Material and Energy Flow Analysis of Hydrometallurgical Recycling for Lithium-Ion Battery Based on Aspen Plus
Abstract
1. Introduction
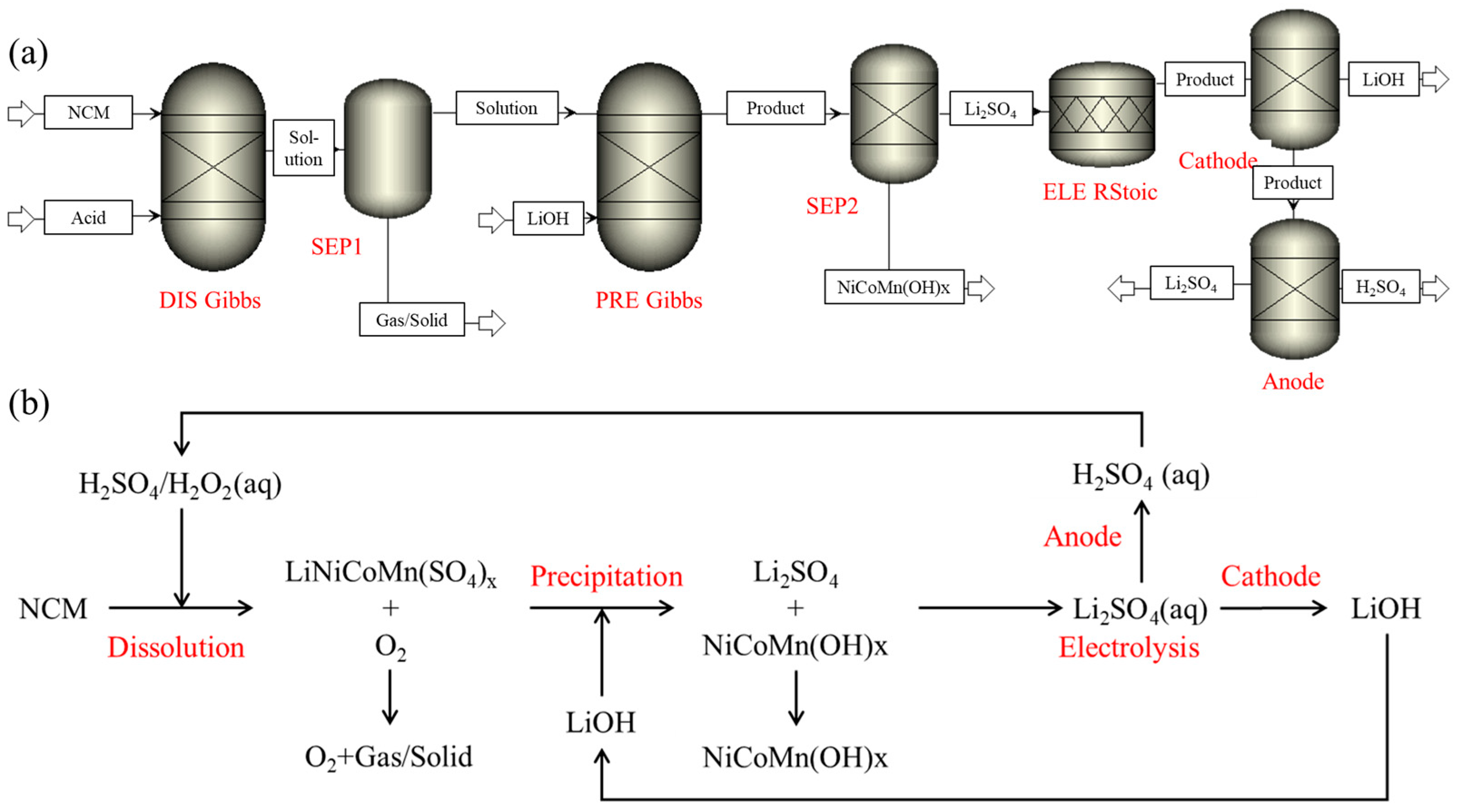
2. Methods
3. Results
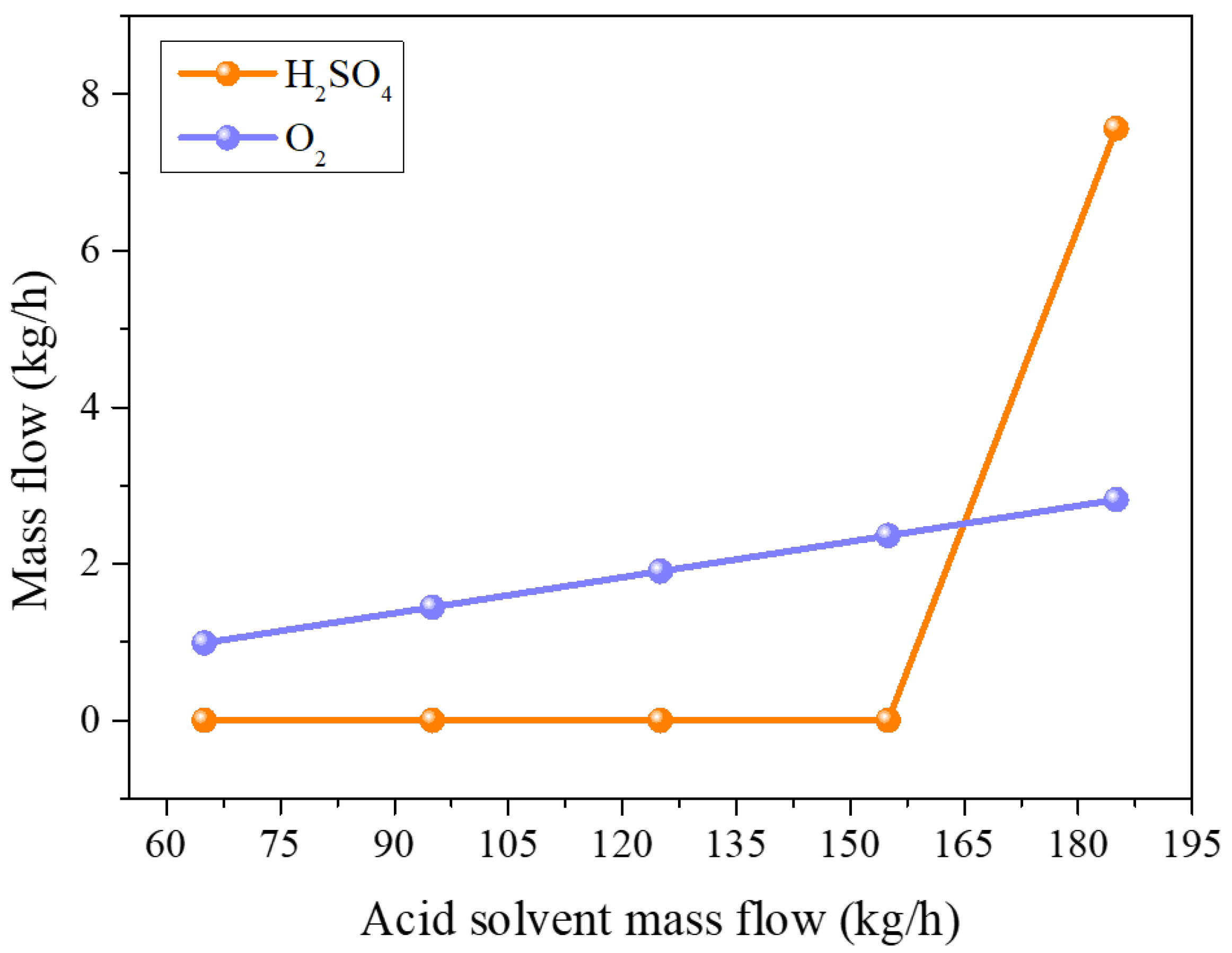
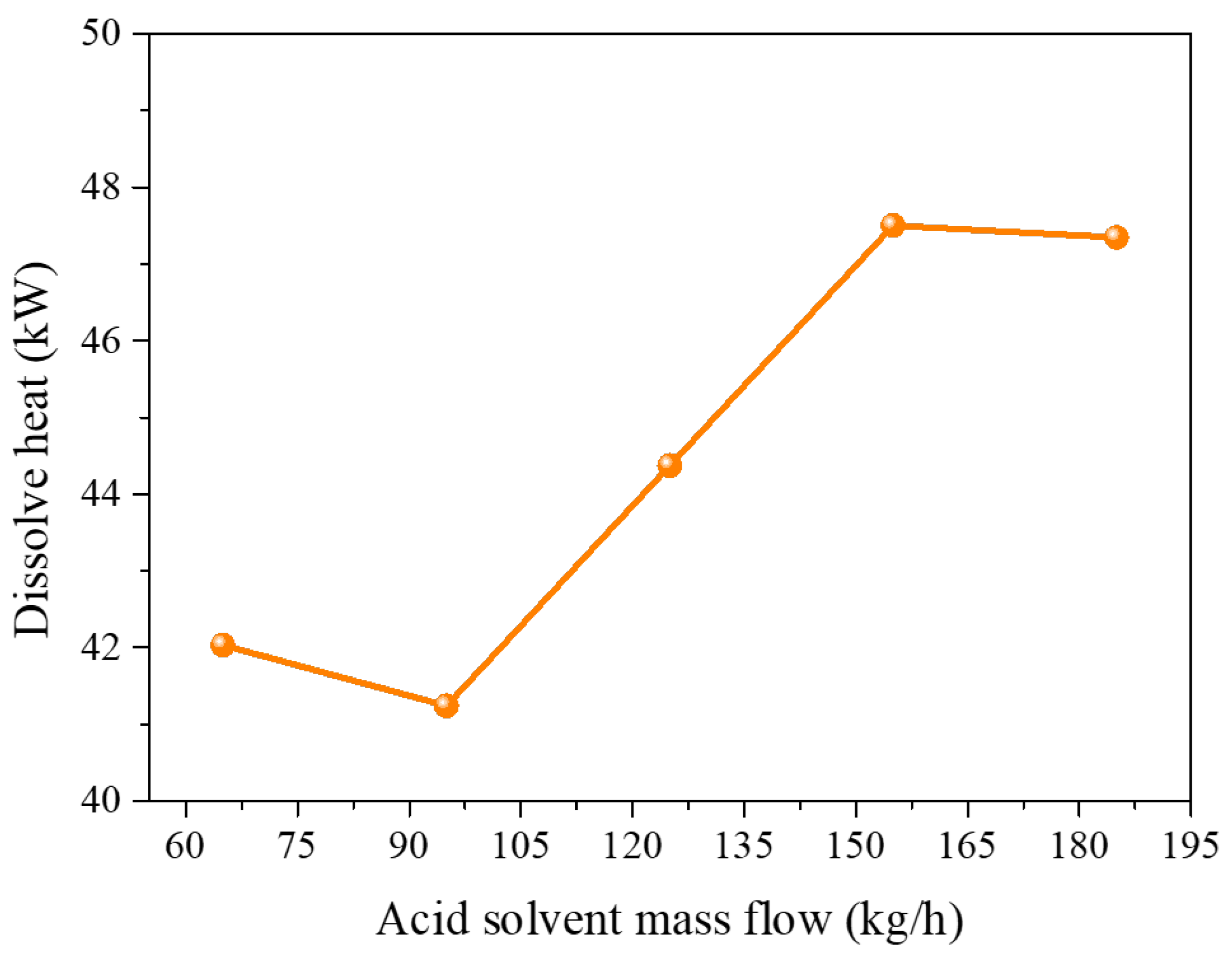
3.1. Process of Dissolution and Leaching
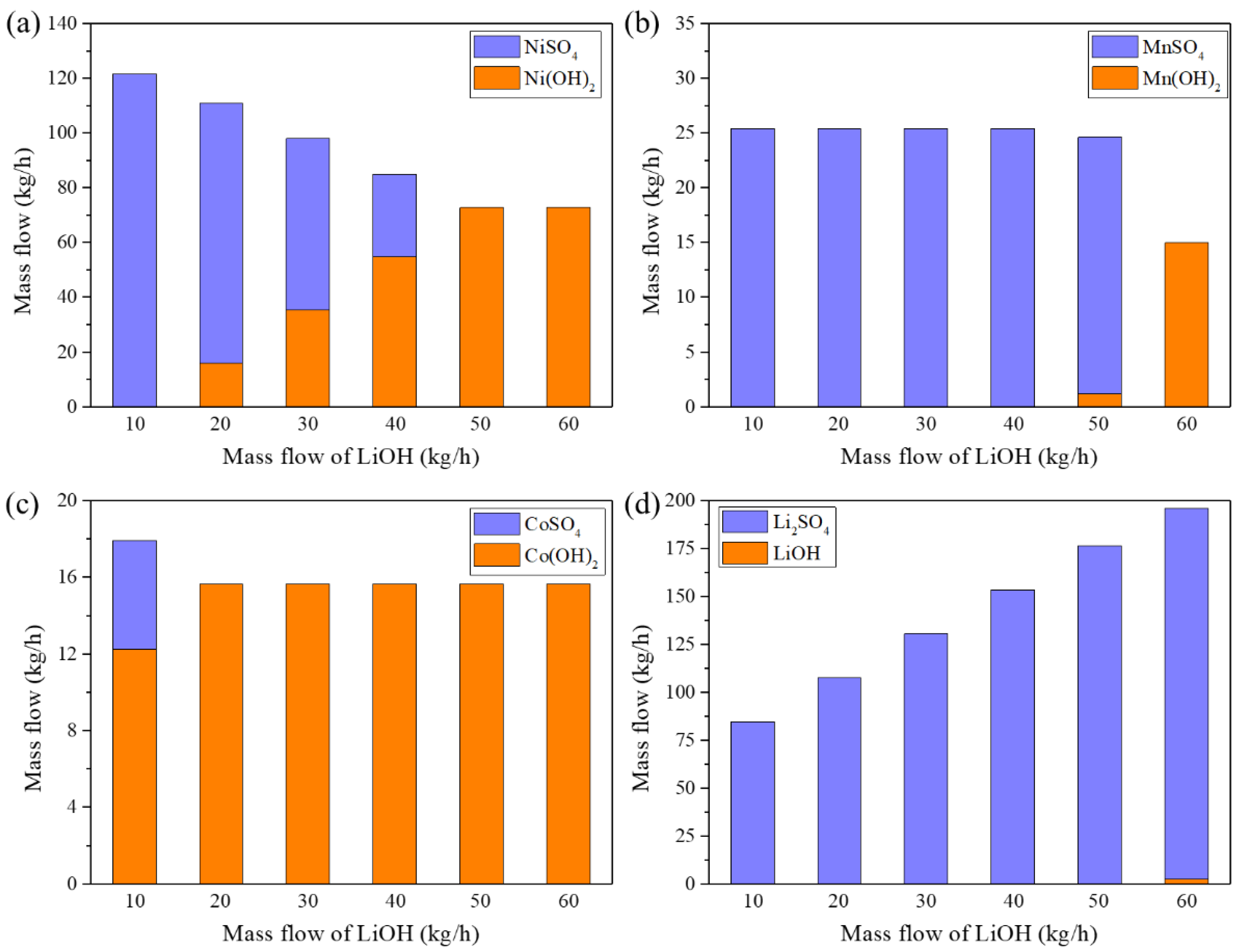
3.2. Process of Precipitation
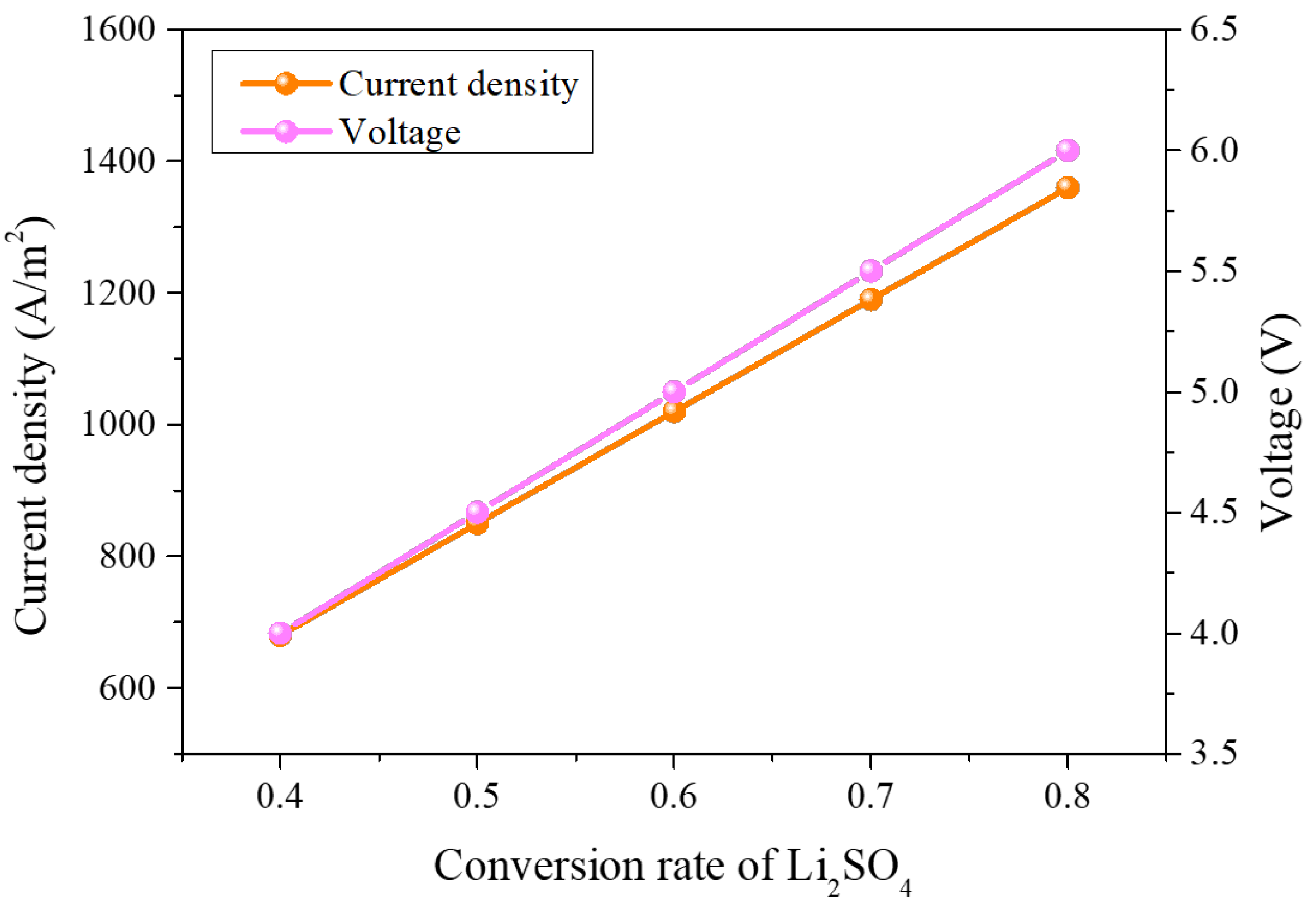
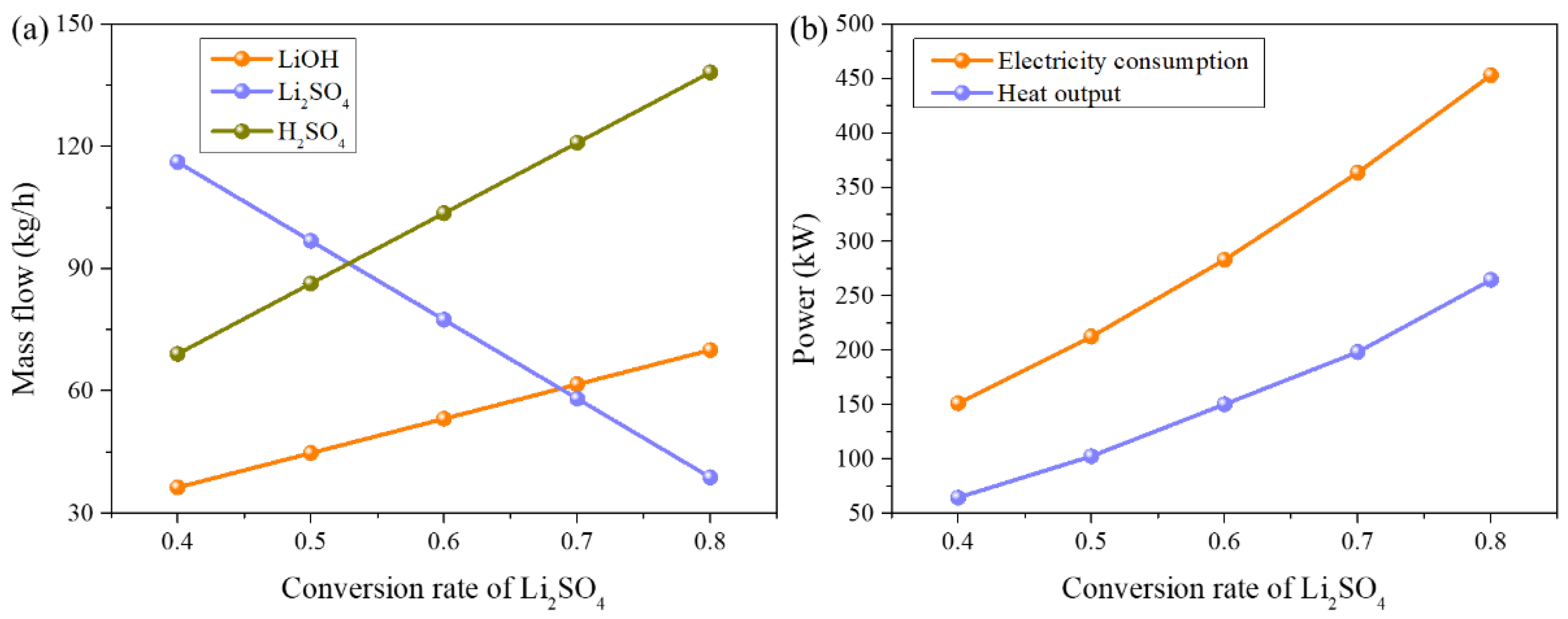
3.3. Process of Electrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasan, H.A.; Togun, H.; Mohammed, H.I.; Abed, A.M.; Homod, R.Z. CFD simulation of effect spacing between lithium-ion batteries by using flow air inside the cooling pack. J. Energy Storage 2023, 72, 108631–108644. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Lin, Y.; Xiao, J.; Xia, X.; Liu, X.; Wang, L.; He, X. Fundamentals of the recycling of spent lithium-ion batteries. Chem. Soc. Rev. 2024, 53, 11967–12013. [Google Scholar] [CrossRef]
- Arshad, F.; Lin, J.; Manurkar, N.; Fan, E.; Ahmad, A.; Tariq, M.-U.; Wu, F.; Chen, R.; Li, L. Life Cycle Assessment of Lithium-ion Batteries: A Critical Review. Resour. Conserv. Recycl. 2022, 180, 106164–106184. [Google Scholar] [CrossRef]
- Ma, X.; Meng, Z.; Bellonia, M.V.; Spangenberger, J.; Harper, G.; Gratz, E.; Olivetti, E.; Arsenault, R.; Wang, Y. The evolution of lithium-ion battery recycling. Nat. Rev. Clean Technol. 2025, 1, 75–94. [Google Scholar] [CrossRef]
- Porzio, J.; Scown, C.D. Life-Cycle Assessment Considerations for Batteries and Battery Materials. Adv. Energy Mater. 2021, 11, 2100771. [Google Scholar] [CrossRef]
- Zhang, B.; Xin, Q.; Chen, S.; Wang, B.; Li, H.; Wang, Z.; Bansal, P. Lithium-ion battery recycling relieves the threat to material scarcity amid China’s electric vehicle ambitions. Nat. Commun. 2025, 16, 6661. [Google Scholar] [CrossRef]
- van de Ven, J.J.; Teeuwisse, P.J.; Hendrikx, R.W.; Yang, Y.; Abrahami, S.T. Simultaneous Recycling of Spent LiFePO4 and LiNixMnyCozO2 Li-Ion Batteries Under Mild Leaching Conditions. J. Sustain. Metall. 2025, 11, 1982–1993. [Google Scholar] [CrossRef]
- Salces, A.M.; Kelly, N.; Streblow, G.J.; Temel, E.T.; Rudolph, M.; Chagnes, A.; Vanderbruggen, A. A contribution to understanding ion-exchange mechanisms for lithium recovery from industrial effluents of lithium-ion battery recycling operations. J. Environ. Chem. Eng. 2024, 12, 112951–112961. [Google Scholar] [CrossRef]
- Liu, M.; Pan, X.; Sun, X.; Kim, H.C.; Shen, W.; De Castro Gomez, D.; Wu, Y.; Zhang, S. Lithium-Ion Battery Recycling: Bridging Regulation Implementation and Technological Innovations for Better Battery Sustainability. Environ. Sci. Technol. 2024, 58, 21385–21388. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, F.; Zhang, S.; Xiong, Y.; Liu, Z.; Pan, X.; Lin, G.; Gomez, D.J.D.C.; He, X.; Almoniee, M.A.; et al. Carbon Footprint of Battery-Grade Lithium Chemicals in China. ACS Sustain. Chem. Eng. 2025, 13, 3930–3938. [Google Scholar] [CrossRef]
- Machala, M.L.; Chen, X.; Bunke, S.P.; Forbes, G.; Yegizbay, A.; de Chalendar, J.A.; Azevedo, I.L.; Benson, S.; Tarpeh, W.A. Life cycle comparison of industrial-scale lithium-ion battery recycling and mining supply chains. Nat. Commun. 2025, 16, 988–1001. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, M.; Bai, Z.; Li, H.; Wang, N. Advances in lithium-ion battery recycling: Strategies, pathways, and technologies. ChemPhysMater 2025, 4, 30–47. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Zhang, L.; Liu, Q.; Wang, Y.; Lin, Y.; Xu, C.; Guo, J.; Cheali, P.; Xia, X. Progress, challenges, and prospects of spent lithium-ion batteries recycling: A review. J. Energy Chem. 2024, 89, 144–171. [Google Scholar] [CrossRef]
- Kallitsis, E.; Korre, A.; Kelsall, G.H. Life cycle assessment of recycling options for automotive Li-ion battery packs. J. Clean. Prod. 2022, 371, 133636–133648. [Google Scholar] [CrossRef]
- Rezaei, M.; Nekahi, A.; MR, A.K.; Nizami, A.; Li, X.; Deng, S.; Nanda, J.; Zaghib, K. A review of lithium-ion battery recycling for enabling a circular economy. J. Power Sources 2025, 630, 236157–236178. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Kang, X.; Ge, Q. Acid-resistant membranes for efficient metal recovery from acidic wastewater in spent lithium-ion battery recycling. Chem. Eng. J. 2025, 515, 163499–163509. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Wang, W.; Chen, M.; Zheng, W.; Yang, W.; Zou, H.; Chen, S. LCA for lithium battery recycling technology-recent progress. Ionics 2024, 30, 4417–4428. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J. Life Cycle Assessment for Spent Lithium-Ion Batteries’ Recycling Process: Environmental Impact, Energy Consumption, and Sensitivity Analysis. ACS Sustain. Chem. Eng. 2024, 12, 12966–12975. [Google Scholar] [CrossRef]
- Wang, R.; Bulati, A.; Zhan, L.; Xu, Z. Complicated pollution characteristics (particulate matter, heavy metals, microplastics, VOCs) of spent lithium-ion battery recycling at an industrial level. Sci. Total Environ. 2025, 962, 178406–178413. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, X.-W.; Liu, X.; Gu, Q.; Mu, J.; Luo, W.-B. Economical and Ecofriendly Lithium-Ion Battery Recycling: Material Flow and Energy Flow. ACS Sustain. Chem. Eng. 2024, 12, 2511–2530. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Xia, Y.; Li, Q. Enhanced reducing capacity of citric acid for lithium-ion battery recycling under microwaveassisted leaching. Waste Manag. 2024, 189, 23–33. [Google Scholar] [CrossRef]
- Dai, X.; Qi, T.; Li, X.; Peng, Z.; Liu, G.; Zhou, Q.; Wang, Y.; Shen, L. High-temperature hydrothermal reduction for selective extraction of lithium from spent lithium-ion batteries. J. Ind. Eng. Chem. 2025, in press. [CrossRef]
- Wang, H.; Li, F.; Zhu, C.; Chen, C.; Ding, Z.; Chen, X. Chromatographic separation of Li, Ni, Co and Mn by functionalized fiber towards spent lithium-ion battery recycling. Chem. Eng. J. 2025, 505, 159417–159428. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Cui, D.; Yoon, S.-J.; Bae, Y.-S.; Park, B.; Wu, Y.; Zhou, F.; Pan, C.; Xiao, R. Energy and CO2 emission analysis of a Bio-Energy with CCS system: Biomass gasification-solid oxide fuel cell-mini gas turbine-CO2 capture. Fuel Process. Technol. 2022, 238, 107476–107486. [Google Scholar] [CrossRef]
- Asadi, A.; Kang, D.; Harandi, H.B.; Jung, J.C.; Sui, P.C. Utilization of lithium sulphate electrodialysis for closed-loop LIB recycling: Experimental study and process simulation. Sep. Purif. Technol. 2024, 343, 126989–127002. [Google Scholar] [CrossRef]
- Kim, J.; Moon, I.; Kim, J. Integration of wastewater electro-electrodialysis and CO2 capture for sustainable LIB recycling: Process design and economic analyses. J. Clean. Prod. 2023, 391, 136241–136249. [Google Scholar] [CrossRef]
- Chen, H.; Hu, P.; Wang, D.; Liu, Z. Selective leaching of Li from spent LiNi0.8Co0.1Mn0.1O2 cathode material by sulfation roast with NaHSO4·H2O and water leach. Hydrometallurgy 2022, 210, 105865–105874. [Google Scholar] [CrossRef]
- Porvali, A.; Shukla, S.; Lundström, M. Low-acid leaching of lithium-ion battery active materials in Fe-catalyzed Cu-H2SO4 system. Hydrometallurgy 2020, 195, 105408–105412. [Google Scholar] [CrossRef]
- Qing, J.; Wu, X.; Zeng, L.; Guan, W.; Cao, Z.; Li, Q.; Wang, M.; Zhang, G.; Wu, S. Novel approach to recycling of valuable metals from spent lithium-ion batteries using hydrometallurgy, focused on preferential extraction of lithium. J. Clean. Prod. 2023, 431, 139645–139656. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Ghassa, S.; Farzanegan, A.; Gharabaghi, M.; Abdollahi, H. Novel bioleaching of waste lithium ion batteries by mixed moderate thermophilic microorganisms, using iron scrap as energy source and reducing agent. Hydrometallurgy 2020, 197, 105465–105476. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Mussehl, V.; Piao, D. Material and Energy Flow Analysis of Hydrometallurgical Recycling for Lithium-Ion Battery Based on Aspen Plus. Coatings 2025, 15, 990. https://doi.org/10.3390/coatings15090990
Zhang Y, Mussehl V, Piao D. Material and Energy Flow Analysis of Hydrometallurgical Recycling for Lithium-Ion Battery Based on Aspen Plus. Coatings. 2025; 15(9):990. https://doi.org/10.3390/coatings15090990
Chicago/Turabian StyleZhang, Yifei, Valentin Mussehl, and Dequan Piao. 2025. "Material and Energy Flow Analysis of Hydrometallurgical Recycling for Lithium-Ion Battery Based on Aspen Plus" Coatings 15, no. 9: 990. https://doi.org/10.3390/coatings15090990
APA StyleZhang, Y., Mussehl, V., & Piao, D. (2025). Material and Energy Flow Analysis of Hydrometallurgical Recycling for Lithium-Ion Battery Based on Aspen Plus. Coatings, 15(9), 990. https://doi.org/10.3390/coatings15090990




