Hydrophobic, Durable, and Reprocessable PEDOT:PSS/PDMS-PUa/SiO2 Film with Conductive Self-Cleaning and De-Icing Functionality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
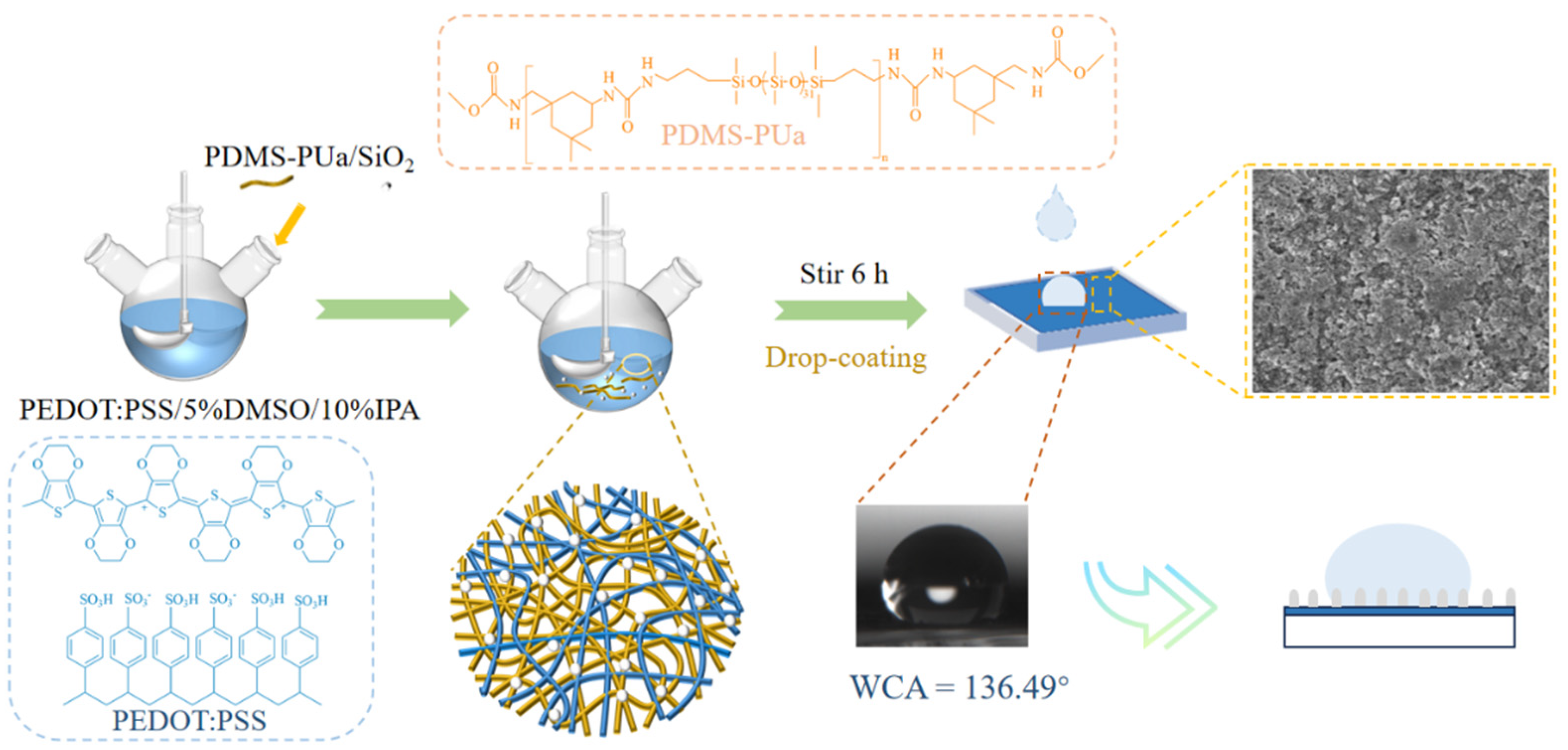
2.2. Synthesis of PDMS-PUa
2.3. Fabrication of PEDOT:PSS/PDMS-PUa/SiO2 Films
2.4. Characterization of PEDOT:PSS and PEDOT:PSS/PDMS-PUa/SiO2 Films
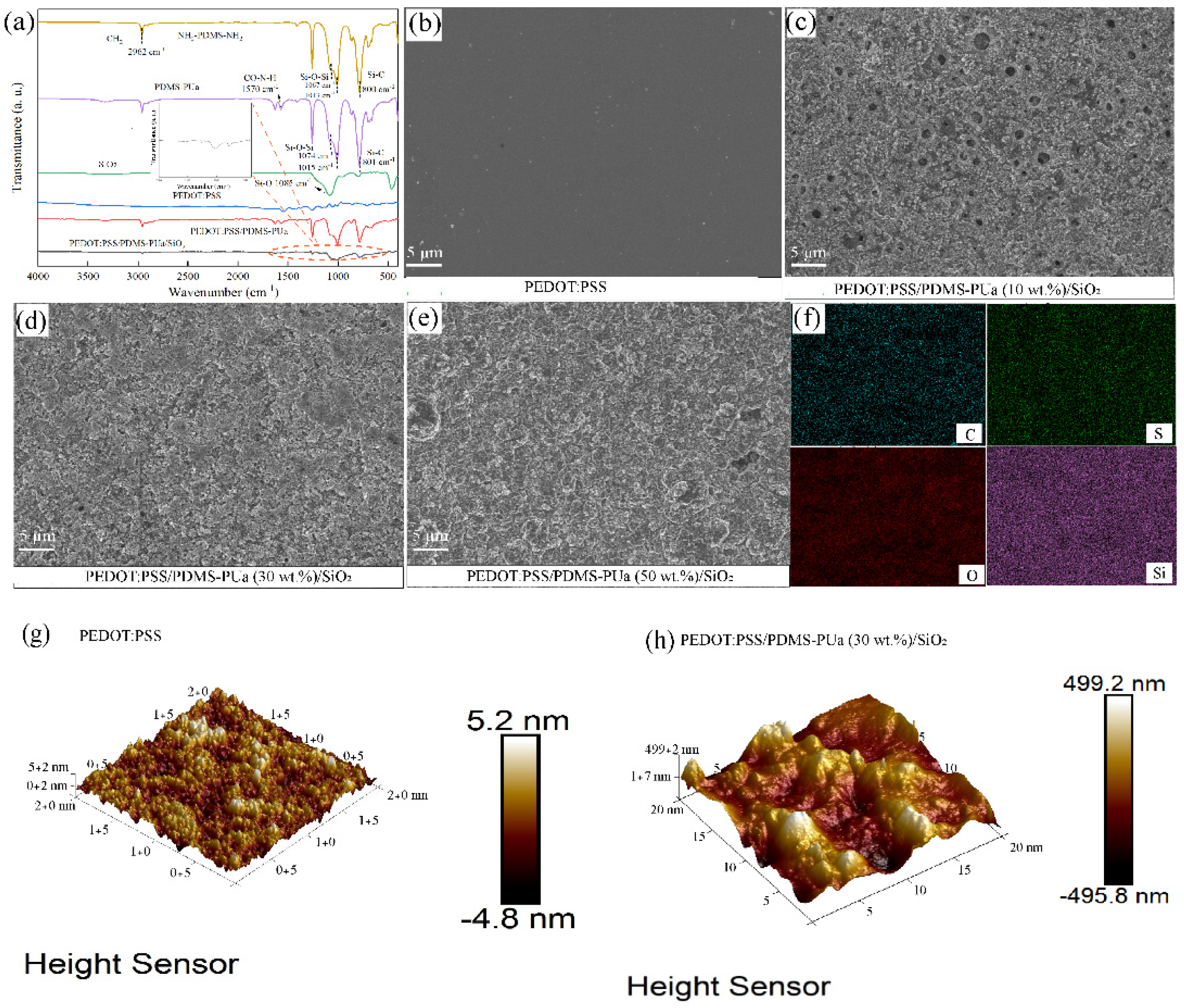
2.4.1. Structure and Morphology
2.4.2. Spectral Absorbency
2.4.3. Electrical Capability
2.4.4. Surface Wettability
2.4.5. Surface Abradability
2.4.6. Interfacial Adhesion
2.4.7. Thermostability
2.4.8. UV Aging
2.4.9. Chemical Corrosion
2.4.10. Contamination
2.4.11. Icing
2.4.12. Redispersion
3. Results and Discussion
3.1. Structure and Morphology Analysis of PEDOT:PSS/PDMS-PUa/SiO2 Films
3.2. Optoelectronic Properties of PEDOT:PSS/PDMS-PUa/SiO2 Films
3.2.1. Color and UV-Vis Absorption Spectra
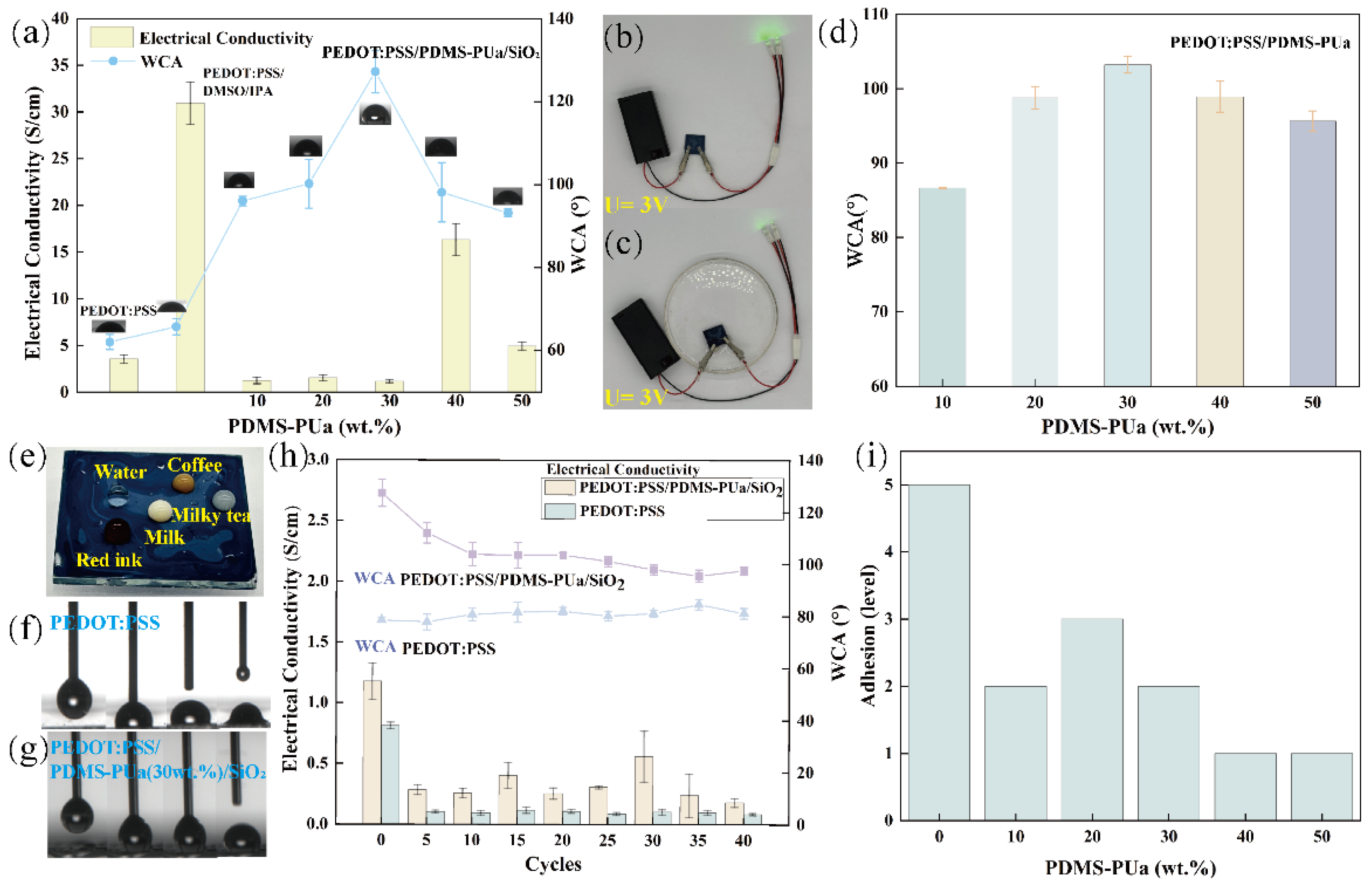
3.2.2. Surface Resistance and Electrical Conductivity
3.3. Surface-Interface Properties of PEDOT:PSS/PDMS-PUa/SiO2 Films
3.3.1. Surface Hydrophobicity
3.3.2. Surface Abradability
3.3.3. Interfacial Adhesion
3.4. Durability of the Hydrophobic PEDOT:PSS/PDMS-PUa (30 wt.%)/SiO2 Films
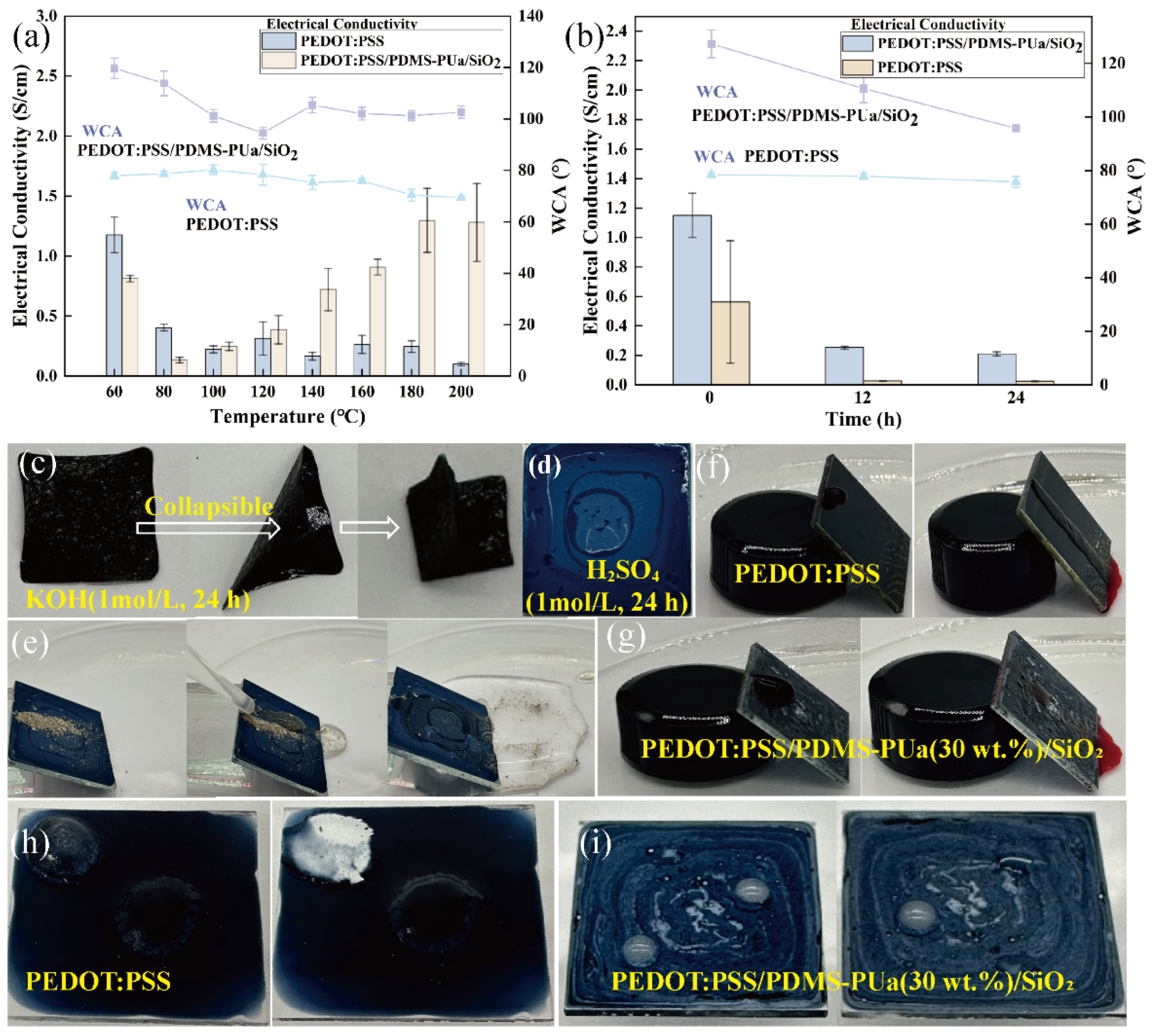
3.4.1. Thermostability
3.4.2. Anti-UV Aging Capability
3.4.3. Resistance to Acid and Alkali Corrosion
3.4.4. Self-Cleaning Capability
3.4.5. De-Icing Capability
3.5. Redisperse Processing of PEDOT:PSS/PDMS-PUa (30 wt.%)/SiO2 Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goyal, M.; Singh, K.; Bhatnagar, N. Conductive polymers: A multipurpose material for protecting coating. Prog. Org. Coat. 2024, 187, 108083. [Google Scholar] [CrossRef]
- Wei, J.F.; Liang, W.D.; Mao, M.Y.; Li, B.C.; Zhang, B.B.; Zhang, J.P. Impalement-resistant, mechanically stable, and anti-static superamphiphobic coatings enabled by solvent regulation and their application in anti-icing. Langmuir 2023, 40, 1109–1116. [Google Scholar] [CrossRef]
- Tao, B.; Cheng, L.; Wang, J.Y.; Zhang, X.L.; Liao, R.J. A review on mechanism and application of functional coatings for overhead transmission lines. Front. Mater. 2022, 9, 995290. [Google Scholar] [CrossRef]
- Dube, A.; Malode, S.J.; Alodhayb, A.N.; Mondal, K.; Shetti, N.P. Conducting polymer-based electrochemical sensors: Progress, challenges, and future perspectives. Talanta Open 2025, 11, 100395. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Aly, S.A.M.; Khan, M.Z.H. Chemical and electrochemical synthesis of doped conducting polymers and their application in supercapacitors: An overview. Chem. Eng. J. 2025, 507, 160444. [Google Scholar] [CrossRef]
- Shahid, M.A.; Rahman, M.M.; Hossain, M.T.; Hossain, I.; Sheikh, M.S.; Rahman, M.S.; Uddin, N.; Donne, S.W.; Hoque, M.I.U. Advances in conductive polymer-based flexible electronics for multifunctional applications. J. Compos. Sci. 2025, 9, 42. [Google Scholar] [CrossRef]
- Gayathri, V.; Khan, T.; Gowtham, M.; Balan, R.; Sebaey, T.A. Functionalized conductive polymer composites for tissue engineering and biomedical applications—A mini review. Front. Bioeng. Biotechnol. 2025, 13, 2025. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, S.; Zhang, Z. PEDOT and PEDOT:PSS thin-film electrodes: Patterning, modification and application in stretchable organic optoelectronic devices. J. Mater. Chem. C 2023, 11, 10435–10454. [Google Scholar] [CrossRef]
- Zheng, W.Q.; Wang, L.N.; Jiao, H.; Wu, Z.X.; Zhao, Q.; Lin, T.; Ma, H.D.; Zhang, Z.L.; Xu, X.Y.; Cao, J.; et al. A cost-effective, fast cooling, and efficient anti-inflammatory multilayered topological hydrogel patch for burn wound first aid. Chem. Eng. J. 2023, 455, 140553. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Chen, G.D.; Xue, Y.; Duan, Q.F.; Liang, X.Y.; Lin, T.; Wu, Z.X.; Tan, Y.; Zhao, Q.; Zheng, W.Q.; et al. Fatigue-resistant conducting polymer hydrogels as strain sensor for underwater robotics. Adv. Funct. Mater. 2023, 33, 2305705. [Google Scholar] [CrossRef]
- Faruk, O.; Adak, B. Recent advances in PEDOT:PSS integrated graphene and MXene-based composites for electrochemical supercapacitor applications. Synth. Met. 2023, 297, 117384. [Google Scholar] [CrossRef]
- Goyal, M.; Agarwal, S.N.; Singh, K.; Bhatnaga, N. Synthesis and characterization of poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) for energy storage device application. J. Appl. Polym. Sci. 2023, 140, e53830. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, W.S.; Hwang, J.S.; Pak, N.Y.; Choi, Y.J.; Chung, D. Study on binders for preparing antistatic films of PEDOT/PSS. Appl. Chem. Eng. 2015, 26, 458–462. [Google Scholar] [CrossRef]
- Chen, S.; Liang, L.S.; Zhang, Y.Q.; Lin, K.W.; Yang, M.N.; Zhu, L.; Yang, X.M.; Zang, L.; Lu, B.Y. PEDOT: PSS-based electronic materials: Preparation, performance tuning, processing, applications, and future prospect. Prog. Polym. Sci. 2025, 166, 101990. [Google Scholar] [CrossRef]
- Lee, I.; Park, S.; Lee, Y.S.; Kim, Y.; Kang, M.H.; Yun, C. Gradual morphological change in PEDOT:PSS thin films immersed in an aqueous solution. Langmuir 2023, 39, 1600–1610. [Google Scholar] [CrossRef]
- Si, Y.; Dong, Z.; Jiang, L. Bioinspired designs of superhydrophobic and superhydrophilic materials. ACS Cent. Sci. 2018, 4, 1102–1112. [Google Scholar] [CrossRef]
- Li, H.; Duan, Y.J.; Shao, Y.L.; Ren, L.Q.; Zhang, Z.H. Advances in organic adsorption on hydrophilic hierarchical structures for bionic superhydrophobicity–from fundamentals to applications. J. Mater. Chem. A 2024, 12, 14885–14939. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, Y.; Liu, A.; Zhu, Y.; Liu, S.; Jiang, H. Bio-inspiredly fabricating the hierarchical 3D porous structure superhydrophobic surfaces for corrosion prevention. Mater. Des. 2016, 103, 300–307. [Google Scholar] [CrossRef]
- Song, W.J.; Major, Z.; Guo, Y.Q.; Karsch, S.; Guo, H.B.; Ferenc, K.; Fukumoto, M.; Dingwell, D.B. Biomimetic super “silicate” phobicity and superhydrophobicity of ceramic material. Adv. Mater. Interfaces 2022, 9, 2201267. [Google Scholar] [CrossRef]
- Liao, X.Q.; Goh, K.L.; Liao, Y.L.; Wang, R.L.; Razaqpur, A.G. Bio-inspired super liquid-repellent membranes for membrane distillation: Mechanisms, fabrications and applications. Adv. Colloid Interface Sci. 2021, 297, 102547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Wang, D.H.; Sun, Z.N.; Song, J.N.; Deng, X. Robust superhydrophobicity: Mechanisms and strategies. Chem. Soc. Rev. 2021, 50, 4031–4061. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.L.; Li, W.; Amirfazli, A.; Yu, S. Recent advances in shape memory superhydrophobic surfaces: Concepts, mechanism, classification, applications and challenges. Polymer 2022, 256, 125193. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, J.; Li, H. Robust superhydrophobic surface with excellent corrosion resistance based on wet chemical etching method. Surf. Interfaces 2025, 64, 106363. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.S.; Xing, X.T.; Wang, N. Progress in fabrication and applications of micro/nanostructured superhydrophobic surfaces. Surf. Innov. 2021, 10, 89–110. [Google Scholar] [CrossRef]
- Ren, Z.C.; Liu, M.M.; Yan, P.C.; Hou, Y.Y.; Wu, Y.L.; Zheng, H.Y. Laser texture-electrochemical deposition composite preparation of superhydrophobic L-Al@Ni-SA with anti/de-icing property. Surf. Interfaces 2025, 69, 106778. [Google Scholar] [CrossRef]
- Chang, Y.M.; Wang, Y.S.; Chen, H.Y. Controlling superhydrophobicity on complex substrates based on a vapor-phase sublimation and deposition polymerization. ACS Appl. Mater. Interfaces 2023, 15, 48754–48763. [Google Scholar] [CrossRef]
- Li, J.Q.; Han, X.; Li, W.; Yang, L.; Li, X.; Wang, L.Q. Nature-inspired reentrant surfaces. Prog. Mater. Sci. 2023, 133, 101064. [Google Scholar] [CrossRef]
- Wan, T.; Wang, B.; Han, Q.; Chen, J.S.; Li, B.C.; Wei, S.C. A review of superhydrophobic shape-memory polymers: Preparation, activation, and applications. Appl. Mater. Today 2022, 29, 101665. [Google Scholar] [CrossRef]
- Xu, S.S.; Wang, Q.; Wang, N. Chemical fabrication strategies for achieving bioinspired superhydrophobic surfaces with micro and nanostructures: A review. Adv. Eng. Mater. 2021, 23, 2001083. [Google Scholar] [CrossRef]
- Oechsle, A.L.; Schöner, T.; Geiger, C.; Tu, S.; Wang, P.; Cubitt, R.; Müller-Buschbaum, P. Unraveling the humidity influence on the electrical properties of ionic liquid posttreated poly(3,4-ethylene dioxythiophene):poly(styrenesulfonate) films. Macromolecules 2023, 56, 9117–9126. [Google Scholar] [CrossRef]
- Zhao, B.H.; Li, Z.; Zheng, L.; Ye, Z.C.; Yuan, Y.Y.; Zhang, S.S.; Liang, B.; Li, T.Y. Recent progress in the biomedical application of PEDOT:PSS hydrogels. Chinese Chem. Lett. 2024, 35, 109810. [Google Scholar] [CrossRef]
- Fung, D.D.; Qiao, L.; Choy, W.C.; Wang, C.D.; Sha, W.E.I.; Xie, F.X.; He, S.l. Optical and electrical properties of efficiency enhanced polymer solar cells with Au nanoparticles in a PEDOT:PSS layer. J. Mater. Chem. 2011, 21, 16349–16356. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Song, Z.; Wang, Y.X.; Hu, W. PEDOT-based stretchable optoelectronic materials and devices for bioelectronic interfaces. Chem. Soc. Rev. 2024, 53, 10575. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.; Ramos, J.R.; Guzmán, D.J.; Fernández, M.D.; Fernández, M.J. PCL/POSS nanocomposites: Effect of POSS derivative and preparation method on morphology and properties. Polymer 2018, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Yu, J.R.; Gao, N.; Xie, X.W.; Chen, S.; Zhong, J.; Xu, J.K. Effects of POSS composition on PEDOT:PSS conductive film. Synth. Met. 2021, 282, 116947. [Google Scholar] [CrossRef]
- Li, Z.Q.; Xie, X.W.; Zhou, M.; Zhu, L.; Fu, C.Q.; Chen, S. High water-stable, hard and strong-adhesive antistatic films from waterborne PEDOT:PSS composites. Synth. Met. 2023, 293, 117290. [Google Scholar] [CrossRef]
- Ma, S.; Qiao, W.; Cheng, T. Optical–electrical–chemical engineering of PEDOT:PSS by incorporation of hydrophobic nafion for efficient and stable perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 3902–3911. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.Y.; Tsai, H.; Wang, N.X.; Huang, H.H.; Xia, Y.G. PEDOT:PSS for flexible and stretchable electronics: Modifications, strategies, and applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Shen, H.; Shuai, D.C.; Xiong, X.Y.; Wang, Y.; Huang, H.J.; Li, Y. Superior self-cleaning surfaces via the synergy of superhydrophobicity and photocatalytic activity: Principles, synthesis, properties, and applications. J. Clean. Prod. 2023, 428, 139430. [Google Scholar] [CrossRef]
- Xie, Q.Y.; Liu, C.; Lin, X.B.; Ma, C.F.; Zhang, G.Z. Nanodiamond reinforced poly(dimethylsiloxane)-based polyurea with self-healing ability for fouling release coating. ACS Appl. Polym. Mater. 2020, 2, 3181–3188. [Google Scholar] [CrossRef]
- Fu, Y.H.; Xu, F.C.; Weng, D.H.; Li, X.; Li, Y.; Sun, J.Q. Superhydrophobic foams with chemical-and mechanical-damage-healing abilities enabled by self-healing polymers. ACS Appl. Mater. Interfaces 2019, 11, 37285–37294. [Google Scholar] [CrossRef]
- Wang, F.J.; Xie, T.X.; Ou, J.F.; Xue, M.S.; Li, W. Cement based superhydrophobic coating with excellent robustness and solar reflective ability. J. Alloy. Compo. 2020, 823, 153702. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Si, H.F.; Liu, Y. Superhydrophobic polyurea coating with excellent adhesion and abrasion resistance prepared via convenient microphase separation. Prog. Org. Coat. 2022, 170, 106994. [Google Scholar] [CrossRef]
- Du, H.; Zhang, M.; Liu, K.; Parit, M.; Jiang, Z.; Zhang, X.; Li, B.; Si, C. Conductive PEDOT:PSS/cellulose nanofibril paper electrodes for flexible supercapacitors with superior areal capacitance and cycling stability. Chem. Eng. J. 2022, 428, 131994. [Google Scholar] [CrossRef]
- Eshaghi, A. Fabrication of transparent silica-silica nanotube/PFTS nano-composite thin films with superhydrophobic, oleophobic, self-cleaning and anti-icing properties. Opt. Quant. Electron. 2020, 52, 516. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Younes, E.M.; Namkoong, G.; El-Maghraby, E.M.; Elsayed, A.H.; Abo Elazm, A.H. Solvents effects on the hole transport layer in organic solar cells performance. Sol. Energy 2016, 137, 337–343. [Google Scholar] [CrossRef]
- Mahato, S.; Puigdollers, J.; Voz, C.; Mukhopadhyay, M.; Mukherjee, M.; Hazra, S. Near 5% DMSO is the best: A structural investigation of PEDOT:PSS thin films with strong emphasis on surface and interface for hybrid solar cell. Appl. Surf. Sci. 2020, 499, 143967. [Google Scholar] [CrossRef]
- Tounakti, C.; Decorse, P.; Kouki, F.; Philippe, L. Relationship between enhancement of PEDOT:PSS conductivity by solvent treatment and PSS chain reorganization. J. Polym. Sci. 2023, 61, 582–603. [Google Scholar] [CrossRef]
- Chou, T.R.; Chen, S.H.; Chiang, Y.T.; Lin, Y.T.; Chao, C.Y. Highly conductive PEDOT: PSS films by post-treatment with dimethyl sulfoxide for ITO-free liquid crystal display. J. Mater. Chem. C 2015, 3, 3760–3766. [Google Scholar] [CrossRef]
- Lattach, Y.; Deniset-Besseau, A.; Guigner, J.M.; Remita, S. Radiation chemistry as an alternative way for the synthesis of PEDOT conducting polymers under “soft” conditions. Radiat. Phys. Chem. 2013, 82, 44–53. [Google Scholar] [CrossRef]
- Ahmad, D.; Van Den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Neves, L.B.; Afonso, I.S.; Nobrega, G.; Barbosa, L.G.; Lima, R.A.; Ribeiro, J.E. A review of methods to modify the PDMS surface wettability and their applications. Micromachines 2024, 15, 670. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective approaches to improve the electrical conductivity of PEDOT: PSS: A review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Sharma, K.; Hooda, A.; Goyat, M.S.; Rai, R.; Mittal, A. A review on challenges, recent progress and applications of silica nanoparticles based superhydrophobic coatings. Ceram. Int. 2022, 48, 5922–5938. [Google Scholar] [CrossRef]
- Wang, D.; Yang, K.; Cheng, S. Harsh environment resistible and recyclable thermoplastic polyurea adhesivebased on stable and density hydrogen bonds. Chem. Eng. J. 2024, 482, 148663. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Q.Y.; Ma, C.F.; Zhang, G.Z. Fouling release property of polydimethylsiloxane-based polyurea with improved adhesion to substrate. IEC Res. 2016, 55, 6671–6676. [Google Scholar]
- Vitoratos, E.; Sakkopoulos, S.; Dalas, E.; Paliatsas, N.; Karageorgopoulos, D. Thermal degradation mechanisms of PEDOT:PSS. Org. Electron. 2009, 10, 61–66. [Google Scholar] [CrossRef]
- Smoleń, J.; Stępień, K.; Mrowiec, A.; Mendala, B.; Wilczyńska, W.; Czakiert, J.; Kozioł, M. Study of resin coating adhesion on GFRP laminate surfaces after UV degradation. Int. J. Adhes. 2024, 135, 103841. [Google Scholar] [CrossRef]
- Schultheiss, A.; Gueye, M.; Carella, A.; Benayad, A.; Pouget, S.; Faure-Vincent, J.; Demadrille, R.; Revaux, A.; Simonato, J.P. Insight into the degradation mechanisms of highly conductive poly(3,4-ethylenedioxythiophene) thin films. ACS Appl. Polym. Mater. 2020, 2, 2686–2695. [Google Scholar] [CrossRef]
- Fan, Z.; Li, P.C.; Du, D.H.; Ouyang, J.Y. Significantly enhanced thermoelectric properties of PEDOT:PSS films through sequential post-treatments with common acids and bases. Adv. Energy Mater. 2017, 7, 1602116. [Google Scholar] [CrossRef]
- He, S.H.; Chen, J.R.; Lu, Y.; Huang, S.; Feng, K. Enhanced waterproof performance of superhydrophobic SiO2/PDMS coating. Prog. Org. Coat. 2024, 197, 108845. [Google Scholar] [CrossRef]
- Park, J.; Jang, J.G.; Kang, K.; Kim, S.H.; Kwak, J. High thermoelectric performance in solution-processed semicrystalline PEDOT:PSS films by strong acid-base treatment: Limitations and potential. Adv. Sci. 2024, 11, 2308368. [Google Scholar] [CrossRef]
- Chen, B.X.; Sun, J.P. A radiative cooling, anti-corrosion multifunctional composite coating derived from Jatropha (Jatropha curcas L.) oil. Polym. Eng. Sci. 2022, 62, 3652–3661. [Google Scholar] [CrossRef]
- Hosseini, E.; Kollath, V.O.; Karan, K. The key mechanism of conductivity in PEDOT:PSS thin films exposed by anomalous conduction behaviour upon solvent-doping and sulfuric acid post-treatment. J. Mater. Chem. C 2020, 8, 3982–3990. [Google Scholar] [CrossRef]
- Wu, B.; Cui, X.; Jiang, H.; Wu, N.; Peng, C.; Hu, Z.; Liang, X.; Yan, Y.; Huang, J.; Li, D. A superhydrophobic coating harvesting mechanical robustness, passive anti-icing and active de-icing performances. J. Colloid Interface Sci. 2021, 590, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Yu, J.; Gao, N.; Xue, Z.; Zhang, W.; Xu, J.; Chen, S. Freeze-drying and mechanical redispersion of aqueous PEDOT:PSS. J. Appl. Polym. Sci. 2020, 138, e49774. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Dong, R.; Zhou, M.; Liang, L.; Yang, M.; Xing, H.; Qiao, Y.; Chen, S. Hydrophobic, Durable, and Reprocessable PEDOT:PSS/PDMS-PUa/SiO2 Film with Conductive Self-Cleaning and De-Icing Functionality. Coatings 2025, 15, 985. https://doi.org/10.3390/coatings15090985
Fang J, Dong R, Zhou M, Liang L, Yang M, Xing H, Qiao Y, Chen S. Hydrophobic, Durable, and Reprocessable PEDOT:PSS/PDMS-PUa/SiO2 Film with Conductive Self-Cleaning and De-Icing Functionality. Coatings. 2025; 15(9):985. https://doi.org/10.3390/coatings15090985
Chicago/Turabian StyleFang, Jie, Rongqing Dong, Meng Zhou, Lishan Liang, Mingna Yang, Huakun Xing, Yongluo Qiao, and Shuai Chen. 2025. "Hydrophobic, Durable, and Reprocessable PEDOT:PSS/PDMS-PUa/SiO2 Film with Conductive Self-Cleaning and De-Icing Functionality" Coatings 15, no. 9: 985. https://doi.org/10.3390/coatings15090985
APA StyleFang, J., Dong, R., Zhou, M., Liang, L., Yang, M., Xing, H., Qiao, Y., & Chen, S. (2025). Hydrophobic, Durable, and Reprocessable PEDOT:PSS/PDMS-PUa/SiO2 Film with Conductive Self-Cleaning and De-Icing Functionality. Coatings, 15(9), 985. https://doi.org/10.3390/coatings15090985








