Microstructure and Corrosion Behavior of Cold-Sprayed Zn-Al Composite Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Raw Powder Materials
2.2. Cold Spray Process
2.3. Coating Characterization
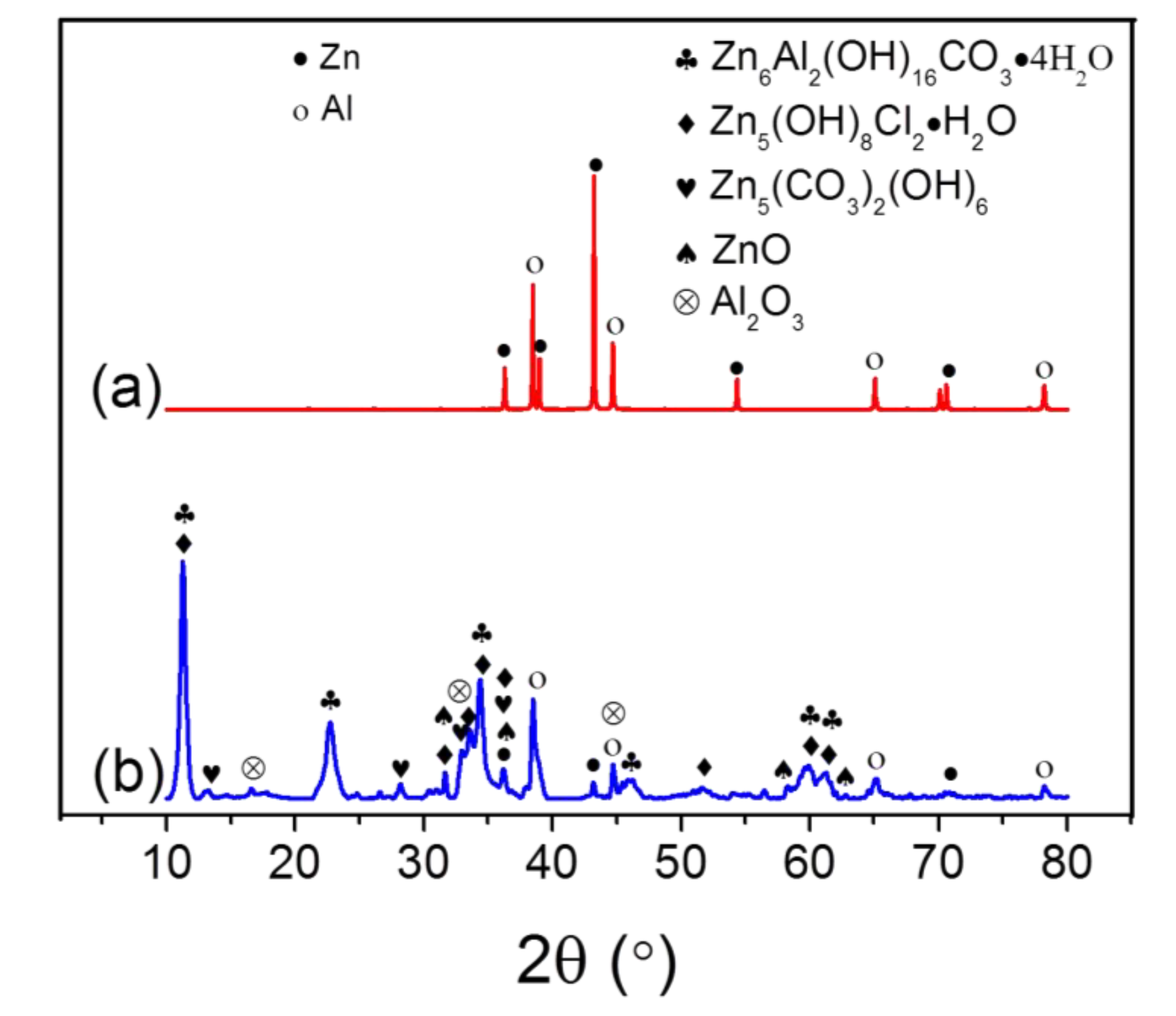
3. Results and Discussion
4. Conclusions
- The prepared cold-sprayed composite coating was dense with a porosity of 0.4%. Zn and Al phases were distributed uniformly in the composite coating;
- The composite coating showed uniform corrosion attack instead of local corrosion during the salt spray test, which is beneficial for achieving the long-term corrosion of the coating in marine environment;
- The main corrosion products of the Zn-Al composite coating were Zn6Al2(OH)16CO3·4H2O, Zn5(OH)8Cl2·H2O, Zn5(CO3)2(OH)6, ZnO and Al2O3;
- There was a synergistic corrosion effect between the Zn and Al phases, promoted by the flow of the droplets, which looks similar to uniform corrosion.
Author Contributions
Funding
Conflicts of Interest
References
- Alcantara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Diaz, I.; Morcillo, M. Marine Atmospheric Corrosion of Carbon Steel: A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, C.G.; Garbatov, Y.; Zayed, A.; Wang, G. Influence of environmental factors on corrosion of ship structures in marine atmosphere. Corros. Sci. 2009, 51, 2014–2026. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. The atmospheric corrosion kinetics of low carbon steel in a tropical marine environment. Corros. Sci. 2010, 52, 1796–1800. [Google Scholar] [CrossRef]
- Adedipe, O.; Brennan, F.; Kolios, A. Review of corrosion fatigue in offshore structures: Present status and challenges in the offshore wind sector. Renew. Sustain. Energy Rev. 2016, 61, 141–154. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.; Karthikeyan, J.; Prakash, S. Cold spray coating process for corrosion protection: A review. Surf. Eng. 2014, 30. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Jungwirth, S.; Seely, N.; Fang, Y.; Shi, X. The Application of Anti-Corrosion Coating for Preserving the Value of Equipment Asset in Chloride-Laden Environments: A Review. Int. J. Electrochem. Sci. 2015, 10, 10756–10780. [Google Scholar]
- Moon, K.-M.; Kim, Y.-H.; Lee, M.-H.; Baek, T.-S. Polarization characteristics of four types of coating films by thermal spray in seawater solution. Mod. Phys. Lett. B 2015, 29. [Google Scholar] [CrossRef]
- Niu, L.; Chang, S.-H.; Su, Y.; Han, D.; Li, G. A aluminum coating with chromium-free passivating film formed on AZ91D magnesium alloy. J. Alloy. Compd. 2015, 635, 11–15. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Bartolome, M.J.; Gonzalez, J.A.; Lopez, V.; Feliu, S. Passivating oxide film and growing characteristics of anodic coatings on aluminium alloys. Appl. Surf. Sci. 2008, 254, 2755–2762. [Google Scholar] [CrossRef] [Green Version]
- Bonabi, S.F.; Ashrafizadeh, F.; Sanati, A.; Nahvi, S.M. Structure and Corrosion Behavior of Arc-Sprayed Zn-Al Coatings on Ductile Iron Substrate. J. Therm. Spray Technol. 2018, 27, 524–537. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Mozafari, A.; Omidvar, H.; Javanbakht, M. Preparation and corrosion resistance of pulse electrodeposited Zn and Zn-SiC nanocomposite coatings. Appl. Surf. Sci. 2014, 300, 1–7. [Google Scholar] [CrossRef]
- Persson, D.; Thierry, D.; Karlsson, O. Corrosion and corrosion products of hot dipped galvanized steel during long term atmospheric exposure at different sites world-wide. Corros. Sci. 2017, 126, 152–165. [Google Scholar] [CrossRef]
- Su, F.; Zhang, P.; Wei, D.; Chen, X.; Ding, F.; Wang, B. Corrosion behavior of hot-dip Al-Zn coating doped with Si, RE, and Mg during exposure to sodium chloride containing environments. Mater. Corros. Werkst. Korros. 2018, 69, 714–724. [Google Scholar] [CrossRef]
- Su, X.; Zhou, J.; Wang, J.; Wu, C.; Liu, Y.; Tu, H.; Peng, H. Thermodynamic analysis and experimental study on the oxidation of the Zn-Al-Mg coating baths. Appl. Surf. Sci. 2017, 396, 154–160. [Google Scholar] [CrossRef]
- Wood, R.J.K.; Speyer, A.J. Erosion-corrosion of candidate HVOF aluminium-based marine coatings. Wear 2004, 256, 545–556. [Google Scholar] [CrossRef]
- Torres, B.; Taltavull, C.; Lopez, A.J.; Campo, M.; Rams, J. Al/SiCp and Al11Si/SiCp coatings on AZ91 magnesium alloy by HVOF. Surf. Coat. Technol. 2015, 261, 130–140. [Google Scholar] [CrossRef]
- Katayama, H.; Kuroda, S. Long-term atmospheric corrosion properties of thermally sprayed Zn, Al and Zn-Al coatings exposed in a coastal area. Corros. Sci. 2013, 76, 35–41. [Google Scholar] [CrossRef]
- De Rincon, O.; Rincon, A.; Sanchez, M.; Romero, N.; Salas, O.; Delgado, R.; Lopez, B.; Uruchurtu, J.; Marroco, M.; Panosian, Z. Evaluating Zn, Al and Al-Zn coatings on carbon steel in a special atmosphere. Constr. Build. Mater. 2009, 23, 1465–1471. [Google Scholar] [CrossRef]
- Lee, H.S.; Ismail, M.A.; Choe, H.B. Arc thermal metal spray for the protection of steel structures: An overview. Corros. Rev. 2015, 33, 31–61. [Google Scholar] [CrossRef]
- Yang, H.Q.; Yao, Z.J.; Wei, D.B.; Zhou, W.B.; Yin, G.X.; Feng, L.X. Anticorrosion of thermal sprayed Al-Zn-Si coating in simulated marine environments. Surf. Eng. 2014, 30, 801–805. [Google Scholar] [CrossRef]
- Odhiambo, J.G.; Li, W.; Zhao, Y.; Li, C. Porosity and Its Significance in Plasma-Sprayed Coatings. Coatings 2019, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, Y.; Miyazaki, F.; Yamasaki, M.; Yamagata, Y.; Kobayashi, N.; Muraoka, K. Coating Qualities Deposited Using Three Different Thermal Spray Technologies in Relation with Temperatures and Velocities of Spray Droplets. Coatings 2017, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Chen, C.; Suo, X.; Lupoi, R. Cold-Sprayed Metal Coatings with Nanostructure. Adv. Mater. Sci. Eng. 2018. [Google Scholar] [CrossRef] [Green Version]
- Grujicic, M.; Zhao, C.L.; DeRosset, W.S.; Helfritch, D. Adiabatic shear instability based mechanism for particles/substrate bonding in the cold-gas dynamic-spray process. Mater. Des. 2004, 25, 681–688. [Google Scholar] [CrossRef]
- Yin, S.; Cavaliere, P.; Aldwell, B.; Jenkins, R.; Liao, H.; Li, W.; Lupoi, R. Cold spray additive manufacturing and repair: Fundamentals and applications. Addit. Manuf. 2018, 21, 628–650. [Google Scholar] [CrossRef]
- Gaertner, F.; Schmidt, T.; Stoltenhoff, T.; Kreye, H. Recent developments and potential applications of cold spraying. Adv. Eng. Mater. 2006, 8, 611–618. [Google Scholar] [CrossRef]
- Champagne, V.; Helfritch, D. The unique abilities of cold spray deposition. Int. Mater. Rev. 2016, 61, 437–455. [Google Scholar] [CrossRef]
- Schmidt, T.; Gartner, F.; Assadi, H.; Kreye, H. Development of a generalized parameter window for cold spray deposition. Acta Mater. 2006, 54, 729–742. [Google Scholar] [CrossRef]
- Schmidt, T.; Assadi, H.; Gaertner, F.; Richter, H.; Stoltenhoff, T.; Kreye, H.; Klassen, T. From Particle Acceleration to Impact and Bonding in Cold Spraying. J. Therm. Spray Technol. 2009, 18, 794–808. [Google Scholar] [CrossRef] [Green Version]
- Assadi, H.; Gartner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Balani, K.; Laha, T.; Agarwal, A.; Karthikeyan, J.; Munroe, N. Effect of carrier gases on microstructural and electrochemical behavior of cold-sprayed 1100 aluminum coating. Surf. Coat. Technol. 2005, 195, 272–279. [Google Scholar] [CrossRef]
- Zahiri, S.H.; Fraser, D.; Gulizia, S.; Jahedi, M. Effect of Processing Conditions on Porosity Formation in Cold Gas Dynamic Spraying of Copper. J. Therm. Spray Technol. 2006, 15, 422–430. [Google Scholar] [CrossRef]
- Luo, X.-T.; Wei, Y.-K.; Wang, Y.; Li, C.-J. Microstructure and mechanical property of Ti and Ti6Al4V prepared by an in-situ shot peening assisted cold spraying. Mater. Des. 2015, 85, 527–533. [Google Scholar] [CrossRef]
- Lu, X.; Wang, S.; Xiong, T.; Wen, D.; Wang, G.; Du, H. Anticorrosion Properties of Zn-Al Composite Coating Prepared by Cold Spraying. Coatings 2019, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Haixiang, L.I.; Xiangbo, L.I.; Mingxian, S.U.N.; Hongren, W.; Guosheng, H. Corrosion resistance of cold-sprayed Zn-50Al coatings in seawater. J. Chin. Soc. Corros. Prot. 2010, 30, 62–66. [Google Scholar]
- Wong, W.; Vo, P.; Irissou, E.; Ryabinin, A.N.; Legoux, J.G.; Yue, S. Effect of Particle Morphology and Size Distribution on Cold-Sprayed Pure Titanium Coatings. J. Therm. Spray Technol. 2013, 22, 1140–1153. [Google Scholar] [CrossRef]
- Jodoin, B.; Ajdelsztajn, L.; Sansoucy, E.; Zúñiga, A.; Richer, P.; Lavernia, E.J. Effect of particle size, morphology, and hardness on cold gas dynamic sprayed aluminum alloy coatings. Surf. Coat. Technol. 2006, 201, 3422–3429. [Google Scholar] [CrossRef]
- Li, H.; Sun, M.; Li, X.; Wang, H.; Huang, G. Depositing characteristic of 65% Zn-Al coatings produced by cold gas dynamic spray. Chin. J. Nonferrous Met. 2010, 20, 1353–1359. [Google Scholar]
- Lou, K. The Organizational Structure of the Cold Spraying Al Composite Coating and Corrosion Behavior Research. Master’s Thesis, JiMei University, Fujian, China, 2017. [Google Scholar]
- Wang, X.; Zhang, B.; Lv, J.; Yin, S. Investigation on the Clogging Behavior and Additional Wall Cooling for the Axial-Injection Cold Spray Nozzle. J. Therm. Spray Technol. 2015, 24, 696–701. [Google Scholar] [CrossRef]
- Cole, I.S.; Ganther, W.D.; Furman, S.A.; Muster, T.H.; Neufeld, A.K. Pitting of zinc: Observations on atmospheric corrosion in tropical countries. Corros. Sci. 2010, 52, 848–858. [Google Scholar] [CrossRef]

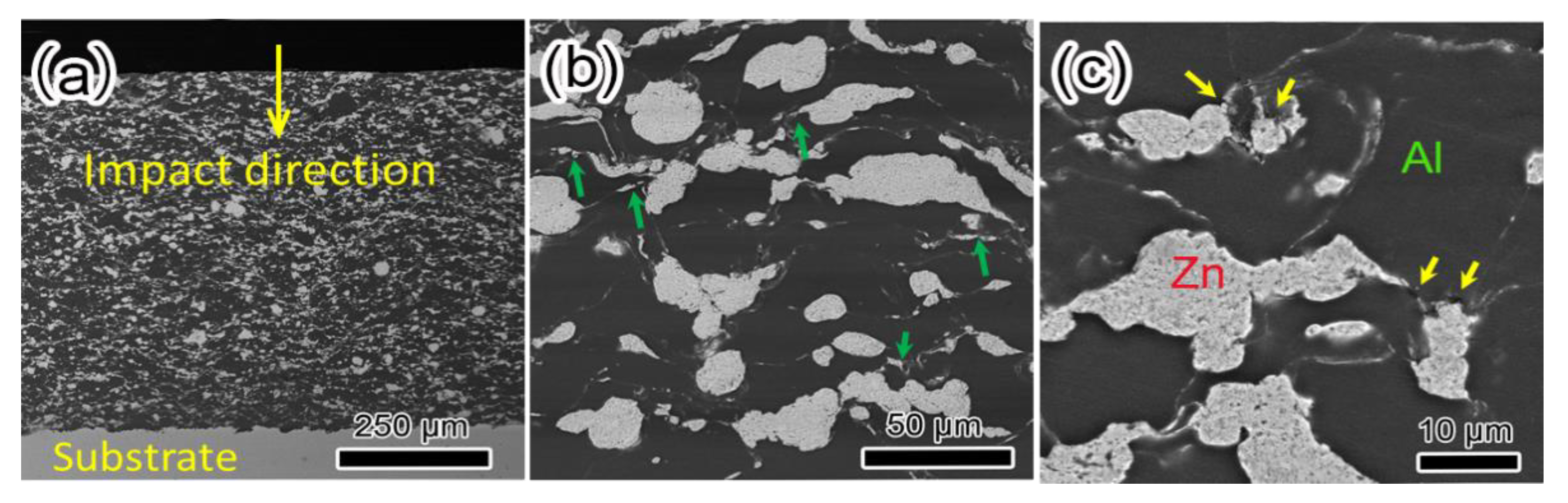
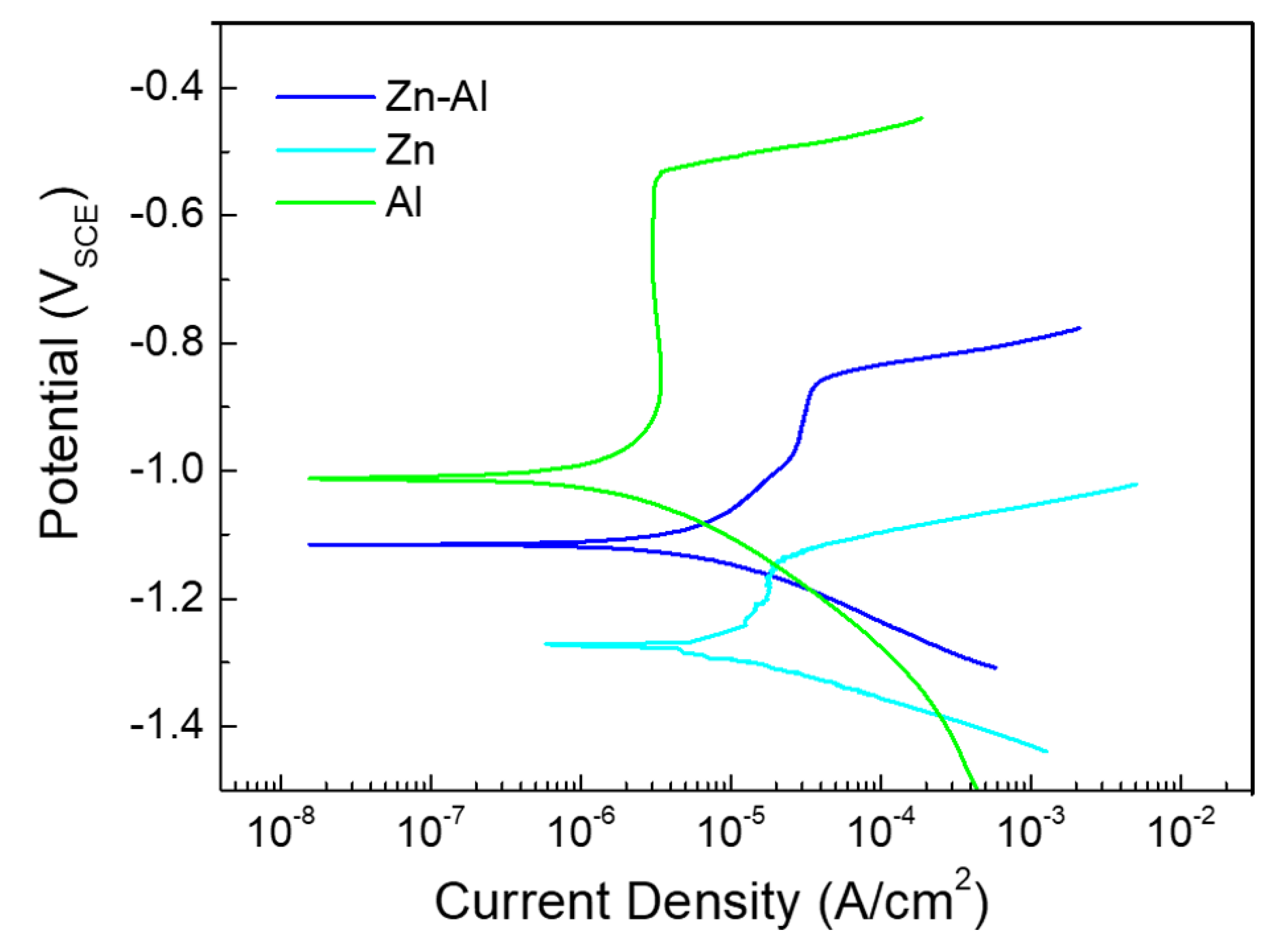
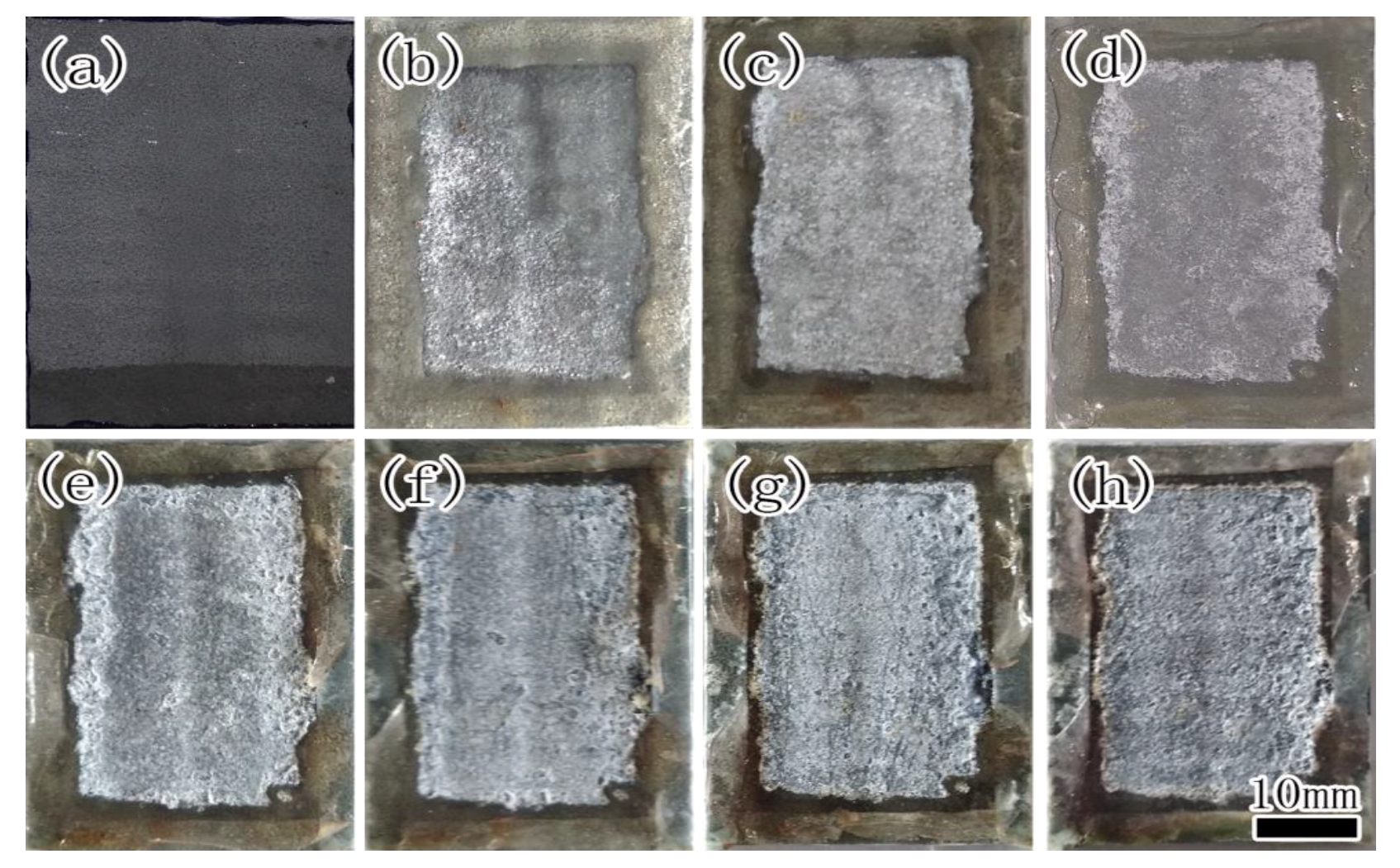
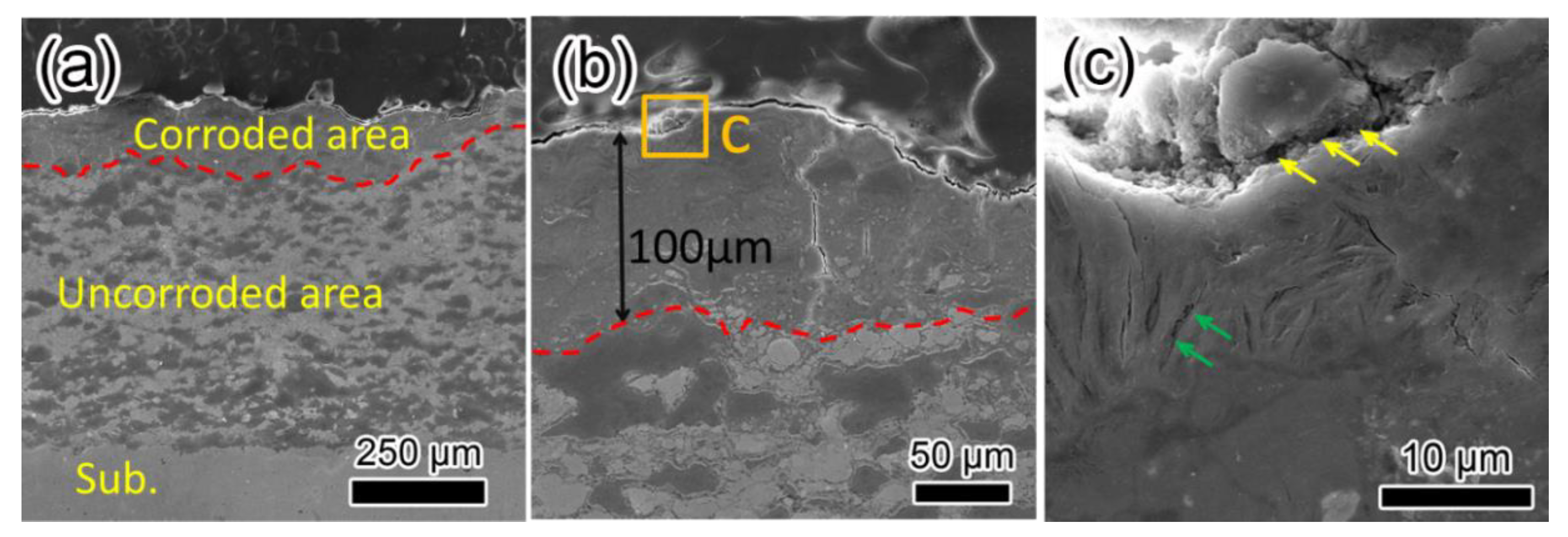


| Element | Fe | C | Mn | Si | S | P |
|---|---|---|---|---|---|---|
| Content (wt.%) | balance | 0.173 | 0.465 | 0.287 | 0.036 | 0.027 |
| Sample | Ecorr (VSCE) | Icorr (μA/cm2) | Epit (VSCE) | Corrosion Rate (mpy) |
|---|---|---|---|---|
| Al | −1.00 | 0.32 | −0.54 | 0.18 |
| Zn | −1.27 | 3.80 | −1.15 | 1.64 |
| Zn-Al | −1.12 | 0.77 | −0.86 | 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Tang, J.; Tariq, N.u.H.; Wang, J.; Cui, X.; Xiong, T. Microstructure and Corrosion Behavior of Cold-Sprayed Zn-Al Composite Coating. Coatings 2020, 10, 931. https://doi.org/10.3390/coatings10100931
Zhao Z, Tang J, Tariq NuH, Wang J, Cui X, Xiong T. Microstructure and Corrosion Behavior of Cold-Sprayed Zn-Al Composite Coating. Coatings. 2020; 10(10):931. https://doi.org/10.3390/coatings10100931
Chicago/Turabian StyleZhao, Zhipo, Junrong Tang, Naeem ul Haq Tariq, Jiqiang Wang, Xinyu Cui, and Tianying Xiong. 2020. "Microstructure and Corrosion Behavior of Cold-Sprayed Zn-Al Composite Coating" Coatings 10, no. 10: 931. https://doi.org/10.3390/coatings10100931
APA StyleZhao, Z., Tang, J., Tariq, N. u. H., Wang, J., Cui, X., & Xiong, T. (2020). Microstructure and Corrosion Behavior of Cold-Sprayed Zn-Al Composite Coating. Coatings, 10(10), 931. https://doi.org/10.3390/coatings10100931




