1. Introduction
Transparent metal oxide thin films have been among the most extensively studied materials over the past decade, owing to their broad application prospects—for instance, in the fabrication of technical components for optical technologies, solar cells, and gas sensors [
1]. Optical transparency and electrical conductivity are the key properties for their use as transparent conductive electrodes in various optoelectronic devices, which depend on their sheet resistance range [
2]. However, it is challenging to simultaneously stabilize optical transparency and electrical conductivity in a single host material, as there is a trade-off between these two critical properties. For example, glass is transparent but intrinsically insulating, while metals are conductive yet opaque [
3].
Currently, indium tin oxide (ITO) is the most commonly and widely researched and applied oxide electrode material, and it is also the one that has been commercially produced on a large scale [
4,
5]. ITO thin films exhibit numerous excellent properties, demonstrating remarkable advantages in terms of light transmittance, resistivity, adhesion, and corrosion resistance [
6,
7]. Nevertheless, ITO still has several drawbacks: first, its sheet resistance is relatively high, typically reaching tens or even hundreds of ohms [
8], making it difficult to adapt to the rapid development of modern technology; second, ITO has poor or no flexibility, and according to the frontier orbital theory, the work function of ITO electrodes cannot match the energy levels of organic semiconductors, leading to a large interfacial contact potential difference that impairs the performance of optoelectronic devices; finally, indium (In) in ITO transparent conductive oxides has a scarce natural reserve but a high actual demand—scarcity drives up prices and manufacturing costs, which fails to meet the requirements of long-term large-scale production and market demand.
Therefore, research on indium-free transparent conductive oxides is particularly crucial. SnO
2 thin films are renowned for their chemical and thermal stability in atmospheric environments and high optical transmittance. Thanks to these characteristics, SnO
2 thin films have diverse applications, such as in gas sensing, lithium-ion batteries, solar cells, and catalysts for various organic reactions [
9]. These versatile applications are associated with their photophysical, chemical, and electronic properties, which can be tailored by regulating factors like the size, shape, crystallinity, and electronic state of tin oxide nanoparticles [
10].
Today, the most intensively studied and technically widely applied indium-free transparent metal oxide materials are zinc oxide (ZnO), SnO
2, and titanium oxide (TiO
2)—the latter at least attracting attention in the research field. We comparatively analyzed the characteristics of the aforementioned materials as well as In
2O
3 in
Table 1.
Based on the data in
Table 1 and the characteristics of SnO
2, the average content of its metallic constituent (tin) in the Earth’s crust is 40 ppm. Although this is lower than that of ZnO (132 ppm) and TiO
2 (4100 ppm), it is far more abundant than In
2O
3 (0.1 ppm). With a direct band gap of 3.6 eV at 300 K, SnO
2 is a wide-bandgap semiconductor, which endows it with excellent transparency in the visible light range while retaining semiconductor properties, making it suitable for optoelectronic device applications. It has a tetragonal rutile crystal structure with a space group of P4
2/mm (No. 136), a structure that imparts good physicochemical stability and strong weather resistance to the material. SnO
2 itself has a melting point of 1620 °C, much higher than that of metallic tin (232 °C), indicating good structural stability in high-temperature environments.
More importantly, SnO2 can be mass-produced via chemical and physical methods with a high degree of process maturity. In the future, researchers will further advance the applications of SnO2 by controlling doping elements, heterojunction structures, and grain sizes. Therefore, this paper selects SnO2 as the research object and systematically reviews SnO2 thin films in terms of their physical and chemical preparation methods as well as transparent conductive properties.
2. Structure of SnO2
The high transparency of SnO
2 thin films in the visible light region and high reflectivity in the infrared region make them highly attractive as transparent thermally reflective materials [
12]. Typically, SnO
2 thin films are n-type semiconductors with a wide band gap (the band gap of bulk materials at room temperature is 3.6 eV) and are known to have a tetragonal rutile structure [
13,
14], with a space group of P4
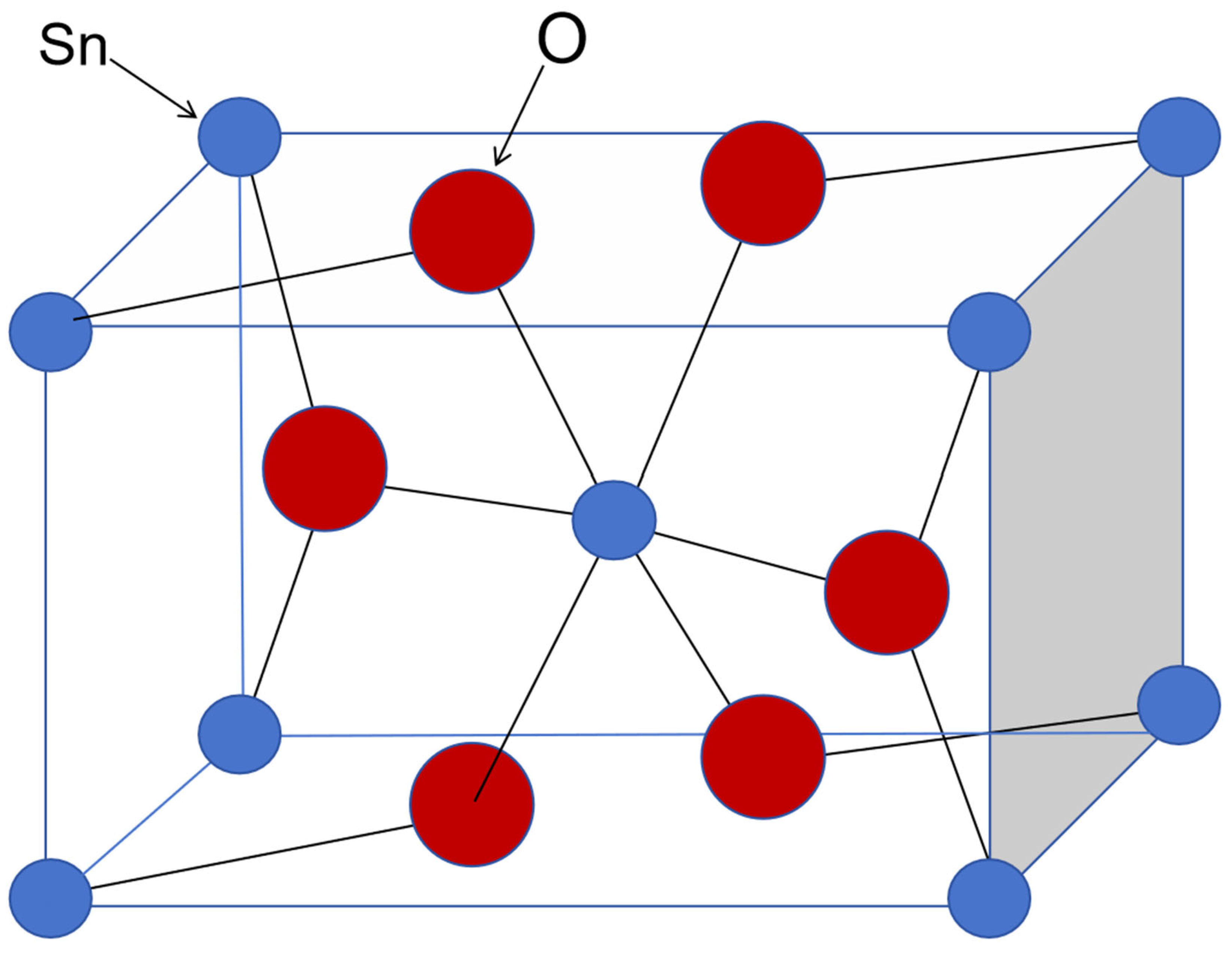
2/mnm. The unit cell contains 6 atoms, namely 2 tin atoms and 4 oxygen atoms, as shown in
Figure 1. Each tin atom is located at the center of 6 oxygen atoms, which are approximately at the vertices of a regular octahedron; each oxygen atom is surrounded by 3 tin atoms, which are approximately at the vertices of an equilateral triangle [
15].
This crystal structure endows SnO2 thin films with unique physical and chemical properties. Owing to the tetragonal rutile structure of SnO2, the interatomic bonding is strong, which imparts high hardness and excellent chemical stability to the films. This stability enables SnO2 thin films to maintain their performance under various environmental conditions and remain less susceptible to external factors. Meanwhile, the wide band gap of SnO2 thin films gives them high transparency in the visible light region and high reflectivity in the infrared region—characteristics that render SnO2 thin films highly promising for applications in fields such as optical devices and thermally reflective coatings. In addition, the n-type semiconductor property of SnO2 thin films also opens up possibilities for their use in electronic devices, such as transparent conductive electrodes.
3. Optoelectronic Properties of SnO2 Transparent Conductive Thin Films
The electrical conductivity of SnO2 thin films is mainly derived from free electrons within the films, which are primarily provided by oxygen vacancies and doping elements. The presence of oxygen vacancies leads to local charge imbalance in the films, thereby generating free electrons; these electrons can move freely under an electric field, endowing the films with electrical conductivity. Meanwhile, doping with other elements (e.g., fluorine (F), antimony (Sb), etc.) can further increase the concentration of free electrons in the films and enhance their electrical conductivity.
In terms of transparency, the wide band gap of SnO2 thin films gives them high transmittance for visible light. When the photon energy is lower than the band gap energy, photons cannot be absorbed and thus can transmit through the films. This enables SnO2 thin films to exhibit excellent transparency in the visible light region, making them suitable for optoelectronic devices that require transparent conductive electrodes.
In addition, the transparent conductive properties of SnO2 thin films are also affected by factors such as preparation processes, film thickness, and surface morphology. Different preparation processes result in differences in the internal structure, defect states, and distribution of doping elements in the films, which in turn affect their transparent conductive performance.
3.1. Optical Properties of SnO2
Film thickness and surface morphology also affect the scattering and absorption of light, thereby altering the transparency of the films. Therefore, when preparing SnO
2 transparent conductive films, these factors need to be comprehensively considered to obtain film materials with excellent performance. To calculate the direct band gap of the samples, the following formula was used [
16]:
where α, hν, and Eg represent the absorption coefficient, incident radiation energy, and optical band gap, respectively, and A is a constant. The band gap energy can be obtained by plotting the curve of (αhν)
2 versus hν and extrapolating the linear portion of the curve to the point where the energy is zero. The absorption coefficient α can be estimated by the following equation:
where T is the optical transmittance of the film at a wavelength of 550 nm, and t is the thickness of the film. The transmission coefficient is calculated by the following formula:
where V(λ) is the luminous spectral efficiency, and T(λ) is the measured transmittance of the film system.
3.2. Electrical Properties of SnO2
Resistivity, a key indicator for evaluating the electrical conductivity of thin films, can be accurately measured by the four-probe method. Sheet resistance reflects the resistance characteristics of a thin film per unit area and is closely related to the film’s thickness and resistivity.
The resistivity
ρ of each film was calculated using the following formula:
where t denotes the thickness of the film. To evaluate the optoelectronic performance of transparent conductive films, the Haacke formula was used to determine the figure of merit (FOM), which is a combination of sheet resistance and optical transmittance [
17].
where T and R
sh represent the transmittance at 550 nm and sheet resistance of each film, respectively. To characterize the carrier transport capability of transparent conductive films, the calculation of carrier concentration and carrier mobility is also essential.
where n is the carrier concentration, q is the elementary charge, and μ is the carrier mobility. The electrical conductivity σ of a material (the reciprocal of resistivity, σ = 1/ρ) is determined by the carrier transport capability, with the conductivity formula given as:
These formulas provide strong theoretical support for an in-depth understanding and optimization of the transparent conductive properties of SnO2 thin films.
3.3. Physical Conduction Mechanism of SnO2
Native oxygen vacancy (Ov) defects exist in pristine SnO2. When oxygen atoms escape from the lattice, free electrons are generated (Ov → Ov+ + e−), endowing the film with intrinsic n-type conductivity. Doping serves as a crucial strategy to enhance the conductive performance of transparent conductive films, which enables the regulation of carrier concentration and defect structure by introducing dopants such as Sb and F. Taking Sb as an example, when Sb acts as a donor impurity, Sb3+ ions substitute for Sn4+ ions in the lattice. To maintain electrical neutrality, one free electron is released per substitution, thus significantly increasing the carrier concentration.
The electron transport process of SnO2 transparent conductive films is jointly affected by intragranular scattering and grain boundary scattering. Intragranular scattering includes ionized impurity scattering and lattice vibration scattering. An increase in doping concentration introduces more ionized impurity centers, thereby enhancing intragranular scattering. Elevated temperatures intensify lattice vibration scattering, which in turn impacts the Hall mobility. At grain boundaries, the disordered atomic arrangement and high defect density form a “potential barrier region” for electron transport. Electrons have to overcome the potential barrier when crossing grain boundaries, resulting in hindered movement, namely grain boundary scattering. This is the dominant limiting factor for carrier mobility in nanocrystalline SnO2 films.
To better explain the electrical conductivity of SnO
2, we need to introduce Matthiessen’s Rule:
In the formula, μhall refers to the Hall mobility, which we define as the total mobility. In contrast, μhall describes the total scattering intensity, namely the sum of intragranular scattering and grain boundary scattering. μopt denotes the optical mobility, where μopt is used to characterize intragranular scattering; μg.b. represents the grain boundary mobility, with 1/μg.b. specifically quantifying the effect of grain boundary scattering. The grain size increases with the rise in doping concentration, leading to a reduction in grain boundary scattering and a corresponding increase in grain boundary mobility. Although a higher doping concentration introduces more ionized impurity centers and thus enhances intragranular scattering, the negative impact is far outweighed by the positive effect of reduced grain boundary scattering.
When the carrier concentration reaches a sufficiently high level, SnO
2 enters a degenerate semiconductor state. A large number of electrons fill the empty states at the bottom of the conduction band, which shifts the Fermi level to a higher energy [
18]. This results in a slight increase in the apparent band gap observed in optical experiments, a phenomenon known as the Burstein–Moss effect [
19]. The magnitude of the Burstein–Moss effect (band gap widening ΔE
g) serves as a direct characterization of carrier concentration and band structure in the conduction mechanism:
m
eh is the reduced mass of electron–hole pairs, and N
opt is the optical carrier concentration. This effect leads to the “filling” of the conduction band, causing the motion states of electrons in the conduction band to deviate from the parabolic type (i.e., the non-parabolicity of the conduction band is enhanced), thereby affecting the effective mass of electrons.
m is the electron effective mass, while m
0 and P are the effective mass at the conduction band edge and the non-parabolicity parameter, respectively. In the physical conduction mechanism, m is the core parameter affecting carrier mobility.
τ is the electron relaxation time. It can be seen that the variation in m induced by the Burstein–Moss effect is an important contributing factor to the “competition of scattering mechanisms” in the conduction mechanism.
Hyeon Seob So et al. [
20] systematically investigated the effects of the Burstein–Moss effect and temperature on the conductive properties of SnO
2 via Sb doping. It was found that an increase in Sb doping concentration widened the optical band gap due to the Burstein–Moss effect, increased the grain size from 5.7 nm to 7.8 nm, and elevated the effective mass from 0.245 m
0 to 0.4 m
0. The carrier concentration and mobility increased from 2.6 × 10
19 cm
−3 and 1.0 cm
2/(V·s) to 2.0 × 10
20 cm
−3 and 7.2 cm
2/(V·s), respectively. The band diagrams under different temperatures and doping concentrations are shown in
Figure 2.
4. Research Progress on SnO2 Transparent Conductive Thin Films
As is well known, the fabrication of thin films is of great importance in modern micro- and nano-devices, as thin film materials can be easily assembled into complex structures to meet the requirements of different applications. For this reason, SnO2 thin films have been extensively investigated and exhibit enormous potential in the miniaturization of optoelectronic, photovoltaic, and solar cell devices. In the subsequent sections, we will review the various physical and chemical methods employed by different researchers to fabricate SnO2 thin films in the past, and discuss the relationship between these deposition methods and the optoelectronic properties of SnO2 thin films.
Due to the significant heterogeneity in preparation processes and measurement conditions, direct horizontal comparison of the optoelectronic performance data of SnO
2-based transparent conductive films across different studies is fundamentally limited. Therefore, we will introduce an additional thickness normalization term, with 100 nm as the standard thickness, and the formula is as follows:
FOM
norm is the thickness-normalized figure of merit, and t is the actual film thickness (nm), which enables the comparison of the comprehensive optoelectronic properties of films with different thicknesses. To eliminate the interference of thickness on the evaluation of conductive properties, the formula is introduced as follows:
4.1. Research Progress on SnO2 Transparent Conductive Thin Films Prepared by Magnetron Sputtering
In recent years, the mainstream physical methods for depositing SnO2 transparent conductive films include magnetron sputtering, pulsed laser deposition (PLD), and atomic layer deposition (ALD)—all of which belong to physical vapor deposition (PVD).
PVD is a technology that converts solid material sources into a gaseous phase through physical processes (rather than chemical reactions) and then deposits the vapor onto substrate surfaces to form thin films; it is one of the core technologies in the field of thin film fabrication.
Magnetron sputtering, as the most widely used thin film deposition technique within PVD, operates on the core principle of bombarding the target surface with high-energy ions to sputter target atoms/molecules, which then deposit onto the substrate surface to form thin films. Compared with conventional sputtering, it introduces a magnetic field to constrain electron movement, significantly increasing the deposition rate and reducing substrate temperature rise. It is a mainstream industrial/research method for fabricating high-quality, large-area thin films with high adhesion, and is commonly used in the preparation of transparent conductive films such as SnO2, ZnO, and TiO2.
Based on the power supply mode and target type, magnetron sputtering is mainly classified into the following types: direct current (DC) magnetron sputtering, radio frequency (RF) magnetron sputtering, pulsed magnetron sputtering, and reactive magnetron sputtering.
Compared with other methods, magnetron sputtering enables large-area (e.g., coated glass, wafer-scale) and continuous production, and the uniformity of the prepared films is far superior to that of PLD and sol–gel methods. It is the only method that can simultaneously meet the needs of laboratory research and industrial mass production. Additionally, the films exhibit strong adhesion, high density, controllable oxygen vacancy concentration, uniform distribution of doping elements, and a stable resistivity in the range of 10
−3~10
−4 Ω·cm. Wissal Belayachi et al. [
21] prepared undoped SnO
2 films at substrate temperatures ranging from 100 °C to 400 °C via RF magnetron sputtering technology, among which the films deposited at 100 °C exhibited the optimal performance. The undoped SnO
2 films showed a minimum resistivity of 4.45 × 10
−3 Ω·cm, an average visible light transmittance of 83.6%, an optical band gap of 3.86 eV, a work function of 5.02 eV, and a FOM of 5.77 × 10
−4 Ω
−1.
4.1.1. Multi-Structured SnO2 Transparent Conductive Thin Films
In 1955, Gillham et al. proposed the oxide/metal/oxide (OMO) multilayer structure, which exhibits a transmittance of >80% and a low sheet resistance [
22]. In addition, the OMO multilayer structure is thinner than traditional transparent conductive oxides, thus reducing the production cost. The sheet resistance of the OMO multilayer structure is lower than that of the single-layer structure. However, when the thickness of the metal layer increases, the transmittance decreases due to reflection and absorption, i.e., there exists a trade-off between optical transmittance and electrical conductivity [
12]. Therefore, it is crucial to determine the optimal thickness of the metal layer.
In 2020, Min-Ho Hwang et al. [
23] sputtered SnO
2/Ag-Ni/SnO
2 multilayer films with different Ni contents on quartz and p-type Si substrates using a magnetron sputtering system, where the metal layer was deposited via an RF and DC magnetron co-sputtering system. The FOM of the as-deposited SnO
2/Ag/SnO
2 multilayer structure was 14.5 × 10
3 Ω
−1, and it decreased with the increase in annealing temperature. However, the FOM of the SnO
2/Ag–Ni/SnO
2 multilayer structure increased when the annealing temperature was raised to 550 °C. The highest FOM value of 23.8 × 10
3 Ω
−1 was achieved for the SnO
2/Ag–Ni (3.2 at%)/SnO
2 multilayer structure annealed at 550 °C. The Ag-Ni (3.2 at%) multilayer films annealed at 550 °C exhibited a maximum transmittance of 83% in the wavelength range of 400–700 nm.
In 2020, Shahidi et al. [
24] fabricated SnO
2/Ag/SnO
2 multilayer structures with different Ag layer thicknesses (15 nm, 25 nm) on glass substrates using a dual-target RF/DC magnetron sputtering system. They comparatively investigated the effects of glancing angle deposition (GLAD) technology (substrate tilted at 75°) and normal deposition on the performance of the structures. The results showed that GLAD technology could significantly enhance the transmission coefficient and FOM in the visible light region. For the sample with a 15 nm Ag layer, the transmission coefficient increased from 86.40% to 88.19%, and its FOM increased from 1.38 × 10
−2 Ω
−1 to 2.46 × 10
−2 Ω
−1. The sample with a 25 nm Ag layer exhibited a minimum sheet resistance of 5.19 Ω/□.
In 2021, Wang et al. [
25] successfully prepared SnO
2/Ag/SnO
2 three-layer transparent conductive films on float glass via magnetron sputtering technology. The total thickness of the films was only 120 nm, much lower than that of commercial fluorine-doped tin oxide (FTO). These films demonstrated excellent optoelectronic properties: visible light transmittance of 85.0%, sheet resistance of 6.71 Ω/□, carrier concentration of 8.28 × 10
21 cm
−3, and resistivity of 7.38 × 10
−5 Ω·cm. After annealing optimization, single-step annealing at 200 °C yielded the optimal film performance: sheet resistance of 5.92 Ω/□, visible light transmittance of 87.0%, carrier concentration of 8.32 × 10
21 cm
−3, and mobility of 13.51 cm
2/(V·s).
4.1.2. Doped SnO2 Transparent Conductive Thin Films
Nguyen Thi Kim Chung et al. [
26] prepared Zn-doped SnO
2(ZTO) and Zn-N co-doped SnO
2 (ZNTO) films on quartz/Si substrates via direct current (DC) magnetron sputtering. Among these films, the ZNTO-8 film with 8 wt% ZnO doping exhibited the best comprehensive performance, featuring a visible light transmittance of over 85%, a minimum resistivity of 6.3 × 10
−3 Ω·cm, a hole mobility of 7.66 cm
2/(V·s), a hole concentration of 1.3 × 10
20 cm
−3, and a band gap of 3.92 eV. This study represents an emerging exploration in the field of p-type SnO
2: the synergistic effect of Zn and N is employed to suppress charge compensation, endowing the films with p-type conductive characteristics and thus providing a new strategy for breaking through the bottleneck of p-type conductivity. However, it should be clarified that this study is only an exploratory attempt for p-type SnO
2. Its technological maturity, stability, and repeatability still require further verification. There exists an essential distinction between this approach and the mature application of standard n-type SnO
2, and these two should not be regarded as equally mature technical routes for transparent conductive oxides.
Wenbo Gong et al. [
27] prepared ITO films by pulsed DC magnetron sputtering, using rotationally sintered ceramic targets of In
2O
3 doped with 3 wt% and 5 wt% Sb as raw materials, under an argon-oxygen atmosphere (O
2 content: 0%–4%) and a substrate temperature of 150 °C. The ITO film doped with 3 wt% Sb achieved a maximum Hall mobility of 32.2 cm
2/(V·s), while the film doped with 5 wt% Sb had a minimum resistivity of 4.5 × 10
−4 Ω·cm. All films exhibited an average transmittance of over 88% in the wavelength range of 400–1100 nm. B.L. Zhu et al. [
28] prepared antimony-doped tin dioxide (ATO) films at different RF powers (PRF) under two oxygen flow rates (FO
2) via magnetron sputtering, followed by rapid thermal annealing (RTA) treatment. After RTA treatment, the crystallinity of the films was significantly improved, the compressive stress was converted to tensile stress, and no significant changes were observed in the surface morphology. Meanwhile, their electrical conductivity was enhanced remarkably, and the band gap increased significantly. The ATO films exhibited a visible light transmittance of 76.35%–80.86%, a sheet resistance of 51.7–91.9 Ω/□, and a FOM of 1.03–1.67 × 10
−3 Ω
−1.
4.2. Research Progress on SnO2 Transparent Conductive Thin Films Prepared by Pulsed Laser Deposition
Pulsed Laser Deposition (PLD) is an important branch of Physical Vapor Deposition (PVD). Its core principle is to use high-energy pulsed laser beams to focus and bombard the target surface, causing the target to undergo instantaneous melting, vaporization, and even plasma formation (ablation); the plasma plume is then transported toward the substrate and deposited to form thin films. This deposition technology has been widely applied to various oxides, nitrides, and carbides, and is also used for fabricating metal systems, even polymers or fullerenes [
29].
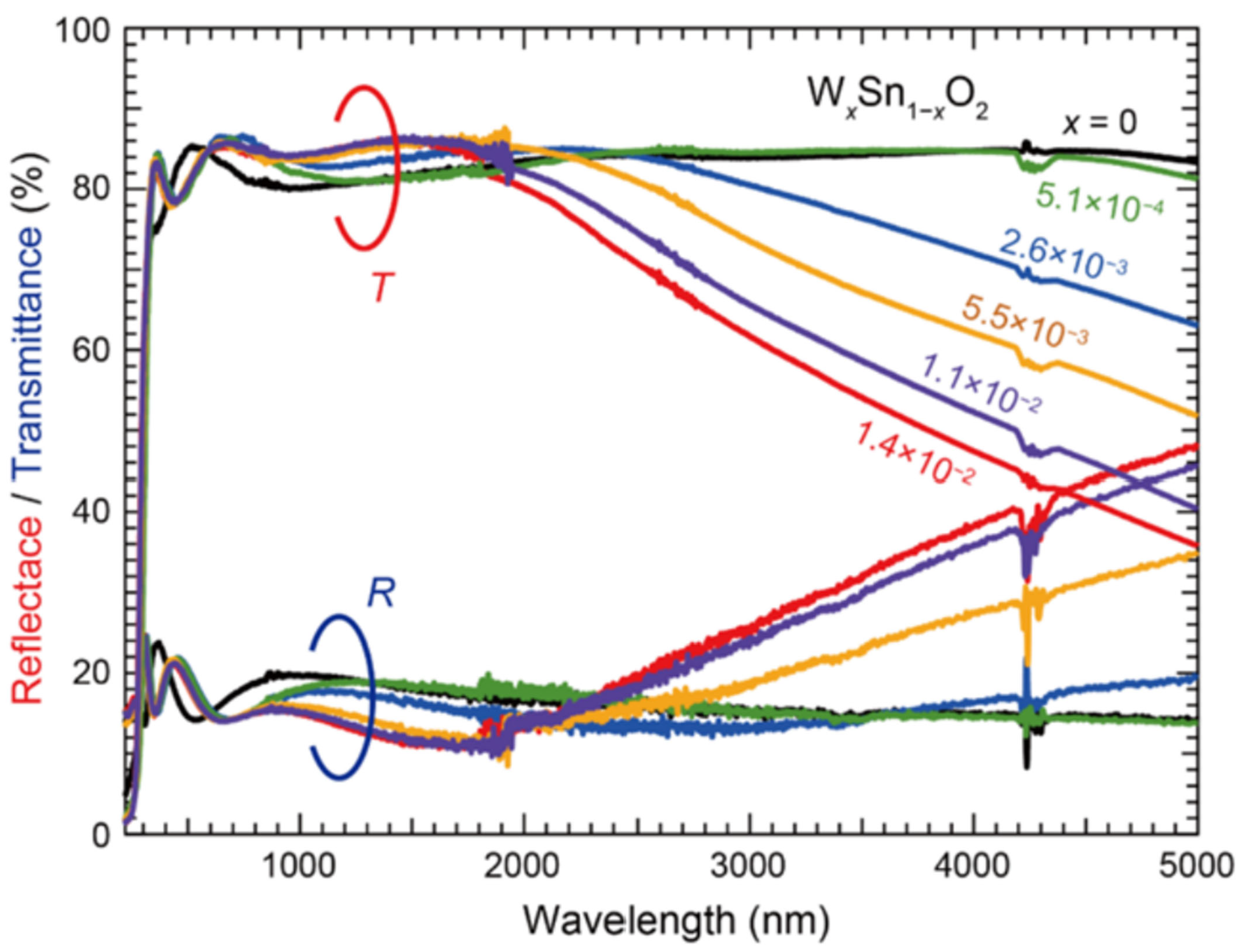
Compared with other methods for preparing SnO2 transparent conductive films, PLD technology has the advantages of precise doping element ratio, extremely high crystallinity, and a defect density from 1016 cm−3 to 1019 cm−3, making it the optimal method for investigating doping mechanisms and grain boundary effects. It can be used to fabricate SnO2 films with high-entropy doping and multilayer heterostructures, which are suitable for novel optoelectronic devices. However, its disadvantages—such as poor large-area uniformity of the films and a low deposition rate of only 0.1–1 nm/min—prevent it from being adapted for industrial continuous production.
In 2022, Michitaka Fukumoto et al. [
30] fabricated W-doped SnO
2 (WTO) epitaxial films on TiO
2 (001)/Al
2O
3(10_10) substrates via PLD technology. The optimal WTO film (W content x = 1.4 × 10
−2) exhibited a Hall mobility of 136 cm
2/(V·s), a carrier concentration of 2.1 × 10
20 cm
−3, and a sheet resistance of 13.0 Ω/□, with excellent transmittance in the wavelength range of 300–2000 nm as shown in
Figure 3.
In 2023, Harisha Kumar K et al. [
31] successfully fabricated cadmium (Cd)-doped tin oxide (Cd:SnO
2, CTO) transparent conductive films with different Cd doping concentrations on glass substrates via PLD technology, and systematically analyzed their structural, optical, and electrical properties. Among them, the film doped with 3 wt% Cd exhibited the optimal performance: it had the lowest sheet resistance (300.9 Ω/□) and resistivity (2.04 × 10
−3 Ω·cm), a transmittance of over 80% in the visible and near-infrared regions, and an optical band gap ranging from 3.40 to 3.49 eV. The film possessed a tetragonal rutile structure (with lattice parameters a = 4.742 Å and c = 3.177 Å), and with the increase in Cd doping concentration, the surface roughness decreased while the grain size increased.
4.3. Research Progress on SnO2 Transparent Conductive Thin Films Prepared by Atomic Layer Deposition
ALD is a vapor-phase thin film deposition technology based on self-limiting surface reactions. Its core principle is to alternately introduce precursors into the reaction chamber in the form of pulses, enabling precise growth of thin films through layer-by-layer, atomic/molecular-level surface chemical reactions. Between each precursor pulse, the reaction chamber is purged with an inert gas. By appropriately adjusting the experimental conditions, this process can be carried out via saturated steps. Under such conditions, the growth process is stable, and the thickness increment per deposition cycle remains constant. This self-limiting growth mechanism facilitates the growth of conformal films with precise thickness over large areas [
32]. The growth of different multilayer structures is also relatively straightforward [
33]. These advantages make the ALD method highly attractive in the microelectronics field, and it can be used to fabricate next-generation integrated circuits [
34].
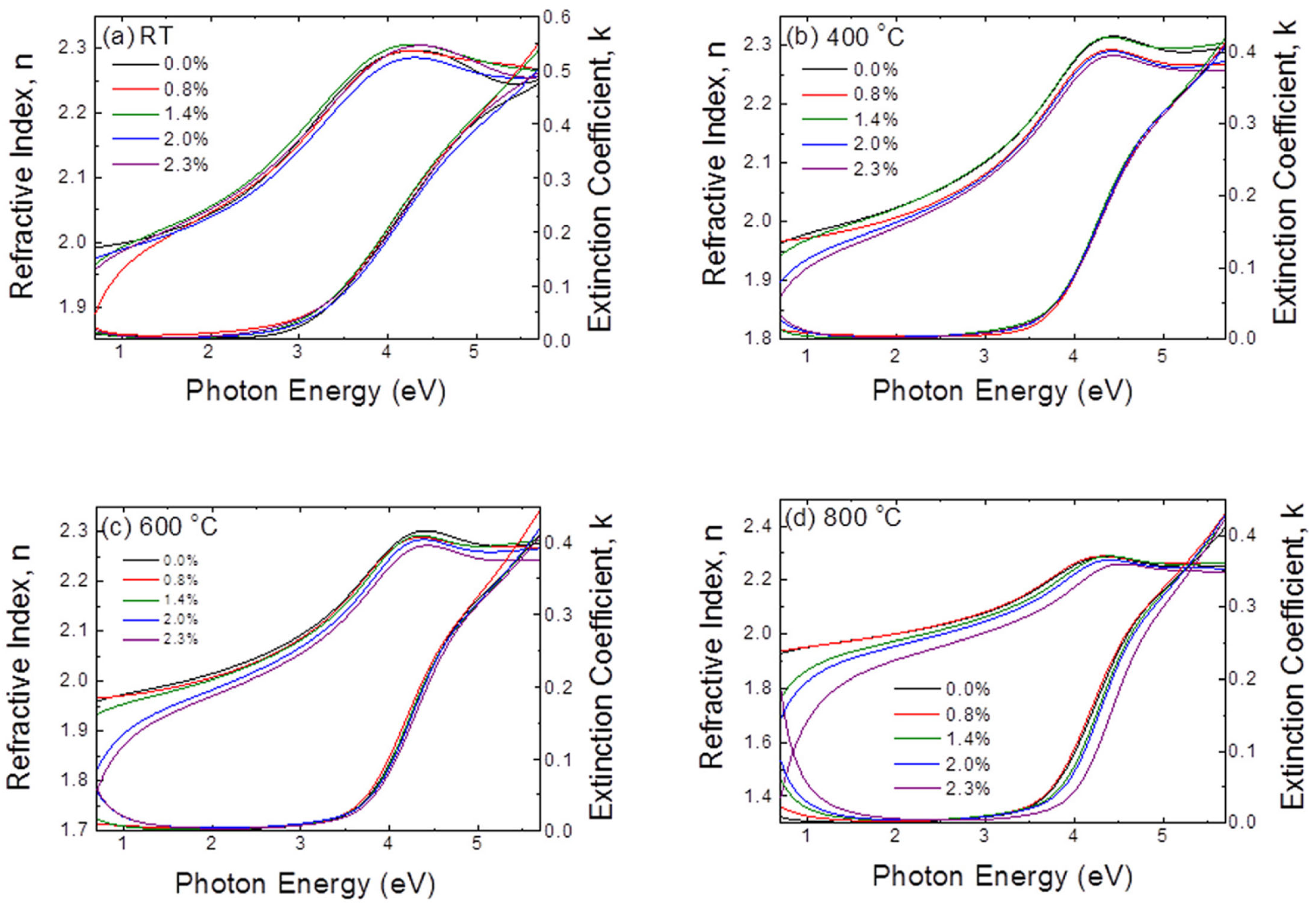
Jung-Hoon Lee et al. [
35] fabricated undoped SnO
2 transparent conductive electrodes (TCEs) via low-temperature ALD technology. At a deposition temperature of 250 °C, the SnO
2 films exhibited a sheet resistance of 98 Ω/□, a resistivity as low as 1.6 × 10
−3 Ω·cm, a carrier concentration of 7.3 × 10
20 cm
−3, and a carrier mobility of 5.3 cm
2/(V·s). The transmittance and Tauc plots of SnO
2 films at different deposition temperatures are shown in
Figure 4.
Umme Farva et al. [
36] successfully deposited low-temperature, high-quality SnO
2 films via ALD using tetrakis (dimethylamino) tin (TDMASn) and ozone plasma as precursors. With the increase in deposition temperature, the optical band gap energy of the SnO
2 films decreased from 3.4 eV to 3.1 eV. At a relatively low deposition temperature of 115 °C, the obtained films exhibited a high resistivity of 7.3 × 10
2 Ω·cm; in contrast, at a higher growth temperature of 200 °C, nearly impurity-free films with low resistivity (8.9 × 10
−4 Ω·cm) were achieved, along with a high carrier concentration (1.4 × 10
21 cm
−3) and a mobility of 12.5 cm
2/(V·s). The prepared films exhibited an optical transmittance of 81%–97% in the visible light region at all growth temperatures.
Gyeong Ryul Lee et al. [
37] prepared SnO
2−x films via thermal ALD using water (H
2O) as the reactant. For the 18 nm-thick n-type SnO
2−x films, the mobility reached 21.55 cm
2/(V·s) and the carrier concentration reached 3.3 × 10
19 cm
−3. However, the study also found that the mobility of SnO
2−x films annealed in nitrogen decreased to approximately 50% of the initial value after being placed in a humid environment for 2–3 days.
Liangge Xu et al. [
38] prepared tin oxide (SnO
x) films at 250 °C by ALD combined with layer-by-layer argon plasma treatment. After plasma treatment, the Hall mobility of the tin oxide films decreased from 18.25 cm
2/(V·s) to 12.06 cm
2/(V·s), the sheet resistance was 139.6 Ω/□, the carrier concentration increased from 5.12 × 10
20 cm
−3 to 4.425 × 10
20 cm
−3, and the resistivity was 1.117 × 10
−3 Ω·cm. The tin oxide films maintained good optical transparency over a broad wavelength range from 400 nm to 2500 nm, with a visible light transmittance of over 82.3%.
4.3.1. Multi-Structured SnO2 Transparent Conductive Films
Hyunwoo Park et al. [
39] fabricated SnO
2/Au/SnO
2 multilayer structures via ALD by depositing upper and lower SnO
2 film layers with a thickness of 20 nm each, combined with inserting a Au intermediate layer of 3~12 nm thickness via electron beam evaporation. With the increase in Au thickness, the carrier concentration increased from 1.99 × 1020 cm
−3 to 2.37 × 10
22 cm
−3, the Hall mobility increased from 1.26 cm
2/(V·s) to 11.96 cm
2/(V·s), and the resistivity decreased from 2.49 × 10
−2 Ω·cm to 2.21 × 10
−5 Ω·cm. The sample with a 6 nm Au layer achieved a transmittance of 82.7% at 550 nm.
Namgue Lee et al. [
40] applied the synergistic effect of microwave (MW) radiation and gold nanoparticles (NPs) to SnO
2 films grown via ALD. For the SnO
2 films with gold nanoparticles added during microwave radiation, their carrier concentration, Hall mobility, and resistivity were 1.45 × 10
22 cm
−3, 5.4 cm
2/(V·s), and 7.93 × 10
−5 Ω·cm, respectively. These electrical properties were significantly enhanced compared with the as-deposited SnO
2 films. The optical transmittance of SnO
2 films with Au NPs after microwave irradiation was 70.0%.
4.3.2. Doped SnO2 Transparent Conductive Films
Jangho Bae et al. [
41] doped aluminum (Al) at three different positions (bottom, middle, and top) of SnO
2 films via super-cycle ALD technology. Al doping at the middle position most significantly reduced the carrier concentration, which was as low as 1.31 × 10
20 cm
−3.
4.4. Research Progress on SnO2 Transparent Conductive Thin Films Prepared by Spray Pyrolysis
Chemical methods [
42,
43,
44,
45,
46] are low-cost and easy-to-operate routes for preparing SnO
2 transparent conductive films, which do not require vacuum equipment. Their core principle is to realize film formation through the conversion of liquid-phase precursors, with the mainstream methods including spray pyrolysis, sol–gel method, and others.
Spray pyrolysis is characterized by one-step film formation, a high deposition rate (on the order of μm/h), and the ability to prepare large-area films [
47,
48]. It does not require a vacuum, and the equipment cost is only 1/10 that of magnetron sputtering and PLD. Its core principle is to atomize a solution containing tin precursors and doping salts into microdroplets, which are sprayed onto a preheated substrate via a carrier gas (e.g., N
2); the droplets directly form SnO
2 films through solvent evaporation, thermal decomposition, and oxidation reactions [
49].G. Kiruthiga et al. [
50] prepared SnO
2 TCO films with molar concentrations ranging from 0.1 M to 0.5 M via nebulized spray pyrolysis technology at a deposition temperature of 350 ± 10 °C. After annealing some of the samples at 300 °C for 3 h, the crystallinity and grain size of the films were significantly improved (e.g., the grain size of the 0.1 M sample increased from 74.83 nm to 85.36 nm), the optical band gap increased from 3.71 eV to 3.75 eV, and the transmittance in the visible light region (400–800 nm) was approximately 70%. Taking the 0.5 M concentration as an example, the resistivity was 3.65 × 10
−3 Ω·cm, the carrier mobility was 83.65 cm
2/(V·s), and the carrier concentration was 1.91 × 10
19 cm
−3.
4.4.1. Sb Doped SnO2 Transparent Conductive Thin Films
Nandarapu Purushotham Reddy et al. [
51] prepared 5 wt% ATO films on glass substrates via a low-cost spray pyrolysis method. The ATO films maintained a tetragonal rutile phase (JCPDS 41-1445). Optically, the transmittance at 550 nm reached 80%, and the optical band gap decreased from 3.87 eV (pure SnO
2) to 3.82 eV. Electrically, the ATO films exhibited a sheet resistance as low as 12.18 Ω/□, a resistivity of 6.09 × 10
−4 Ω·cm, a carrier concentration of 6.20 × 10
20 cm
−3, a mobility of 16.49 cm
2/(V·s), and a FOM of 8.23 × 10
−3 Ω
−1.
R. Ramarajan et al. [
52] deposited highly conductive barium (Ba) and Sb co-doped SnO
2 films on glass via an economical and efficient spray pyrolysis method. The film co-doped with 2 wt% Ba and 5 wt% Sb (BATO2) exhibited a relatively high average transmittance of approximately 85% in the visible light region, with an estimated direct band gap value of 3.71 eV. The BATO2 film also showed a relatively low resistivity (4.415 × 10
−4 Ω·cm) and sheet resistance (23.14 Ω/□). The carrier concentration of the BATO2 film was 47.3 × 10
20 cm
−3, the carrier mobility was 22.20 cm
2/(V·s), and the FOM was 1.05 × 10
−2 Ω
−1. Compared with films with other doping concentrations, it possessed superior optoelectronic properties.
Md. Faruk Hossain et al. [
53] prepared pure SnO
2 and ATO films with Sb doping concentrations of 5% and 10% on glass substrates at 400 °C via low-cost nebulized spray pyrolysis. The average visible light transmittance of pure SnO
2 was over 85%, which decreased to above 73% after Sb doping. Sb doping reduced the resistivity from 1.61 × 10
−3 Ω·cm to 3.92 × 10
−4 Ω·cm, increased the carrier concentration from 8.30 × 10
19 cm
−3 to 7.02 × 10
21 cm
−3, but decreased the mobility from 54.74 cm
2/(V·s) to 2.26 cm
2/(V·s). The sheet resistance decreased from 134.41 Ω/□ to 32.73 Ω/□. Ultimately, the film doped with 5% Sb achieved the highest FOM of 1.38 × 10
−3 Ω
−1, while the FOM decreased at 10% Sb doping due to the reduction in transmittance.
D. Paul Joseph et al. [
54] prepared undoped SnO
2, 5 wt% Sb-doped SnO
2, and SnO
2 films co-doped with 5 wt% Sb and different Li concentrations (1, 3, 5 wt%) (LATO1, LATO3, LATO5) on glass substrates via spray pyrolysis, and systematically investigated the effect of Li-Sb co-doping on their properties. All films were polycrystalline tetragonal SnO
2. The LATO5 film exhibited an average transmittance of 87.9% at 550 nm and a direct optical band gap of 4.06 eV; electrically, the LATO5 film showed the optimal performance: a carrier concentration of 7.54 × 10
20 cm
−3, a mobility of 7.221 cm
2/(V·s), a resistivity as low as 1.57 × 10
−3 Ω·cm, and a sheet resistance of 32.38 Ω/□. Additionally, the FOM of the LATO5 film reached 8.56 × 10
−3 Ω
−1. Li-Sb co-doped SnO
2 films are potential alternative materials for transparent conductive electrodes.
4.4.2. F Doped SnO2 Transparent Conductive Thin Films
Roy G. Gordon [
55] systematically reviewed the selection criteria and applications of transparent conductors, with a focus on comparing the physicochemical properties of various materials. Among these, fluorine-doped SnO
2 films prepared by spray pyrolysis exhibit low production cost, high optoelectronic performance, as well as excellent chemical and mechanical durability. The films feature a sheet resistance of 8 Ω/□, a FOM of 3 Ω
−1, and a resistivity of 200 Ω·cm. This work has laid a foundation for subsequent research on SnO
2-based transparent conductive films.
Adithya Nath R et al. [
56] prepared highly a-axis-oriented FTO films on glass substrates via a low-cost spray pyrolysis method. The results showed that a molar concentration of 1.2 M (sample FM3) was the optimal doping condition, and the corresponding films exhibited the best performance: a tetragonal rutile structure, a grain size of 47.90 nm, a resistivity of 0.47 × 10
−3 Ω·cm, a carrier concentration of 71.32 × 10
19 cm
−3, a mobility of 31.06 cm
2/(V·s), and a sheet resistance of 266 Ω/□.
When preparing FTO films via ultrasonic spray pyrolysis, Myeong-Hun Jo et al. [
57] innovatively introduced NaOH as a functional additive (without the need for additional NH
4F). The chemical reaction between NaOH and HF accelerated F
− dissociation, thereby increasing the F doping concentration. The results showed that 5 vol% NaOH (5NaOH-FTO) was the optimal additive dosage. Under this condition, the FTO film exhibited an F doping concentration of 3.57 at%, a carrier concentration increased to 7.81 × 10
20 cm
−3, a Hall mobility of 27.18 cm
2/(V·s), a sheet resistance reduced to 5.3 ± 0.16 Ω/□, and a resistivity of 2.94 Ω·cm.
P. Karthick et al. [
58] prepared FTO films at a substrate temperature of 350 °C via atomized spray pyrolysis, using HF as the fluorine source and SnCl
2·2H
2O as the tin source. The F doping concentration reached 6.1 at%, the maximum visible light transmittance was 81%, the minimum room-temperature resistivity was 9.0 × 10
−4 Ω·cm, the carrier concentration was 7.34 × 10
20 cm
−3, and the Hall mobility was 11.8 cm
2/(V·s).
Indrajit Mondal et al. [
59] fabricated Al/SnO
2 hybrid transparent conductive electrodes via crack template method combined with spray pyrolysis, and obtained Al/F-SnO
2 electrodes through fluorination with Selectfluor solution. The electrode adopted an Al mesh with a line width of 10 μm as the conductive framework, and 200 nm-thick SnO
2 was spray-deposited to fill the non-conductive regions; an FTO-like surface was formed after fluorination. It exhibited excellent core properties: a transmittance of ~83% at 550 nm, a sheet resistance of 5.5 Ω/□, a resistivity of 2.8 × 10
−5 Ω·cm, and high-temperature stability up to 500 °C.
4.4.3. Other Elements Doped SnO2 Transparent Conductive Films
R. Ramarajan et al. [
60] further deposited niobium-doped tin oxide (NTO) films on large-area (10 × 10 cm
2) glass substrates via a facile chemical spray pyrolysis technique. The films achieved an average visible light transmittance of 75%, a room-temperature carrier concentration of 9.33 × 10
19 cm
−3, a mobility of 39.4 cm
2/(V·s), a resistivity of 1.69 × 10
−3 Ω·cm, and a minimum sheet resistance of 26.5 Ω/□ in the central region.
This group also [
61] prepared 4 wt% tantalum (Ta)-doped SnO
2 (TTO) films on 10 × 10 cm
2 glass substrates via chemical spray pyrolysis. The films exhibited a visible light transmittance of 81% at 550 nm and a band gap of 3.77 eV; they also showed outstanding electrical properties: a room-temperature carrier concentration of 5.42 × 10
21 cm
−3, a mobility of 41.7 cm
2/(V·s), a resistivity of 4.55 × 10
−4 Ω·cm, a sheet resistance of 26 Ω/□, and FOM of 6.15 × 10
−3 Ω
−1.
E. R. Mawarnis et al. [
62] prepared SnO
2 films with different aluminum (Al) doping concentrations (0, 3, 5, 7, 10 wt%) on glass substrates via ultrasonic spray pyrolysis, and applied them to transparent thin-film heaters (TTFH). It was found that 5 wt% Al doping was the optimal concentration, under which the films exhibited the best performance: a visible light transmittance of 87% at 550 nm, a resistivity as low as 3.54 × 10
−4 Ω·cm, and a maximum FOM of 54.5 × 10
−3 Ω
−1.
Ramarajan Ramanathan et al. [
63] prepared Ag-doped SnO
2 (ASO) films with different Ag doping concentrations (0, 0.1, 0.2, 0.3, 0.4, 0.5 wt%) via spray pyrolysis, aiming to develop indium-free n-type TCOs to replace ITO/FTO electrodes for application in dye-sensitized solar cells. It was found that 0.4 wt% (ASO4) was the optimal concentration, and the films exhibited excellent performance: a visible light transmittance of 88.7%, a band gap of 3.73 eV, a resistivity as low as 2.26 × 10
−4 Ω·cm, a sheet resistance of 18.6 Ω/□, and stable resistance at 400 °C.
4.5. Research Progress on SnO2 Transparent Conductive Thin Films Prepared by Sol–Gel Method
Sol–gel technology has been developed over the past 50 years as an alternative method for preparing glasses and ceramics at significantly lower temperatures. The initial system is a solution in which various polymerization and polycondensation processes gradually form a solid-phase network. The formed sol first undergoes a series of operations: gelation, drying, pressing, drawing, and casting, which result in various structural and phase transformations. This enables the formation of powders, fibers, coatings, bulk monolithic products, etc., from the same initial composition [
64]. Various reactions that affect the composition and structure of xerogels occur at each stage of the sol–gel process. Studies have shown that the core of the sol–gel method is to prepare a homogeneous sol from precursors and convert it into a gel. Subsequently, the solvent in the gel is removed from the gel structure, and the remaining gel is dried.
Among various synthetic methods for preparing tin oxide, the sol–gel method has numerous advantages over other methods. In addition, this method is characterized by reduced processing temperature, better uniformity, controllable stoichiometry, and ease of forming dense bulk materials, thin films, or nanoparticles [
65].
Different Elements Doped SnO2 Transparent Conductive Thin Films
Sivakumar et al. [
66] prepared pure SnO
2 and Ga-doped SnO
2 (GTO) films with 1, 3, and 5 at% Ga concentrations on glass substrates via the sol–gel spin-coating method, and systematically investigated the effect of Ga doping on their properties. Ga doping caused the visible light transmittance to decrease from 85% to 74% and the band gap to narrow from 3.92 eV to 3.68 eV. In terms of electrical properties, Ga doping increased the sheet resistance from 62.8 Ω/□ to 76.2 Ω/□ and the resistivity from 2.63 × 10
−3 Ω·cm to 3.2 × 10
−3 Ω·cm. The pure SnO
2 film exhibited the best comprehensive optoelectronic properties, with FOM of 3.3 × 10
−3 Ω
−1, while the 5 at% Ga-doped sample had the lowest FOM of 0.7 × 10
−3 Ω
−1. Although Ga doping can regulate the properties of SnO
2, pure SnO
2 is more suitable for the requirements of optoelectronic devices.
This group also [
67] prepared undoped SnO
2 films and SnO
2 films with Ti doping concentrations of 1–5 at% on glass substrates via the sol–gel spin-coating method. All films possessed a polycrystalline tetragonal rutile structure with the main orientation along the (110) direction; Ti doping enhanced the (211) orientation and reduced the grain size from 28.33 nm to 10.62 nm. The average visible light transmittance of undoped SnO
2 was over 85%, which decreased to 77% for the 5 at% Ti-doped sample, and the optical band gap decreased from 3.91 eV to 3.73 eV. When the Ti doping concentration reached 3 at%, the sheet resistance and resistivity decreased to the minimum values of 38.6 Ω/□ and 1.62 × 10
−3 Ω·cm, respectively. The 2 at% Ti-doped SnO
2 film achieved the highest FOM (3.66 × 10
−3 Ω
−1).
Harish Sharma Akkera et al. [
68] prepared pure SnO
2 and Bi-doped SnO
2 (Bi:SnO
2) TCO films with different bismuth (Bi) doping concentrations on glass substrates via the sol–gel spin-coating method. The results showed that all films exhibited a polycrystalline tetragonal rutile structure with the (110) crystal plane as the preferred orientation; with the increase in Bi doping concentration, the grain size and crystallinity decreased, while the lattice parameters and dislocation density increased. Optically, all films had an average transmittance of over 76% in the visible light region, and the optical band gap energy reached a maximum value of 4.22 eV at 3 at% Bi doping. In terms of electrical properties, the 3 at% Bi-doped film exhibited the lowest sheet resistance (38.8 Ω/□) and resistivity (1.62 × 10
−3 Ω·cm). And then, they [
69] prepared pure SnO
2 and Sm
3+:SnO
2 transparent conductive oxide films with different samarium (Sm) doping concentrations (1, 2, 3, 4, 5 at%) on glass substrates via the sol–gel spin-coating method, and systematically characterized their structural, optical, and electrical properties. With the increase in Sm doping concentration, the crystallite size decreased from 13.6 nm to 6.6 nm, the grain size reduced from 54 nm to 16 nm, and the surface roughness increased to 20.4 nm. Optically, the visible light transmittance of pure SnO
2 reached 86%, which decreased to 77% (5 at% Sm doping) after Sm doping, and the optical band gap reached a maximum value of 4.23 eV at 3 at% Sm doping. In terms of electrical properties, the 3 at% Sm-doped film exhibited the lowest sheet resistance (36.2 Ω/□) and resistivity (1.73 × 10
−3 Ω·cm), with FOM of 2.45 × 10
−3 Ω
−1.
Vedaste Uwihoreye et al. [
70] prepared a series of tantalum-doped tin dioxide films (Sn
1−xTa
xO
2, x = 0.001, 0.01, 0.02, 0.03) via a low-cost sol–gel spin-coating method. Among them, the Sn
0.98Ta
0.02O
2 film exhibited the highest conductivity of 855 S/cm and a resistivity of 1.17 × 10
−3 Ω·cm, with a carrier concentration of 2.3 × 10
20 cm
−3 and a mobility as high as 23 cm
2/(V·s). This series of films showed extremely high optical transparency of 89.5% in the visible light region. The optical band gap of the films increased from 3.96 eV (pure tin dioxide) to 4.24 eV (Sn
0.98Ta
0.02O
2 film).
Harish Sharma Akkera et al. [
71] grew pure and strontium (Sr)-doped SnO
2 films on glass substrates via sol–gel spin-coating technology. The Sr-doped SnO
2 films exhibited an optical transmittance of over 76% in the visible light region. The optical Eg of the pure SnO
2 film was 3.89 eV, while the band gap energy decreased with the increase in Sr doping concentration, with the minimum band gap of 3.78 eV obtained in the 5 at% Sr:SnO
2 film. The minimum sheet resistance of 32 Ω/□ and FOM of 3.8 × 10
−3 Ω
−1 were observed in the 3 at% Sr:SnO
2 film.
In 2025, Wang et al. [
72] proposed a novel sol–gel evaporation method to prepare ATO transparent conductive electrodes under atmospheric pressure without vacuum conditions. By optimizing the Sb doping content to 5 at%, heating temperature to 500 °C, and holding time to 30 min, the prepared ATO films exhibited a visible light transmittance of >80%, a sheet resistance as low as 12 Ω/□, a resistivity of 3.22 × 10
−4 Ω·cm, a carrier concentration of 1.79 × 10
21 cm
−3, a mobility of 10.84 cm
2/(V·s), and FOM of 1.14 × 10
−2 Ω
−1.
The
Table 2 has collected Optoelectronic characteristic parameters of SnO
2 transparent conductive films by different methods.
5. The Influence of Different Preparation Methods on the Optoelectronic Properties of SnO2 Transparent Conductive Thin Films
5.1. The Influence of Physical Methods on the Optoelectronic Properties of SnO2 Transparent Conductive Thin Films
We summarized the effects of different physical methods on the optoelectronic properties of the prepared SnO
2 transparent conductive films, as well as their typical structural characteristics, processes, and the advantages, disadvantages, and application prospects of different preparation methods. It can be seen from
Table 3 that the core breakthrough of physical methods for preparing SnO
2 transparent conductive films lies in the precise regulation of the film microstructure achievable by these methods, thereby significantly enhancing their transmittance and electrical conductivity. Via sputtering deposition, ALD, PLD, and other methods, researchers have successfully prepared SnO
2 films with high density, excellent uniformity, and controllable crystallinity.
Each physical method has its own advantages and disadvantages. Magnetron sputtering has prominent advantages: it enables large-area continuous production, achieves a balanced resistivity and transmittance, and allows flexible process regulation. However, its crystallinity is inferior to that of PLD, and high-performance systems rely on metal composite layers, resulting in a complex structure. PLD delivers top-tier crystallization quality with a defect density of <1015 cm−3, precise doping, and excellent broadband transmittance. Nevertheless, it is difficult to achieve mass production due to its low deposition rate (0.1–1 nm/min) and poor large-area uniformity. ALD features atomic-level precision and low-temperature compatibility, making it suitable for complex structures. However, it suffers from large resistivity fluctuations, poor environmental stability of some films, and low efficiency.
In the future, targeted breakthroughs are required for the three methods. For magnetron sputtering, it is feasible to develop high-FoM pure SnO2 systems without metal composite layers to simplify the structure, while optimizing the process to improve crystallinity. For PLD, technologies such as magnetic field assistance can be integrated to enhance the deposition rate and uniformity, expanding its specialized applications in high-precision optoelectronic devices. For ALD, it is necessary to optimize precursors and modification processes to reduce resistivity fluctuations and improve stability, and explore multi-chamber continuous deposition to adapt to the mass production of small and medium-sized devices. In addition, the cross-integration of the three methods (e.g., ALD for seed layer preparation + MS for thickness enhancement) may break through performance bottlenecks and promote the application of SnO2 films in more fields.
5.2. The Influence of Chemical Methods on the Optoelectronic Properties of SnO2 Transparent Conductive Thin Films
We have summarized the influence of different chemical methods on the optoelectronic properties of the prepared SnO
2 transparent conductive films, as well as the preparation processes of SnO
2 transparent conductive films, and the advantages, disadvantages and application prospects of different chemical preparation methods. The specific contents are shown in
Table 4.
Chemical methods are low-cost routes for preparing SnO2 transparent conductive films, which do not require vacuum equipment and facilitate large-scale fabrication. Spray pyrolysis features a high deposition rate and enables large-area film formation, with a minimum resistivity of 2.8 × 10−5 Ω·cm and a maximum transmittance of up to 89.68%. However, high doping levels lead to a decrease in transmittance, and the stability is insufficient under extreme conditions. The sol–gel method has the advantages of low processing temperature and excellent compositional uniformity, and can produce SnO2 products with diverse morphologies; nevertheless, it suffers from low deposition efficiency, high-concentration doping reduces film crystallinity, and the sheet resistance is generally high. Overall, although chemical methods have significant cost advantages, the consistency and stability of optoelectronic properties are still inferior to those of physical methods, and high-performance systems rely on precise doping and process regulation.
In the future, chemical methods need to focus on performance optimization and process upgrading. For spray pyrolysis, it is feasible to develop novel doping systems (e.g., multi-element co-doping) to balance the relationship between doping concentration and transmittance, while optimizing the atomization and substrate temperature processes to improve the environmental stability of films. For the sol–gel method, new precursors or composite processes (e.g., precise regulation via spin-coating + annealing) can be introduced to reduce the crystallinity loss caused by high doping, and rapid film-forming technologies can be explored to improve production efficiency. In addition, the combination of chemical and physical methods (e.g., seed layer preparation via chemical methods + thickness enhancement via physical methods) can retain the low-cost advantage while improving the electrical properties of films, which is expected to promote the large-scale application of SnO2 films in low-cost scenarios such as civilian photovoltaic and flexible optoelectronic devices.
5.3. Trade-Off of Scattering Mechanisms in the Preparation of SnO2 Transparent Conductive Films: Grain Boundary Scattering vs. Ionized Impurity Scattering
The electrical properties of SnO2 transparent conductive films depend crucially on carrier transport efficiency, while scattering mechanisms are the key factors restricting carrier mobility. In different preparation processes, there exists a significant trade-off between grain boundary scattering dominated by chemical methods (e.g., spray pyrolysis, sol–gel method) and ionized impurity scattering dominated by high-quality films prepared via physical methods (e.g., magnetron sputtering, PLD, ALD). This difference stems from the essential distinctions in film microstructure, defect states, and doping uniformity, which directly affect the optimization direction of film optoelectronic properties and their adaptability to application scenarios.
5.3.1. Essence of Grain Boundary Scattering and Ionized Impurity Scattering
Chemical methods realize film formation through the conversion of liquid-phase precursors, which tend to produce polycrystalline structures with small grain sizes and high grain boundary density. As disordered regions between grains, grain boundaries disrupt lattice periodicity and thus impede the carrier transport path. Carriers undergo elastic scattering at grain boundaries due to lattice mismatch and defect enrichment (e.g., oxygen vacancies, impurity segregation), resulting in reduced mobility. The scattering intensity is positively correlated with grain boundary density: the smaller the grain size, the more the grain boundaries, and the more pronounced the scattering effect.
Physical methods achieve direct film deposition via vapor-phase deposition, enabling precise control over film microstructure and the fabrication of high-quality films with high crystallinity and large grains, where grain boundary scattering is significantly suppressed. At this point, ionized impurities introduced by doping become the primary source of carrier scattering. Doping elements (e.g., F, Sb, W) form charged centers after ionization, exerting Coulomb attraction or repulsion on carriers and causing deflection of carrier motion trajectories. The scattering intensity is related to the concentration of ionized impurities and carriers; high doping levels enhance Coulomb interaction and thus intensify the scattering effect.
5.3.2. Trade-Off Between Mobility and Carrier Concentration
Chemical methods: Grain boundary scattering limits the improvement of mobility. To ensure conductivity, it is necessary to increase carrier concentration through high doping, but high doping further exacerbates grain boundary scattering, leading to a compromise of “low mobility–high concentration”. For instance, the 5 at% Sb-doped SnO
2 film prepared by the sol–gel method [
72] achieves a carrier concentration of 1.79 × 10
21 cm
−3, but its mobility is only 10.84 cm
2/(V·s), and it has to rely on high carrier concentration to compensate for the conductivity loss caused by low mobility.
Physical methods: Low grain boundary scattering maintains high mobility, enabling low resistivity without extremely high doping levels, and thus achieving a more optimal match between carrier concentration and mobility. For example, the SnO
2/Ag/SnO
2 multilayer film prepared by magnetron sputtering [
25] exhibits a carrier concentration of 8.32 × 10
21 cm
−3 and a mobility of 13.51 cm
2/(V·s), with a resistivity as low as 7.38 × 10
−5 Ω·cm, demonstrating the performance advantages dominated by ionized impurity scattering.
5.3.3. Trade-Off Between Process Cost and Performance Stability
Chemical methods: Although grain boundary scattering leads to relatively low mobility, these processes do not require vacuum equipment, with a cost only 1/10 of that of physical methods. Moreover, the high carrier concentration brought by high doping ensures sufficient conductivity for films applied in medium-and low-demand scenarios. However, defect enrichment at grain boundaries reduces film stability. For example, SnO2 films prepared by spray pyrolysis are prone to redox reactions at grain boundaries under high-temperature and high-humidity conditions, resulting in resistivity drift.
Physical methods: Ionized impurity scattering can be optimized by precise control of doping concentration. The films feature high crystallinity and good stability, meeting the requirements of high-precision optoelectronic devices (e.g., flexible displays, infrared detectors). Nevertheless, physical methods involve expensive equipment and low deposition rates, leading to high costs for large-scale production.
5.3.4. Synergistic Restriction Between Transmittance and Conductivity
Chemical methods: High doping increases carrier concentration but intensifies light scattering and impurity absorption at grain boundaries, resulting in decreased transmittance. For example, the transmittance of spray pyrolysis-prepared SnO
2 films doped with 5 wt% Sb decreases from 85% (pure SnO
2) to 80%; when the Sb doping level reaches 10%, the transmittance further drops to 73%, reflecting the synergistic negative effects of grain boundary scattering and light absorption [
51,
53].
Physical methods: High crystallinity reduces grain boundary light scattering, and the light absorption effect of ionized impurities is more controllable, enabling films to achieve both high transmittance and high conductivity simultaneously. For instance, the SnO
2/Ag-Ni/SnO
2 multilayer film prepared by magnetron sputtering achieves a transmittance of 83% and an FOM as high as 23.8 × 10
3 Ω
−1, which is far superior to similar films prepared by chemical methods [
23].
6. SnO2 Transparent Conductive Films in Devices
SnO
2 exhibits excellent stability in environments such as oxygen and humidity [
73], which is highly beneficial for enhancing device stability when it is employed as a charge transport layer. Caruso et al. [
74] demonstrated that compared with TiO
2 nanosheets, the device incorporating SnO
2 nanosheet electron transport layer combined with C
60 shows superior storage stability. Below, we discuss the stability of SnO
2 films prepared by different methods and with doping, as well as the performance analysis of indium-free devices and ITO-based devices.
6.1. Preparation via Physical Methods
Mengjia Li et al. [
75] fabricated high-quality SnO
2 electron transport layers (ETLs) at room temperature using electron beam (E-beam) evaporation technology, and achieved dual passivation of SnO
2/perovskite interface defects with 11-maleimidoundecanoic acid (11MA). The rigid device achieved an optimal performance of 22.08% power conversion efficiency (PCE); the unencapsulated device retained 80% of its initial efficiency after storage for 2000 h in an environment with 10%–30% relative humidity (RH). The flexible device exhibited a PCE of 15.73% with stable performance after 1000 bending cycles, and large-area devices with a size of 10–600 mm
2 were successfully prepared simultaneously.
Nulati Yesibolati et al. [
76] deposited an amorphous HfO
2 passivation layer on the surface of SnO
2/reduced graphene oxide (rGO) anodes using atomic layer deposition (ALD) technology. The anode coated with 6 ALD cycles delivered a capacity of 853 mAh/g after 100 cycles at a current density of 150 mA/g, with the initial Coulombic efficiency increased from 53.6% to 61.7% and the charge transfer resistance (Rct) significantly reduced.
Peng Sun et al. [
77] proposed an oxygen-assisted glancing angle deposition technology to fabricate oxygen-vacancy-free SnO
2 vertical nanopillar (NP) electron transport layers (ETLs) on FTO and ITO-PET substrates in one step, which were further applied in flexible perovskite solar cells (fPSCs). The large-area flexible device with an area of 1 cm
2 achieved a champion PCE of 14.9%, retaining 90% of its initial efficiency after storage for 800 h in a 75% RH environment, and only 20% efficiency attenuation after 400 manual bending cycles with a bending radius of 12 mm.
Matthew Kam et al. [
78] prepared SnO
2 electron transport layers by room-temperature RF magnetron sputtering technology, which were successfully applied in perovskite solar cells (PSCs) on rigid FTO glass substrates and flexible ITO-PEN substrates with the device structure of FTO/ITO-PEN → SnO
2 → MAPbI
3 → Spiro-OMeTAD → Au. The optimized rigid device (with 40 nm SnO
2, sputtering pressure of 0.25 Pa, and O
2:Ar ratio of 5:50) achieved a champion PCE of 12.82%, retaining 93% of its initial efficiency after 192 h storage in dry air. The flexible device exhibited a PCE of 5.88%, maintaining >90% of its initial performance after 100 bending cycles with a bending radius of 2 cm. Notably, all devices were fabricated in an air environment with >65% RH.
6.2. Preparation via Chemical Methods
Fengjiu Yang et al. [
79] prepared SnO
2 electron transport layers (ETMs) on PEN/ITO substrates by a low-temperature solution method, and successfully developed fPSCs with both high PCE and excellent bending durability. The device achieved a PCE of 17.1% and a stabilized power output (SPO) of 17.0% with a photoactive area of 0.1 cm
2; the large-area device with 1.0 cm
2 still maintained a PCE of 16.2%, retaining 76.5% of its initial PCE after 2000 bending cycles with a small bending radius of 4.0 mm.
Yuran Zhang et al. [
80] fabricated SnO
2/silver nanowire (AgNWs)/colorless polyimide (CPI) composite flexible transparent conductive films via solution processing. The core performance parameters were as follows: light transmittance over 80% at 550 nm, minimum sheet resistance of only 10.0 Ω sq
−1, and surface roughness as low as 13.3 nm. The film showed no peeling after 10 tape peeling tests, with the resistance increased by only 10% after 4000 bending cycles with a bending radius of 3 mm. When applied as the top electrode in bifacial perovskite solar cells (PSCs), it achieved a PCE of 6.34% under illumination (close to 8.72% of the ITO side). Meanwhile, it could serve as a flexible transparent heater, heating up to 85 °C within 250 s under a voltage of 6 V with good heating stability.
Lei Ning et al. [
81] doped water-soluble DAC-AA into SnO
2 colloids to prepare SnO
2 nanocrystal electron transport layers on PEN/ITO substrates. The device structure (from bottom to top) was PEN/ITO substrate → SnO
2-DAC-AA ETL → α-FAPbI
3 perovskite photoactive layer → Spiro-OMeTAD hole transport layer (HTL) → MoO
3/Ag electrode. The champion device achieved a PCE of 23.87% with an active area of 0.092 cm
2, and a PCE of 22.41% with a large area of 1 cm
2. The encapsulated device retained 91.1% of its initial efficiency after storage for 900 h under 85 °C/85% RH conditions, and maintained 92.5% of its initial performance after 10,000 bending cycles with a bending radius of 6 mm.
6.2.1. Doping Synergistic Passivation
Patrick Mwinzi Mwathe et al. [
82] prepared undoped SnO
2, FTO, and palladium-doped SnO
2 films via spray pyrolysis technology, and systematically investigated their optoelectronic properties through a post-treatment process of 450 °C air annealing combined with 450 °C nitrogen passivation. The optimal F doping concentration was 16.41 at%; annealing further improved conductivity (increased carrier mobility), while passivation slightly increased resistivity but enhanced stability. These results indicate that the combined strategy of doping + annealing + passivation can prepare SnO
2-based films with high conductivity, high transparency, and high stability.
Xing Guo et al. [
83] realized synergistic doping and passivation of SnO
2/perovskite interface defects by doping InCl
3 into SnO
2 ETLs via a two-step spin-coating method. The champion PCE was improved from 19.1% to 20.8%, with the short-circuit current density (Jsc) reaching 24.12 mA/cm
2. The unencapsulated device retained 78% of its initial PCE after storage for 960 h in a 30% RH environment, and the defect density was reduced from 7.76 × 10
14 cm
−3 to 5.46 × 10
14 cm
−3.
Fengxuan Chen et al. [
84] achieved synergistic optimization of defects by doping SnF
2 into SnO
2 ETLs. The PCE was enhanced from 21.43% to 22.65%, the fill factor (FF) increased from 80.01% to 82.12%, with the open-circuit voltage (V
oc) reaching 1.14 V and Jsc reaching 24.15 mA/cm
2. The unencapsulated device retained 90% of its initial efficiency after storage for 1000 h in a nitrogen environment.
6.2.2. Undoped Interface Passivation
Buried interface engineering is an effective method to suppress heterointerfacial defects. Organic amine salts with permeation selectivity have been used for the post-treatment of perovskites to passivate halide defects and charged defects [
85].
Xu et al. [
86] introduced reduced glutathione (GSH) small molecules on the surface of SnO
2 ETLs to achieve dual passivation of SnO
2/perovskite heterointerfacial defects. The champion device achieved a PCE of 21.66% (11.3% higher than that of the unmodified device with 19.47% PCE), and the hysteresis index was reduced from 0.087 to 0.012. The unencapsulated device retained more than 80% of its initial PCE after storage for 672 h in a 30%–40% RH environment.
Tongle Bu et al. [
87] proposed a universal potassium ion (K
+) interface passivation strategy, which treated slot-die printed SnO
2 ETLs with KOH to successfully solve the hysteresis phenomenon and improve the performance of SnO
2-based PSCs. This strategy can promote perovskite grain growth and passivate SnO
2/perovskite interface defects. The unencapsulated device only showed 5% efficiency degradation after dark storage for 30 days, with performance loss no more than 30% after 1800 bending cycles.
Ershuai Jiang et al. [
88] modified SnO
2 ETLs via passivation with phosphoric acid. When the optimized phosphoric acid doping concentration was 7.4 at%, the electron mobility of SnO
2 was increased by about 3 times, the visible light transmittance was improved, and photon loss was reduced. The perovskite solar cell based on this phosphoric acid-passivated SnO
2 ETL achieved a champion PCE of 21.02%, with Jsc of 23.20 mA/cm
2, V
oc of 1.17 V, and FF of 77.40%.
6.2.3. SnO2 Thin-Film Transistors
Won-Yong Lee et al. [
89] prepared SnO
2 thin-film transistors (TFTs) via the sol–gel method, using Y
2O
3 as the passivation layer, and systematically investigated the effect of annealing time (5/15/30 min) on device performance. The optimized SnO
2:Y
2O
3 TFTs (with 15 min annealing) exhibited excellent comprehensive performance, including a field-effect mobility of 7.59 (cm
2/(V·s), a threshold voltage (VTH) of 9.16 V, a subthreshold swing (SS) of 0.88 V/decade, an on-off current ratio of approximately 1 × 10
8, excellent bias stability, and good performance retention after storage for 90 days in air.
Changmin Lee et al. [
90] fabricated SnO
2 TFTs via the sol–gel method with Y
2O
3 as the passivation layer. The devices showed a field-effect mobility of 5.7 ± 0.3 cm
2/(V·s), a subthreshold swing of 1.1 ± 0.2 V/decade, a threshold voltage of +6.8 ± 0.4 V, an on-off current ratio of 6.1 ± 0.3 × 10
7, and excellent bias stability.
6.3. Comparison of Stability of SnO2 Films Prepared by Chemical and Physical Methods
In summary, SnO
2 films prepared by physical methods have greater advantages in environmental and mechanical stability, while chemical methods excel in process cost, device efficiency improvement, and scalability potential. Both methods enhance stability through “structure optimization/defect passivation” and are suitable for different application scenarios. The comparison of stability of SnO
2 films prepared by chemical and physical methods is shown in
Table 5.
6.4. Performance Comparison of Indium-Free Tin-Based Films/Devices vs. ITO Counterparts
Whether tin dioxide can replace ITO depends on its integration feasibility into practical devices. Dye-sensitized solar cells (DSSCs), as third-generation solar cells based on the “dye-sensitization” mechanism, offer advantages including simple structure, low fabrication cost, flexible design, and excellent low-light response. Their core principle involves light energy absorption by dye molecules to achieve charge separation and transport, converting solar energy into electrical energy. Indium-free SnO2-based materials are the core alternative electrode choice for DSSCs to realize low-cost and large-scale applications, with their significance reflected in four key dimensions: resource availability, performance, cost, and application compatibility, directly addressing the critical drawbacks of traditional ITO electrodes.
This section systematically compares indium-free tin-based films with conventional ITO films in terms of key device application performance metrics, with specific parameters shown in the
Table 6 below.
As the conventional transparent electrode for DSSCs, ITO demonstrates exceptional core photovoltaic performance and charge transport efficiency. Its open-circuit voltage Voc reaches 730 mV, short-circuit current density Jsc is 15.1 mA/cm2, fill factor (FF) is 65.0%, and the final power conversion efficiency η attains 7.15%, which is significantly superior to that of various indium-free SnO2-based devices. In terms of charge transport, ITO exhibits a series resistance Rs of only 12.94 Ω, with the photoanode-electrolyte interface resistance R1 and counter electrode-electrolyte interface resistance (R2) as low as 1.68 Ω and 1.81 Ω, respectively, enabling smooth charge transport and minimal recombination loss.
In contrast, among indium-free SnO2-based devices, the top-performing silver-doped SnO2 exhibits a slightly higher Voc (820 mV) than ITO and an Rs (12.6 Ω) close to that of ITO, but its Jsc (9.29 mA/cm2), FF (61.5%), and η (4.69%) are still markedly inferior. Furthermore, its R1 (76 Ω) and R2 (98 Ω) are much higher than those of ITO. For TTO and ATO, their Voc values are comparable to those of ITO, but their Jsc is less than 62% of ITO’s level, with Rs as high as 71.69 Ω and 60.93 Ω, respectively, and relatively high R1, resulting in power conversion efficiencies of only 3.26% and 4.05%. Undoped SnO2 lags behind across all performance parameters, with an η of merely 0.29%, which barely meets the practical application requirements of DSSCs.
In the dimensions of cost and practicality, the performance advantages of ITO are offset by its resource scarcity and high preparation cost. Indium, the key constituent of ITO, has extremely low crustal abundance and volatile market prices, coupled with complex high-vacuum fabrication processes, which restrict the large-scale application of DSSCs. In contrast, indium-free SnO2-based devices take tin—a highly abundant and widely distributed element—as the core raw material, completely eliminating the dependence on indium. Additionally, they can be deposited over large areas via low-cost processes such as spray pyrolysis and sol–gel method. Some materials (e.g., TTO) also possess favorable high-temperature stability and compatibility with flexible substrates, making them more suitable for large-scale production and diversified application scenarios. Overall, ITO still dominates in terms of performance, but indium-free SnO2-based devices have emerged as an important alternative electrode candidate for DSSCs due to their advantages in resource availability and cost-effectiveness, as well as the potential of certain performance parameters. Future optimization of doping strategies and reduction in interface resistance are expected to further narrow the performance gap with ITO.
7. Conclusions
This review systematically elaborates on the research progress of SnO2 thin films as TCOs, clarifying their core advantages and application potential in replacing ITO. SnO2 is an n-type wide-bandgap semiconductor with a bandgap of 3.6 eV at room temperature and a tetragonal rutile crystal structure, exhibiting excellent physicochemical stability. Moreover, the content of tin in the Earth’s crust is much higher than that of indium. Its optoelectronic properties mainly depend on the regulation of free electron concentration by oxygen vacancies and doping elements (e.g., F, Sb), while also being affected by the microstructure of the thin film and preparation processes.
In terms of preparation technologies, physical and chemical methods have their respective focuses: among physical methods, magnetron sputtering has become the mainstream due to its large-area continuous production capacity and balanced performance; PLD can achieve high crystallinity and precise doping; ALD offers atomic-level precision but needs improvement in stability. Chemical methods (spray pyrolysis, sol–gel method) feature low cost and no need for vacuum equipment, yet high doping tends to cause a decrease in light transmittance and damage to crystallinity, which requires improvement through multi-element co-doping and process optimization. By regulating the crystallinity and defect density of thin films, both types of methods can produce high-performance thin films with a resistivity as low as 2.8 × 10−5 Ω·cm and visible light transmittance exceeding 89%.
At the device application level, SnO2 thin films fabricated by physical methods exhibit superior environmental stability and mechanical stability, whereas those prepared via chemical methods demonstrate great potential in large-scale devices due to their low cost and solution processability. Through modification strategies including doping synergistic passivation and interface passivation, SnO2 thin films have achieved a maximum photoelectric conversion efficiency (PCE) of 23.87% in perovskite solar cells, along with excellent bending durability in flexible devices. When comparing indium-free tin-based devices with ITO-based counterparts, although ITO still holds advantages in photovoltaic performance and charge transport efficiency, indium-free SnO2-based devices have emerged as a crucial alternative owing to their resource and cost benefits. Moreover, certain parameters have shown the potential to surpass those of ITO-based devices.
Future research needs to specifically break through technical bottlenecks: physical methods should simplify the structure of magnetron-sputtered metal composite layers and improve PLD efficiency; chemical methods need to balance the relationship between doping and light transmittance; meanwhile, promote the cross integration of physical–chemical methods (e.g., chemical seed layer + physical thickening) to balance cost and performance.
First, priority should be given to establishing a unified performance reporting template and data standardization processing scheme, clarifying key parameters such as film thickness and measurement conditions to improve the comparability of data from different studies. Second, it is necessary to conduct stability tests under practical scenarios such as high temperature and high humidity, and hydrogen plasma exposure in accordance with International Electrotechnical Commission (IEC) standards, so as to establish an industry-recognized rating system. Meanwhile, efforts should be made to develop new doping systems with high mobility and low absorption, screen high-valence and small atomic radius dopants through first-principles calculations, and optimize carrier transport efficiency. In addition, research and development of scalable Atomic Layer Deposition (ALD) and Continuous Atomic Layer Deposition (C-ALD) technologies should be promoted to improve deposition rate and uniformity, meet the requirements of large-scale production, and further tap the application potential of SnO2 thin films in solar cells, flexible displays and other fields.
In summary, relying on indium-free characteristics, tunable optoelectronic properties, and mature processes, SnO2 thin films have clear prospects for large-scale applications in photovoltaic, flexible optoelectronic devices and other fields, providing key technical support for the development of next-generation transparent conductive electrodes.
Author Contributions
Conceptualization, Z.L., F.T. and J.Z.; methodology, Y.Q., Z.G. and C.Z.; writing—original draft preparation, X.L. and F.T.; writing—review and editing, Y.Y. and Y.X.; visualization, L.L. and Y.F.; supervision, X.C. and C.H.; funding acquisition, Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by specific research fund for Innovation Platform for Academicians of Hainan Province under Grant YSPTZX202513; in part by the Key Research and Development Projects in Hainan Province under Grant ZDYF2025GXJS007; in part by Hainan Normal University Graduate Students Innovative Scientific Research Project under Grant S202511658042, Grant S202511658046; Grant CXCYXJ2025015, Grant CXCYXJ2025025, Grant CXCYXJ2025036; in part by Hainan Normal University College Students’ Innovation and Entrepreneurship Open Fund (Banyan Tree Fund) Project under Grant RSXH20231165803X, Grant RSXH20231165811X, Grant RSYH20231165806X, Grant RSYH20231165824X; Grant RSYH20231165833X; in part by Hainan Province International Science and Technology Cooperation R&D Project under Grant GHYF2025030; and in part by the National Natural Science Foundation of China under Grant 62464006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable. No new data were created or analyzed in this study.
Acknowledgments
Thanks all the authors’ contribution to this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramanathan, S. (Ed.) Thin Film Metal-Oxides: Fundamentals and Applications in Electronics and Energy; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Ramarajan, R.; Joseph, D.P.; Thangaraju, K.; Kovendhan, M. Metal and Metal Oxides for Energy and Electronics; Springer: Berlin/Heidelberg, Germany, 2021; pp. 149–183. [Google Scholar]
- Guillen, C.; Herrero, J. TCO/metal/TCO structures for energy and flexible electronics. Thin Solid Film. 2011, 520, 1–17. [Google Scholar] [CrossRef]
- Liu, J.; Yi, Y.; Zhou, Y.; Cai, H. Highly stretchable and flexible graphene/ITO hybrid transparent electrode. Nanoscale Res. Lett. 2016, 11, 108–121. [Google Scholar] [CrossRef]
- Ali, A.H.; Hassan, Z.; Shuhaimi, A. Enhancement of optical transmittance and electrical resistivity of post-annealed ITO thin films RF sputtered on Si. Appl. Surf. Sci. 2018, 443, 544–547. [Google Scholar] [CrossRef]
- Park, C.Y.; Lee, J.H.; Choi, B.H. Effects of surface treatment of ITO anode layer patterned with shadow mask technology on characteristics of organic light-emitting diodes. Org. Electron. 2013, 14, 3172–3179. [Google Scholar] [CrossRef]
- Shin, Y.H.; Kang, S.B.; Lee, S.; Kim, J.J.; Kim, H.K. Near infra-red transparent Mo-doped In2O3 by hetero targets sputtering for phosphorescent organic light emitting diodes. Org. Electron. 2013, 14, 926–933. [Google Scholar] [CrossRef]
- Ham, J.; Kim, S.; Jung, G.H.; Dong, W.J.; Lee, J.L. Design of broadband transparent electrodes for flexible organic solar cells. J. Mater. Chem. A 2013, 1, 3076–3082. [Google Scholar] [CrossRef]
- Wang, H.; Rogach, A.-L. Hierarchical SnO2 nanostructures: Recent advances in design, synthesis, and applications. Chem. Mater. 2014, 26, 123–133. [Google Scholar] [CrossRef]
- Periyasamy, M.; Kar, A. Modulating the properties of SnO2 nanocrystals: Morphological effects on structural, photoluminescence, photocatalytic, electrochemical and gas sensing properties. J. Mater. Chem. C 2020, 8, 4604–4635. [Google Scholar] [CrossRef]
- Ellmer, K.; Mientus, R.; Seeger, S. Metallic Oxides (ITO, ZnO, SnO2, TiO2). In Transparent Conductive Materials, 1st ed.; Levy, D., Castellón, E., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 31–80. [Google Scholar] [CrossRef]
- Roy Gordon, J. Chemical vapor deposition of coatings on glass. J. Non-Cryst. Solids 1997, 218, 81–91. [Google Scholar] [CrossRef]
- Göpel, W.; Schierbaum, K.D. SnO2 sensors: Current status and future prospects. Sens. Actuators B Chem. 1995, 26, 1–12. [Google Scholar] [CrossRef]
- Chopra, K.L.; Major, S.; Pandya, D.K. Transparent conductors—A status review. Thin Solid Films 1983, 102, 1–46. [Google Scholar] [CrossRef]
- Jarzebski, Z.M.; Marton, J.P. Physical properties of SnO2 materials: I. preparation and defect structure. J. Electrochem. Soc. 1976, 123, 199C. [Google Scholar] [CrossRef]
- Mohamed, S. Effects of Ag layer and ZnO top layer thicknesses on the physical properties of ZnO/Ag/ZnO multilayer system. J. Phys. Chem. Solids 2008, 69, 2378–2384. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Ivchenko, V. Theory of Burstein-Moss effect in semiconductors with anisotropic energy bands. Phys. Scr. 2024, 99, 035952. [Google Scholar] [CrossRef]
- Burstein, E. Anomalous optical absorption limit in InSb. Phys. Rev. B 1954, 93, 632. [Google Scholar] [CrossRef]
- So, H.S.; Park, J.W.; Jung, D.H.; Ko, K.H.; Lee, H. Optical properties of amorphous and crystalline Sb-doped SnO2 thin films studied with spectroscopic ellipsometry: Optical gap energy and effective mass. J. Appl. Phys. 2015, 118, 085303. [Google Scholar] [CrossRef]
- Belayachi, W.; Ferblantier, G.; Fix, T.; Schmerber, G.; Rehspringer, J.L.; Heiser, T.; Slaoui, A.; Abd-Lefdil, M.; Dinia, A. SnO2 Films Elaborated by Radio Frequency Magnetron Sputtering as Potential Transparent Conducting Oxides Alternative for Organic Solar Cells. ACS Appl. Energy Mater. 2022, 5, 170–177. [Google Scholar] [CrossRef]
- Gillham, E.J.; Preston, J.S.; Williams, B.E. CXIX. A study of transparent, highly conducting gold films. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2010, 46, 1051–1068. [Google Scholar] [CrossRef]
- Hwang, M.H.; Kong, H.; Jeong, J.W.; Lee, H.Y. Thermal stability enhancement of SnO2/Ag/SnO2 transparent electrodes using Ni-doped Ag. Superlattices Microstruct. 2020, 141, 106503. [Google Scholar] [CrossRef]
- Shahidi, M.M.; Rezagholipour Dizaji, H.; Ehsani, M.H.; Ghazi, M.E. Effect of GLAD technique on optical and electrical properties of SnO2/Ag/SnO2 structure. Infrared Phys. Technol. 2020, 106, 103263. [Google Scholar] [CrossRef]
- Wang, W.; Wei, X.; Wang, C.; Zhou, W.; Zhu, B.; Wang, C.; Liu, L. Fabrication and thermo stability of the SnO2/Ag/SnO2 tri-layer transparent conductor deposited by magnetic sputtering. Ceram. Int. 2021, 47, 3548–3552. [Google Scholar] [CrossRef]
- Chung, N.T.K.; Dang, H.P.; Nguyen, T.P.; Le, T. Effects of Zn and Zn–N doping on optical, electrical, and structural properties of p-type SnO2 films. J. Photochem. Photobiol. A Chem. 2021, 418, 113436. [Google Scholar] [CrossRef]
- Gong, W.; Wang, G.; Gong, Y.; Zhao, L.; Mo, L.; Diao, H.; Tian, H.; Wang, W.; Zong, J.; Wang, W. Investigation of In2O3:SnO2 films with different doping ratio and application as transparent conducting electrode in silicon heterojunction solar cell. Sol. Energy Mater. Sol. Cells 2022, 234, 111404. [Google Scholar] [CrossRef]
- Zhu, B.L.; Cai, X.J.; Wang, C.C.; Wu, J.; Yao, J.L.; Sun, W.Q. Optimization of transparent conductive properties of magnetron sputtered Sb-doped SnO2 films by RF power: Towards application in polymer-dispersed liquid crystal (PDLC) films. Opt. Mater. 2024, 150, 115262. [Google Scholar] [CrossRef]
- Krebs, H.U.; Weisheit, M.; Faupel, J.; Süske, E.; Scharf, T.; Fuhse, C.; Störmer, M.; Sturm, K.; Seibt, M.; Kijewski, H.; et al. Pulsed laser deposition (PLD)—A versatile thin film technique. In Advances in Solid State Physics; Springer: Berlin/Heidelberg, Germany, 2003; pp. 505–518. [Google Scholar] [CrossRef]
- Fukumoto, M.; Hirose, Y.; Williamson, B.A.D.; Nakao, S.; Kimura, K.; Hayashi, K.; Sugisawa, Y.; Sekiba, D.; Scanlon, D.O.; Hasegawa, T. Ligand Field-Induced Exotic Dopant for Infrared Transparent Electrode: W in Rutile SnO2. Adv. Funct. Mater. 2022, 32, 2110832. [Google Scholar] [CrossRef]
- Kumar, K.H.; Dharmaprakash, S.M. Transparent and conductive thin films of cadmium doped tin oxide fabricated by pulsed laser deposition technique. Phys. B Condens. Matter 2023, 665, 415059. [Google Scholar] [CrossRef]
- Ritala, M.; Leskela, M.; Dekker, J.-P.; Mutsaers, C.; Soininen, P.J.; Skarp, J. Perfectly conformal TiN and Al2O3 films deposited by atomic layer deposition. Chem. Vap. Depos. 1999, 5, 7–9. [Google Scholar] [CrossRef]
- Kukli, K.; Ihanus, J.; Ritala, M.; Leskela, M. Tailoring the dielectric properties of HfO2–Ta2O5 nanolaminates. Appl. Phys. Lett. 1996, 66, 3737–3739. [Google Scholar] [CrossRef]
- Ritala, M.; Kukli, K.; Rahtu, A.; Raisanen, P.I.; Leskela, M.; Sajavaara, T.; Keinonen, J. Atomic layer deposition of oxide thin films with metal alkoxides as oxygen sources. Science 2000, 288, 319–321. [Google Scholar] [CrossRef]
- Lee, J.H.; You, Y.J.; Saeed, M.A.; Kim, S.H.; Choi, H.S.; Kim, S.; Lee, S.Y.; Park, J.S.; Shim, J.W. Undoped tin dioxide transparent electrodes for efficient and cost-effective indoor organic photovoltaics (SnO2 electrode for indoor organic photovoltaics). NPG Asia Mater. 2021, 13, 43. [Google Scholar] [CrossRef]
- Farva, U.; Kim, J. Growth temperature-dependent morphological, optical, and electrical study of SnO2 thin film by atomic layer deposition. Mater. Chem. Phys. 2021, 267, 124584. [Google Scholar] [CrossRef]
- Lee, G.R.; Seong, M.; Kim, S.; Pyeon, K.; Chung, R.B. Conductive SnO2−x thin films deposited by thermal ALD with H2O reactant. Vacuum 2022, 200, 111018. [Google Scholar] [CrossRef]
- Xu, L.; He, L.; Yang, L.; Zhang, Z.; Guo, S.; Yang, Z.; Wang, P.; Geng, F.; Gao, G.; Sun, C.; et al. Plasma treatment to tailor growth and photoelectric performance of plasma-enhanced atomic layer deposition SnOx infrared transparent conductive thin films. Surf. Coat. Technol. 2020, 403, 126414. [Google Scholar] [CrossRef]
- Park, H.; Choi, H.; Lee, N.; Jung, C.; Choi, Y.; Park, S.; Kim, B.; Yuk, H.; Choi, Y.; Kim, K. Tuning properties of SnO2/Au/SnO2 multilayer with variable Au thicknesses as transparent conductive oxides. Jpn. J. Appl. Phys. 2020, 59, 105502. [Google Scholar] [CrossRef]
- Lee, N.; Bang, J.H.; Kim, H.W.; Jeon, H. New approach to SnO2-based transparent conducting oxides incorporating synergistic effects of Au nano particles and microwave irradiation. Ceram. Int. 2021, 47, 10628–10634. [Google Scholar] [CrossRef]
- Bae, J.; Jeon, H. Characteristics of aluminum-doped SnO2 in various positions using super-cycle ALD. Nanotechnology 2025, 36, 185701. [Google Scholar] [CrossRef]
- Pires, F.I.; Joanni, E.; Savu, R.; Zaghete, M.A.; Longo, E.; Varela, J.A. Microwaveassisted hydrothermal synthesis of nanocrystalline SnO powders. Mater. Lett. 2008, 62, 239–242. [Google Scholar] [CrossRef]
- Wu, D.-S.; Han, C.-Y.; Wang, S.-Y.; Wu, N.-L.; Rusakova, I.A. Microwave-assisted solution synthesis of SnO nanocrystallites. Mater. Lett. 2002, 53, 155–159. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Pinna, N.; Kumari, K.P.; Perumal, K.; Jayaprakash, R. Microwaveassisted synthesis and characterization of tin oxide nanoparticles. Mater. Lett. 2008, 62, 3437–3440. [Google Scholar] [CrossRef]
- Wang, H.E.; Xi, L.J.; Ma, R.G.; Lu, Z.G.; Chung, C.Y.; Bello, I.; Zapien, J.A. Microwaveassisted hydrothermal synthesis of porous SnO2 nanotubes and their lithium ion storage properties. J. Solid State Chem. 2012, 190, 104–110. [Google Scholar] [CrossRef]
- Chetri, P.; Choudhury, A. Investigation of optical properties of SnO2 nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2013, 47, 257–263. [Google Scholar] [CrossRef]
- Kumar, K.D.A.; Ganesh, V.; Valanarasu, S.; Shkir, M.; Kulandaisamy, I.; Kathalingam, A.; Al Faify, S. Effect of solvent on the key properties of Al doped ZnO films prepared by nebulized spray pyrolysis technique. Mater. Chem. Phys. 2018, 212, 167–174. [Google Scholar] [CrossRef]
- Kumar, K.D.A.; Valanarasu, S.; Jeyadheepan, K.; Kim, H.S.; Vikraman, D. Evaluation of the physical, optical, and electrical properties of SnO2:F thin films prepared by nebulized spray pyrolysis for optoelectronics. J. Mater. Sci. Mater. Electron. 2018, 29, 3648–3656. [Google Scholar] [CrossRef]
- Ramarajan, R.; Kovendhan, M.; Thangarajua, K.; Josepha, D.P.; Babu, R.R. Facile deposition and characterization of large area highly conducting and transparent Sb-doped SnO2 thin film. Appl. Surf. Sci. 2019, 487, 1385–1393. [Google Scholar] [CrossRef]
- Kiruthiga, G.; Rajni, K.S.; Geethanjali, N.; Raguram, T.; Nandhakumar, E.; Senthilkumar, N. SnO2: Investigation of optical, structural, and electrical properties of transparent conductive oxide thin films prepared by nebulized spray pyrolysis for photovoltaic applications. Inorg. Chem. Commun. 2022, 145, 109968. [Google Scholar] [CrossRef]
- Reddy, N.P.; Muniramaiah, R.; Santhosh, R.; Fernandes, J.M.; Padmanaban, D.B.; Maharana, G.; Kovendhan, M.; Joseph, D.P.; Murali, B. Cost-effective Sb-doped SnO2 films as stable and efficient alternative transparent conducting electrodes for dye-sensitized solar cells. J. Mater. Chem. C 2022, 10, 7997–8008. [Google Scholar] [CrossRef]
- Ramarajan, R.; Kovendhan, M.; Thangaraju, K.; Joseph, D.P.; Babu, R.R.; Elumalai, V. Enhanced optical transparency and electrical conductivity of Ba and Sb co-doped SnO2 thin films. J. Alloys Compd. 2020, 823, 153709. [Google Scholar] [CrossRef]
- Hossain, F.; Shah, A.H.; Islam, A.; Hossain, S. Transparent conducting SnO2 thin films synthesized by nebulized spray pyrolysis technique: Impact of Sb doping on the different physical properties. Mater. Sci. Semicond. Process. 2021, 121, 105346. [Google Scholar] [CrossRef]
- Joseph, D.P.; Devarajan, U.; Fernandes, J.M.; Ramarajan, R.; Kovendhan, M.; Purushothamreddy, N.; Muniramaiah, R.; Venkateswaran, C. Lithium-antimony co-doping induced morphology transition in spray deposited SnO2 thin films. Surf. Interfaces 2021, 23, 100918. [Google Scholar] [CrossRef]
- Gordon, R.G. Criteria for Choosing Transparent Conductors. MRS Bull. 2000, 25, 52–57. [Google Scholar] [CrossRef]
- Nath, A.; Salam, J.A.; Anand, A.M.; Raj, A.; Jayakrishnan, R. Solar cells using spray coated a-axis grown F: SnO2 thin films. Opt. Mater. 2025, 159, 116612. [Google Scholar] [CrossRef]
- Jo, M.H.; Koo, B.R.; Ahn, H.J. Accelerating F-doping in transparent conducting F-doped SnO2 films for electrochromic energy storage devices. Ceram. Int. 2020, 46, 25066–25072. [Google Scholar] [CrossRef]
- Karthick, P.; Saravanakumar, K.; Sanjeeviraja, C.; Jeyadheepan, K. Realization of highly conducting and transparent SnO2 thin films by optimizing F/Sn molar ratio for electrochemical applications. Thin Solid Films 2020, 713, 138362. [Google Scholar] [CrossRef]
- Mondal, I.; Bahuguna, G.; Ganesha, M.K.; Verma, M.; Gupta, R.; Singh, A.K.; Singh, G.U. Scalable Fabrication of Scratch-Proof Transparent Al/F–SnO2 Hybrid Electrodes with Unusual Thermal and Environmental Stability. ACS Appl. Mater. Interfaces 2020, 12, 54203–54211. [Google Scholar] [CrossRef]
- Ramarajan, R.; Kovendhan, M.; Thangaraju, K.; Joseph, D.P. Indium-free large area Nb-doped SnO2 thin film as an alternative transparent conducting electrode. Ceram. Int. 2020, 46, 12224–12231. [Google Scholar] [CrossRef]
- Ramarajan, R.; Purushothamreddy, N.; Dileep, R.K.; Kovendhan, M.; Veerappan, G.; Thangaraju, K.; Joseph, D.P. Large-area spray deposited Ta-doped SnO2 thin film electrode for DSSC application. Sol. Energy 2020, 211, 547–559. [Google Scholar] [CrossRef]
- Mawarnis, E.R.; Roza, L.; Fauzia, V.; Khaira, K.; Rahman, M.Y.A. Spray pyrolyzed Al-doped SnO2 films with desirable type inversion and physical properties for use in transparent thin-film heater. J. Mater. Sci. Mater. Electron. 2024, 35, 275. [Google Scholar] [CrossRef]
- Ramanathan, R.; Kumar, A.; Rao, H.; Samudrala, R.K. Enhancement of Optoelectronic Properties of Ag-Doped SnO2 Thin Film-Based Electrodes for DSSC Application. ACS Omega 2025, 10, 52492–52506. [Google Scholar] [CrossRef] [PubMed]
- Dimitriev, Y.; Ivanova, Y.; Iordanova, R. History of sol-gel science and technology. J. Univ. Chem. Technol. Metall. 2008, 43, 181–192. [Google Scholar] [CrossRef]
- Choudhary, M.; Mishra, V.N.; Dwivedi, R. Preparation of nanosized tin oxide powder by sol-gel method. In Proceedings of the 2012 Students Conference on Engineering and Systems, Allahabad, Uttar Pradesh, India, 16–18 March 2012; pp. 1–5. [Google Scholar] [CrossRef]
- Sivakumar, P.; Akkera, H.S.; Ranjeth Kumar Reddy, T.; Srinivas Reddy, G.; Kambhala, N.; Nanda Kumar Reddy, N. Influence of Ga doping on structural, optical and electrical properties of transparent conducting SnO2 thin films. Optik 2021, 226, 165859. [Google Scholar] [CrossRef]
- Sivakumar, P.; Akkera, H.S.; Kumar Reddy, T.R.; Bitla, Y.; Ganesh, V.; Kumar, P.M.; Reddy, G.S.; Poloju, M. Effect of Ti doping on structural, optical and electrical properties of SnO2 transparent conducting thin films deposited by sol-gel spin coating. Opt. Mater. 2021, 113, 110845. [Google Scholar] [CrossRef]
- Akkera, H.S.; Sivakumar, P.; Bitla, Y.; Vanga, G.; Kambhala, N.; Naveen, N.S.; Ranjeth Kumar Reddy, T.; Reddy, G.S. Structural, electrical, and optical properties of spin-coated Bi:SnO2 transparent conducting oxide thin films. Phys. B Condens. Matter 2022, 638, 413839. [Google Scholar] [CrossRef]
- Akkera, H.S.; Kumar, Y.; Kumar, M.D.; Reddy, G.S.; Kumar, B.R.; Pasha, U.M.; Bitla, Y.; Ganesh, V. Structural, electrical, and optical properties of rare-earth Sm3+ doped SnO2 transparent conducting oxide thin films for optoelectronic device applications: Synthesized by the spin coating method. Opt. Mater. 2022, 133, 112993. [Google Scholar] [CrossRef]
- Uwihoreye, V.; Yang, Z.; Zhang, J.Y.; Lin, Y.M.; Liang, X.; Yang, L.; Zhang, K.H. Transparent conductive SnO2 thin films via resonant Ta doping. Sci. China Mater. 2023, 66, 264–271. [Google Scholar] [CrossRef]
- Akkera, H.S.; Mann, V.; Varalakshmi, B.N.; Ploloju, M.; Kambhala, N.; Venkatesh, G. Effect of Sr-doped on physical and photoluminescence properties of SnO2 transparent conducting oxide thin films. J. Mater. Sci. Mater. Electron. 2023, 34, 1044. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Q.; Li, Y.; Lu, X.; Liu, S. High-performance Sb-doped SnO2 transparent conducting electrode via novel sol-gel evaporation approach for Sb2S3 solar cells. Mater. Sci. Semicond. Process. 2025, 199, 109852. [Google Scholar] [CrossRef]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron Mobility and Injection Dynamics in Mesoporous ZnO, SnO2, and TiO2 Films Used in Dye-Sensitized Solar Cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Q.; Chen, D.; Chen, Y.B.; Caruso, R.A. Thin Films of Tin Oxide Nanosheets Used as the Electron Transporting Layer for Improved Performance and Ambient Stability of Perovskite Photovoltaics. Solar RRL 2017, 1, 1700117. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Zhang, X.; Wang, C.; Gao, D.; Han, J.; Chen, C.; Song, H.; Xu, S.; Chen, C. Highly efficient and stable perovskite solar cells based on E-beam evaporated SnO2 and rational interface defects passivation. Nano Sel. 2022, 3, 956–964. [Google Scholar] [CrossRef]
- Yesibolati, N.; Shahid, M.; Chen, W.; Hedhili, M.N.; Reuter, M.C.; Ross, F.M.; Alshareef, H.N. SnO2 Anode Surface Passivation by Atomic Layer Deposited HfO2 Improves Li-Ion Battery Performance. Small 2014, 10, 2849–2858. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Qu, G.; Hu, Q.; Ma, Y.; Liu, H.; Xu, Z.X.; Huang, Z. Highly Efficient Large-Area Flexible Perovskite Solar Cells Containing Tin Oxide Vertical Nanopillars without Oxygen Vacancies. ACS Appl. Energy Mater. 2022, 5, 3568–3577. [Google Scholar] [CrossRef]
- Kam, M.; Zhang, Q.; Zhang, D.; Fan, Z. Room-Temperature Sputtered SnO2 as Robust Electron Transport Layer for Air-Stable and Efficient Perovskite Solar Cells on Rigid and Flexible Substrates. Sci. Rep. 2019, 9, 6963. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, J.; Lim, H.E.; Ishikura, Y.; Shinokita, K.; Miyauchi, Y.; Wakamiya, A.; Murata, Y.; Matsuda, K. High Bending Durability of Efficient Flexible Perovskite Solar Cells Using Metal Oxide Electron Transport Layer. J. Phys. Chem. C 2018, 122, 17088–17095. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Xiao, X.; Xu, Y.; Xue, S. SnO2/Silver Nanowire/Polyimide Flexible Transparent Conductive Films for Double-Sided Perovskite Solar Cells and Heaters. ACS Appl. Nano Mater. 2024, 7, 20745–20754. [Google Scholar] [CrossRef]
- Ning, L.; Yao, Z.; Zha, L.; Song, L.; Du, P.; Chen, W.H.; Xiong, J. High-Oriented SnO2 Nanocrystals for Air-Processed Flexible Perovskite Solar Cells with an Efficiency of 23.87%. Adv. Mater. 2025, 37, 2418791. [Google Scholar] [CrossRef]
- Mwinzi Mwathe, P. Effect of Annealing and Surface Passivation on Doped SnO2 Thin Films Prepared by Spray Pyrolysis Technique. Adv. Mater. 2015, 4, 51–58. [Google Scholar] [CrossRef]
- Guo, X.; Du, J.; Lin, Z.; Su, J.; Feng, L.; Zhang, J.; Hao, Y.; Chang, J. Enhanced efficiency and stability of planar perovskite solar cells using SnO2:InCl3 electron transport layer through synergetic doping and passivation approaches. Chem. Eng. J. 2021, 407, 127997. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X.; Jisi, L.; Su, L.; Zhao, H.; Wei, Y.; Zhou, R.; Chen, Y.; Qu, J.; Xiong, Y.; et al. Passivating defects in SnO2 electron transport layer through SnF2 incorporation in perovskite solar cells. Mater. Today Commun. 2024, 38, 108552. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhao, L.; Yang, X.; Hou, C.-H.; Wu, J.; Su, R.; Jia, S.; Shyue, J.-J.; Luo, D.; et al. Depth-dependent defect manipulation in perovskites for high-performance solar cells. Energy Environ. Sci. 2021, 14, 6526–6535. [Google Scholar] [CrossRef]
- Xu, Y.; Rui, Y.; Wang, X.; Li, B.; Jin, Z.; Wang, Y.; An, W.; Zhang, Q. Dual passivation of SnO2/Perovskite heterogeneous interfacial defects for efficient perovskite solar cells. Sol. Energy Mater. Sol. Cells 2023, 250, 112088. [Google Scholar] [CrossRef]
- Bu, T.; Li, J.; Zheng, F.; Chen, W.; Wen, X.; Ku, Z.; Peng, Y.; Zhong, J.; Cheng, Y.B.; Huang, F. Universal passivation strategy to slot-die printed SnO2 for hysteresis-free efficient flexible perovskite solar module. Nat. Commun. 2018, 9, 4609. [Google Scholar] [CrossRef]
- Jiang, E.; Ai, Y.; Yan, J.; Li, N.; Lin, L.; Wang, Z.; Shou, C.; Yan, B.; Zeng, Y.; Sheng, J.; et al. Phosphate-Passivated SnO2 Electron Transport Layer for High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 36727–36734. [Google Scholar] [CrossRef]
- Lee, W.Y.; Kim, D.W.; Kim, H.J.; Kim, K.; Lee, S.H.; Bae, J.H.; Kang, I.M.; Kim, K.; Jang, J. Environmentally and Electrically Stable Sol–Gel-Deposited SnO2 Thin-Film Transistors with Controlled Passivation Layer Diffusion Penetration Depth That Minimizes Mobility Degradation. ACS Appl. Mater. Interfaces 2022, 14, 10558–10565. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, W.Y.; Kim, D.W.; Kim, H.J.; Bae, J.H.; Kang, I.N.; Lim, D.; Kim, K.; Jang, J. Extremely bias stress stable enhancement mode sol–gel-processed SnO2 thin-film transistors with Y2O3 passivation layers. Appl. Surf. Sci. 2021, 559, 149971. [Google Scholar] [CrossRef]
- Su, H.; Zhang, M.; Chang, Y.H.; Zhai, P.; Hau, N.Y.; Huang, Y.T.; Liu, C.; Soh, A.K.; Feng, S.P. Highly conductive and low cost Ni-PET flexible substrate for efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 5577–5584. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L.; Liska, P.; Zakeeruddin, S.M.; Gratzel, M. Optically transparent FTO-free cathode for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 22343–22350. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |