ATP-Responsive ZIF-90 Nanocontainers Encapsulating Natural Antifoulants for Intelligent Marine Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction, Isolation, and Purification of Natural Organic Compounds
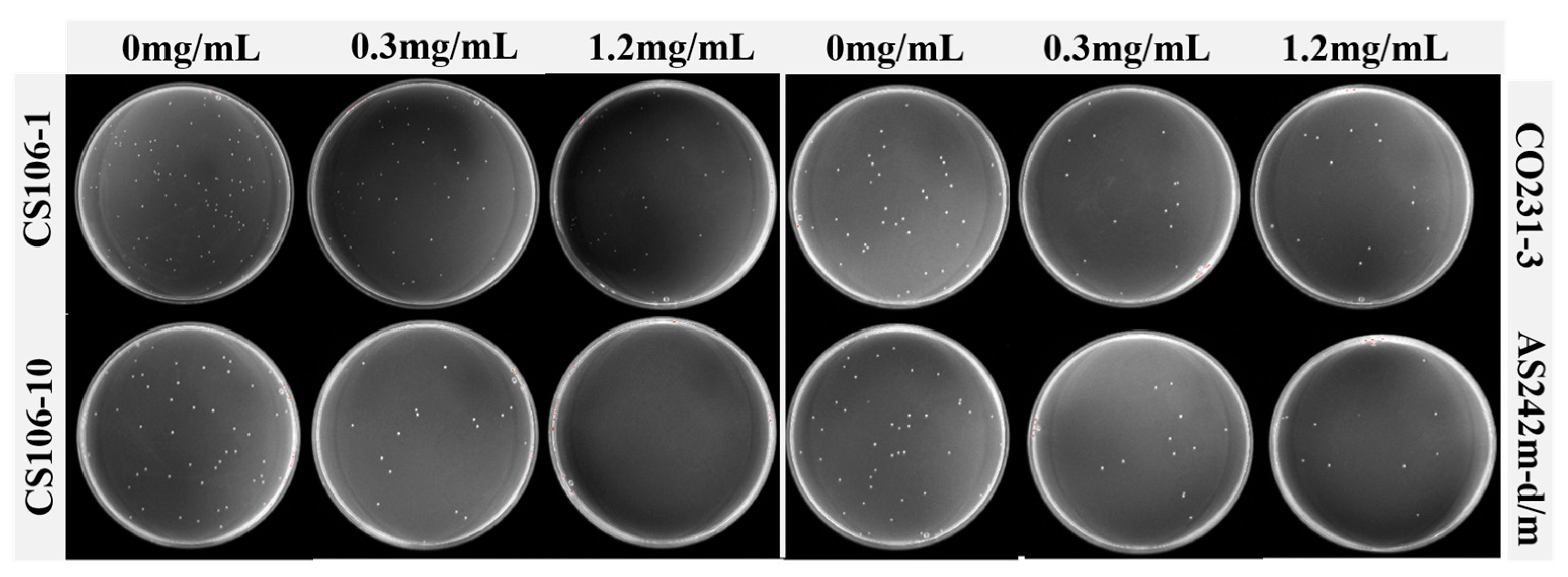
2.2.1. Separation and Purification of Compounds CS106-1 and CS106-10
2.2.2. Separation and Purification of Compound CO231-3
2.2.3. Separation and Purification of Compound AS242m-d/m
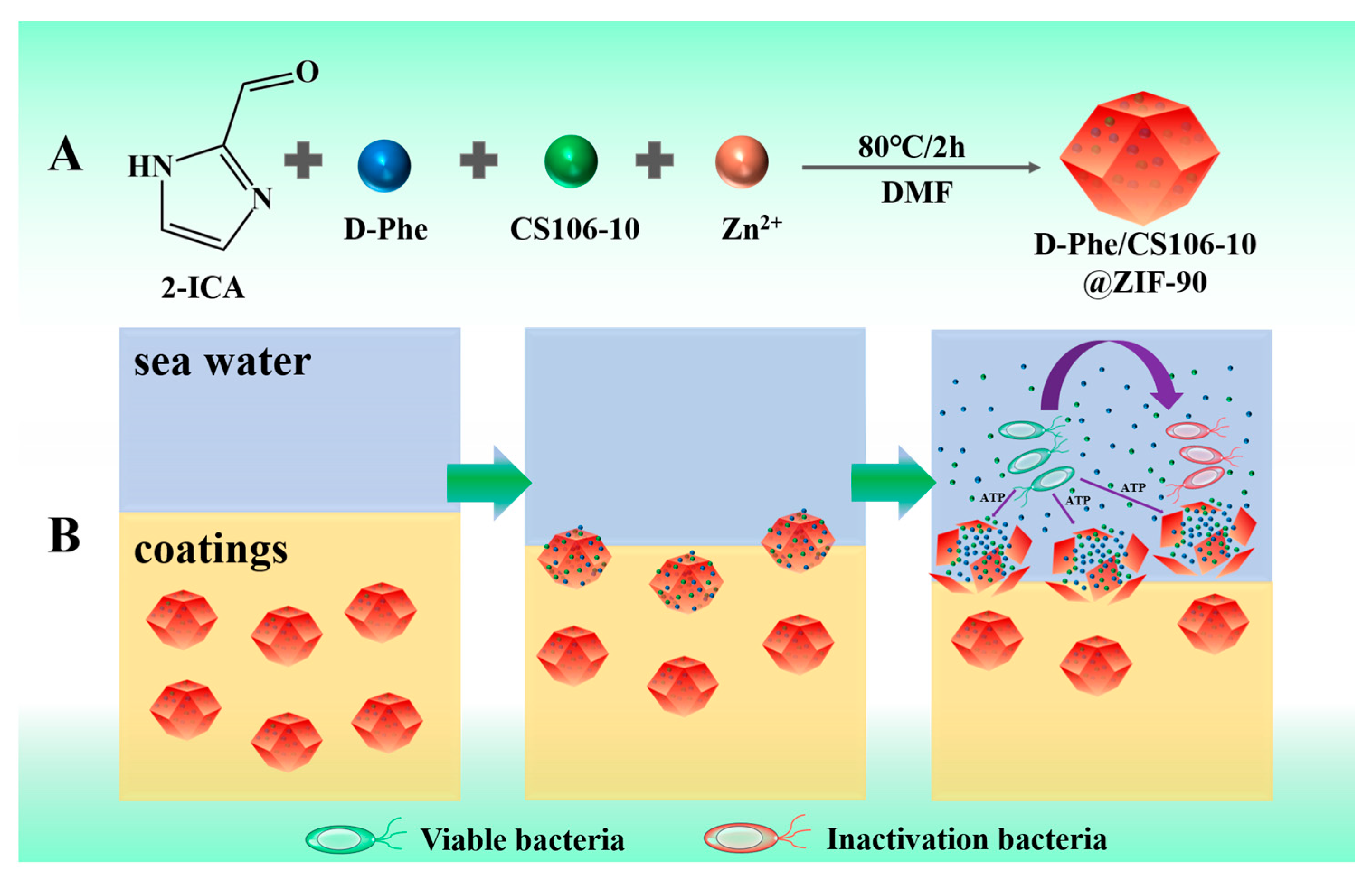
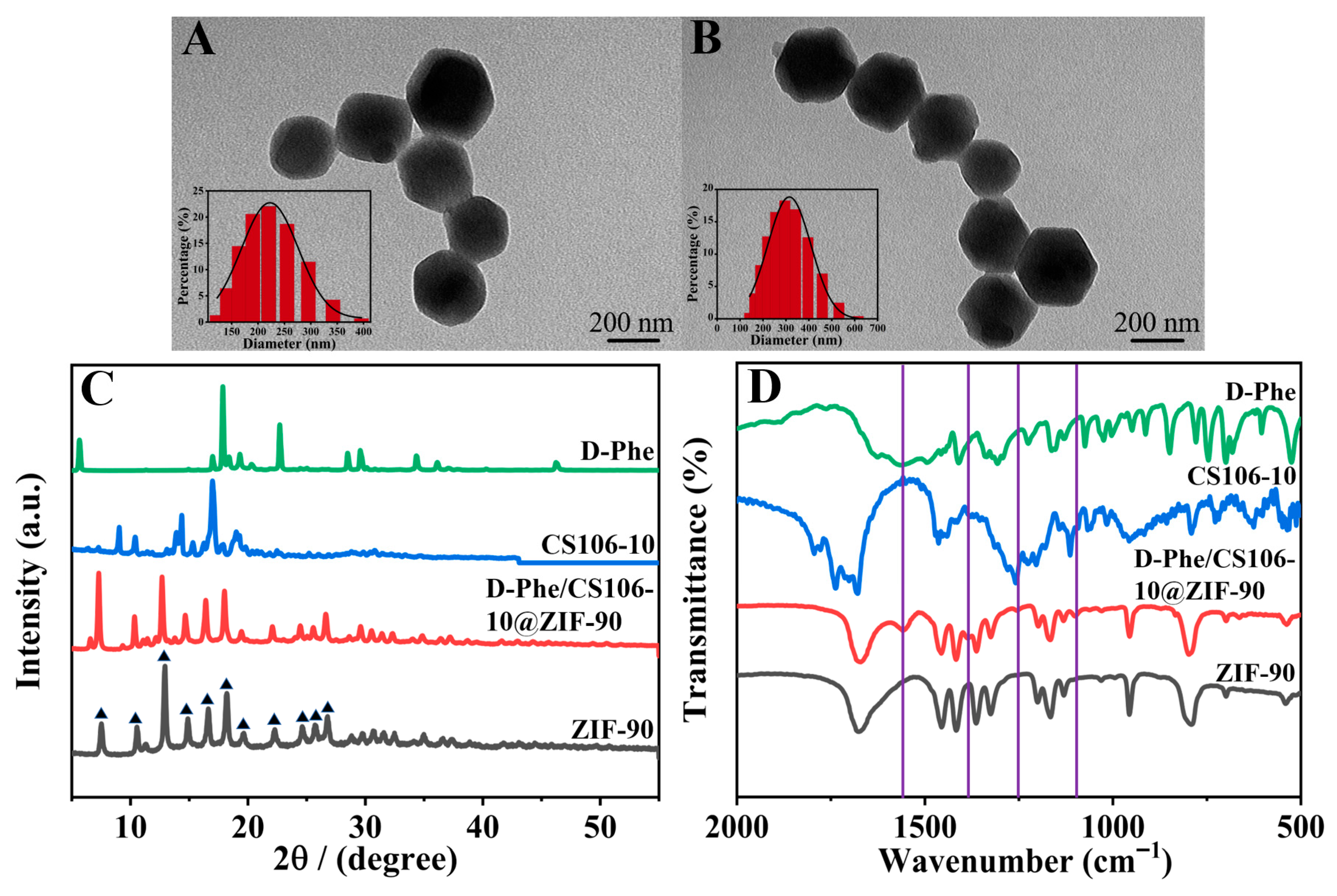
2.3. Preparation of D-Phe/CS106-10@ZIF-90 Nanoparticles
2.4. Preparation of Intelligent Self-Polishing Antifouling Coating
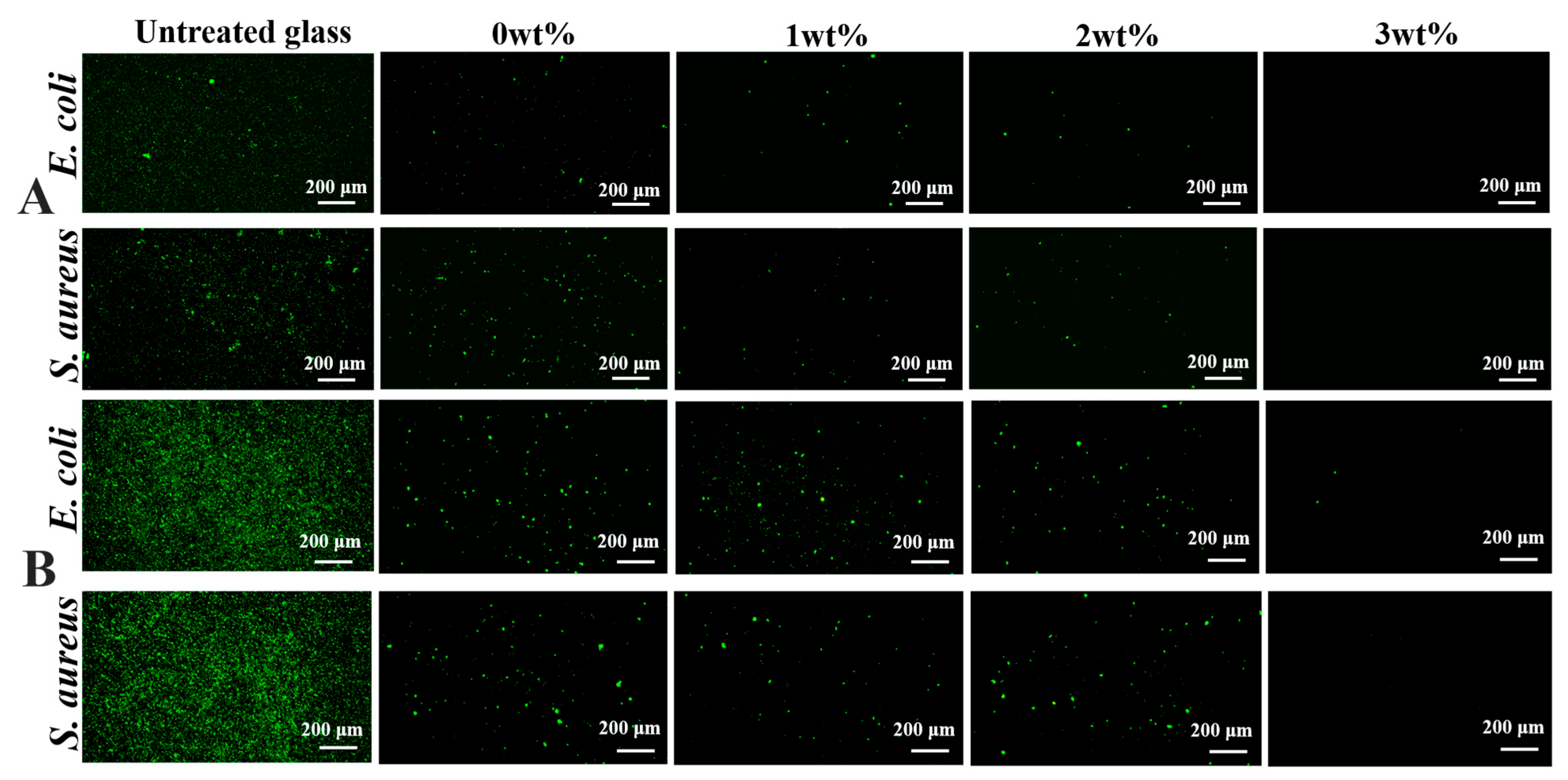
2.5. Bacterial Adhesion Inhibition of Antifouling Coating
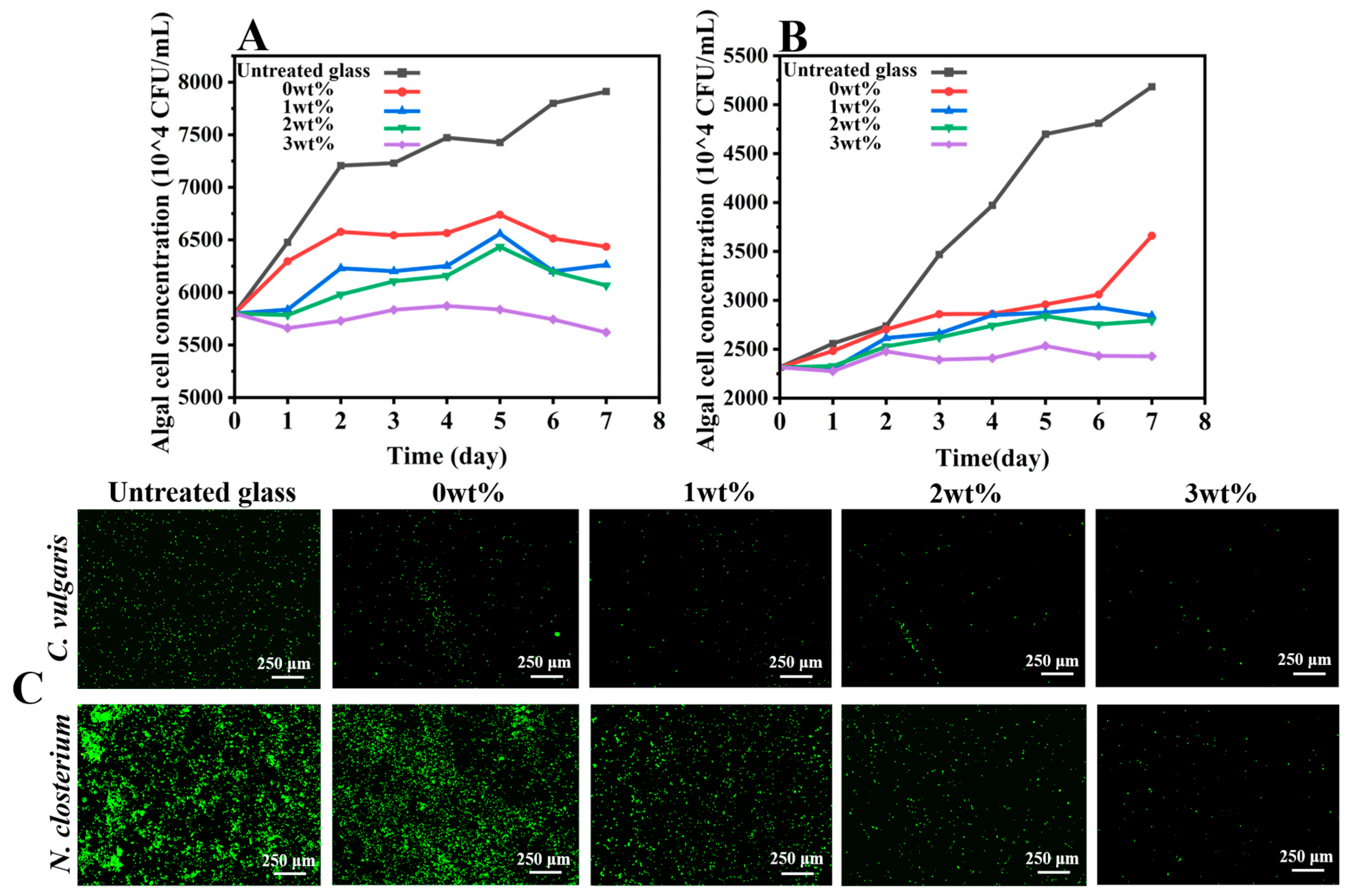
2.6. Antialgal Activity Evaluation of Antifouling Coating
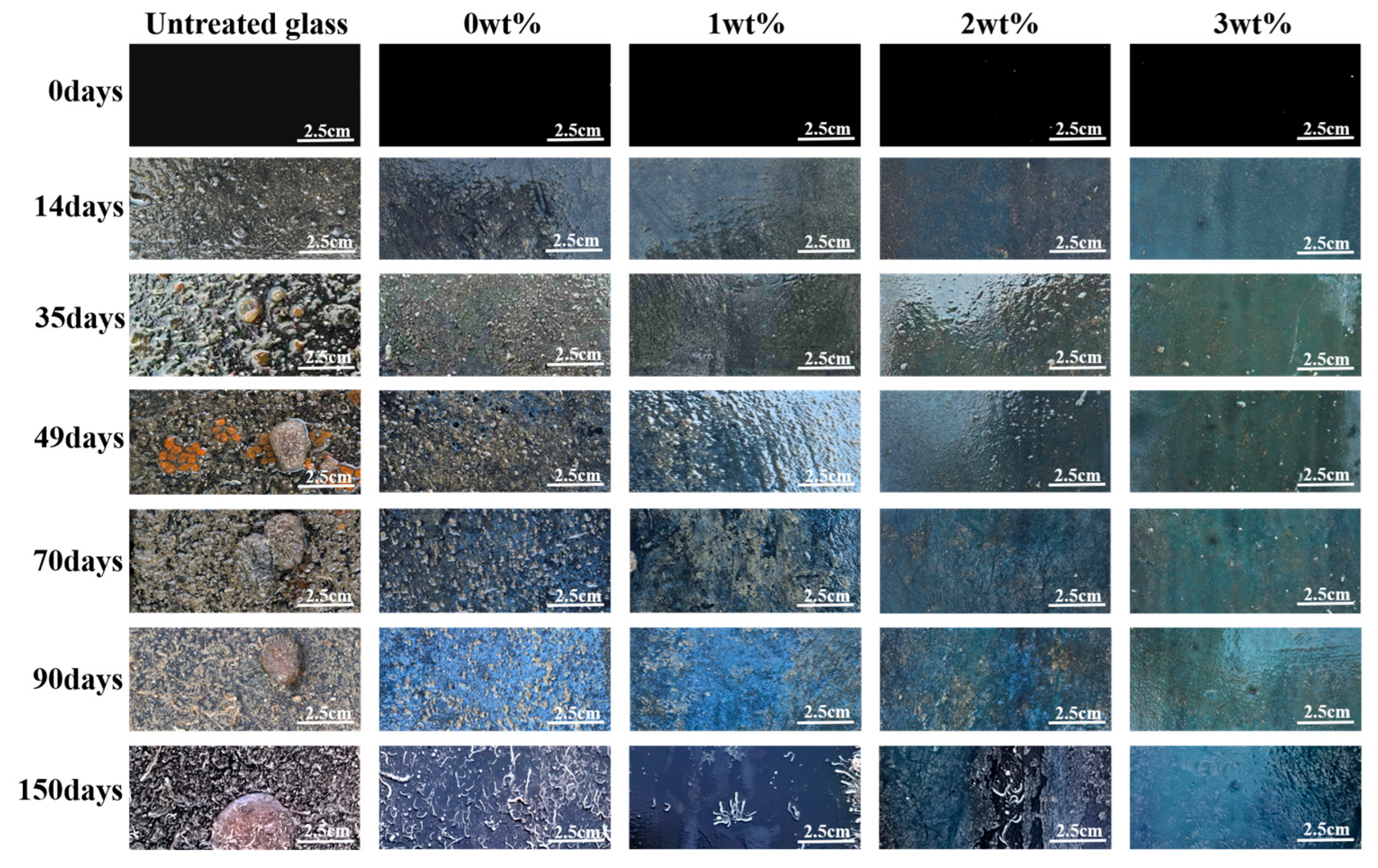
2.7. Antifouling Experiment in Marine Environment
3. Results
3.1. Screening of Natural Antifouling Agents
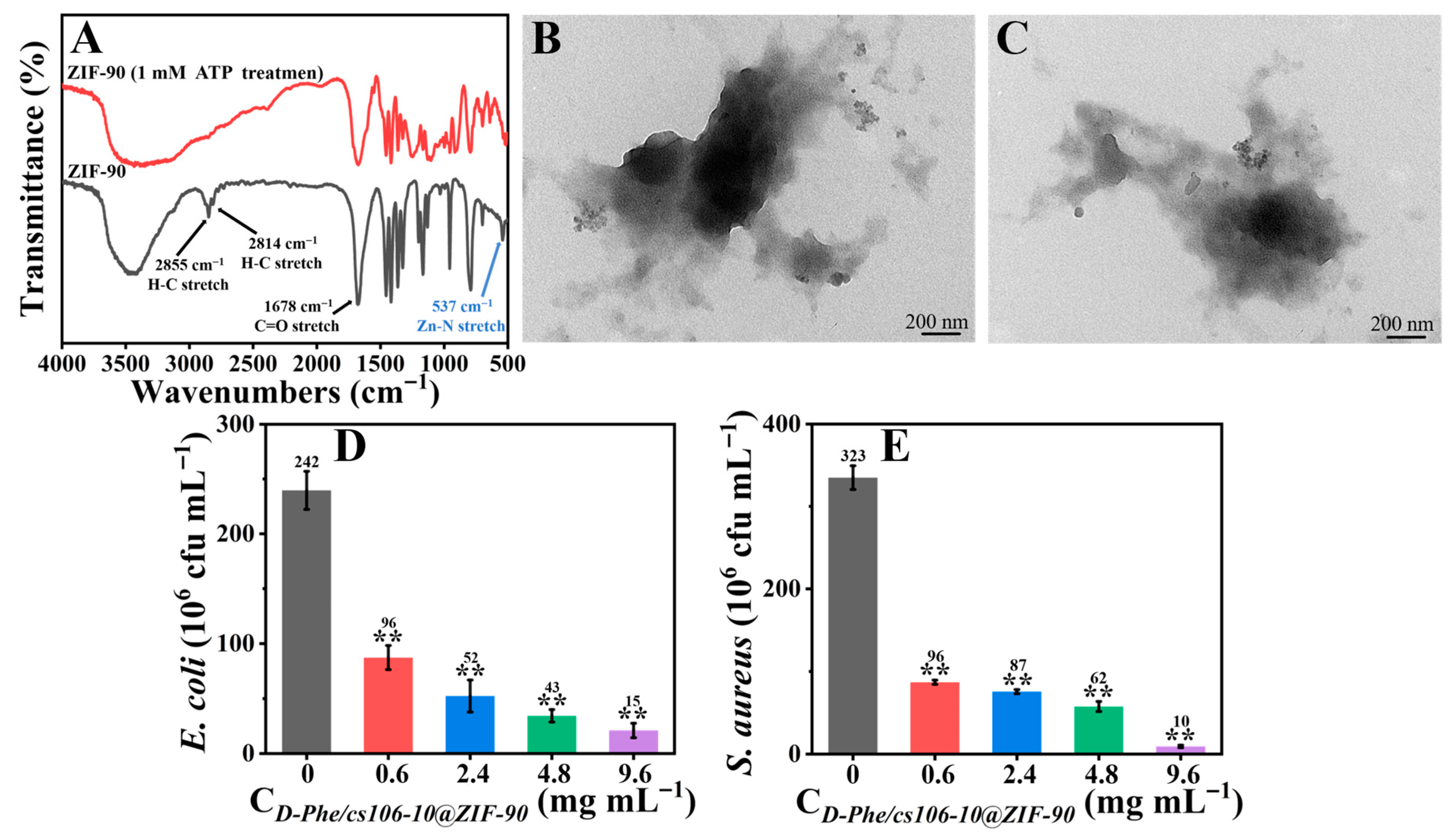
3.2. Characterization of D-Phe/CS106-10@ZIF-90 (B) Nanoparticles
3.3. Performance of the Intelligent, Self-Polishing Antifouling Coating
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, P.-Y.; Cheng, A.; Wang, R.; Zhang, R. Marine biofilms: Diversity, interactions and biofouling. Nat. Rev. Microbiol. 2022, 20, 671–684. [Google Scholar] [CrossRef]
- Qiu, H.; Feng, K.; Gapeeva, A.; Meurisch, K.; Kaps, S.; Li, X.; Yu, L.; Mishra, Y.K.; Adelung, R.; Baum, M. Functional polymer materials for modern marine biofouling control. Prog. Polym. Sci. 2022, 127, 101516. [Google Scholar] [CrossRef]
- Eskhan, A.; Johnson, D. Microscale characterization of abiotic surfaces and prediction of their biofouling/anti-biofouling potential using the AFM colloidal probe technique. Adv. Colloid Interface Sci. 2022, 310, 102796. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, Y.F. An intelligent coating doped with inhibitor-encapsulated nanocontainers for corrosion protection of pipeline steel. Chem. Eng. J. 2017, 315, 537–551. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Q.; Han, D.; Chen, Y.; Liu, W.; Pei, Y.; Duan, J.; Hou, B. Progress in anti-biofouling materials and coatings for the marine environment. J. Environ. Sci. 2026, 161, 494–510. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Y.; Zhang, D.; Chen, C. 3D printing technology meets marine biofouling: A study on antifouling resin for protecting marine sensors. Addit. Manuf. 2023, 73, 103697. [Google Scholar] [CrossRef]
- Kaidarova, A.; Geraldi, N.R.; Wilson, R.P.; Kosel, J.; Meekan, M.G.; Eguíluz, V.M.; Hussain, M.M.; Shamim, A.; Liao, H.; Srivastava, M.; et al. Wearable sensors for monitoring marine environments and their inhabitants. Nat. Biotechnol. 2023, 41, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Demirel, Y.K.; Atlar, M. An investigation into the effect of biofouling on the ship hydrodynamic characteristics using CFD. Ocean Eng. 2019, 175, 122–137. [Google Scholar] [CrossRef]
- Simões, L.A.R.; Vogt, É.L.; da Costa, C.S.; de Amaral, M.; Hoff, M.L.M.; Graceli, J.B.; Vinagre, A.S. Effects of tributyltin (TBT) on the intermediate metabolism of the crab Callinectes sapidus. Mar. Pollut. Bull. 2022, 182, 114004. [Google Scholar] [CrossRef]
- Vanavermaete, D.; Hostens, K.; Everaert, G.; Parmentier, K.; Janssen, C.; De Witte, B. Assessing the risk of booster biocides for the marine environment: A case study at the Belgian part of the North Sea. Mar. Pollut. Bull. 2023, 197, 115774. [Google Scholar] [CrossRef]
- de Campos, B.G.; do Prado e Silva, M.B.M.; Avelelas, F.; Maia, F.; Loureiro, S.; Perina, F.; Abessa, D.M.S.; Martins, R. Toxicity of innovative antifouling additives on an early life stage of the oyster Crassostrea gigas: Short- and long-term exposure effects. Environ. Sci. Pollut. Res. 2022, 29, 27534–27547. [Google Scholar] [CrossRef]
- Qiu, Q.; Gu, Y.; Ren, Y.; Ding, H.; Hu, C.; Wu, D.; Mou, J.; Wu, Z.; Dai, D. Research progress on eco-friendly natural antifouling agents and their antifouling mechanisms. Chem. Eng. J. 2024, 495, 153638. [Google Scholar] [CrossRef]
- Ghattavi, S.; Homaei, A.; Fernandes, P. Marine natural products for biofouling elimination in marine environments. Biocatal. Agric. Biotechnol. 2024, 61, 103385. [Google Scholar] [CrossRef]
- Xu, X.; Guo, S.; Vancso, G.J. Perceiving and countering marine biofouling: Structure, forces, and processes at surfaces in sea water across the length scales. Langmuir 2025, 41, 7996–8018. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R. Marine biofilms: A successful microbial strategy with economic implications. Front. Mar. Sci. 2018, 5, 126. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Coelho, A.V.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and bioactive natural products from marine invertebrates: From basic research to innovative applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, P.; Loiselle, G.; Darragh, R.; Slipski, C.; Bay, D.C. Reviewing the complexities of bacterial biocide susceptibility and in vitro biocide adaptation methodologies. npj Antimicrob. Resist. 2025, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef]
- Wu, X.; Yue, H.; Zhang, Y.; Gao, X.; Li, X.; Wang, L.; Cao, Y.; Hou, M.; An, H.; Zhang, L.; et al. Packaging and delivering enzymes by amorphous metal-organic frameworks. Nat. Commun. 2019, 10, 5165. [Google Scholar] [CrossRef]
- Kong, Y.; Li, H.; Li, B.; Li, D.; Guo, J.; Zhou, Q.; Wang, Z.; Ma, W.; Yuan, J. Narrow bandgap non-fused polymers enable efficient organic/quantum dot hybrid solar cells. J. Energy Chem. 2025, 109, 1–7. [Google Scholar] [CrossRef]
- Canossa, S.; Ji, Z.; Gropp, C.; Rong, Z.; Ploetz, E.; Wuttke, S.; Yaghi, O.M. System of sequences in multivariate reticular structures. Nat. Rev. Mater. 2023, 8, 331–340. [Google Scholar] [CrossRef]
- Han, D.; Liu, X.; Wu, S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chem. Soc. Rev. 2022, 51, 7138–7169. [Google Scholar] [CrossRef]
- Li, J.; Fang, Z.; Chen, H.; Liu, K.; Pan, Y.; Li, X.; Lin, D.; Wang, N.; Guo, C.; Han, C.; et al. Synergistic construction of in situ self-polymerized interface and localized pH buffer zone for high-performance aqueous zinc–iodine batteries. Angew. Chem. Int. Ed. 2025, 64, e202511490. [Google Scholar] [CrossRef]
- Falcaro, P.; Okada, K.; Hara, T.; Ikigaki, K.; Tokudome, Y.; Thornton, A.W.; Hill, A.J.; Williams, T.; Doonan, C.; Takahashi, M. Centimetre-scale micropore alignment in oriented polycrystalline metal–organic framework films via heteroepitaxial growth. Nat. Mater. 2017, 16, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Hou, M.J.; Chen, J.T.; Jiang, W.L.; Liu, X.; Zhang, Y.; Yu, L.; Wang, Y.; Fu, Y. ATP fluorescent nanoprobe based on ZIF-90 and near-infrared dyes for imaging in tumor mice. Sens. Actuators B Chem. 2022, 369, 132286. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, M. In-vivo fluorescence imaging of adenosine 5′-triphosphate. Trends Anal. Chem. 2016, 80, 190–203. [Google Scholar] [CrossRef]
- Rubino, F.A.; Mollo, A.; Kumar, S.; Shaffer, C.L.; Herbert, S.; Hopkins, O.; Romero, J.; Walker, S. Detection of transport intermediates in the peptidoglycan flippase MurJ identifies residues essential for conformational cycling. J. Am. Chem. Soc. 2020, 142, 5482–5486. [Google Scholar] [CrossRef]
- Borisova, B.; Nocheva, H.; Iliev, I.; Laronze-Cochard, M.; Gérard, S.; Petrin, S.; Danalev, D. Synthesis and analgesic activity of new analogs of FELL tetrapeptide containing D-phe in the first position. Curr. Res. Biotechnol. 2024, 8, 100249. [Google Scholar] [CrossRef]
- Yuill, J.L. The acids produced from sugar by a penicillium parasitic upon Aspergillus niger. Biochem. J. 1934, 28, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, T.-M.; Shen, M.-H.; Yang, M.-Q.; Feng, Q.-Y.; Tang, X.-M.; Li, X.-M. Spiculisporic acids B-D, three new γ-butenolide derivatives from a sea urchin-derived fungus Aspergillus sp. HDf2. Molecules 2012, 17, 13175–13182. [Google Scholar] [CrossRef]
- Meng, L.; Awakawa, T.; Li, X.; Zhang, Y.; Zhang, J.; Wang, J.; Abe, I. Discovery of (±)-penindolenes reveals an unusual indole ring cleavage pathway catalyzed by P450 monooxygenase. Angew. Chem. Int. Ed. 2024, 63, e202403963. [Google Scholar] [CrossRef]
- Cui, W.; Lu, X.; Bian, J.; Chen, Y.; Wang, Y.; Li, Z.; Zhang, Z. Curvularia spicifera and curvularia muehlenbeckiae causing leaf blight on Cunninghamia lanceolata. Plant Pathol. 2020, 69, 1139–1147. [Google Scholar] [CrossRef]
- Zhang, A.; Shi, X.; Wang, B.; Meng, L.; Pineda, L.M.; Li, Y.; Wang, J.; Abe, I.; Liu, Y.; Zhang, C. New curvularin and norlignanolide derivatives from cocultures of marine mangrove endophytic fungus Penicillium brocae MA-231 and phytopathogen Curvularia spicifera QA-26. Chem. Biodivers. 2025, 22, e202500761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.-M.; Song, N.; Meng, L.-H.; Li, X.; Wang, B.-G. Secondary metabolites with fungicide potentials from the deep-sea seamount-derived fungus talaromyces scorteus AS-242. Bioorg. Chem. 2024, 147, 107417. [Google Scholar] [CrossRef] [PubMed]
- GB 5370-2007; Test Method for Shallow Sea Immersion of Antifouling Paint Panels. General Administration of Quality Supervision, Inspection and Quarantine. Standardization Administration: Beijing, China, 2007.
- Brown, S.P.; Goodwin, N.C.; MacMillan, D.W.C. The first enantioselective organocatalytic mukaiyama−michael reaction: A direct method for the synthesis of enantioenriched γ-butenolide architecture. J. Am. Chem. Soc. 2003, 125, 1192–1194. [Google Scholar] [CrossRef]
- Yu, F.; Du, Y.; Guo, M.; Chen, X.; Liu, H.; Zhang, Y. Application of biosurfactant surfactin for the removal of heavy metals from contaminated water and soil via a micellar-enhanced ultrafiltration process. Sep. Purif. Technol. 2023, 327, 124947. [Google Scholar] [CrossRef]
- Li, X.; Yan, B.; Huang, W.; Zhang, Y.; Liu, Y.; Wang, J. Room-temperature synthesis of hydrophobic/oleophilic ZIF-90-CF3/melamine foam composite for the efficient removal of organic compounds from wastewater. Chem. Eng. J. 2022, 428, 132501. [Google Scholar] [CrossRef]
- Mei, D.C.; Li, H.; Liu, L.J.; Jiang, L.C.; Zhang, C.H.; Wu, X.R.; Dong, H.X.; Ma, F.Q. Efficient uranium adsorbent with antimicrobial function: Oxime functionalized ZIF-90. Chem. Eng. J. 2021, 425, 130468. [Google Scholar] [CrossRef]
- Chen, S.; Pang, H.; Sun, J.; Li, K. Research advances and applications of ZIF-90 metal–organic framework nanoparticles in the biomedical field. Mater. Chem. Front. 2024, 8, 1195–1211. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Q.; Jiang, Y.; Zhang, M.; Wang, M.; Mao, L. Nanoscale ATP-responsive zeolitic imidazole framework-90 as a general platform for cytosolic protein delivery and genome editing. J. Am. Chem. Soc. 2019, 141, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, Y.; Sun, L.; Yuan, B.; Tian, Y.; Xiang, L.; Li, Y.; Li, Y.; Li, J.; Wu, A. Dual ATP and pH responsive ZIF-90 nanosystem with favorable biocompatibility and facile post-modification improves therapeutic outcomes of triple negative breast cancer in vivo. Biomaterials 2019, 197, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Vuong, P.; McKinley, A.; Kaur, P. Understanding biofouling and contaminant accretion on submerged marine structures. npj Mater. Degrad. 2023, 7, 50. [Google Scholar] [CrossRef]
- Alam, F.; Kumar, S.; Varadarajan, K.M. Quantification of adhesion force of bacteria on the surface of biomaterials: Techniques and assays. ACS Biomater. Sci. Eng. 2019, 5, 2093–2110. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.M.R.; Lopes, P.R.M.; Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Shi, X.L.; Liang, H.; Li, Y.Z. Review of Progress in Marine Anti-Fouling Coatings: Manufacturing Techniques and Copper- and Silver-Doped Antifouling Coatings. Coatings 2024, 14, 1454. [Google Scholar] [CrossRef]
- Li, L.; Hong, H.; Cao, J.; Yang, Y. Progress in Marine Antifouling Coatings: Current Status and Prospects. Coatings 2023, 13, 1893. [Google Scholar] [CrossRef]
- Yan, H.; Wu, Q.; Yu, C.; Zhao, T.; Liu, M. Recent Progress of Biomimetic Antifouling Surfaces in Marine. Adv. Mater. Interfaces 2020, 7, 2000966. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Ferreira, S.S.; Correia, A.; Vilanova, M.; Silva, T.H.; Coimbra, M.A.; Nunes, C. Reserve, structural and extracellular polysaccharides of Chlorella vulgaris: A holistic approach. Algal Res. 2020, 45, 101757. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Chao, Y.; Feng, X.; Wang, B.; Meng, L.; Qi, P.; Zeng, Y.; Wang, P. ATP-Responsive ZIF-90 Nanocontainers Encapsulating Natural Antifoulants for Intelligent Marine Coatings. Coatings 2026, 16, 7. https://doi.org/10.3390/coatings16010007
Chao Y, Feng X, Wang B, Meng L, Qi P, Zeng Y, Wang P. ATP-Responsive ZIF-90 Nanocontainers Encapsulating Natural Antifoulants for Intelligent Marine Coatings. Coatings. 2026; 16(1):7. https://doi.org/10.3390/coatings16010007
Chicago/Turabian StyleChao, Yanrong, Xingyan Feng, Bingui Wang, Linghong Meng, Peng Qi, Yan Zeng, and Peng Wang. 2026. "ATP-Responsive ZIF-90 Nanocontainers Encapsulating Natural Antifoulants for Intelligent Marine Coatings" Coatings 16, no. 1: 7. https://doi.org/10.3390/coatings16010007
APA StyleChao, Y., Feng, X., Wang, B., Meng, L., Qi, P., Zeng, Y., & Wang, P. (2026). ATP-Responsive ZIF-90 Nanocontainers Encapsulating Natural Antifoulants for Intelligent Marine Coatings. Coatings, 16(1), 7. https://doi.org/10.3390/coatings16010007








