Review on Durability Deterioration and Mitigation of Concrete Structures
Abstract
1. Introduction
2. Durability Degradation Mechanisms of Concrete Structures
2.1. Concrete Carbonation
2.2. Concrete Crack
2.3. Reinforcement Corrosion
2.4. Freeze–Thaw Damage
3. Durability Prevention Strategies for Concrete Structures
3.1. Carbonation
3.2. Crack
3.3. Reinforcement Corrosion
3.4. Freeze–Thaw Damage
3.5. Evaluation Criteria for Durability Prevention Strategies
3.5.1. Specificity to Deterioration Mechanisms
3.5.2. Long-Term Performance in Field Conditions
3.5.3. Structural Compatibility
3.5.4. Monitoring and Quantifiability
3.5.5. Economic Feasibility and Constructability
3.5.6. Environmental Impact and Regulatory Compliance
4. Durability Enhancement Strategies at the Whole Life Cycle Level for Concrete Structures
5. Conclusions
- 1.
- Based on existing studies, concrete carbonation, as a long-standing and widely studied durability deterioration mechanism, fundamentally involves the reaction between atmospheric carbon dioxide and alkaline substances within the concrete. This process reduces the alkalinity of the concrete, thereby weakening the stability of the passive film on the reinforcement surface and indirectly triggering reinforcement corrosion. The carbonation rate and its impact on structural performance are influenced by multiple factors, including the water-to-binder ratio, cover thickness, environmental humidity, and temperature. In current engineering practice, carbonation is regarded as one of the critical factors affecting the long-term service performance of reinforced concrete structures and serves as an important basis for durability design, material selection, and subsequent maintenance strategies.
- 2.
- Cracking not only indicates the degradation of the structure’s mechanical integrity but also serves as a pathway for the ingress of aggressive agents such as moisture, chloride ions, and carbon dioxide. This accelerates carbonation, freeze–thaw damage, and reinforcement corrosion. Crack formation is complex and multi-factorial, commonly associated with material shrinkage, construction practices, and load-induced stresses.
- 3.
- Reinforcement corrosion is one of the most prevalent and severe durability issues in concrete bridges. The expansive corrosion products can cause concrete cover spalling, crack propagation, and a significant reduction in load-bearing capacity, thus posing a direct threat to structural safety. Corrosion is primarily driven by chloride ingress and carbonation, with a concealed and time-delayed progression that makes it difficult to detect in early stages.
- 4.
- Freeze–thaw damage predominantly occurs in cold or seasonally freezing regions. The expansion of pore water during freezing disrupts the concrete’s density and integrity, promoting crack initiation and propagation and accelerating overall structural degradation. Enhancing the freeze–thaw resistance of concrete requires a comprehensive approach involving material design, optimization of air-void structure, and waterproofing protection.
- 5.
- In response to these durability challenges, this study proposes a holistic prevention and control framework encompassing the design, construction, operation, and maintenance phases. Studies have shown that novel materials such as ultra-high-performance concrete (UHPC) have been widely applied in bridges and related structures, significantly enhancing their durability and service life. The appropriate selection of these advanced materials, in combination with optimized structural detailing, strict construction quality control, and the integration of intelligent monitoring technologies, remains a key approach to improving the long-term performance of bridges. Although existing durability models have partially incorporated the unique properties of new materials such as UHPC—particularly in terms of modified diffusion coefficients, permeability, and damage-healing mechanisms—most remain at the stage of theoretical exploration or experimental fitting and lack mature, widely accepted generalized models.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Collura, D.; Nascimbene, R. Comparative Assessment of Variable Loads and Seismic Actions on Bridges: A Case Study in Italy Using a Multimodal Approach. Appl. Sci. 2023, 13, 2771. [Google Scholar] [CrossRef]
- Zhong, X.; Jin, W.; Zhang, B. Durability design method for concrete structures in chloride salt environments. J. Build. Mater. 2016, 19, 544–549. [Google Scholar]
- Wang, J.; Wang, M.; Men, P.; Qin, F.; Peng, X.; Di, J. Experimental investigation on mechanical behavior of Push-out Perfobond connectors. Eng. Struct. 2025, 341, 120828. [Google Scholar] [CrossRef]
- Zhao, D. Durability design of highway concrete structures based on carbonation numerical model. Shanghai Highw. 2006, 25–28. [Google Scholar] [CrossRef]
- Jiang, T.; Ai, L.; Yang, Z. Analysis of typical durability diseases and causes in concrete bridges. World Build. Mater. 2013, 34, 23–26. [Google Scholar]
- Zhou, X.; Zhang, X. Thoughts on the Development of Bridge Technology in China. Engineering 2019, 5, 1120–1130. [Google Scholar] [CrossRef]
- Qin, F.; Han, Y.; Wei, X.; Wang, X.; Zhang, Z.; Zhang, X. Flexural behavior of engineered cementitious composites (ECC) slabs with different strength grades. Materials 2025, 18, 2047. [Google Scholar] [CrossRef]
- Wei, X.; Li, X.; Di, J.; Qin, F.; Zhang, Z.; Liang, F. Flexural performance of U-shaped high-strength ECC perma-nent formwork concrete composite beams. Structures 2025, 74, 108641. [Google Scholar] [CrossRef]
- Aktan, A.E.; Farhey, D.N.; Brown, D.L.; Dalal, V.; Helmicki, A.J.; Hunt, V.J.; Shelley, S.J. Condition Assessment for Bridge Management. J. Infrastruct. Syst. 1996, 2, 108–117. [Google Scholar] [CrossRef]
- Vu, K.A.T.; Stewart, M.G. Structural reliability of concrete bridges including improved chloride-induced corrosion models. Struct. Saf. 2000, 22, 313–333. [Google Scholar] [CrossRef]
- Stewart, M.G.; Rosowsky, D.V. Structural Safety and Serviceability of Concrete Bridges Subject to Corrosion. J. Infrastruct. Syst. 1998, 4, 146–155. [Google Scholar] [CrossRef]
- Wang, H.; Wang, A.; Wang, J.; Zhang, J.; Du, T.; Shen, J. Optimizing quaternary chemical additives and steel fiber dispersion in CSA cement based HPC for rapid repair engineering. Constr. Build. Mater. 2025, 489, 142265. [Google Scholar] [CrossRef]
- Men, P.; Chen, F.; Qin, F.; Peng, X.; Di, J.; Jiao, H. Behavior of composite beams with UHPC-concrete composite slabs under negative bending moment. J. Constr. Steel Res. 2025, 227, 109415. [Google Scholar] [CrossRef]
- Vassie, P.; TRRL. Reinforcement Corrosion and the Durability of Concrete Bridges. Proc. Inst. Civ. Eng. 1984, 76, 713–723. [Google Scholar] [CrossRef]
- Sarja, A. Durability design of concrete structures—Committee report 130-CSL. Mater. Struct. 2000, 33, 14–20. [Google Scholar] [CrossRef]
- Lei, J. Detection and Evaluation of Disease in Jinghuai Bridge. Master’s Thesis, Hefei University of Technology, Hefei, China, 2008. [Google Scholar]
- Tang, D.; Zhang, X. Diagnosis and treatment measures of durability defects in in-service concrete bridges. Heilongjiang Sci. Technol. Inf. 2010, 225, 236. [Google Scholar]
- Abdel-Jaber, H.; Glisic, B. Monitoring of prestressing forces in prestressed concrete structures—An overview. Struct. Control Health Monit. 2019, 26, e2374. [Google Scholar] [CrossRef]
- Rozière, E.; Loukili, A.; Cussigh, F. A performance based approach for durability of concrete exposed to carbonation. Constr. Build. Mater. 2009, 23, 190–199. [Google Scholar] [CrossRef]
- Glasser, F.P.; Marchand, J.; Samson, E. Durability of concrete—Degradation phenomena involving detrimental chemical reactions. Cem. Concr. Res. 2008, 38, 226–246. [Google Scholar] [CrossRef]
- Chen, X. Factors Affecting the Durability of Bridge Structures and Their Optimization Design. Eng. Constr. Des. 2023, 85–87. [Google Scholar] [CrossRef]
- Chen, Z. Study on influencing factors and optimization design of bridge structure durability. Transp. Sci. Manag. 2023, 4, 143–145. [Google Scholar]
- Ding, W.; Huang, W. Discussion on field detection of durability of bridge concrete structures. Transp. World 2022, 99–101. [Google Scholar] [CrossRef]
- Cusson, D.; Lounis, Z.; Daigle, L. Durability Monitoring for Improved Service Life Predictions of Concrete Bridge Decks in Corrosive Environments. Comput.-Aided Civ. Infrastruct. Eng. 2011, 26, 524–541. [Google Scholar] [CrossRef]
- Hooton, R.D.; Bickley, J.A. Design for durability: The key to improving concrete sustainability. Constr. Build. Mater. 2014, 67, 422–430. [Google Scholar] [CrossRef]
- Li, C. Study on Factors Affecting Bridge Structure Durability and Its Optimization Design. Transp. Sci. Manag. 2023, 4, 147–149. [Google Scholar]
- Liu, B. Analysis of common diseases and reinforcement of expressway bridges. Transp. Sci. Manag. 2023, 4, 75–77. [Google Scholar]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Silva, R.V.; Silva, A.; Neves, R.; Brito, J. Statistical Modeling of Carbonation in Concrete Incorporating Recycled Aggregates. J. Mater. Civ. Eng. 2016, 28, 04015082. [Google Scholar] [CrossRef]
- Czarnecki, L.; Woyciechowski, P. Concrete Carbonation as a Limited Process and Its Relevance to Concrete Cover Thickness. ACI Mater. J. 2012, 109, 275–282. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Li, D. Design and key construction technologies of protective coating systems for concrete bridge durability. Highway 2021, 66, 178–183. [Google Scholar]
- Wang, W.; Yang, L.; Wu, F.; He, X.; Tan, G.; Liu, B.; Wang, H.; Lv, D.; Liu, H. Compressive behavior deterioration mechanism of concrete under carbonization and freeze-thaw cycles and time-varying structural reliability evaluation. Constr. Build. Mater. 2025, 466, 140359. [Google Scholar] [CrossRef]
- Zheng, B. Analysis of disease treatment techniques in road and bridge engineering construction. Hous. Real Estate 2021, 235–236. [Google Scholar] [CrossRef]
- Parameswaran, L.; Kumar, R.; Sahu, G.K. Effect of Carbonation on Concrete Bridge Service Life. J. Bridge Eng. 2008, 13, 75–82. [Google Scholar] [CrossRef]
- Song, X. Discussion on bridge structure defects and maintenance strengthening measures. Urban Constr. Theory Res. (Electron. Ed.) 2020, 62. [Google Scholar] [CrossRef]
- Ann, K.Y.; Pack, S.W.; Hwang, J.P.; Song, H.W.; Kim, S.H. Service life prediction of a concrete bridge structure subjected to carbonation. Constr. Build. Mater. 2010, 24, 1494–1501. [Google Scholar] [CrossRef]
- Du, M. Comprehensive Analysis and Remedial Strategies for Crack Occurrences in Concrete Bridge Structural Systems. Heilongjiang Transp. Sci. Technol. 2021, 44, 108–109. [Google Scholar] [CrossRef]
- Wang, J. Causes and treatment technologies of cracks in concrete beam bridges on expressways. Transp. World 2019, 131–132. [Google Scholar] [CrossRef]
- Chen, S.; Duffield, C.; Miramini, S.; Nasim Khan Raja, B.; Zhang, L. Life-cycle modelling of concrete cracking and reinforcement corrosion in concrete bridges: A case study. Eng. Struct. 2021, 237, 112143. [Google Scholar] [CrossRef]
- Golewski, G.L. The Phenomenon of Cracking in Cement Concretes and Reinforced Concrete Structures: The Mechanism of Cracks Formation, Causes of Their Initiation, Types and Places of Occurrence, and Methods of Detection—A Review. Buildings 2023, 13, 765. [Google Scholar] [CrossRef]
- Cai, C.; Chen, S.; Liu, L. Detection of Fatigue Cracks for Concrete Structures by Using Carbon Ink-Based Conductive Skin and Electrical Resistance Tomography. Sensors 2023, 23, 8382. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Palevičius, P.; Landauskas, M.; Orinaitė, U.; Timofejeva, L.; Ragulskis, M. An Overview of Challenges Associated with Automatic Detection of Concrete Cracks in the Presence of Shadows. Appl. Sci. 2021, 11, 11396. [Google Scholar] [CrossRef]
- Abdel-Qader, I.; Abudayyeh, O.; Kelly, M.E. Analysis of Edge-Detection Techniques for Crack Identification in Bridges. J. Comput. Civ. Eng. 2003, 17, 255–263. [Google Scholar] [CrossRef]
- Gribniak, V.; Kaklauskas, G.; Bacinskas, D. State-Of-Art Review of Shrinkage Effect on Cracking and Deformations of Concrete Bridge Elements. Balt. J. Road Bridge Eng. 2007, 2, 183–193. [Google Scholar]
- Flah, M.; Suleiman, A.R.; Nehdi, M.L. Classification and quantification of cracks in concrete structures using deep learning image-based techniques. Cem. Concr. Compos. 2020, 114, 103781. [Google Scholar] [CrossRef]
- Li, Z. Study on formation and repair methods of concrete structural cracks. Jiangxi Build. Mater. 2022, 95–97. [Google Scholar] [CrossRef]
- Xue, C. Control of cast-in-place box girder concrete cracks in bridge structures. Urban Constr. Theory Res. (Electron. Ed.) 2024, 151–153. [Google Scholar] [CrossRef]
- Issa, M.A. Investigation of Cracking in Concrete Bridge Decks at Early Ages. J. Bridge Eng. 1999, 4, 116–124. [Google Scholar] [CrossRef]
- Raupach, M. Models for the propagation phase of reinforcement corrosion—An overview. Mater. Corros. 2006, 57, 605–613. [Google Scholar] [CrossRef]
- Chen, D.; Mahadevan, S. Chloride-induced reinforcement corrosion and concrete cracking simulation. Cem. Concr. Compos. 2008, 30, 227–238. [Google Scholar] [CrossRef]
- Capozucca, R. Damage to reinforced concrete due to reinforcement corrosion. Constr. Build. Mater. 1995, 9, 295–303. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction—A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahmad, S.; Al-Gahtani, H.J. Chloride-Induced Corrosion of Steel in Concrete: An Overview on Chloride Diffusion and Prediction of Corrosion Initiation Time. Int. J. Corros. 2017, 2017, 5819202. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Wu, C.; Jiao, Y. Experimental and Simulation Study on Diffusion Behavior of Chloride Ion in Cracking Concrete and Reinforcement Corrosion. Adv. Mater. Sci. Eng. 2018, 2018, 8475384. [Google Scholar] [CrossRef]
- Li, Z. Seismic vulnerability analysis of large-span high-pier rigid frame bridges under chloride ion erosion. Fujian Transp. Sci. Technol. 2024, 31–35. [Google Scholar]
- Bažant, Z.P. Physical Model for Steel Corrosion in Concrete Sea Structures—Application. J. Struct. Div. 1979, 105, 1155–1166. [Google Scholar] [CrossRef]
- Zheng, Z. Study on deterioration mechanisms and protection measures of reinforced concrete bridge durability. Fujian Transp. Sci. Technol. 2021, 90–94, 125. [Google Scholar]
- Ren, J. Investigation into the Influencing Factors and Optimization Design of Bridge Structure Durability. Transp. World 2024, 205–207. [Google Scholar] [CrossRef]
- Yu, D. Experimental Study on Durability of Precast Segmental Beam Joints of Bridges Under Freeze-Thaw and Carbonation in Northern Hebei. Master’s Thesis, Hebei University of Architecture, Zhangjiakou, China, 2020. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, R.; Wang, W.; Zhang, S. Effect of load on bonding properties and salt freeze-thaw resistance of bridge expansion joint concrete. Constr. Build. Mater. 2024, 411, 134680. [Google Scholar] [CrossRef]
- Ma, Z.; Zhu, F.; Ba, G. Effects of freeze-thaw damage on the bond behavior of concrete and enhancing measures. Constr. Build. Mater. 2019, 196, 375–385. [Google Scholar] [CrossRef]
- Tian, J.; Hong, J. Influence of freeze-thaw cycles on the service life of concrete pavements. Shanxi Archit. 2008, 287–289. [Google Scholar] [CrossRef]
- Peng, R.; Qiu, W.; Teng, F. Investigation on seawater freeze-thaw damage deterioration of marine concrete structures in cold regions from multi-scale. Ocean. Eng. 2022, 248, 110867. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhang, P. Influence of freeze-thaw cycles on the service life of concrete bridges. J. Hebei Univ. Technol. 2009, 38, 107–110. [Google Scholar]
- Xue, F.; Zhao, S.; Liu, J. Effects of freeze-thaw erosion on concrete bridges in plateau mountainous areas and protective measures. Urban Roads Bridges Flood Control 2018, 199-201+231+21-22. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.M.; Yan, H.D.; Mu, R. Damage and damage resistance of high strength concrete under the action of load and freeze-thaw cycles. Cem. Concr. Res. 1999, 29, 1519–1523. [Google Scholar] [CrossRef]
- Liu, D.; Tu, Y.; Sas, G.; Elfgren, L. Freeze-thaw damage evaluation and model creation for concrete exposed to freeze–thaw cycles at early-age. Constr. Build. Mater. 2021, 312, 125352. [Google Scholar] [CrossRef]
- Li, X.; Guo, M.; Man, G. Analysis of pavement diseases and maintenance technologies in road and bridge construction. Urban Hous. 2019, 26, 173–174. [Google Scholar]
- Li, Y. Common diseases and protective measures of urban roads and bridges. Smart City 2017, 3, 154. [Google Scholar] [CrossRef]
- Yang, F. Common diseases and durability analysis of concrete bridges. Eng. Constr. 2016, 48, 95-97+101. [Google Scholar] [CrossRef]
- Basheer, L.; Basheer, P.A.M.; Long, A.E. Influence of coarse aggregate on the permeation, durability and the microstructure characteristics of ordinary Portland cement concrete. Constr. Build. Mater. 2005, 19, 682–690. [Google Scholar] [CrossRef]
- Zhou, Y.; Gencturk, B.; Willam, K.; Attar, A. Carbonation-Induced and Chloride-Induced Corrosion in Reinforced Concrete Structures. J. Mater. Civ. Eng. 2015, 27, 04014245. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, Z.; Zhang, W.; Gu, X.; Dou, X. Numerical analysis of the effect of coarse aggregate distribution on concrete carbonation. Constr. Build. Mater. 2012, 37, 27–35. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, D.; Dong, X. Analysis of causes for cracking of bottom slabs during construction of prestressed concrete continuous box girder bridges. World Bridges 2021, 49, 103–107. [Google Scholar]
- Ruan, X.; Wang, B.; Wu, J.; Zhao, X.; Chen, Y. Identification of spalling and rebar exposure defects in reinforced concrete bridges based on deep learning. World Bridges 2020, 48, 88–92. [Google Scholar]
- Yang, H.; Pan, J. Crack control technology during construction of concrete continuous beams of Hong Kong-Zhuhai-Macao Bridge. Bridge Constr. 2017, 47, 106–111. [Google Scholar]
- Hu, Z.; Yu, S.; Li, G. Cracking analysis in negative moment regions of prestressed steel-concrete box composite continuous beams. Bridge Constr. 2020, 50, 66–71. [Google Scholar]
- Han, W. Study on temperature and shrinkage crack control of large volume concrete in bridge abutment foundations. China Highw. 2021, 96–98. [Google Scholar] [CrossRef]
- Liu, A. Research on repair technology of cracks in highway bridge abutment sidewalls based on mineral admixtures. Transp. World 2023, 130–132. [Google Scholar] [CrossRef]
- Wu, Z. Application and performance testing of polymer cement mortar in crack repair of road and bridge concrete structures. Aging Appl. Compos. Mater. 2022, 51, 88-90+10. [Google Scholar] [CrossRef]
- Tan, Q. Analysis and Treatment Methods for Cracks in Concrete Bridge Structures. Eng. Constr. Des. 2024, 233–235. [Google Scholar] [CrossRef]
- Suo, D. Durability design analysis of highway bridge structures based on multi-factor analysis. Transp. Sci. Manag. 2025, 6, 118–120. [Google Scholar]
- Dhir, R.K.; Jones, M.R. Development of chloride-resisting concrete using fly ash. Fuel 1999, 78, 137–142. [Google Scholar] [CrossRef]
- Hossain, A.B.; Shrestha, S.; Summers, J. Properties of Concrete Incorporating Ultrafine Fly Ash and Silica Fume. Transp. Res. Rec. 2009, 2113, 41–46. [Google Scholar] [CrossRef]
- Hariharan, A.R.; Santhi, A.S.; Ganesh, G.M. Effect of ternary cementitious system on compressive strength and resistance to chloride ion penetration. Int. J. Civ. Struct. Eng. 2011, 1, 695–706. [Google Scholar]
- Thomas, M.D.A.; Bamforth, P.B. Modelling chloride diffusion in concrete: Effect of fly ash and slag. Cem. Concr. Res. 1999, 29, 487–495. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takewaka, K. Experimental studies on epoxy coated reinforcing steel for corrosion protection. Int. J. Cem. Compos. Lightweight Concr. 1984, 6, 99–116. [Google Scholar] [CrossRef]
- Swamy, R.; Koyama, S. Epoxy coated rebars the panacea for steel corrosion in concrete. Constr. Build. Mater. 1989, 3, 86–91. [Google Scholar] [CrossRef]
- Alsayed, S.H.; Al-Salloum, Y.A.; Almusallam, T.H. Performance of glass fiber reinforced plastic bars as a reinforcing material for concrete structures. Compos. Part B Eng. 2000, 31, 555–567. [Google Scholar] [CrossRef]
- Shehata, M.H.; Thomas, M.D.A. The role of alkali content of Portland cement on the expansion of concrete prisms containing reactive aggregates and supplementary cementing materials. Cem. Concr. Res. 2010, 40, 569–574. [Google Scholar] [CrossRef]
- Taha, B.; Nounu, G. Using lithium nitrate and pozzolanic glass powder in concrete as ASR suppressors. Cem. Concr. Compos. 2008, 30, 497–505. [Google Scholar] [CrossRef]
- Xu, G.J.Z.; Watt, D.F.; Hudec, P.P. Effectiveness of mineral admixtures in reducing ASR expansion. Cem. Concr. Res. 1995, 25, 1225–1236. [Google Scholar] [CrossRef]
- Gonen, T.; Yazicioglu, S. The influence of mineral admixtures on the short and long-term performance of concrete. Build. Environ. 2007, 42, 3080–3085. [Google Scholar] [CrossRef]
- Shashiprakash, S.G.; Thomas, M.D.A. Sulfate resistance of mortars containing high-calcium fly ashes and combinations of highly reactive pozzolans and fly ash. Spec. Publ. 2001, 199, 221–238. [Google Scholar]
- Collepardi, S.; Corinaldesi, V.; Moriconi, G.; Bonora, G.; Collepardi, M. Durability of high-performance concretes with pozzolanic and composite cements. Spec. Publ. 2000, 192, 159–172. [Google Scholar]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Dong, W.; Tam, V.; Tao, Y. Durability deterioration of concrete under marine environment from material to structure: A critical review. J. Build. Eng. 2021, 35, 102074. [Google Scholar] [CrossRef]
- Niu, F.; Liu, Y.; Xue, F.; Sun, H.; Liu, T. Ultra-high performance concrete: A review of its material properties and usage in shield tunnel segment. Case Stud. Constr. Mater. 2025, 22, e04194. [Google Scholar] [CrossRef]
- Bao, J.; Xue, S.; Zhang, P.; Dai, Z.; Cui, Y. Coupled effects of sustained compressive loading and freeze–thaw cycles on water penetration into concrete. Struct. Concr. 2021, 22, E944–E954. [Google Scholar] [CrossRef]
- Zhang, Q. Analysis of structural damage of bridge concrete under environmental factors. Shanxi Archit. 2011, 37, 177–178. [Google Scholar] [CrossRef]
- Kosior-Kazberuk, M.; Berkowski, P. Surface Scaling Resistance of Concrete Subjected to Freeze-thaw Cycles and Sustained Load. Procedia Eng. 2017, 172, 513–520. [Google Scholar] [CrossRef]
- He, Z.; Tang, S.W.; Zhao, G.S.; Chen, E. Comparison of three and one dimensional attacks of freeze-thaw and carbonation for concrete samples. Constr. Build. Mater. 2016, 127, 596–606. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, R.; Li, R.; Wang, Y.; Cheng, Z.; Li, F.; Ma, Z. Frost resistance of fiber-reinforced blended slag and Class F fly ash-based geopolymer concrete under the coupling effect of freeze-thaw cycling and axial compressive loading. Constr. Build. Mater. 2020, 250, 118831. [Google Scholar] [CrossRef]
- Ren, J.; Lai, Y. Study on the durability and failure mechanism of concrete modified with nanoparticles and polypropylene fiber under freeze-thaw cycles and sulfate attack. Cold Reg. Sci. Technol. 2021, 188, 103301. [Google Scholar] [CrossRef]
- Ebrahimi Besheli, A.; Samimi, K.; Moghadas Nejad, F.; Darvishan, E. Improving concrete pavement performance in relation to combined effects of freeze–thaw cycles and de-icing salt. Constr. Build. Mater. 2021, 277, 122273. [Google Scholar] [CrossRef]
- Tian, J.; Wang, W.; Du, Y. Damage behaviors of self-compacting concrete and prediction model under coupling effect of salt freeze-thaw and flexural load. Constr. Build. Mater. 2016, 119, 241–250. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Li, S.; Zhao, Y.; Qin, Y. Resistance of recycled aggregate concrete containing low- and high-volume fly ash against the combined action of freeze–thaw cycles and sulfate attack. Constr. Build. Mater. 2018, 166, 23–34. [Google Scholar] [CrossRef]
- Li, N.; Long, G.; Fu, Q.; Wang, X.; Ma, K.; Xie, Y. Effects of freeze and cyclic flexural load on mechanical evolution of filling layer self-compacting concrete. Constr. Build. Mater. 2019, 200, 198–208. [Google Scholar] [CrossRef]
- Hao, L.; Liu, Y.; Wang, W.; Zhang, J.; Zhang, Y. Effect of salty freeze-thaw cycles on durability of thermal insulation concrete with recycled aggregates. Constr. Build. Mater. 2018, 189, 478–486. [Google Scholar] [CrossRef]
- Pang, B.; Zhou, Z.; Cheng, X.; Du, P.; Xu, H. ITZ properties of concrete with carbonated steel slag aggregate in salty freeze-thaw environment. Constr. Build. Mater. 2016, 114, 162–171. [Google Scholar] [CrossRef]
- Monkman, S.; Shao, Y. Carbonation Curing of Slag-Cement Concrete for Binding CO2 and Improving Performance. J. Mater. Civ. Eng. 2010, 22, 296–304. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Li, Y. Deterioration of concrete under the coupling effects of freeze–thaw cycles and other actions: A review. Constr. Build. Mater. 2022, 319, 126045. [Google Scholar] [CrossRef]
- Luo, S.; Bai, T.; Guo, M.; Wei, Y.; Ma, W. Impact of Freeze–Thaw Cycles on the Long-Term Performance of Concrete Pavement and Related Improvement Measures: A Review. Materials 2022, 15, 4568. [Google Scholar] [CrossRef]
- Tian, J.; Wu, X.; Zheng, Y.; Hu, S.; Ren, W. Investigation of damage behaviors of ECC-to-concrete interface and damage prediction model under salt freeze-thaw cycles. Constr. Build. Mater. 2019, 226, 238–249. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Jiao, Y.; Yang, J. Quantitative analysis of concrete property under effects of crack, freeze-thaw and carbonation. Constr. Build. Mater. 2016, 129, 106–115. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Z.; Li, Y.; Wang, K.; Zhao, H. Review on the deterioration and approaches to enhance the durability of concrete in the freeze–thaw environment. Constr. Build. Mater. 2022, 321, 126371. [Google Scholar] [CrossRef]
- Beeby, A.W.; Narayanan, R.S. Designers’ Guide to Eurocode 2: Design of Concrete Structures: Designers’ Guide to EN 1992-1-1 and EN 1992-1-2 Eurocode 2: Design of Concrete Structures Design of Concrete Structures General Rules and Rules for Buildings and Structural Fire Design; Thomas Telford Publishing: London, UK, 2005. [Google Scholar]
- Wang, X.; Yang, Q.; Peng, X.; Qin, F. A Review of Concrete Carbonation Depth Evaluation Models. Coatings 2024, 14, 386. [Google Scholar] [CrossRef]
- Ibrahim, A.; Abdelkhalek, S.; Zayed, T.; Qureshi, A.H.; Mohammed Abdelkader, E. A comprehensive review of the key deterioration factors of concrete bridge decks. Buildings 2024, 14, 3425. [Google Scholar] [CrossRef]
- Fan, C.; Ding, Y.; Liu, X.; Yang, K. A review of crack research in concrete structures based on data-driven and intelligent algorithms. Structures 2025, 75, 108800. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, C.; Chen, W.; Fan, J. A Review of Research on Bridge Apparent Defect Detection Based on Machine Vision Methods. China J. Highw. Transp. 2024, 37, 1–15. [Google Scholar] [CrossRef]
- Huang, J.; Lu, W.; Yin, C.; Fu, C. A Review of Research on the Impact of Corrosion on the Bonding Performance Between Steel Bars and Concrete. Mater. Rep. 2024, 38, 228–239. [Google Scholar]
- Dong, H.; Li, H.; Yang, Z.; Wen, J.; Huang, F.; Wang, Z.; Yi, Z. Mechanism of Freeze-Thaw Damage in Concrete and Methods for Life Prediction. Mater. Rep. 2024, 38, 143–153. [Google Scholar]
- Guo, J.; Sun, W.; Xu, Y.; Lin, W.; Jing, W. Damage Mechanism and Modeling of Concrete in Freeze–Thaw Cycles: A Review. Buildings 2022, 12, 1317. [Google Scholar] [CrossRef]
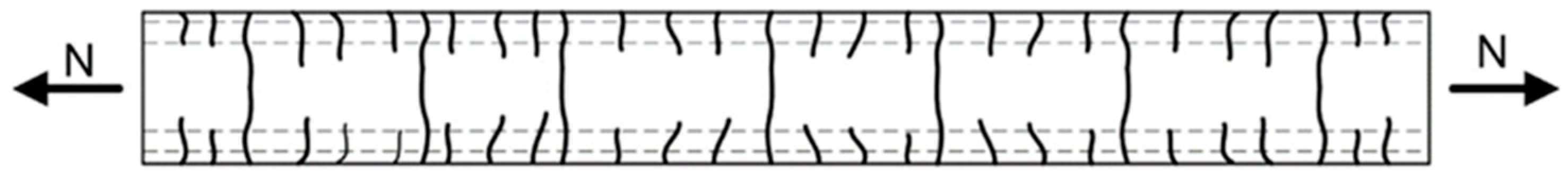
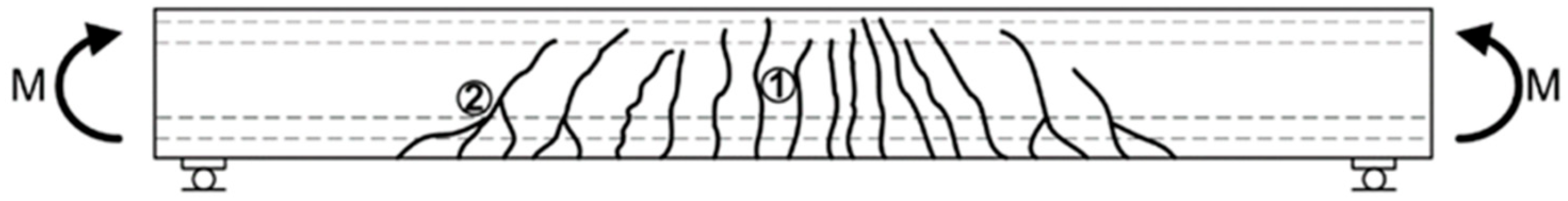
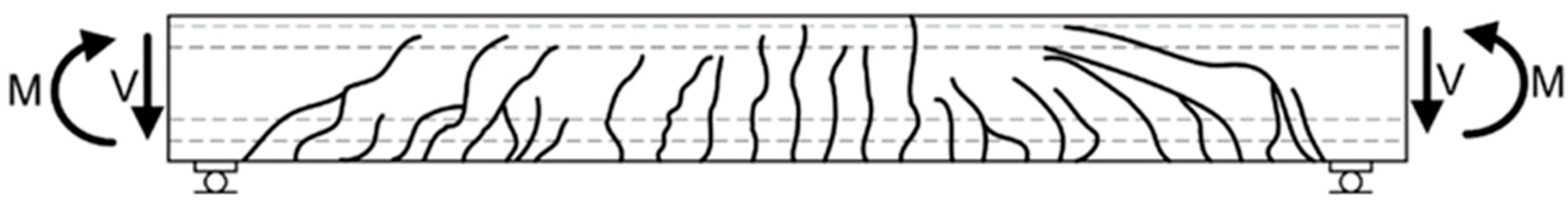

| Category | Specific Factors | Influence Pattern |
|---|---|---|
| Material Properties | Water–cement ratio, cement content, aggregate size, admixtures | Carbonation rate increases with higher water–cement ratio and larger aggregate size; it decreases with higher cement content. Water-reducing agents and air-entraining agents tend to reduce carbonation rate. |
| Environmental Conditions | Relative humidity, temperature, CO2 concentration | Carbonation proceeds most rapidly at a relative humidity of 70%–80%. Higher temperatures accelerate carbonation. The carbonation rate increases proportionally with CO2 concentration. |
| Reference | Research Content/Model | Main Factors | Mechanism/Pattern | Impact on Structural Durability |
|---|---|---|---|---|
| [19,20] | The chemical reaction process of carbonation (Equations (1) and (2)), emphasizing the loss of alkalinity leading to the dissolution of the steel passive film | Micro-scale chemical reactions, CO2 diffusion | pH > 11.5: passive film remains stable; pH < 11.5: passive film deteriorates, increasing steel corrosion susceptibility | Higher risk of reinforcement corrosion and reduced structural durability |
| [21,22,23,24,25] | Influence of environmental factors on carbonation | Temperature, relative humidity, air pollutants, groundwater level fluctuation | Optimal RH: 50%–75%; higher temperature accelerates reactions; SO2 and NOx produce strong acids, corroding concrete; groundwater fluctuations alter pore saturation, affecting CO2 diffusion | Accelerated carbonation, changes in chemical properties, and potential structural damage |
| [21,22,23,24,25] | Influence of material factors on carbonation | Water-to-binder ratio, mineral admixtures, strength grade | Higher w/b ratio → higher porosity → faster carbonation; mineral admixtures may reduce early-age alkalinity reserve; high-strength concrete → lower porosity → slower carbonation | Higher carbonation risk in concretes with high porosity and low alkalinity reserve |
| [26,27] | Alexeyev carbonation model (Equation (3)) | Carbonation depth calculation | Predicts carbonation depth based on CO2 uptake and time | Used for service life and durability prediction |
| [28] | Improved Alexeyev model (Equation (4)) | Concentrations of Ca(OH)2, CSH, C3S, and C2S | Replaces CO2 uptake with concentrations of hydration products to better represent the carbonation process | Improves model accuracy and facilitates material optimization |
| [29] | Carbonation model based on improved Fick’s law (Equation (5)) | 28-day compressive strength, clinker content, CO2 concentration, aggregate water absorption capacity | Incorporates multiple influencing factors for more realistic carbonation depth estimation | More accurate reflection of service environment effects |
| [30] | Influence of water–cement ratio on carbonation (Equation (6)) | Water–cement ratio | Higher w/c ratio → higher porosity → faster carbonation | Provides guidance for mix design optimization |
| [31] | Long-term carbonation case study (Yangtze River Bridge) | Atmospheric exposure, rainwater erosion | Over 50 years of exposure without protection → accelerated carbonation → surface degradation | Safety risks and high maintenance costs |
| [32,33,34,35,36] | Coupled effects of carbonation and other environmental factors | Chloride ingress, freeze–thaw cycles | Carbonation reduces protective capacity → facilitates chloride penetration; cracks + freeze–thaw cause spalling | Reduced load-bearing capacity and impaired functionality |
| Reference | Stage | Frost Damage | Plastic Stage Characteristics | Construction Activities | Physicochemical Factors | Structural Design Factors | Volume Change Factors |
|---|---|---|---|---|---|---|---|
| [40,41,42] | Before Concrete Hardening | Early-age frost damage | Plastic shrinkage cracking | Formwork displacement and deformation | — | — | — |
| Surface scaling, map cracking | Plastic settlement cracking | Uneven foundation settlement | — | — | Autogenous volume shrinkage | ||
| [40,43] | After Concrete Hardening | Freeze–thaw cycles | — | — | Alkali–aggregate reaction | Design load and overloading | Drying shrinkage |
| — | — | — | Reinforcement corrosion | Thermal stress | Thermal expansion and contraction | ||
| — | — | — | — | Material fatigue | Creep deformation |
| Reference | Research Content/Model Formula | Main Influencing Factors | Mechanism/Pattern | Impact on Structural Durability |
|---|---|---|---|---|
| [49,50] | The electrochemical corrosion principles of steel in concrete and the main reaction (Equations (7)–(10)) | Electrolyte solution in concrete pores, steel material heterogeneity, moisture, oxygen | Formation of anodic and cathodic regions on the steel surface, with electron transfer triggering corrosion reactions | Reduction of steel cross-section, loss of bond strength between steel and concrete, decreased structural load-bearing capacity, and durability |
| [51,52] | Destructive effects of chloride ions on steel reinforcement | Chloride ions, moisture, oxygen | Chloride ions disrupt the passive film, form localized corrosion cells, and accelerate electron migration and corrosion rate | Accelerated loss of steel cross-section and reduced bond performance, leading to premature structural deterioration |
| [54] | Prediction model for time to corrosion initiation based on Fick’s Second Law (Equation (11)) | Chloride ion diffusion coefficient, concrete cover thickness, environmental correction factors, surface chloride concentration | Calculates time to corrosion initiation when chloride concentration at the steel surface reaches the critical threshold | Used for service life assessment and durability design |
| [55] | Corrosion model describing uniform reduction in steel diameter over time (Equation (12)) | Steel corrosion rate, time | Assumes uniform corrosion; steel diameter decreases linearly with time | Reduction in cross-sectional area, progressive loss of load-bearing capacity |
| [56] | Material property degradation model of steel reinforcement under chloride-induced corrosion (Equation (13)) | Yield strength, ultimate strength, corrosion rate, strength reduction coefficients | Corrosion leads to reduction in yield and ultimate strength; the model predicts deterioration based on corrosion rate | Decrease in steel load-bearing capacity and reduction in structural safety margin |
| Reference | Research Content/Model Formula | Main Influencing Factors | Mechanism/Pattern | Impact on Structural Durability |
|---|---|---|---|---|
| [32,57,58] | Under freeze–thaw cycles, pore water in concrete freezes and expands, generating tensile stresses. Ice lenses first form in larger pores, followed by gradual freezing in smaller pores, driving unfrozen water toward the freezing front, causing capillary pressure buildup and microcrack formation. | Ambient temperature, pore structure, moisture content | Freezing expansion pressure exceeds tensile strength of concrete → microcrack formation → crack propagation under repeated freeze–thaw cycles | Accumulation of internal microcracks, reduction in structural strength, surface scaling, and cracking |
| [59,60,61] | High moisture content and numerous freeze–thaw cycles lead to cumulative deterioration, significant strength loss, and occurrences of surface scaling, cracking, and spalling. | Moisture content, number of freeze–thaw cycles | Higher moisture content → more ice → greater internal stresses; clear cumulative effect of cycles | Reduction in load-bearing capacity, diminished durability, increased structural safety risks |
| [62,63] | Mechanism and hazards of salt freeze damage | Saline environments (salt lakes, mining areas, coastal regions), temperature variation | Salt freeze damage combines freeze–thaw action and salt attack; salt ions increase osmotic pressure, partially suppressing freezing, but salt crystallization during evaporation generates crystallization pressure → exacerbates structural damage | More harmful than freeze–thaw alone, severe damage to pore structure |
| [64] | Cumulative freeze–thaw damage effects in concretes of different strength grades | Concrete strength grade, number of freeze–thaw cycles | Increasing cycles → continuous decrease in relative dynamic elastic modulus; C30 deteriorates faster than C40/C50 | Strength grade significantly affects frost resistance |
| [65,66,67] | Substructures of bridges (piers, pile foundations) in freezing zones are prone to freeze–thaw damage, often showing cracks, scaling, and exposed reinforcement after 10 years | Location (influenced by surface water/groundwater), service time, temperature variation | Freeze–thaw induces crack propagation, providing pathways for chlorides and other corrosive agents → accelerates steel reinforcement corrosion | Reduction of effective load-bearing cross-section; in severe cases, component instability or failure, threatening overall structural safety and service life |
| Reference | Main Findings/Insights | Protective Measures | Advantages | Disadvantages |
|---|---|---|---|---|
| [68] | Based on field analysis, two corrosion protection schemes were proposed |
| Adding buffering agents is simple and highly effective in protection | Surface coatings have low cost-effectiveness and high construction difficulty |
| [69] | Carbonation is caused by reactions between concrete components and atmospheric substances | Coating to isolate air; addition of corrosion inhibitors during mixing | Corrosion inhibitor strategies are mature, economical, and efficient | Coating method is costly and requires high technical skill |
| [70] | Concrete material design should comprehensively consider multiple factors to delay carbonation |
| Silicate cement slows carbonation; low water–cement ratio reduces porosity | High water–cement ratio accelerates carbonation; poor aggregates may cause alkali–aggregate reaction |
| [71] | Increasing sand content can reduce water–cement ratio and improve protection | Increasing sand reduces carbonation depth and permeability | Reduces carbonation depth and results in a denser structure | Sand content must be controlled properly to avoid affecting other properties |
| [72] | Well-graded fine aggregates perform better than single sand | Use of well-graded fine aggregates; application of thick protective or plaster layers | Delays CO2 penetration and extends service life | Requires strict control of grading during construction |
| [73] | Diffusion and carbonation mainly occur in the mortar phase | Reduce mortar content; use small particle coarse aggregates | Decreases carbonation depth; provides longer diffusion paths | Precise selection of small particle coarse aggregates needed; increases mixing difficulty |
| Crack Width (x/mm) | Treatment Method |
|---|---|
| x < 0.1 | Leave untreated |
| 0.1 ≤ x ≤ 0.2 | Seal with adhesive |
| x > 0.2 | Repair (structural) |
| Reference | Research Object | Cause Analysis | Repair/Preventive Measures | Features |
|---|---|---|---|---|
| [74] | Cracks in precast box girders | – |
| Crack width grading allows for precise repair and improved efficiency |
| [75] | Segmental cracks in box girders |
|
| Improved crack resistance from construction, structural, and curing perspectives |
| [78] | Cracks in mass concrete abutments | Large internal–external temperature difference due to heat of hydration |
| Effective mitigation of thermal shrinkage cracks through temperature control |
| [79] | Cracks in abutment sidewalls |
| Grouting with mineral admixtures; optimize material proportions and grouting process | Restores structural performance; suitable for structural crack repair |
| [80] | Deep structural cracks in road bridges | Cracks are deep; traditional methods lack penetration |
| Reinforces and seals deep cracks; enhances shear and flexural strength |
| [81] | Common bridge cracks (various causes) | Load, temperature variation, foundation deformation, corrosion, etc. |
| Proposes a systematic crack identification, strengthening, and repair technology system |
| Scholar/Research Team | Research Focus | Type and Dosage of SCMs | Technical Highlights | Conclusions and Effects |
|---|---|---|---|---|
| [82] | Chloride diffusion control | Fly ash 5%–10%, silica fume 3%–5% | Controlled water–binder ratio, enhanced compactness | Significantly improved impermeability, delayed chloride diffusion, inhibited corrosion |
| [83] | Pore structure improvement and chloride binding | Low-lime fly ash + silica fume/metakaolin | Optimized mix design | Cement–mineral blends exhibited the best chloride resistance |
| [84] | Role of ultra-fine fly ash | Ultra-fine fly ash (UFFA), silica fume | Enhanced chloride resistance and strength | UFFA notably improved early-age strength and chloride resistance |
| [85] | Performance of ternary SCM systems | Class C fly ash 30%–50%, silica fume 6%–10% | Partial cement replacement | Enhanced compressive strength and reduced chloride permeability |
| [86] | Chloride diffusion modeling | Fly ash, slag | Long-term performance modeling | Limited early improvement, but significant long-term chloride resistance |
| [87,88,89] | Reinforcement protection | Epoxy coating | Surface coating for corrosion protection | Demonstrated excellent anti-corrosion performance |
| [28] | Carbonation resistance | Silica fume and low/high-calcium fly ash replacing aggregate | Altered alkalinity and carbonation path | Improved carbonation resistance, indirectly reducing corrosion risk |
| [93] | Dry–wet cycling and capillary absorption | Silica fume 10% + fly ash 20% (cement replacement) | Enhanced surface density | Significantly enhanced resistance to carbonation and water ingress |
| [94] | Sulfate attack resistance | Low-calcium fly ash, UFFA | Reduced deterioration under aggressive environment | Improved chemical resistance and overall durability |
| [95,96] | Long-term performance and strength development | High-volume fly ash + slag | Combined SCM optimization | Slightly lower early strength, but excellent long-term performance and corrosion control |
| Reference | Mitigation Method | Additive Type | Experimental Condition | Experimental Result |
|---|---|---|---|---|
| [99,100,101] | Air-Entraining Agent | — | Compressive load and freeze–thaw cycle | Reduced concrete mass loss and RDEM loss [99] |
| — | Concrete mixing | Reduced hydrostatic pressure inside concrete pores [100] | ||
| Saponified liquid resin | Salt freeze–thaw cycle and flexural load | Reduced concrete mass loss by 62.8% [101] | ||
| [101,102,103] | Fibers | Polypropylene fibers (PPF) and polyvinyl alcohol fibers (PVAF) | Freeze–thaw cycle and compressive load | Reduced RDEM loss by 77.5% [102] |
| Polypropylene fibers (PPF) | Salt freeze–thaw cycle | Reduced compressive strength loss by 32.4% [103] | ||
| Steel fibers (SF) | Salt freeze–thaw cycle and flexural load | Reduced mass loss of SF concrete by 80% [101] | ||
| [105,106,107] | Pozzolanic Materials | Metakaolin or zeolite | Salt freeze–thaw cycle | Reduced concrete mass loss by 86.7% [105] |
| Fly ash (FA) and blast furnace slag (BFS) | Salt freeze–thaw cycle | Improved concrete density and impermeability [106] | ||
| Fly ash (FA) | Salt freeze–thaw cycle | Reduced RDEM loss by 2.3% and compressive strength loss by 3.2% [107] | ||
| [108,109,110] | Other Methods | Viscosity-modifying agent (VMA) | Flexural load | Reduced RDEM loss [108] |
| Glazed hollow beads (GHB) | Salt freeze–thaw cycle | Reduced mass loss by 74.6% and RDEM loss by 8.5% [109] | ||
| Carbonated steel slag aggregate (CSA) | Salt freeze–thaw cycle | Reduced concrete mass loss by 75.8% [110] |
| Reference | Durability Distress | Design Stage | Construction Stage | Service Stage | Repair Stage |
|---|---|---|---|---|---|
| [68,69,70,71,72,118,119] | Carbonation [118,119] |
|
|
| |
| [74,75,76,77,78,79,80,81,119,120] | Cracking [119,120] |
|
|
|
|
| [82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,122] | Steel Bar Corrosion [122] |
|
|
|
|
| [99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,123,124] | Freeze–thaw Damage [123,124] |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Yang, Q.; Peng, X.; Xia, K. Review on Durability Deterioration and Mitigation of Concrete Structures. Coatings 2025, 15, 982. https://doi.org/10.3390/coatings15090982
Ma J, Yang Q, Peng X, Xia K. Review on Durability Deterioration and Mitigation of Concrete Structures. Coatings. 2025; 15(9):982. https://doi.org/10.3390/coatings15090982
Chicago/Turabian StyleMa, Jiwei, Qiuwei Yang, Xi Peng, and Kangshuo Xia. 2025. "Review on Durability Deterioration and Mitigation of Concrete Structures" Coatings 15, no. 9: 982. https://doi.org/10.3390/coatings15090982
APA StyleMa, J., Yang, Q., Peng, X., & Xia, K. (2025). Review on Durability Deterioration and Mitigation of Concrete Structures. Coatings, 15(9), 982. https://doi.org/10.3390/coatings15090982








