Electrochemical Corrosion Behavior of SiO2 Superhydrophobic Inhibitor in Al7075
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
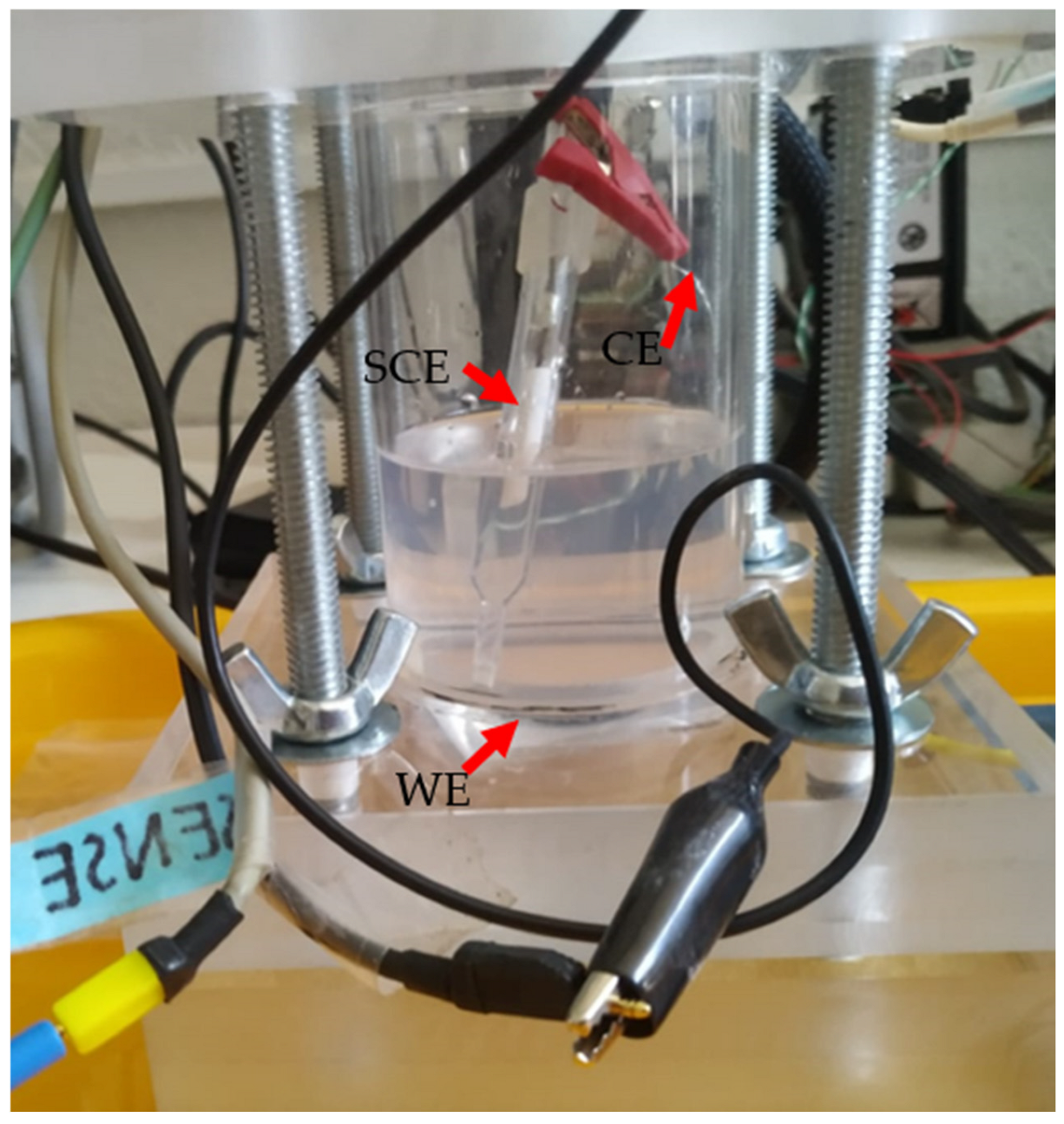
2.2. Equipment
2.3. Synthesis and Coating Procedure
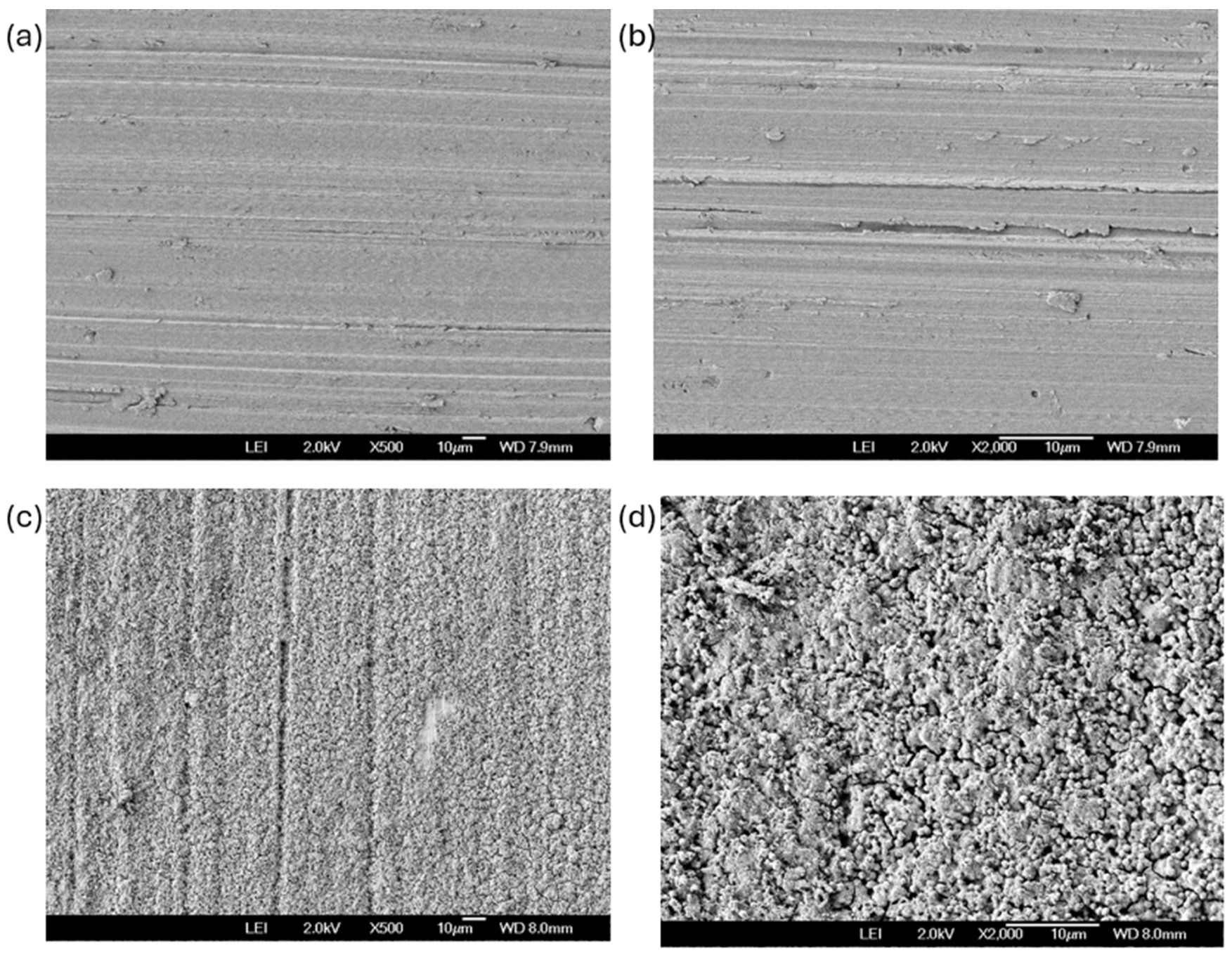
2.4. Scanning Electron Microscopy
2.5. Electrochemical Characterization
3. Results
3.1. SEM Before Corrosion
3.2. Wettability Test
3.3. Electrochemical Test
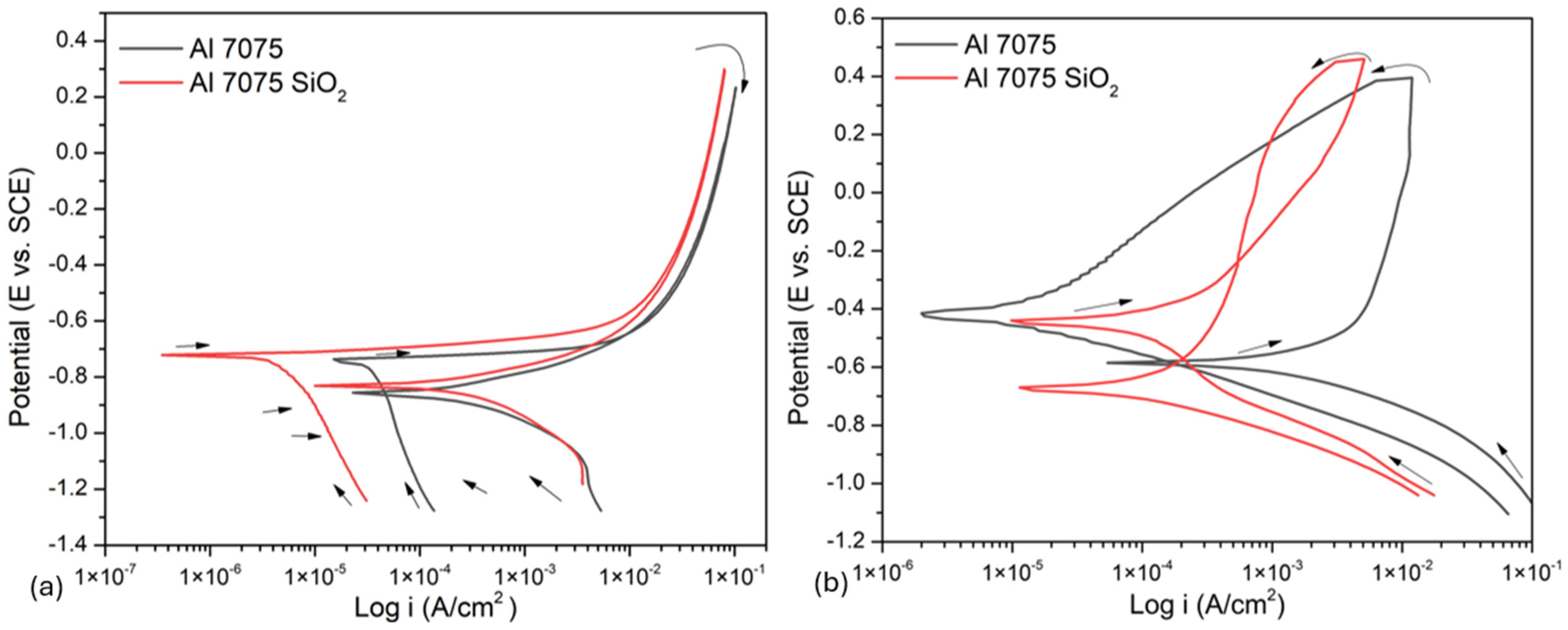
3.3.1. Cyclic Potentiodynamic Polarization
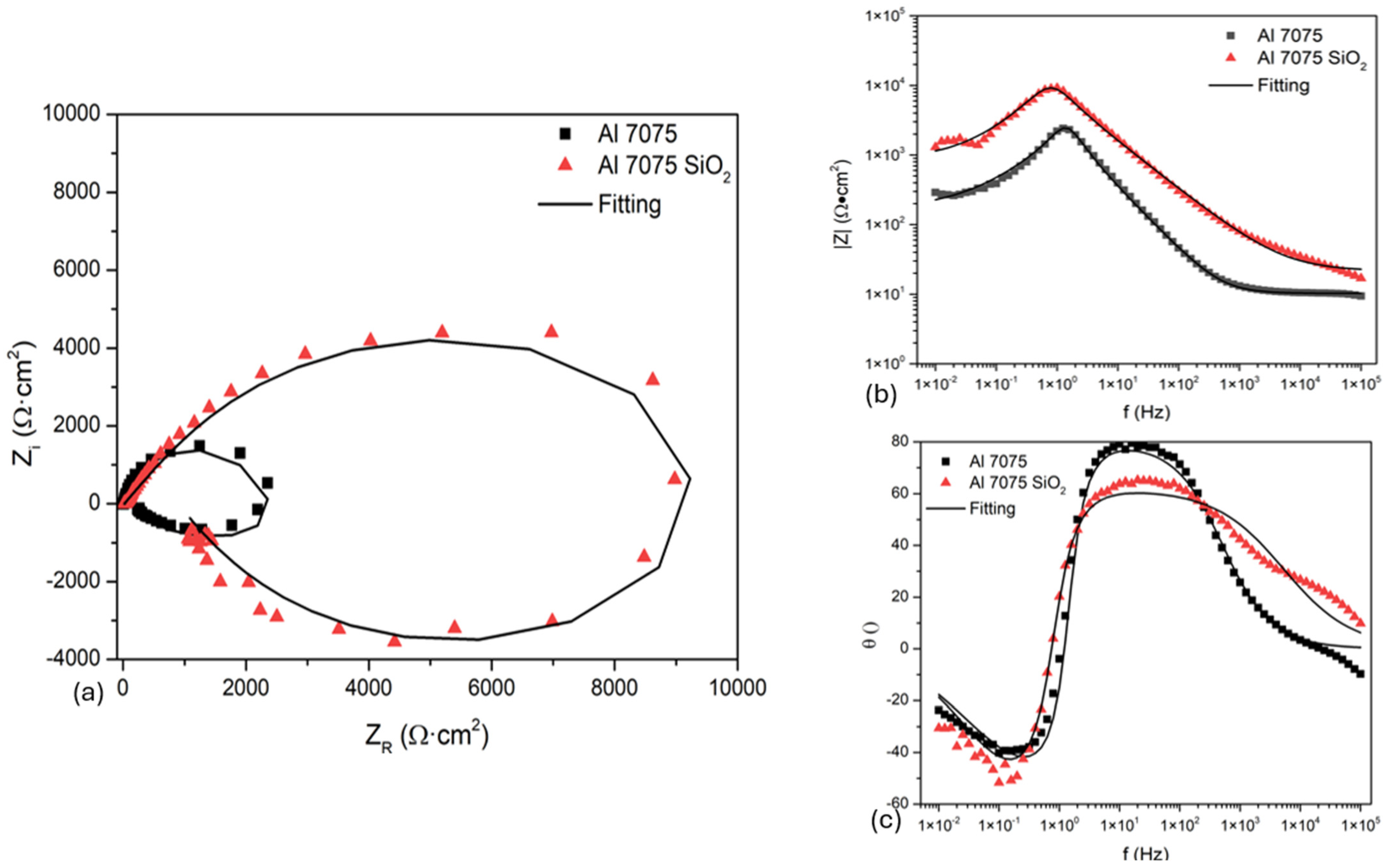
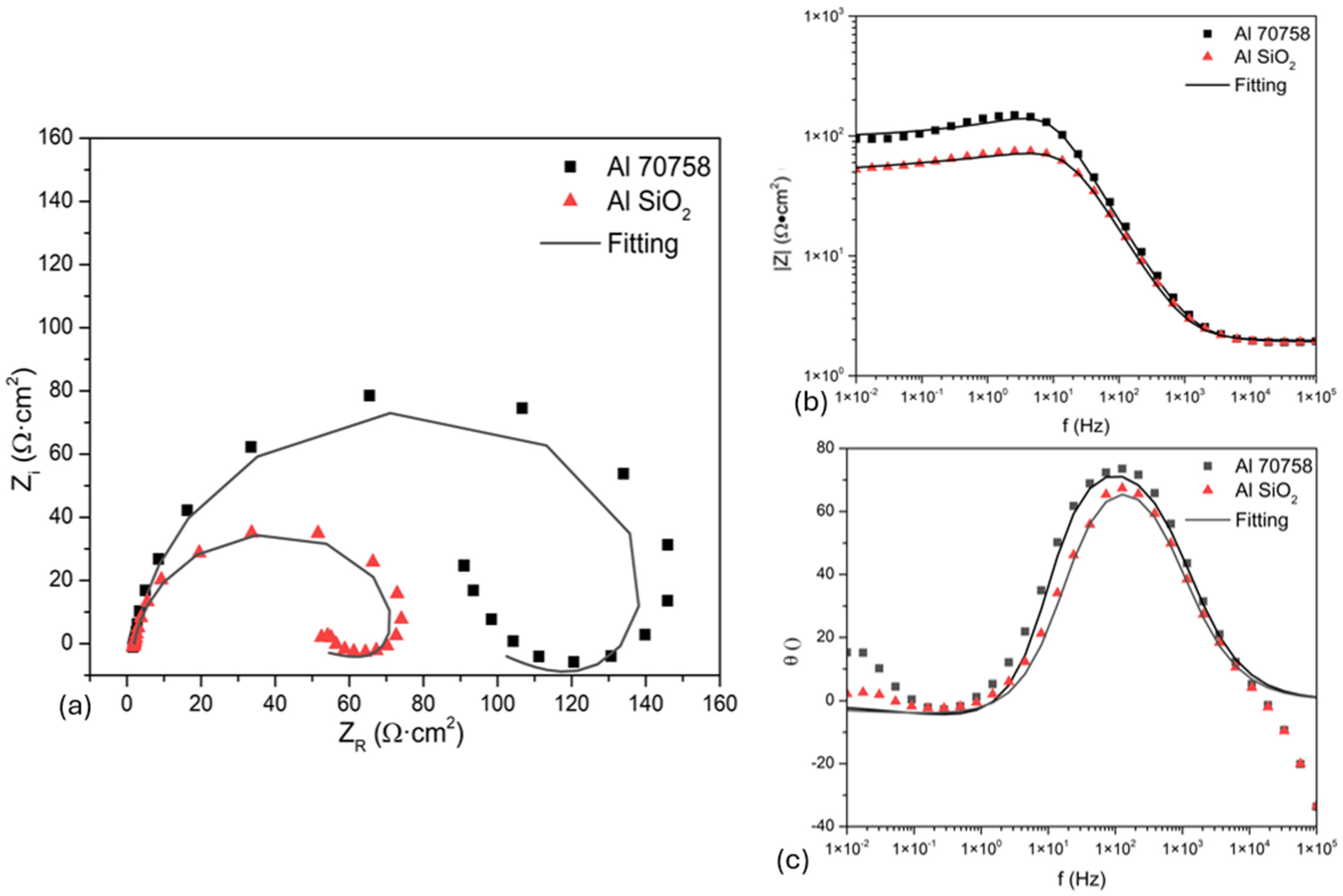
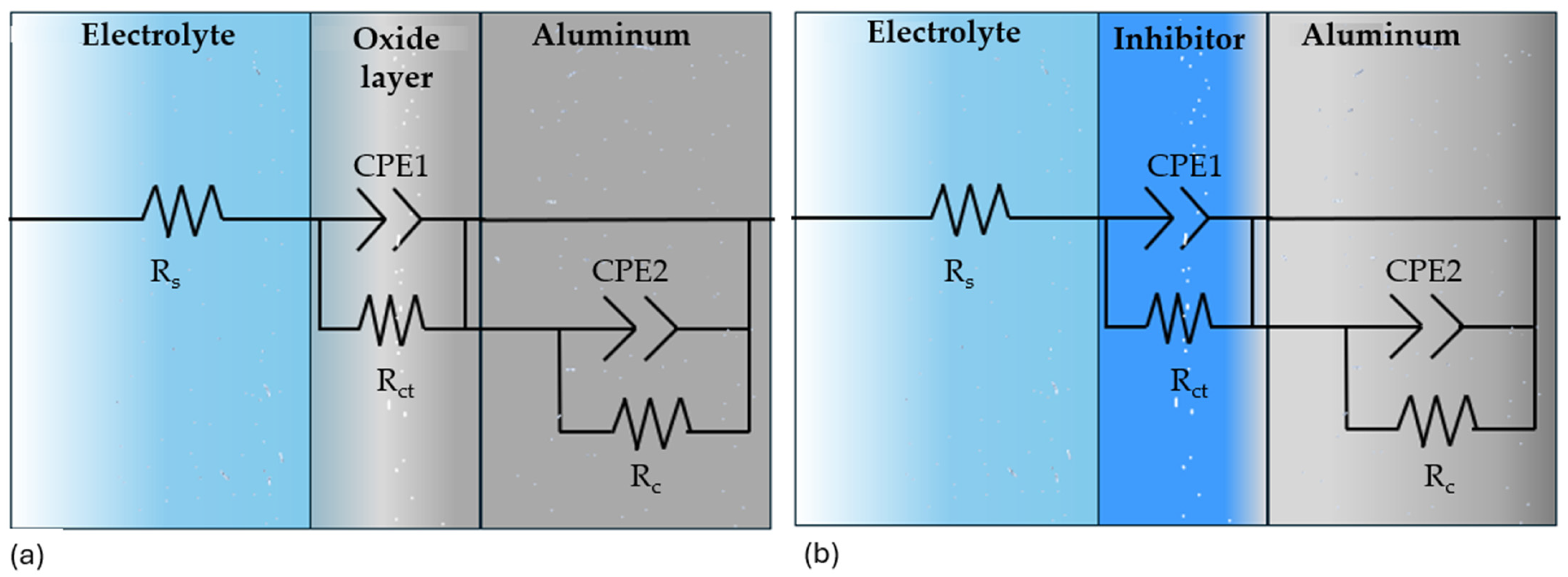
3.3.2. Electrochemical Impedance Spectroscopy
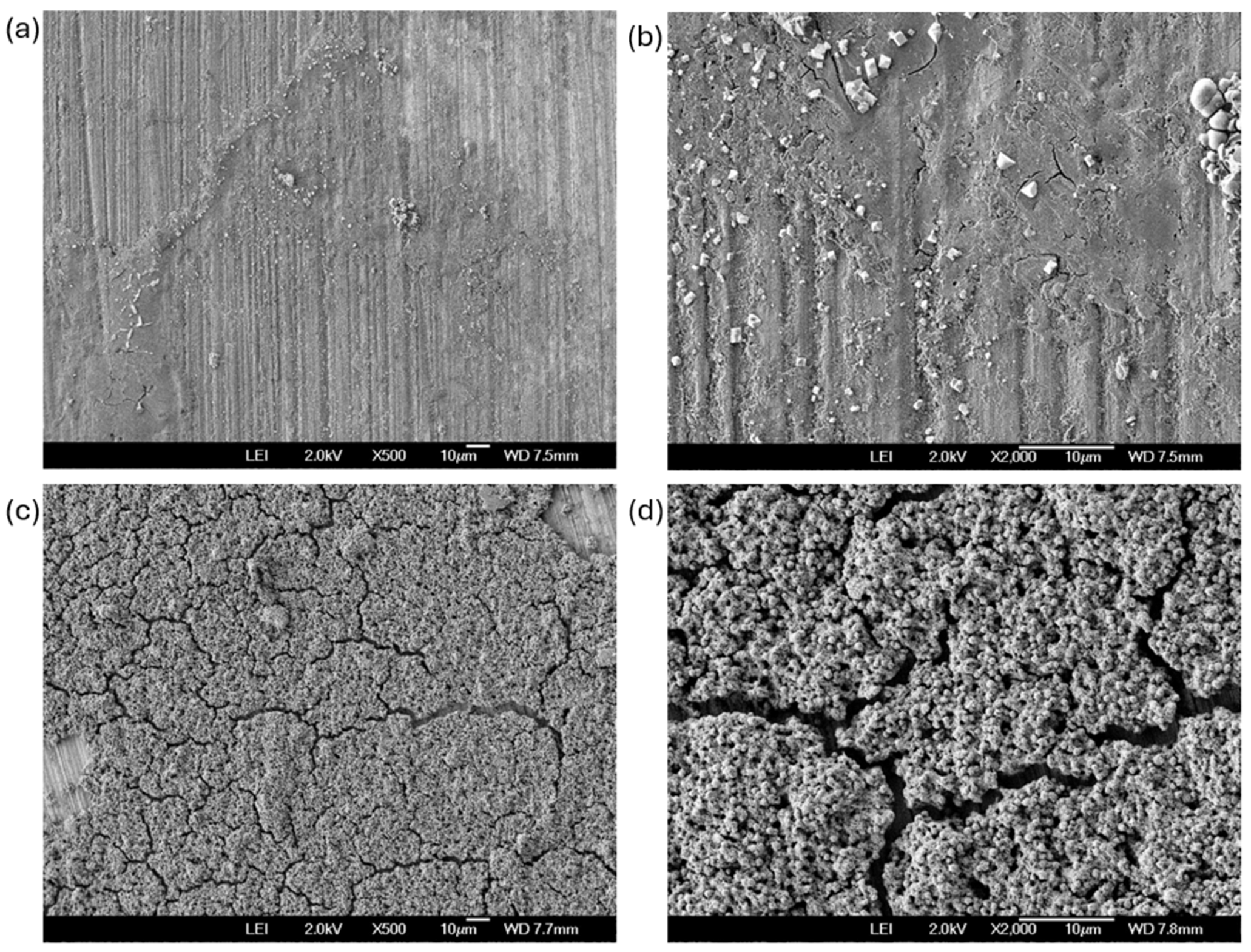
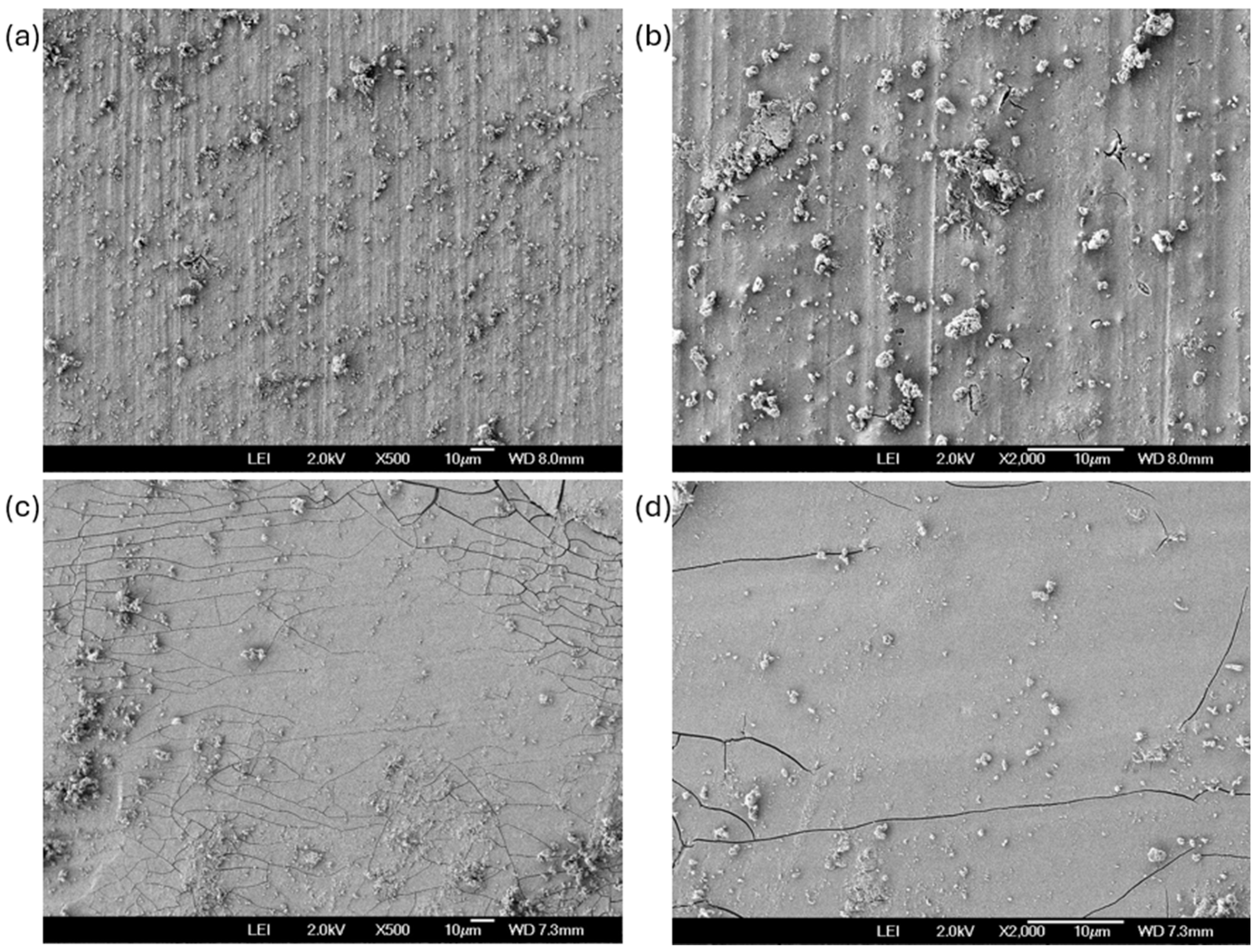
3.4. SEM After Corrosion Test
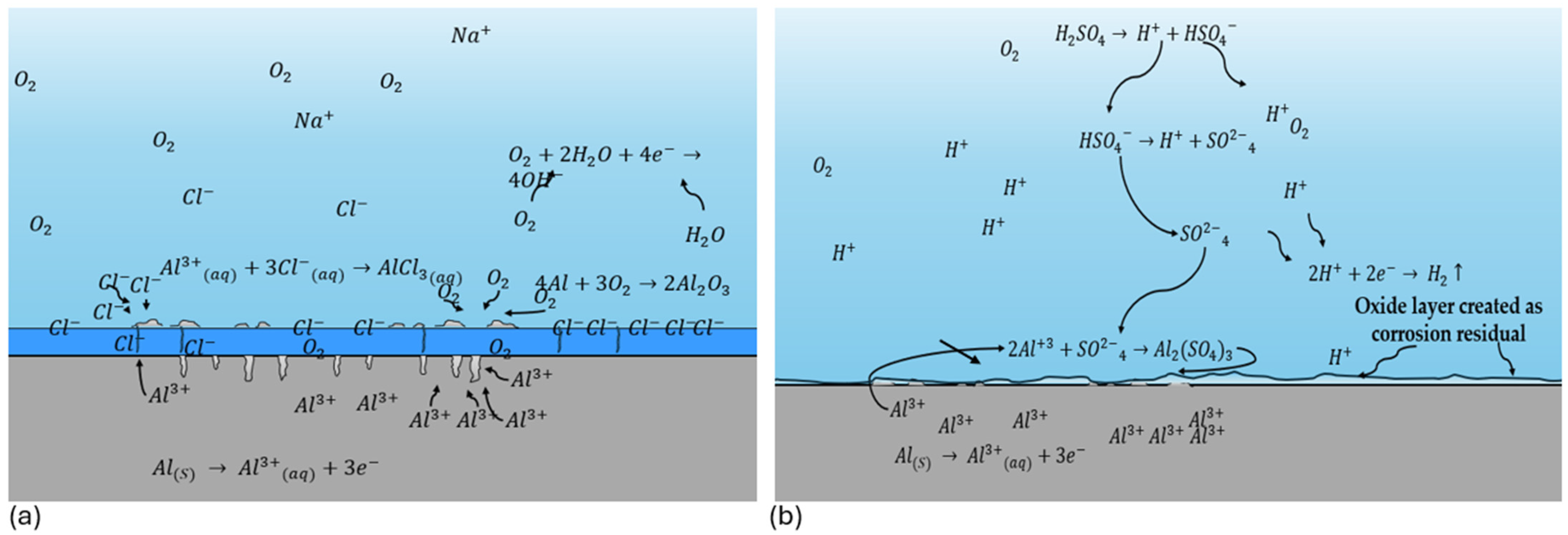
4. Discussion
5. Conclusions
- The coating of SiO2 presented a hierarchical structure with a heterogeneous distribution, yet it was nearly homogeneous; however, it exhibited crack zones that served as corrosion concentrators.
- Samples coated with superhydrophobic coatings showed a reduction in corrosion rate obtained by CPP, indicating that the coating protects the material.
- The Al 7075 coated with SiO2 presented localized corrosion when exposed to NaCl. Cl− ions attacked the cracking zone. On the other hand, the H2SO4 coating presented a uniform corrosion due to the dissolution of the coating in this medium.
- The inhibitor efficiency in NaCl is 81%, indicating higher corrosion resistance. Also, the Rct value of Al 7075 coated by SiO2 exposed to H2SO4 is 70% lower than the Rct in NaCl; therefore, this coating can be employed in marine atmospheres.
- The behavior of Al 7075 coated with SiO2 presented an inductive behavior, indicating that adsorption phenomena are occurring on the surface. This is the natural behavior of aluminum, and the coating did not alter it.
- The imperfections generated by cracking zones help form corrosion concentrators.
- Localized corrosion is occurring on the coating, which initiates the dissolution process of the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Guo, Y.; Wang, J.; Yergin, M. Corrosion Avoidance in Lightweight Materials for Automotive Applications. Npj Mater. Degrad. 2018, 2, 24. [Google Scholar] [CrossRef]
- Vukkum, V.B.; Christudasjustus, J.; Darwish, A.A.; Storck, S.M.; Gupta, R.K. Enhanced Corrosion Resistance of Additively Manufactured Stainless Steel by Modification of Feedstock. Npj Mater. Degrad. 2022, 6, 2. [Google Scholar] [CrossRef]
- Shin, B.H.; Park, J.; Jeon, J.; Heo, S.-B.; Chung, W. Effect of Cooling Rate after Heat Treatment on Pitting Corrosion of Super Duplex Stainless Steel UNS S 32750. Anti-Corros. Methods Mater. 2018, 65, 492–498. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, J. Overview on corrosion in automotive industry and thermal power plant. Proc. Eng. Sci. 2022, 4, 13–22. [Google Scholar] [CrossRef]
- Yin, Z.Z.; Qi, W.C.; Zeng, R.C.; Chen, X.B.; Gu, C.D.; Guan, S.K.; Zheng, Y.F. Advances in Coatings on Biodegradable Magnesium Alloys. J. Magnes. Alloys 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Yamauchi, N.; Ueda, N.; Okamoto, A.; Sone, T.; Tsujikawa, M.; Oki, S. DLC Coating on Mg–Li Alloy. Surf. Coat. Technol. 2007, 201, 4913–4918. [Google Scholar] [CrossRef]
- Sukiman, N.L.; Zhou, X.; Birbilis, N.; Hughes, A.E.; Mol, J.M.C.; Garcia, S.J.; Zhou, X.; Thompson, G.E.; Sukiman, N.L.; Zhou, X.; et al. Durability and Corrosion of Aluminium and Its Alloys: Overview, Property Space, Techniques and Developments. Alum. Alloys New Trends Fabr. Appl. 2012, 5, 47–97. [Google Scholar] [CrossRef]
- Hussain, C.M.; Verma, C.; Aslam, J.; Aslam, R.; Zehra, S. Handbook of Corrosion Engineering: Modern Theory, Fundamentals and Practical Applications; Elsevier: Holland, MI, USA, 2023; ISBN 978-0-323-95185-2. [Google Scholar] [CrossRef]
- Kadhim, A.; Al-Amiery, A.A.; Alazawi, R.; Al-Ghezi, M.K.S.; Abass, R.H. Corrosion Inhibitors. A Review. Int. J. Corros. Scale Inhib. 2021, 10, 54–67. [Google Scholar] [CrossRef]
- Sopha, H.; Norikawa, Y.; Motola, M.; Hromadko, L.; Rodriguez-Pereira, J.; Cerny, J.; Nohira, T.; Yasuda, K.; Macak, J.M. Anodization of Electrodeposited Titanium Films towards TiO2 Nanotube Layers. Electrochem. Commun. 2020, 118, 106788. [Google Scholar] [CrossRef]
- Islam, M.; Azhar, M.R.; Fredj, N.; Burleigh, T.D.; Oloyede, O.R.; Almajid, A.A.; Ismat Shah, S. Influence of SiO2 Nanoparticles on Hardness and Corrosion Resistance of Electroless Ni–P Coatings. Surf. Coat. Technol. 2015, 261, 141–148. [Google Scholar] [CrossRef]
- Sajadi, G.S.; Ali Hosseini, S.M.; Saheb, V.; Shahidi-Zandi, M. Using of Asymmetric Cell to Monitor Corrosion Performance of 304 Austenitic Stainless Steel by Electrochemical Noise Method. J. Mater. Res. Technol. 2023, 22, 107–125. [Google Scholar] [CrossRef]
- Kasturibai, S.; Kalaignan, G.P. Physical and Electrochemical Characterizations of Ni-SiO2 Nanocomposite Coatings. Ionics 2013, 19, 763–770. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/Layered Silicate Nanocomposites: A Review from Preparation to Processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Ruhi, G.; Modi, O.P.; Sinha, A.S.K.; Singh, I.B. Effect of Sintering Temperatures on Corrosion and Wear Properties of Sol–Gel Alumina Coatings on Surface Pre-Treated Mild Steel. Corros. Sci. 2008, 50, 639–649. [Google Scholar] [CrossRef]
- Hosseini, M.; Karapanagiotis, I. Materials with Extreme Wetting Properties: Methods and Emerging Industrial Applications; Springer: Cham, Switzerland, 2021; pp. 1–370. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizão, C.; Farinha, J.P.S. Functional Films from Silica/Polymer Nanoparticles. Materials 2014, 7, 3881–3900. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dong, Y.; Li, X.; Gao, Y.; He, W.; Liu, Y.; Mu, X.; Zhao, Y. Anti-Corrosion Superhydrophobic Micro-TiB2/Nano-SiO2 Based Coating with “Multi-Scale Hard Particles-Embedding-Soft Membrane” Structure Fabricated by Spray Deposition. J. Ind. Eng. Chem. 2025, 144, 496–511. [Google Scholar] [CrossRef]
- Yan, Y.L.; Cai, Y.X.; Liu, X.C.; Ma, G.W.; Lv, W.; Wang, M.X. Hydrophobic Modification on the Surface of SiO2 Nanoparticle: Wettability Control. Langmuir 2020, 36, 14924–14932. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle Decoration with Surfactants: Molecular Interactions, Assembly, and Applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Chen, H.; Fan, H.; Su, N.; Hong, R.; Lu, X. Highly Hydrophobic Polyaniline Nanoparticles for Anti-Corrosion Epoxy Coatings. Chem. Eng. J. 2021, 420, 130540. [Google Scholar] [CrossRef]
- Fuji, M.; Takei, T.; Watanabe, T.; Chikazawa, M. Wettability of Fine Silica Powder Surfaces Modified with Several Normal Alcohols. Colloids Surf. A Physicochem. Eng. Asp. 1999, 154, 13–24. [Google Scholar] [CrossRef]
- Al-Haidary, J.T.; Haddad, J.S.; Alfaqs, F.A.; Zayadin, F.F. Susceptibility of Aluminum Alloy 7075 T6 to Stress Corrosion Cracking. SAE Int. J. Mater. Manuf. 2020, 14, 195–201. [Google Scholar] [CrossRef]
- Kairy, S.K.; Turk, S.; Birbilis, N.; Shekhter, A. The Role of Microstructure and Microchemistry on Intergranular Corrosion of Aluminium Alloy AA7085-T7452. Corros. Sci. 2018, 143, 414–427. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Yang, L.; Du, C. Corrosion Resistance of 316L Stainless Steel in Acetic Acid by EIS and Mott-Schottky. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 574–578. [Google Scholar] [CrossRef]
- Xiong, Y.; Robson, J.D.; Yao, Y.; Zhong, X.; Guarracino, F.; Bendo, A.; Jin, Z.; Hashimoto, T.; Liu, X.; Curioni, M. Effects of Heat Treatments on the Microstructure and Localized Corrosion Behaviors of AA7075 Aluminum Alloy. Corros. Sci. 2023, 221, 111361. [Google Scholar] [CrossRef]
- Gupta, R.; Verma, R.; Kango, S.; Constantin, A.; Kharia, P.; Saini, R.; Kudapa, V.K.; Mittal, A.; Prakash, J.; Chamoli, P. A Critical Review on Recent Progress, Open Challenges, and Applications of Corrosion-Resistant Superhydrophobic Coating. Mater. Today Commun. 2023, 34, 105201. [Google Scholar] [CrossRef]
- Qiao, M.; Ji, G.; Lu, Y.; Zhang, B. Sustainable Corrosion-Resistant Superhydrophobic Composite Coating with Strengthened Robustness. J. Ind. Eng. Chem. 2023, 121, 215–227. [Google Scholar] [CrossRef]
- Mamgain, H.P.; Pati, P.R.; Samanta, K.K.; Brajpuriya, R.; Gupta, R.; Pandey, J.K.; Giri, J.; Sathish, T.; Kanan, M. A Review on Bio-Inspired Corrosion Resistant Superhydrophobic Coating on Copper Substrate: Recent Advances, Mechanisms, Constraints, and Future Prospects. Results Eng. 2025, 25, 103868. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Wang, M.; Yang, C.; Yang, Z.; Liu, X. Insights into the Stability of Fluorinated Superhydrophobic Coating in Different Corrosive Solutions. Prog. Org. Coat. 2021, 151, 106043. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Zhang, D.; Ou, J. Effect of Surface Nanostructure on Enhanced Atmospheric Corrosion Resistance of a Superhydrophobic Surface. Colloids Surf. Physicochem. Eng. Asp. 2022, 647, 129058. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Sánchez-Santamaria, B.; Cornejo-Monroy, D.; Olivas-Armendáriz, I.; Arias-Cerón, J.S.; Villanueva-Montellano, A.; Ordoñez-Casanova, E.; Dávalos-Ramírez, J.O.; Martínez-Gómez, E.A.; Jaquez-Muñoz, J.M. Antibacterial Activity of Superhydrophobic-SiO2 Coatings to Inhibit the Growth of Escherichia coli and Staphylococcus aureus. Coatings 2024, 14, 1211. [Google Scholar] [CrossRef]
- ASTM Standard G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron, Nickel or Cobalt Based Aalloys. ASTM: West Conshohocken, PA, USA, 2010; Volume 86.
- Vázquez Nuñez, L.E.; Cornejo-Monroy, D.; Sánchez-Santamaria, B.; Arias-Cerón, J.S.; Villanueva-Montellano, A.; López-Ibarra, A.A.; Jáquez-Muñoz, J.M. Study of Al7075 Localized Corrosion Inhibition by a SiO2 Superhydrophobic Coating Employing an Electrochemical Noise Technique. Coatings 2025, 15, 131. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Cheng, S.; Tian, J.; Chang, X.; Yin, Y. Corrosion Behavior of Superhydrophobic Surface on Copper in Seawater. Electrochim. Acta 2007, 52, 8003–8007. [Google Scholar] [CrossRef]
- Jin, M.; Xing, Q.; Chen, Z.; Jin, M.; Xing, Q.; Chen, Z. A Review: Natural Superhydrophobic Surfaces and Applications. J. Biomater. Nanobiotechnol. 2020, 11, 110–149. [Google Scholar] [CrossRef]
- Feng, B.L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L. Superhydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Sharma, A.; McQuillan, A.J.; Sharma, L.A.; Waddell, J.N.; Shibata, Y.; Duncan, W.J. Spark Anodization of Titanium–Zirconium Alloy: Surface Characterization and Bioactivity Assessment. J. Mater. Sci. Mater. Med. 2015, 26, 221. [Google Scholar] [CrossRef]
- Shibaeva, T.V.; Laurinavichyute, V.K.; Tsirlina, G.A.; Arsenkin, A.M.; Grigorovich, K.V. The Effect of Microstructure and Non-Metallic Inclusions on Corrosion Behavior of Low Carbon Steel in Chloride Containing Solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Wang, Z.B.; Hu, H.X.; Zheng, Y.G. Synergistic Effects of Fluoride and Chloride on General Corrosion Behavior of AISI 316 Stainless Steel and Pure Titanium in H2SO4 Solutions. Corros. Sci. 2018, 130, 203–217. [Google Scholar] [CrossRef]
- Feizi Mohazzab, B.; Jaleh, B.; Kakuee, O.; Fattah-alhosseini, A. Formation of Titanium Carbide on the Titanium Surface Using Laser Ablation in N-Heptane and Investigating Its Corrosion Resistance. Appl. Surf. Sci. 2019, 478, 623–635. [Google Scholar] [CrossRef]
- Engelhardt, G.R.; Macdonald, D.D. Monte-Carlo Simulation of Pitting Corrosion with a Deterministic Model for Repassivation. J. Electrochem. Soc. 2020, 167, 013540. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, S.I.; Osiceanu, P.; Calderon-Moreno, J.M.; Drob, P.; Vasilescu, E. Characterisation of Passive Film and Corrosion Behaviour of a New Ti-Ta-Zr Alloy in Artificial Oral Media: In Time Influence of PH and Fluoride Ion Content. Mater. Corros. 2015, 66, 971–981. [Google Scholar] [CrossRef]
- Castany, P.; Gordin, D.M.; Drob, S.I.; Vasilescu, C.; Mitran, V.; Cimpean, A.; Gloriant, T. Deformation Mechanisms and Biocompatibility of the Superelastic Ti–23Nb–0.7Ta–2Zr–0.5N Alloy. Shape Mem. Superelast. 2016, 2, 18–28. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An Electrochemical Impedance Spectroscopy Study of the Corrosion Behaviour of PVD Coated Steels in 0.5 N NaCl Aqueous Solution: Part II.: EIS Interpretation of Corrosion Behaviour. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Wicaksono, A.B.; Sutanto, H.; Ruslan, W. Effects of Immersion in the NaCl and H2SO4 Solutions on the Corrosion Rate, Microstructure, and Hardness of Stainless Steel 316L. Res. Eng. Struct. Mat 2023, 9, 1153–1168. [Google Scholar] [CrossRef]
- Wongpanya, P.; Wongpinij, T.; Photongkam, P.; Siritapetawee, J. Improvement in Corrosion Resistance of 316L Stainless Steel in Simulated Body Fluid Mixed with Antiplatelet Drugs by Coating with Ti-Doped DLC Films for Application in Biomaterials. Corros. Sci. 2022, 208, 110611. [Google Scholar] [CrossRef]
- Graedel, T.E. Corrosion Mechanisms for Aluminum Exposed to the Atmosphere. J. Electrochem. Soc. 1989, 136, 204C–212C. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zhang, D. Facile Fabrication of High-Aspect-Ratio Superhydrophobic Surface with Self-Propelled Droplet Jumping Behavior for Atmospheric Corrosion Protection. Appl. Surf. Sci. 2021, 555, 149549. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Yu, T.; Zhang, B. Versatile Nonfluorinated Superhydrophobic Coating with Self-Cleaning, Anti-Fouling, Anti-Corrosion and Mechanical Stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128701. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, L.; Wang, J.; Zhang, S.; Zhang, C.; Ke, W. EIS Evaluation on the Degradation Behavior of Rust-Preventive Oil Coating Exposure to NaCl Electrolyte. Electrochim. Acta 2024, 492, 144359. [Google Scholar] [CrossRef]
- Chávez-Díaz, M.P.; Luna-Sánchez, R.M.; Vazquez-Arenas, J.; Lartundo-Rojas, L.; Hallen, J.M.; Cabrera-Sierra, R. XPS and EIS Studies to Account for the Passive Behavior of the Alloy Ti-6Al-4V in Hank’s Solution. J. Solid State Electrochem. 2019, 23, 3187–3196. [Google Scholar] [CrossRef]
- Macdonald, J.R. Generalizations of ‘Universal Dielectric Response’ and a General Distribution-of-activation-energies Model for Dielectric and Conducting Systems. J. Appl. Phys. 1985, 58, 1971–1978. [Google Scholar] [CrossRef]
- Jafari-Tarzanagh, Y.; Seifzadeh, D.; Khodayari, A.; Samadianfard, R. Active Corrosion Protection of AA2024 Aluminum Alloy by Sol-Gel Coating Containing Inhibitor-Loaded Mesoporous SBA-15. Prog. Org. Coat. 2022, 173, 107166. [Google Scholar] [CrossRef]
- Terashima, A.; Iwai, M.; Kikuchi, T. Nanomorphological Changes of Anodic Aluminum Oxide Fabricated by Anodizing in Various Phosphate Solutions over a Wide PH Range. Appl. Surf. Sci. 2022, 605, 154687. [Google Scholar] [CrossRef]
- de Sousa Araujo, J.V.; Milagre, M.; Costa, I. A Historical, Statistical and Electrochemical Approach on the Effect of Microstructure in the Anodizing of Al Alloys: A Review. Crit. Rev. Solid State Mater. Sci. 2024, 49, 521–581. [Google Scholar] [CrossRef]
- Jaleh, B.; Nasri, A.; Chaharmahali, R.; Kaseem, M.; Fattah-alhosseini, A. Exploring Wear, Corrosion, and Microstructure in PEO Coatings via Laser Surface Treatments on Aluminum Substrates. Opt. Laser Technol. 2025, 181, 111958. [Google Scholar] [CrossRef]
- Tiringer, U.; van Dam, J.P.B.; Abrahami, S.T.; Terryn, H.; Kovač, J.; Milošev, I.; Mol, J.M.C. Scrutinizing the Importance of Surface Chemistry versus Surface Roughness for Aluminium/Sol-Gel Film Adhesion. Surf. Interfaces 2021, 26, 101417. [Google Scholar] [CrossRef]
- Li, B.; Xue, S.; Mu, P.; Li, J. Robust Self-Healing Graphene Oxide-Based Superhydrophobic Coatings for Efficient Corrosion Protection of Magnesium Alloys. ACS Appl. Mater. Interfaces 2022, 14, 30192–30204. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, K.; Hong, F.; Wu, S.; Wang, Z.; Li, M.; Wang, M. The Corrosion Deterioration of Reinforced Passivation Film: The Impact of Defects. Appl. Surf. Sci. 2022, 582, 152408. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Vanithakumari, S.C.; Nanda Gopala Krishna, D.; George, R.P.; Srinivasan, R.; Philip, J. Facile Fabrication of Robust Superhydrophobic Aluminum Surfaces with Enhanced Corrosion Protection and Antifouling Properties. Prog. Org. Coat. 2022, 162, 106560. [Google Scholar] [CrossRef]
- Tran, N.G.; Chun, D.M.; Abd-Elrahim, A.G. Superhydrophobic Aluminum Surfaces with Nano-Micro Hierarchical Composite Structures: A Novel and Sustainable Approach to Corrosion Protection. J. Alloys Compd. 2023, 960, 170907. [Google Scholar] [CrossRef]
- Panda, B. Corrosion Resistant Superhydrophobic Aluminum Alloy: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1017, 012008. [Google Scholar] [CrossRef]

| Sample | Ecorr (mV) | icorr (A/cm2) | Hysteresis |
|---|---|---|---|
| NaCl | |||
| Al 7075 | −750 ± 5 | 1.54 × 10−4–750 ± 5 × 10−5 | Positive |
| Al 7075 SiO2 | −721 ± 4 | 5.91 × 10−5 ± 4 × 10−5 | Positive |
| H2SO4 | |||
| Al 7075 | −589 ± 7 | 1.45 × 10−3 ± 2 × 10−4 | Negative |
| Al 7075 SiO2 | −420 ± 6 | 2.28 × 10−4 ± 5 × 10−5 | Negative |
| Sample | Rs (Ω·cm2) | CPE1-T (F·sn−1/cm2) | n | Rct (Ω·cm2) | CPE2-T (F·sn−1/cm2) | n | RC (Ω·cm2) | IE (%) |
|---|---|---|---|---|---|---|---|---|
| NaCl | ||||||||
| Al 7075 | 21 ± 0.4 | 3.6 × 10−5 ± 2 × 10−6 | 0.7 ± 0.09 | 874 ± 9.1 | 3.42 × 10−4 ± 3.3 × 10−4 | −0.7 ± 0.09 | 1.04 × 103 ± 94 | - |
| Al 7075 SiO2 | 10 ± 1.2 | 6.6 × 10−5 ± 1.5 × 10−6 | 0.9 ± 0.07 | 177 ± 7.2 | 2.09 × 10−3 ± 4.1 × 10−4 | −0.6 ± 0.09 | 1.01 × 104 ± 86 | 81.37 |
| H2SO4 | ||||||||
| Al 7075 | 1.9 ± 0.08 | 1.1 × 10−4 ± 1.7 × 10−5 | 0.9 ± 0.04 | 99 ± 8.4 | 0.066 ± 4.9 × 10−4 | −0.8 ± 0.08 | 52 ± 4.6 | - |
| Al 7075 SiO2 | 1.9 ± 0.09 | 1.5 × 10−4 ± 2.2 × 10−5 | 0.9 ± 0.05 | 52 ± 6.1 | 0.11 ± 1.9 × 10−3 | −0.7 ± 0.09 | 22 ± 1.8 | −102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jáquez-Muñoz, J.M.; Vázquez-Nuñez, L.E.; Sánchez-Santamaria, B.; Arias-Cerón, J.S.; Santana-Esquivel, J.G.; Diaz-Olivares, A.; Arambula-Miranda, L.E.; Carrera-Rámirez, M.G.; López-Ibarra, A.A.; Cornejo-Monroy, D. Electrochemical Corrosion Behavior of SiO2 Superhydrophobic Inhibitor in Al7075. Coatings 2025, 15, 1064. https://doi.org/10.3390/coatings15091064
Jáquez-Muñoz JM, Vázquez-Nuñez LE, Sánchez-Santamaria B, Arias-Cerón JS, Santana-Esquivel JG, Diaz-Olivares A, Arambula-Miranda LE, Carrera-Rámirez MG, López-Ibarra AA, Cornejo-Monroy D. Electrochemical Corrosion Behavior of SiO2 Superhydrophobic Inhibitor in Al7075. Coatings. 2025; 15(9):1064. https://doi.org/10.3390/coatings15091064
Chicago/Turabian StyleJáquez-Muñoz, Jesús Manuel, Luis Eduardo Vázquez-Nuñez, Betania Sánchez-Santamaria, José Saúl Arias-Cerón, Jaime Gonzalo Santana-Esquivel, Abel Diaz-Olivares, Luis Enrique Arambula-Miranda, Martha Guadalupe Carrera-Rámirez, Aurora Abigail López-Ibarra, and Delfino Cornejo-Monroy. 2025. "Electrochemical Corrosion Behavior of SiO2 Superhydrophobic Inhibitor in Al7075" Coatings 15, no. 9: 1064. https://doi.org/10.3390/coatings15091064
APA StyleJáquez-Muñoz, J. M., Vázquez-Nuñez, L. E., Sánchez-Santamaria, B., Arias-Cerón, J. S., Santana-Esquivel, J. G., Diaz-Olivares, A., Arambula-Miranda, L. E., Carrera-Rámirez, M. G., López-Ibarra, A. A., & Cornejo-Monroy, D. (2025). Electrochemical Corrosion Behavior of SiO2 Superhydrophobic Inhibitor in Al7075. Coatings, 15(9), 1064. https://doi.org/10.3390/coatings15091064






