Coatings
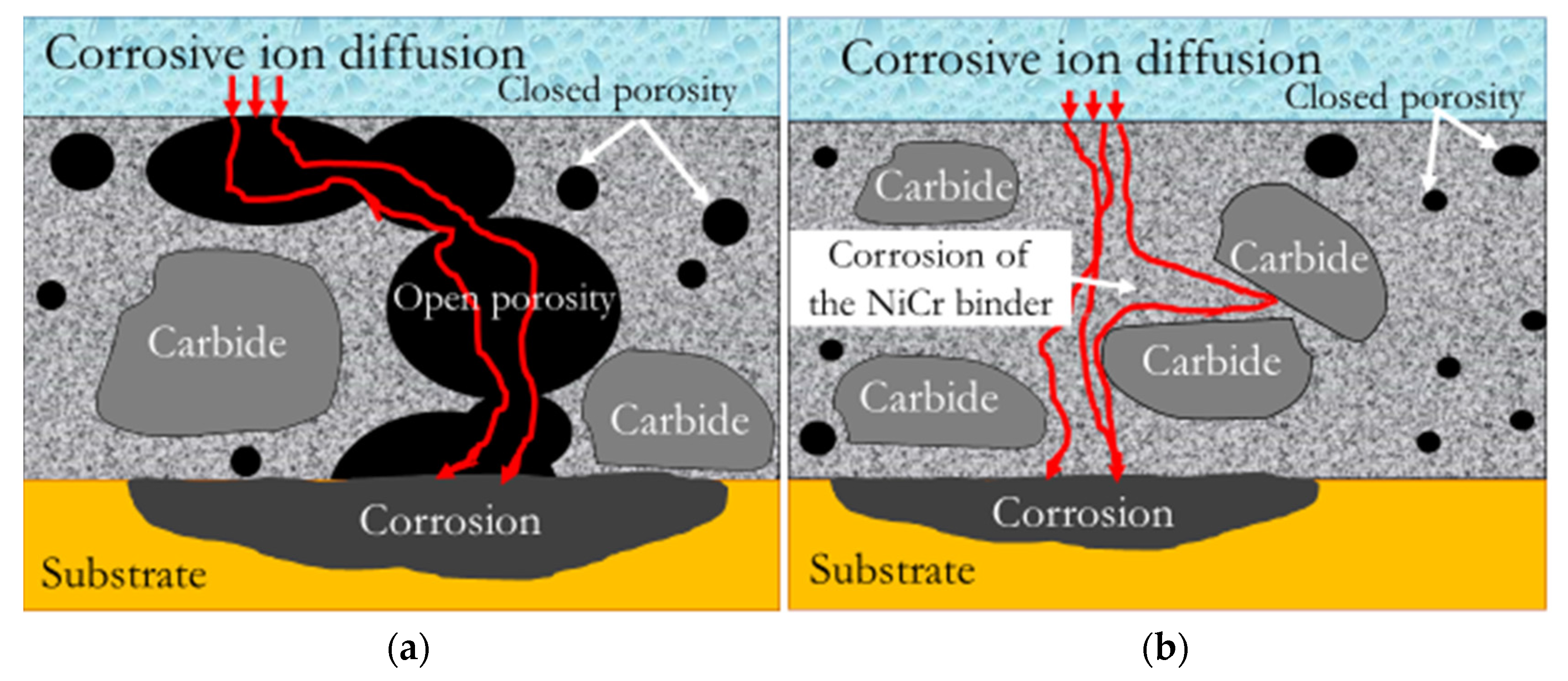
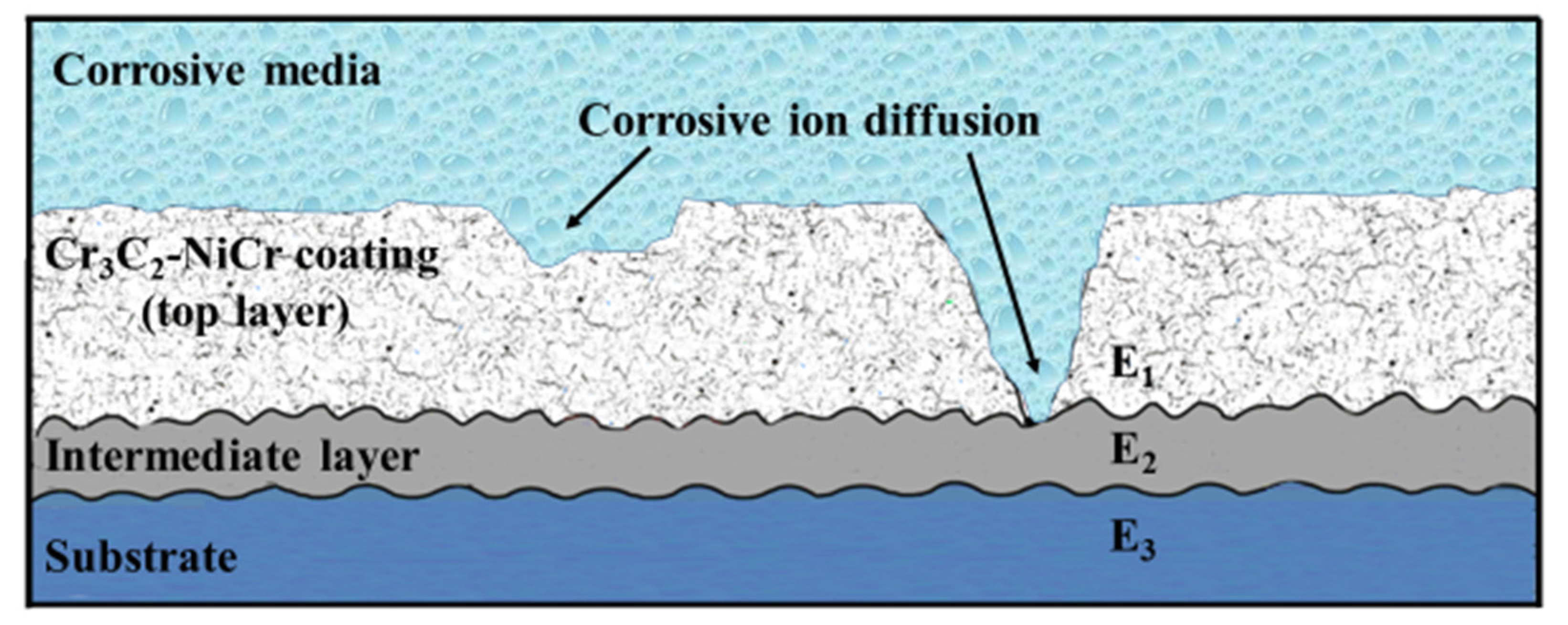
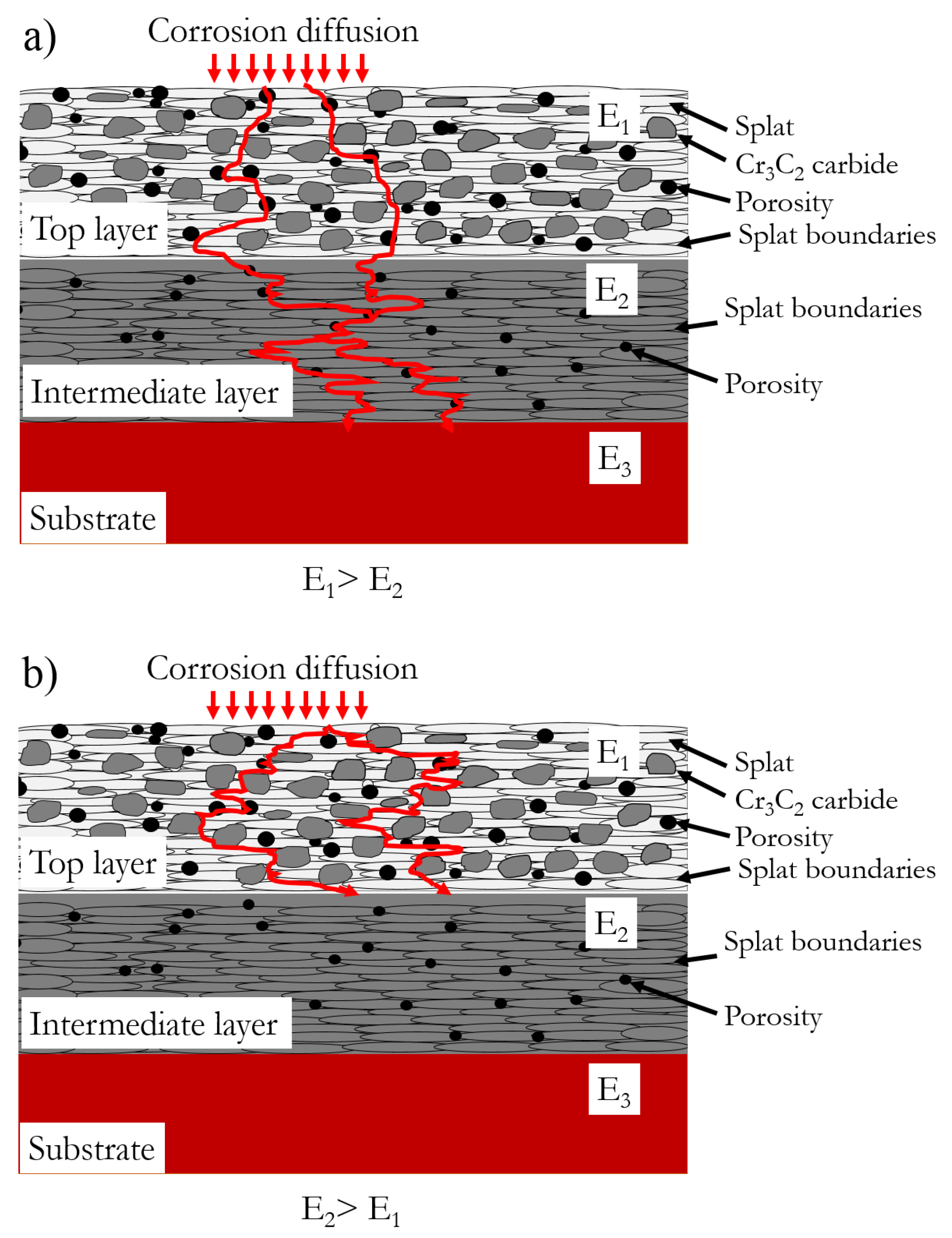
3.2. Proposed Corrosion Mechanism
4. Summary
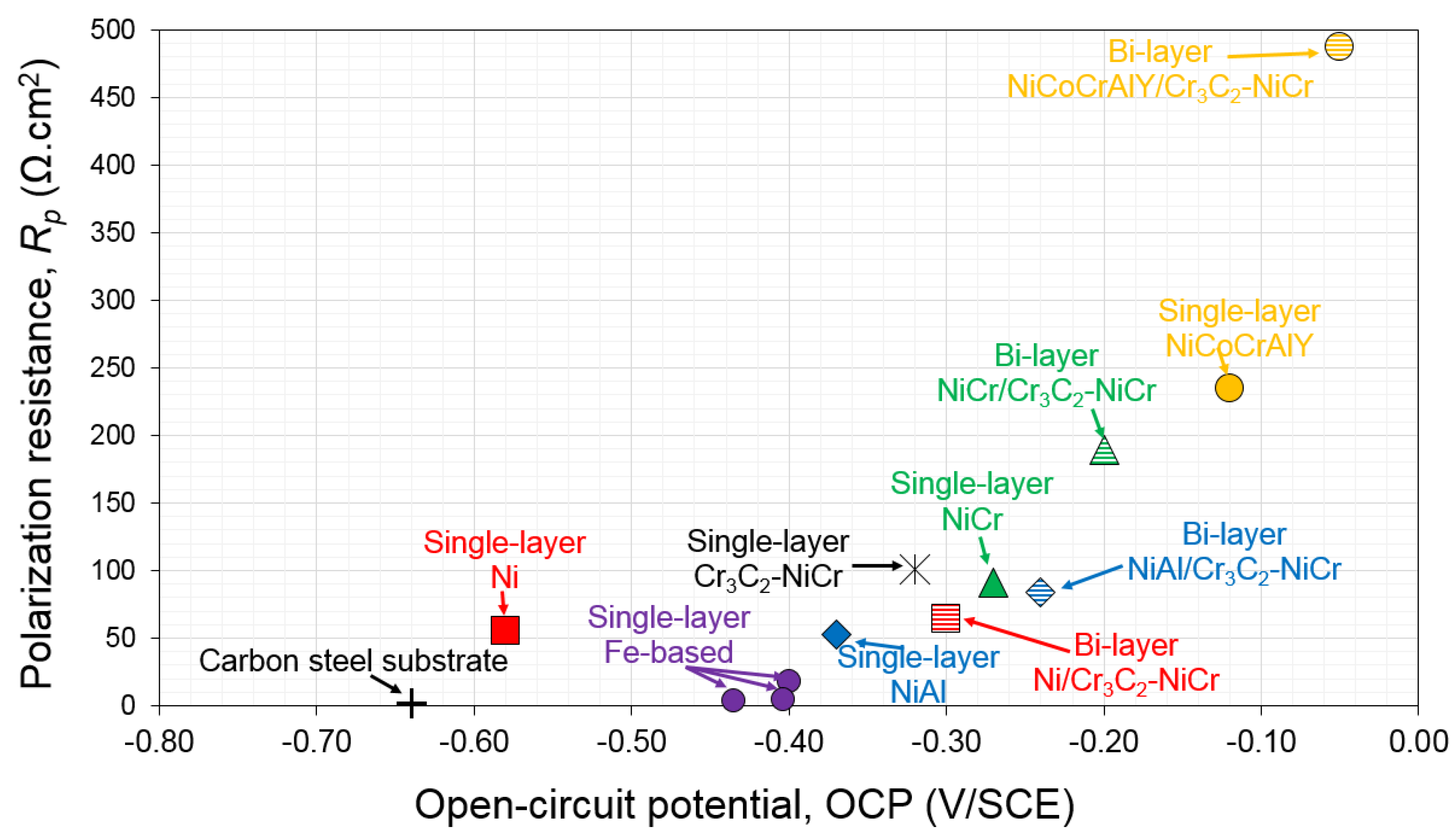
- While high-alloyed Fe-based coatings showed rather porous structures, the corrosion results of all the three coatings showed low Rp as well as low OCP. The study was directed towards the Ni-based coatings, as the Fe-based coatings were unable to provide higher Rp and OCP values.
- Ni-based coatings showed high electrochemical values (Rp and OCP) while having a sufficient level of the passive layer-forming elements, for example, Cr or/and Al. The particles were well-splashed and deformed over each other during the spraying process, and thus the level of porosity was very low.
- The OCP of the intermediate layers determined the corrosion behavior of the bilayer coating. Higher OCP of the intermediate layer than that of the top layer led to a galvanic couple which favored the intermediate layer to be sacrificially protected. In this situation, diffusion of corrosive ions was stopped at the intermediate/top layer interface.
- Both microstructure and chemical composition of the intermediate layer affected the corrosion resistance of the bilayer coatings. The chemical composition had higher contribution to corrosion protection; however, the effect of defects in thermal-spray coatings cannot be neglected.
- Addition of alloying elements such as Cr or Al improved the corrosion behavior of the pure Ni coating. The NiCr or NiCoCrAlY coatings presented higher content of defects, but better corrosion behavior, than the denser Ni coating.
- The most promising coating system used was a Cr3C2-NiCr top layer with an intermediate layer of NiCoCrAlY. However, corrosion protection in the NiCr/Cr3C2-NiCr design could be more economically favored for industrial applications.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P. A comparative study of corrosion resistance for HVAF-sprayed Fe- and Co-based coatings. Coatings 2016, 6, 16. [Google Scholar] [CrossRef]
- Picas, J.A.; Forn, A.; Igartua, A.; Mendoza, G. Mechanical and tribological properties of high velocity oxy-fuel thermal sprayed nanocrystalline CrC-NiCr coatings. Surf. Coat. Technol. 2003, 174, 1095–1100. [Google Scholar] [CrossRef]
- Murthy, J.K.N.; Venkataraman, B. Abrasive wear behaviour of WC–CoCr and Cr3C2–20(NiCr) deposited by HVOF and detonation spray processes. Surf. Coat. Technol. 2006, 200, 2642–2652. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P.; Björklund, S. Corrosion performance of bi-layer Ni/Cr2C3-NiCr HVAF thermal spray coating. Appl. Surf. Sci. 2016, 369, 470–481. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Espallargas, N.; Fernández, J.; Suegama, P.H.; Benedetti, A.V. High-velocity oxyfuel Cr3C2-NiCr replacing hard chromium coatings. J. Therm. Spray Technol. 2005, 14, 335–341. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Espallargas, N.; Suegama, P.H.; Benedetti, A.V. Comparative study of Cr3C2-NiCr coatings obtained by HVOF and hard chromium coatings. Corros. Sci. 2006, 48, 2998–3013. [Google Scholar] [CrossRef]
- Toma, D.; Brandl, W.; Marginean, G. Wear and corrosion behaviour of thermally sprayed cermet coatings. Surf. Coat. Technol. 2001, 138, 149–158. [Google Scholar] [CrossRef]
- Suegama, P.H.; Fugivara, C.S.; Benedetti, A.V.; Fernández, J.; Delgado, J.; Guilemany, J.M. Electrochemical behaviour of thermally sprayed Cr3C2-NiCr coatings in 0.5 M H2SO4 media. J. Appl. Electrochem. 2002, 32, 1287–1295. [Google Scholar] [CrossRef]
- Suarez, M.; Bellayer, S.; Traisnel, M.; Gonzalez, W.; Chicot, D.; Lesage, J.; Puchi-Cabrera, E.S.; Staia, M.H. Corrosion behavior of Cr3C2-NiCr vacuum plasma sprayed coatings. Surf. Coat. Technol. 2008, 202, 4566–4571. [Google Scholar] [CrossRef]
- Poza, P.; Múnez, C.J.; Garrido-Maneiro, M.A.; Vezzù, S.; Rech, S.; Trentin, A. Mechanical properties of Inconel 625 cold-sprayed coatings after laser remelting. Depth sensing indentation analysis. Surf. Coat. Technol. 2014, 243, 51–57. [Google Scholar] [CrossRef]
- García-Rodríguez, S.; López, A.J.; Torres, B.; Rams, J. 316L stainless steel coatings on ZE41 magnesium alloy using HVOF thermal spray for corrosion protection. Surf. Coat. Technol. 2016, 287, 9–19. [Google Scholar] [CrossRef]
- Campo, M.; Carboneras, M.; lópez, M.D.; Torres, B.; Rodrigo, P.; Otero, E.; Rams, J. Corrosion resistance of thermally sprayed Al and Al/SiC coatings on Mg. Surf. Coat. Technol. 2009, 203, 3224–3230. [Google Scholar] [CrossRef]
- Suegama, P.H.; Fugivara, C.S.; Benedetti, A.V.; Guilemany, J.M.; Fernández, J.; Delgado, J. The influence of gun transverse speed on electrochemical behaviour of thermally sprayed Cr3C2-NiCr coatings in 0.5 M H2SO4 solution. Electrochim. Acta 2004, 49, 627–634. [Google Scholar] [CrossRef]
- Lekatou, A.; Zois, D.; Karantzalis, A.E.; Grimanelis, D. Electrochemical behaviour of cermet coatings with a bond coat on Al7075: Pseudopassivity, localized corrosion and galvanic effect considerations in a saline environment. Corros. Sci. 2010, 52, 2616–2635. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. Characterisation and corrosion-erosion behaviour of carbide based thermal spray coatings. J. Miner. Mater. Charact. Eng. 2012, 11, 569. [Google Scholar] [CrossRef]
- Bolelli, G.; Cannillo, V.; Lusvarghi, L.; Rosa, R.; Valarezo, A.; Choi, W.B.; Dey, R.; Weyant, C.; Sampath, S. Functionally graded WC–Co/NiAl HVOF coatings for damage tolerance, wear and corrosion protection. Surf. Coat. Technol. 2012, 206, 2585–2601. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P. Microstructural and electrochemical characterization of Ni-based bi-layer coatings produced by the HVAF process. Surf. Coat. Technol. 2016, 304, 606–619. [Google Scholar] [CrossRef]
- Godoy, C.; Lima, M.M.; Castro, M.M.R.; Avelar-Batista, J.C. Structural changes in high-velocity oxy-fuel thermally sprayed WC–Co coatings for improved corrosion resistance. Surf. Coat. Technol. 2004, 188–189, 1–6. [Google Scholar] [CrossRef]
- Kuroda, S.; Kawakita, J.; Fukushima, T.; Tobe, S. Importance of the adhesion of HVOF sprayed coatings for aqueous corrosion resistance. Mater. Trans. 2003, 44, 381–388. [Google Scholar] [CrossRef]
- Bergant, Z.; Trdan, U.; Grum, J. Effect of high-temperature furnace treatment on the microstructure and corrosion behavior of NiCrBSi flame-sprayed coatings. Corros. Sci. 2014, 88, 372–386. [Google Scholar] [CrossRef]
- Bao, Z.B.; Wang, Q.M.; Li, W.Z.; Gong, J.; Xiong, T.Y.; Sun, C. Corrosion behaviour of AIP NiCoCrAlYSiB coating in salt spray tests. Corros. Sci. 2008, 50, 847–855. [Google Scholar] [CrossRef]
- Bolelli, G.; Börner, T.; Milanti, A.; Lusvarghi, L.; Laurila, J.; Koivuluoto, H.; Niemi, K.; Niemi, P. Tribological behavior of HVOF- and HVAF-sprayed composite coatings based on Fe-Alloy + WC–12% Co. Surf. Coat. Technol. 2014, 248, 104–112. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P. Microstructural characteristics and corrosion behavior of HVAF- and HVOF-sprayed Fe-based coatings. Surf. Coat. Technol. 2017, 318, 365–373. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P. A comparative study on Ni-based coatings prepared by HVAF, HVOF, and APS methods for corrosion protection applications. J. Therm. Spray Technol. 2016, 25, 1604–1616. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Huhtakangas, M.; Joshi, S. Isothermal oxidation of HVAF-sprayed Ni-based chromia, alumina and mixed-oxide scale forming coatings in ambient air. Surf. Coat. Technol. 2017, 316, 10–21. [Google Scholar] [CrossRef]
- ASTM B117-16 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2016.
- Milanti, A.; Matikainen, V.; Koivuluoto, H.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. Effect of spraying parameters on the microstructural and corrosion properties of HVAF-sprayed Fe-Cr-Ni-B-C coatings. Surf. Coat. Technol. 2015, 277, 81–90. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- ASTM E384-11e1 Standard Test Method for Knoop and Vickers Hardness of Materials; ASTM International: West Conshohocken, PA, USA, 2011.
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P. Microstructure effect of intermediate coat layer on corrosion behavior of HVAF-sprayed bi-layer coatings. J. Therm. Spray Technol. 2017, 26, 243–253. [Google Scholar] [CrossRef]
- Zeng, Z.; Sakoda, N.; Tajiri, T.; Kuroda, S. Structure and corrosion behavior of 316L stainless steel coatings formed by HVAF spraying with and without sealing. Surf. Coat. Technol. 2008, 203, 284–290. [Google Scholar] [CrossRef]
- Zhao, W.-M.; Wang, Y.; Dong, L.-X.; Wu, K.-Y.; Xue, J. Corrosion mechanism of NiCrBSi coatings deposited by HVOF. Surf. Coat. Technol. 2005, 190, 293–298. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Hooshyar, H.; Markocsan, N.; Joshi, S.; Nylén, P. Oxidation behavior of HVAF-sprayed NiCoCrAlY coating in H2–H2O environment. Oxid. Met. 2016, 86, 299–314. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Joshi, S. Isothermal oxidation behavior of HVAF-sprayed Ni and NiCr coatings in H2-H2O environment. Surf. Coat. Technol. 2017, 317, 17–25. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P. HVAF thermal spray Fe-based coating : An environmentally acceptable alternative to cobalt-based coating. In Proceedings of the EuroCorr 2015, European Corrosion Congress, Graz, Austria, 6–10 September 2015. [Google Scholar]
- Clement, C.; Sadeghimeresht, E.; Lyphout, C.; Markocsan, N.; Nylén, P. Corrosion behavior of HVAF- and HVOF-sprayed high-chromium Fe-based coatings. In Proceedings of the 7th Rencontres Internationales sur la Projection Thermique, Limoges, France, 9–11 December 2015. [Google Scholar]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P.; Dizdar, S. Corrosion behavior of high-chromium Fe-based coatings produced by HVAF thermal spraying technique. In Proceedings of the 7th Rencontres Internationales sur la Projection Thermique, Limoges, France, 9–11 December 2015. [Google Scholar]
- Milanti, A.; Koivuluoto, H.; Vuoristo, P. Influence of the spray gun type on microstructure and properties of HVAF sprayed fe-based corrosion resistant coatings. J. Therm. Spray Technol. 2015, 24, 1312–1322. [Google Scholar] [CrossRef]
- Xu, C.; Du, L.; Yang, B.; Zhang, W. The effect of Al content on the galvanic corrosion behaviour of coupled Ni/graphite and Ni-Al coatings. Corros. Sci. 2011, 53, 2066–2074. [Google Scholar] [CrossRef]
- Lekatou, A.; Regoutas, E.; Karantzalis, A.E. Corrosion behaviour of cermet-based coatings with a bond coat in 0.5 M H2SO4. Corros. Sci. 2008, 50, 3389–3400. [Google Scholar] [CrossRef]
- Verdian, M.M.; Raeissi, K.; Salehi, M. Corrosion performance of HVOF and APS thermally sprayed NiTi intermetallic coatings in 3.5% NaCl solution. Corros. Sci. 2010, 52, 1052–1059. [Google Scholar] [CrossRef]
- Hoffmeister, H. Modeling of crevice corrosion of pure nickel by coupling of phase and polarization behavior at various pH, chloride, and oxygen levels. Corrosion 2005, 61, 880–888. [Google Scholar] [CrossRef]
- Fan, X.; Zou, B.; Gu, L.; Wang, C.; Wang, Y.; Huang, W.; Zhu, L.; Cao, C. Investigation of the bond coats for thermal barrier coatings on Mg alloy. Appl. Surf. Sci. 2013, 265, 264–273. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Cinca, N.; Dosta, S.; Benedetti, A.V. Corrosion behaviour of thermal sprayed nitinol coatings. Corros. Sci. 2009, 51, 171–180. [Google Scholar] [CrossRef]


| No. | Powder | Chemical Composition (wt %) | Powder Size (µm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Al | Cr | O | C | Co | Mo | Fe | |||
| 1 | Fe-based 1 | 11.0 | – | 30 | 0.2 | 0.6 | – | – | Bal. | 36 ± 20 |
| 2 | Fe-based 2 | 17.0 | – | 28 | 0.1 | 1.8 | – | 4.5 | Bal. | 36 ± 20 |
| 3 | Fe-based 3 | 4.0 | – | 10 | 0.2 | 2.0 | – | 5.5 | Bal. | 36 ± 20 |
| 4 | Ni (Amperit® 176.001) | Bal. | – | – | 0.1 | – | – | – | – | 45 ± 22 |
| 5 | NiCr (Amperit® 251.071) | Bal. | – | 21.1 | 0.1 | – | – | – | – | 45 ± 22 |
| 6 | NiAl (Amperit® 281.003) | Bal. | 5.5 | – | 0.1 | – | – | – | – | 45 ± 5 |
| 7 | NiCoCrAlY (AMDRY 386-0) | Bal. | 8.5 | 20 | 0.2 | – | 23.1 | – | – | 63 ± 5 |
| 8 | Cr3C2-NiCr 75/25 (Amperit® 584.054) | 20.5 | – | Bal. | 0.3 | 8.9 | – | – | – | 45 ± 22 |
| Parameter | Fe-Based 1 | Fe-Based 2 | Fe-Based 3 | Ni | NiCr | NiAl | NiCoCrAlY | Cr3C2-NiCr |
|---|---|---|---|---|---|---|---|---|
| Nozzle | 3L2G | 3L2G | 3L2G | 4L4 | 4L4 | 3L2 | 4L2 | 4L2 |
| Spray distance (mm) | 400 | 400 | 400 | 350 | 300 | 350 | 350 | 350 |
| Gun traverse velocity (m/min) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Powder-feed rate (g/min) | 100 | 100 | 100 | 150 | 50 | 150 | 150 | 150 |
| Nitrogen carrier gas flow (L/min) | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Air pressure (MPa) | 0.8 | 0.8 | 0.8 | 0.82 | 0.82 | 0.8 | 0.8 | 0.8 |
| Fuel 1 (propane) pressure (MPa) | 0.69 | 0.69 | 0.69 | 0.7 | 0.69 | 0.66 | 0.65 | 0.7 |
| Fuel 2 (propane) pressure (MPa) | 0.69 | 0.69 | 0.69 | 0.7 | 0.69 | 0.70 | 0.65 | 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghimeresht, E.; Markocsan, N. Electrochemical Behavior of Bilayer Thermal-Spray Coatings in Low-Temperature Corrosion Protection. Coatings 2017, 7, 162. https://doi.org/10.3390/coatings7100162
Sadeghimeresht E, Markocsan N. Electrochemical Behavior of Bilayer Thermal-Spray Coatings in Low-Temperature Corrosion Protection. Coatings. 2017; 7(10):162. https://doi.org/10.3390/coatings7100162
Chicago/Turabian StyleSadeghimeresht, Esmaeil, and Nicolaie Markocsan. 2017. "Electrochemical Behavior of Bilayer Thermal-Spray Coatings in Low-Temperature Corrosion Protection" Coatings 7, no. 10: 162. https://doi.org/10.3390/coatings7100162
APA StyleSadeghimeresht, E., & Markocsan, N. (2017). Electrochemical Behavior of Bilayer Thermal-Spray Coatings in Low-Temperature Corrosion Protection. Coatings, 7(10), 162. https://doi.org/10.3390/coatings7100162






