Defect Engineering of ZnIn2S4 Photocatalysts for Enhanced Hydrogen Evolution Reaction
Abstract
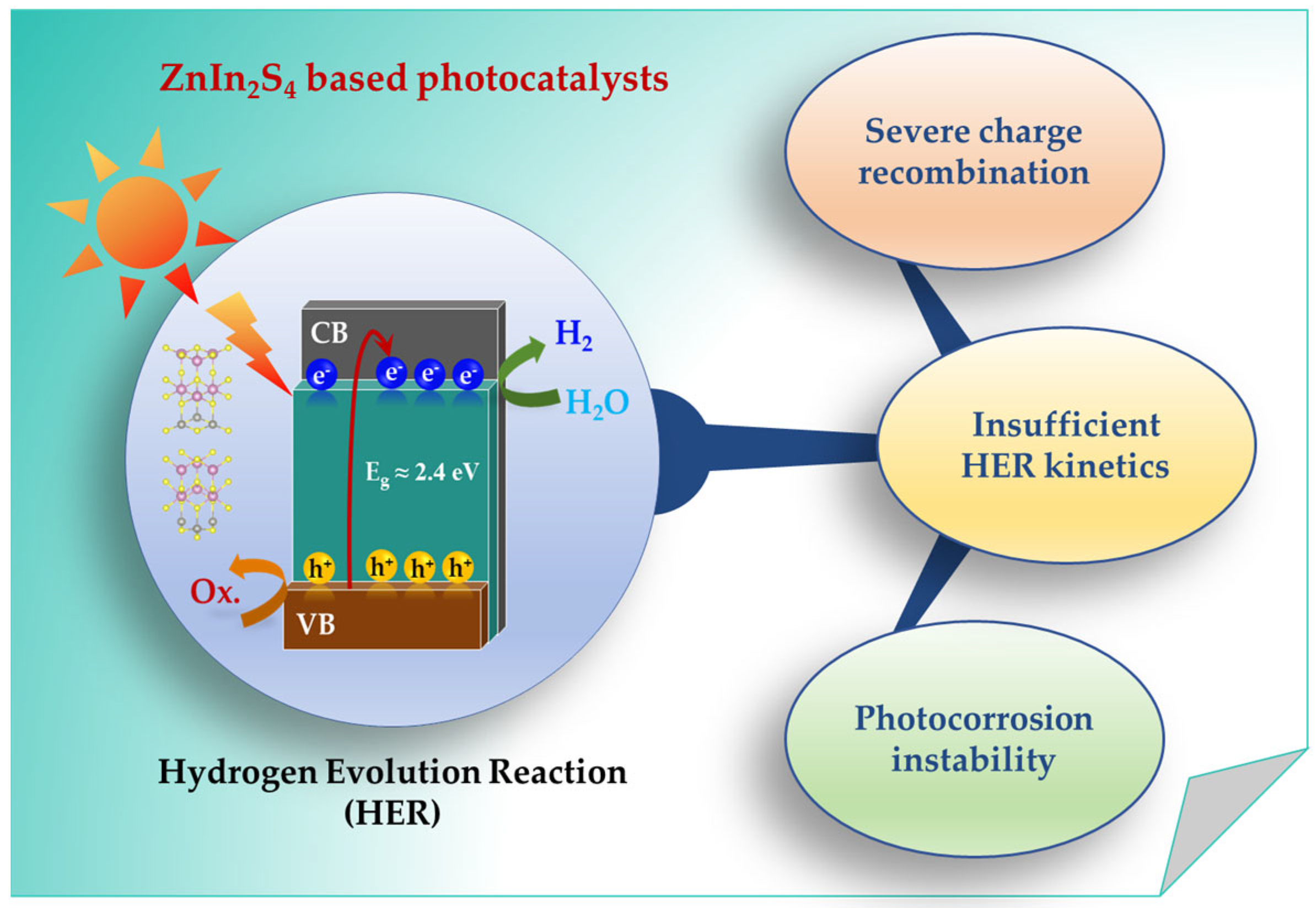
1. Introduction
- (i)
- Severe charge recombination: Due to inter-layer potential barriers (>0.5 eV) and deep-trap states (0.8–1.4 eV below the conduction band minimum) induced by sulfur vacancies (VS), which function as Auger-mediated recombination centers. These constraints confine carrier transport primarily to out-of-plane pathways, culminating in >80% bulk recombination within 5 ns rapid carrier recombination due to inter-layer barriers and inherent defects [15,34,35].
- (ii)
- Insufficient HER kinetics: Arising from low active-site density (<5% S-atom utilization). Density functional theory (DFT) calculations reveal that this originates from suboptimal orbital hybridization: the d-band center of surface In atoms resides at −2.8 eV below EF, weakening S 3p–H 1s coupling. Consequently, the Gibbs free energy of hydrogen adsorption (ΔGH) deviates substantially from thermoneutrality (ΔGH ≈ 0.35 eV), placing ZIS on the weak-adsorption branch of the HER [36,37,38].
- (iii)
- Photocorrosion instability: Mediated by VS sites, which initiate an autocatalytic degradation cycle. Under illumination, VS-derived trap states (0.8 eV below CBM) accumulate photogenerated holes, oxidizing adjacent S atoms and releasing H2S, thereby inducing irreversible structural collapse [39,40,41,42].
2. Classification and Mechanisms of Defect Engineering Strategies
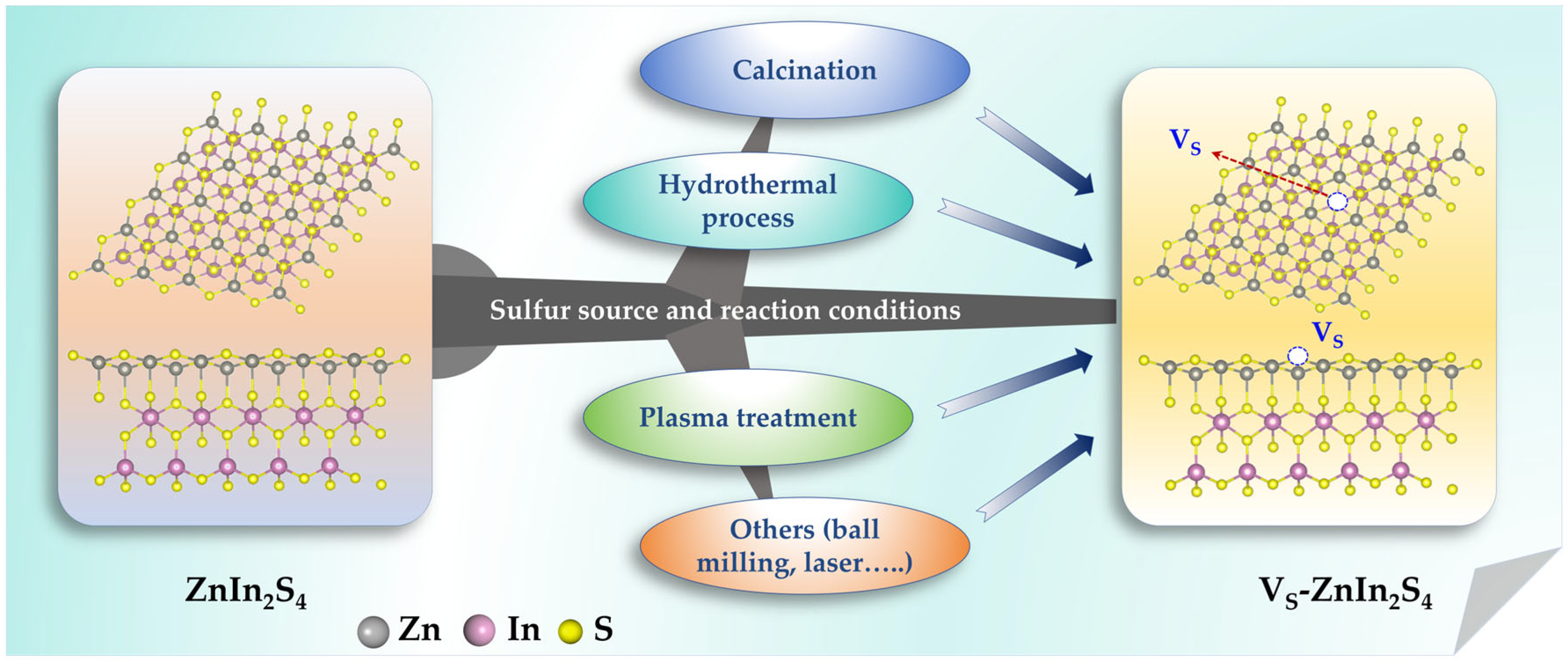
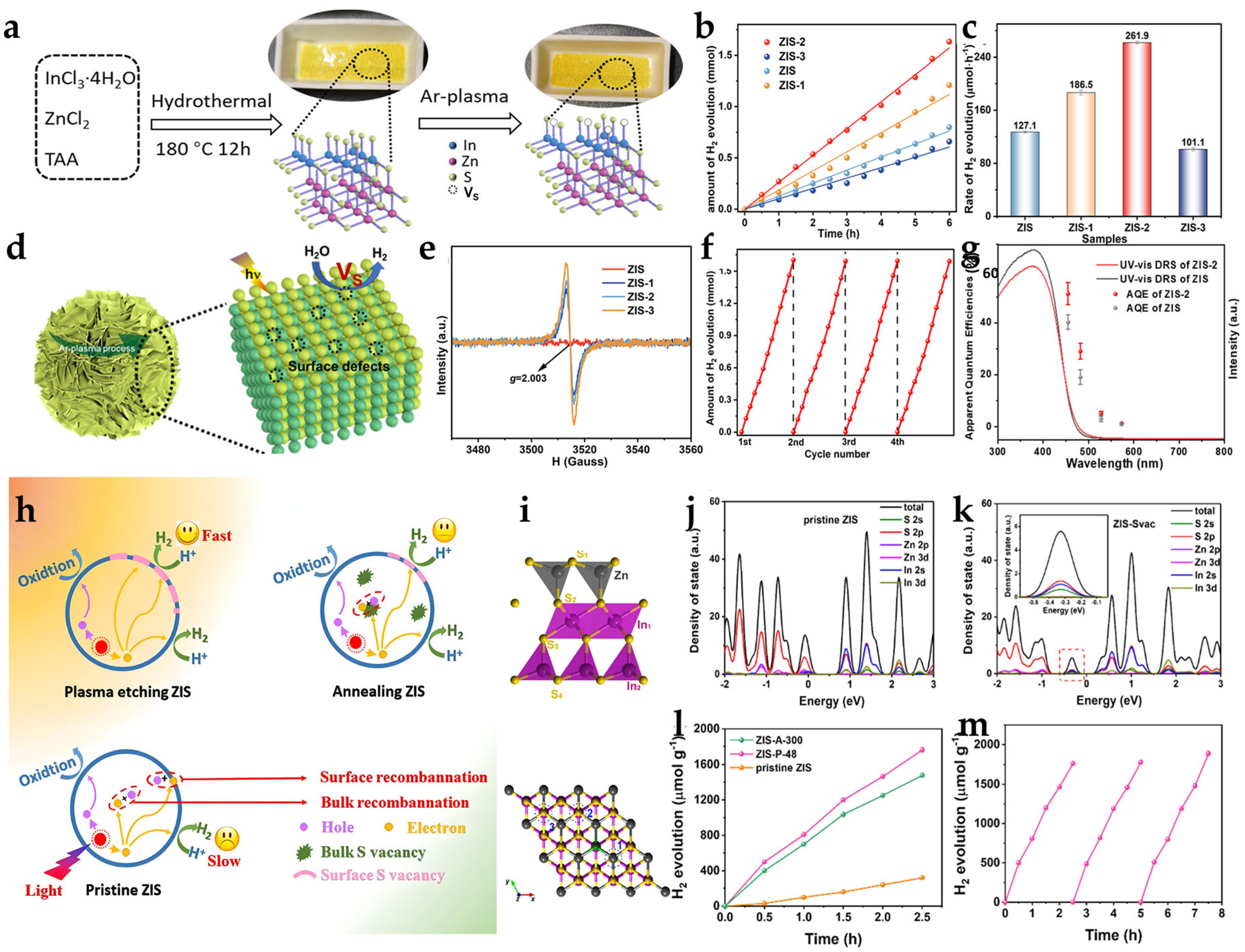
2.1. Sulfur Vacancies (VS)
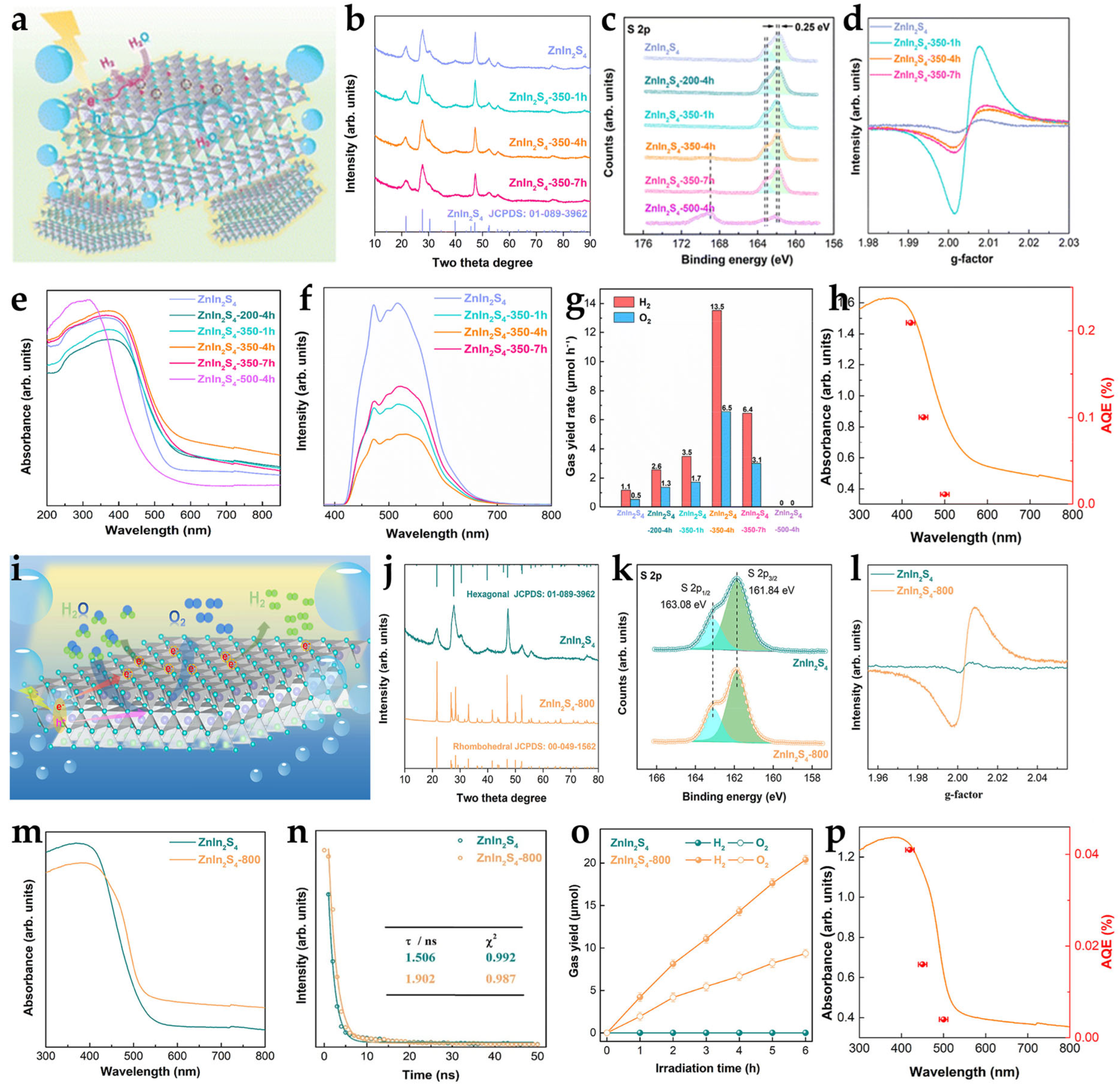
2.1.1. High-Temperature Calcination Method
2.1.2. Hydrothermal/Solvothermal Reduction Method
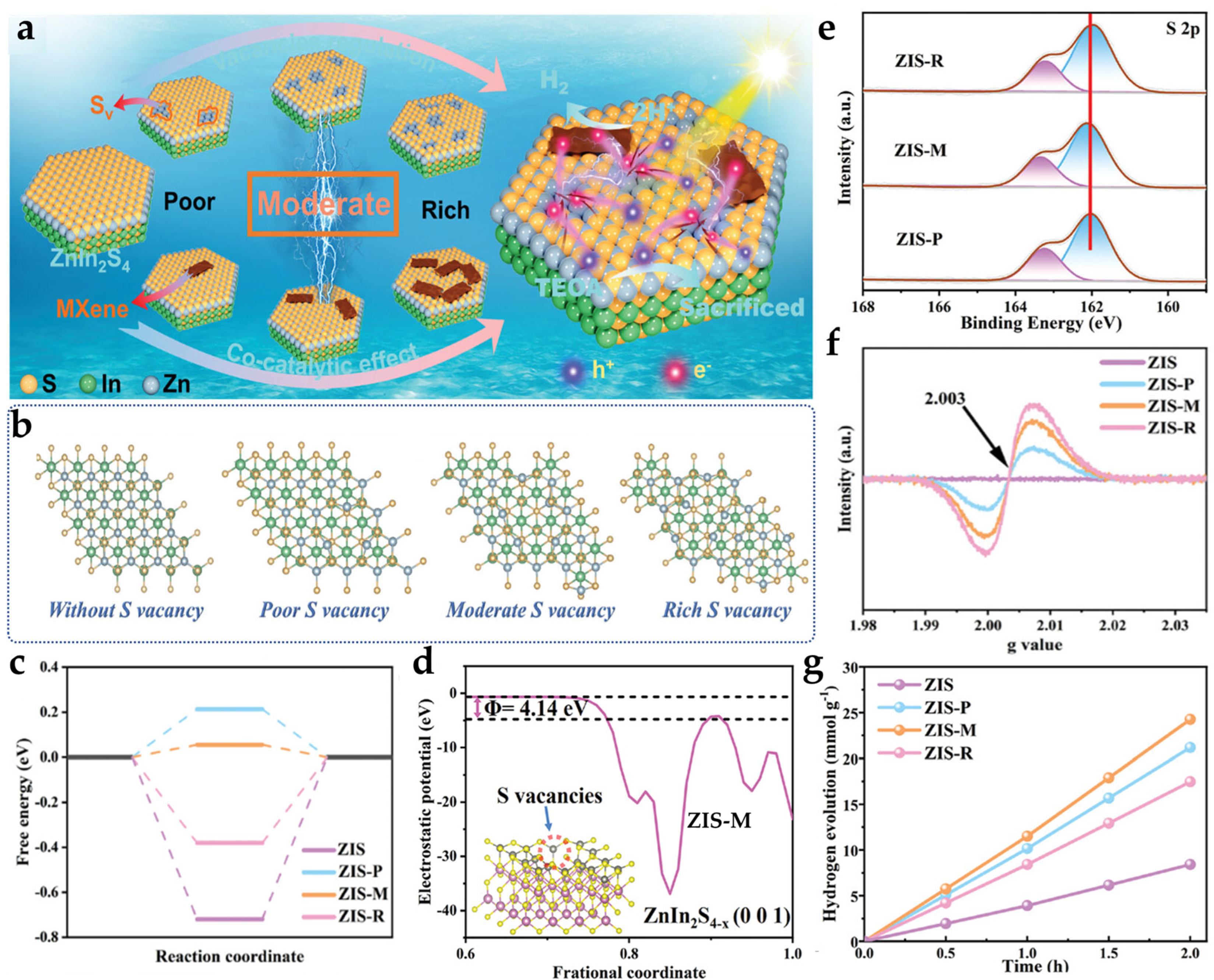
2.1.3. Plasma Treatment Technology
2.1.4. Other Methods
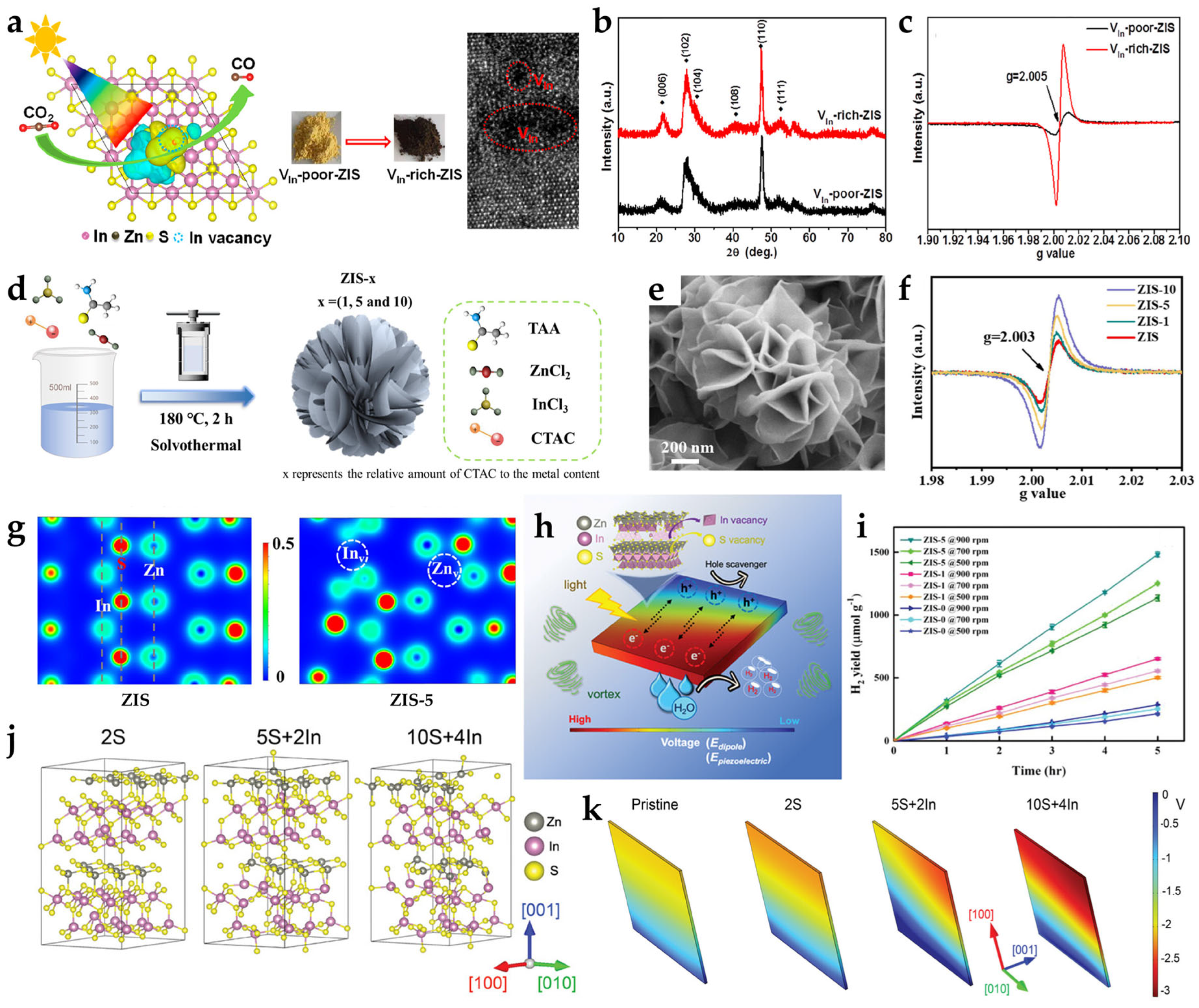
2.2. Zinc or Indium Vacancies
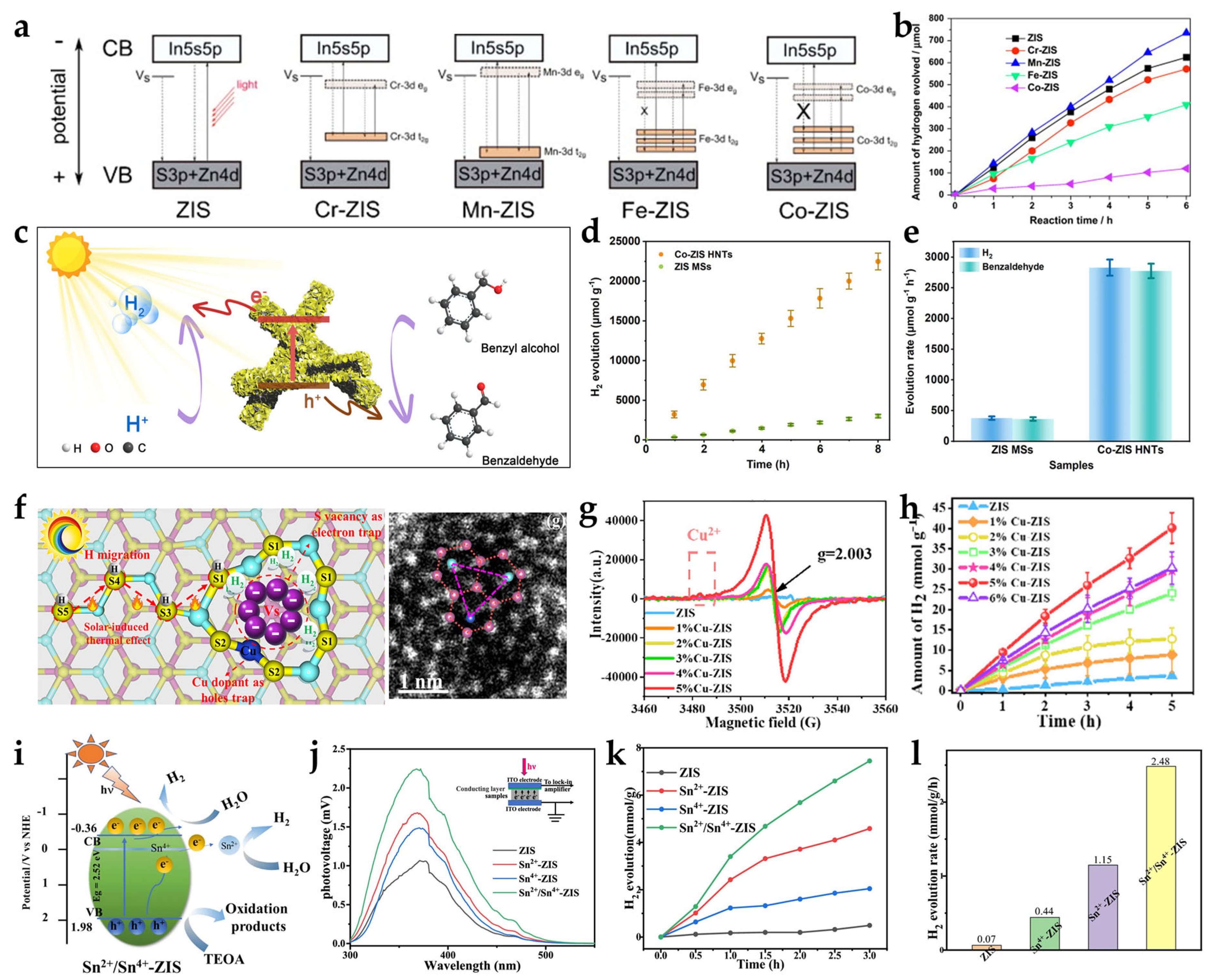
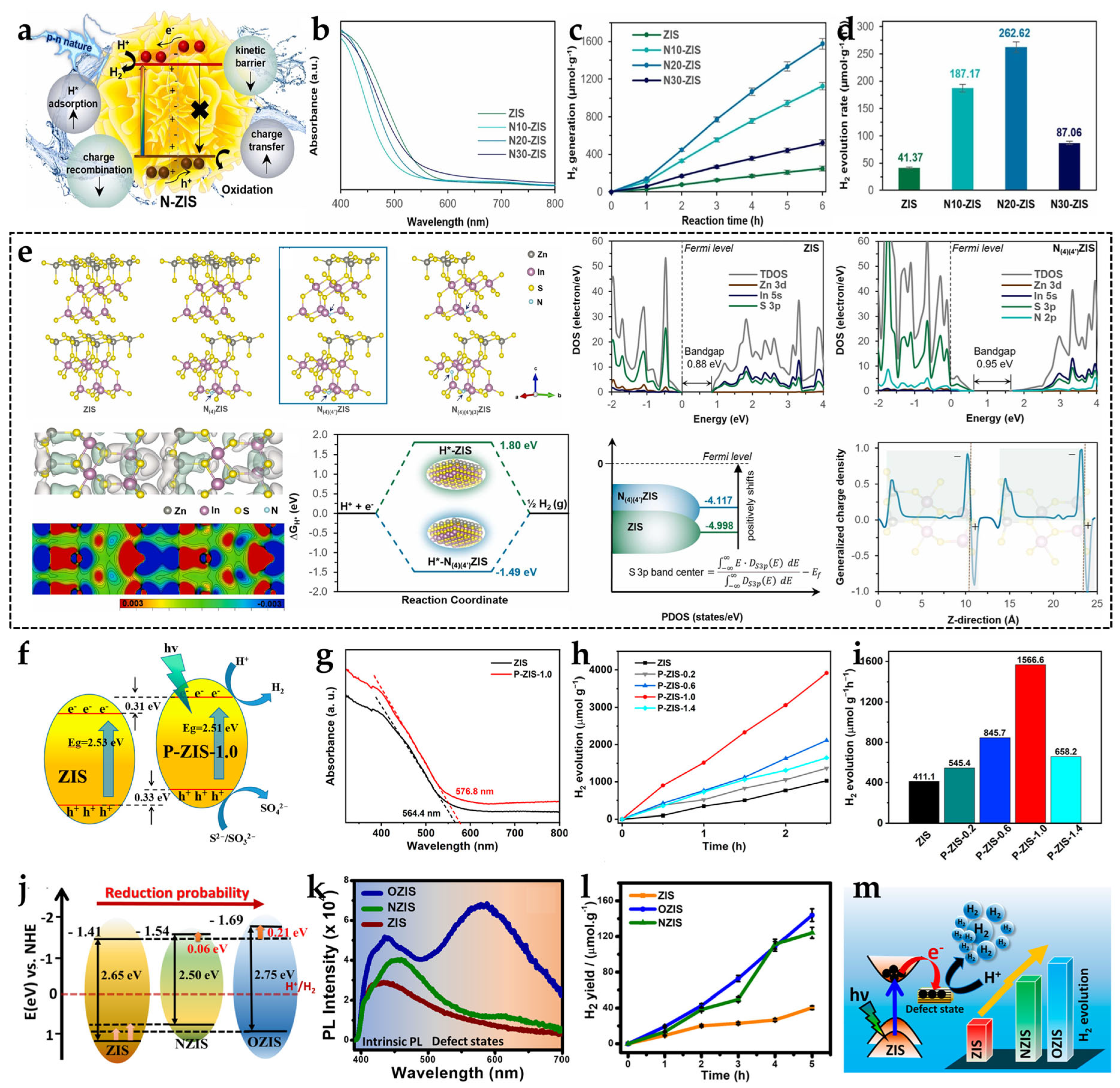
2.3. Doping Engineering (Metal/Non-Metal Doping)
2.4. Performance Enhancements and Mechanistic Insights
3. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Zhang, J.; Li, X.; Guan, S.; Dai, M.; Wang, S. 1D/2D heterostructured photocatalysts: From design and unique properties to their environmental applications. Small 2020, 16, 2005051. [Google Scholar] [CrossRef]
- Zhu, K.; Jie, O.-Y.; Zeng, Q.; Meng, S.; Teng, W.; Song, Y.; Tang, S.; Cui, Y. Fabrication of hierarchical ZnIn2S4OCNO nanosheets for photocatalytic hydrogen production and CO2 photoreduction. Chin. J. Catal. 2020, 41, 454–463. [Google Scholar] [CrossRef]
- Yu, H.; Dai, M.; Zhang, J.; Chen, W.; Jin, Q.; Wang, S.; He, Z. Interface engineering in 2D/2D heterogeneous photocatalysts. Small 2023, 19, 2205767. [Google Scholar] [CrossRef]
- Tian, S.; Feng, Y.; Zheng, Z.; He, Z. TiO2-Based photocatalytic coatings on glass substrates for environmental applications. Coatings 2023, 13, 1472. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, J.; Lu, T.; Zhou, G.-h.; Ren, Y.; Zheng, Z.; Yuan, X.-z.; Wang, S.-g.; He, Z. High-strength TiO2/TPU composite fiber based textiles for organic pollutant removal. npj Clean Water 2024, 7, 98. [Google Scholar] [CrossRef]
- Li, X.; Chang, J.; Zhang, S.; Xiao, L.; Wu, X.; He, Z. Microcystis@TiO2 nanoparticles for photocatalytic reduction reactions: Nitrogen fixation and hydrogen evolution. Catalysts 2021, 11, 1443. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chiao, Y.-C.; Hsu, P.-C. Rapid Microwave-Assisted synthesis of ZnIn2S4 nanosheets for highly efficient photocatalytic hydrogen production. Nanomaterials 2023, 13, 1957. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Meng, Q.; Tian, L.; Cai, Y.; Zhang, Y.; Chen, Y. Engineering of g-C3N4 for Photocatalytic Hydrogen Production: A Review. Int. J. Mol. Sci. 2024, 25, 8842. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, Z. Custom exposed crystal facets: Synergistic effect of optimum crystal facet anisotropy and Ohmic heterojunction boosting photocatalytic hydrogen evolution. Chin. J. Catal. 2025, 74, 294–307. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Lin, L.H.; Jeon, T.H.; Lin, S.; Wang, X.C.; Choi, W. Formation of heterostructures via direct growth CN on h-BN porous nanosheets for metal-free photocatalysis. Nano Energy 2017, 42, 58–68. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Jeon, T.H.; Choi, W. Hydrogenated heterojunction of boron nitride and titania enables the photocatalytic generation of H2 in the absence of noble metal catalysts. Appl. Catal. B Environ. 2018, 237, 772–782. [Google Scholar] [CrossRef]
- Hu, Q.; Niu, J.; Zhang, K.-Q.; Yao, M. One-dimensional CdS/SrTiO3/carbon fiber core-shell photocatalysts for enhanced photocatalytic hydrogen evolution. Coatings 2022, 12, 1235. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, B.; Yang, H.; Li, X.; Qiu, L.; Li, S. Application of metals and their compounds/black phosphorus-based nanomaterials in the direction of photocatalytic hydrogen evolution. Coatings 2024, 14, 1141. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Hao, X. Ni, Co-based selenide anchored g-C3N4 for boosting photocatalytic hydrogen evolution. Acta Phys.-Chim. Sin. 2021, 37, 1912033. [Google Scholar]
- Chen, L.-J.; Liu, T.-T.; Liu, S.-M.; Cai, S.; Zou, X.-X.; Jiang, J.-W.; Mei, Z.-Y.; Zhao, G.-F.; Yang, X.-F.; Guo, H. S vacant CuIn5S8 confined in a few-layer MoSe2 with interlayer-expanded hollow heterostructures boost photocatalytic CO2 reduction. Rare Met. 2022, 41, 144–154. [Google Scholar] [CrossRef]
- Sun, T.; Li, C.; Bao, Y.; Fan, J.; Liu, E. S-scheme MnCo2S4/g-C3N4 heterojunction photocatalyst for H2 production. Acta Phys.-Chim. Sin. 2023, 39, 2212009. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Lin, Z.; Wang, Y.-Q.; Zhang, K.-N.; Zhang, G.-H.; Zhang, J.; Mao, S.S.; Shen, S.-H. A direct polymeric carbon nitride/tungsten oxide Z-scheme heterostructure for efficient photocatalytic hydrogen generation via reforming of plastics into value-added chemicals. Rare Met. 2024, 43, 3771–3783. [Google Scholar] [CrossRef]
- Xiao, L.H.; Li, X.; Zhang, J.; He, Z.L. MgB4 MXene-like nanosheets for photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2021, 4, 12779–12787. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.-Z.; Jin, Z.-L. Graphdiyne (CnH2n-2) as an “electron transfer bridge” boosting photocatalytic hydrogen evolution over Zn0.5Co0.5S/MoS2 S-scheme heterojunction. Rare Met. 2024, 43, 1999–2014. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Cao, Q.; Li, G.-X.; Liu, H.; Zeng, L.-L.; Zhao, L.-L.; Chang, B.; Wang, X.-W.; Liu, H.; Zhou, W.-J. Orientation controlled photogenerated carriers on self-supporting CdS/Ni3S2 paper toward photocatalytic hydrogen evolution and biomass upgrading. Rare Met. 2024, 43, 2015–2025. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Wang, H.; Liu, M.; Xu, Y.; Liu, K.; Zhang, B.; Shi, K.; Zhang, J.; Ma, G. Facet-oriented surface modification for enhancing photocatalytic hydrogen production on Sm2Ti2O5S2 nanosheets. Chin. J. Catal. 2025, 74, 341–351. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Zhang, P.; Li, X.; Wang, S. ZnWO4-ZnIn2S4 S-scheme heterojunction for enhanced photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 122, 231–242. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, F.; Guo, Y.; Guo, F.; Shi, W.; Yang, S. Near-infrared light driven ZnIn2S4-based photocatalysts for environmental and energy applications: Progress and perspectives. Molecules 2023, 28, 2142. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, J.-J.; Liu, Z.-F.; Ma, H.-Y.; Fang, P.-F.; Xiong, R.; Wei, J.-H. Fast charge transfer kinetics in Sv-ZnIn2S4/Sb2S3 S-scheme heterojunction photocatalyst for enhanced photocatalytic hydrogen evolution. Rare Met. 2024, 43, 533–542. [Google Scholar] [CrossRef]
- Yang, W.-N.; Yang, J.; Yang, H.; Sun, L.; Li, H.-X.; Li, D.-C.; Dou, J.-M.; Li, X.-G.; Cao, G.-D. In-situ construction of tubular core-shell noble-metal-free CMT@TiO2/ZnIn2S4 S-scheme heterojunction for superior photothermal-photocatalytic hydrogen evolution. Rare Met. 2025, 44, 2474–2488. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Cao, W.; Zhang, J.; Chen, W.; Jin, Q.; Que, W.; Wang, S. Rational construction of S-scheme BN/MXene/ZnIn2S4 heterojunction with interface engineering for efficient photocatalytic hydrogen production and chlorophenols degradation. Sep. Purif. Technol. 2023, 309, 123004. [Google Scholar] [CrossRef]
- Zheng, X.L.; Song, Y.M.; Liu, Y.H.; Yang, Y.Q.; Wu, D.X.; Yang, Y.J.; Feng, S.Y.; Li, J.; Liu, W.F.; Shen, Y.J.; et al. ZnIn2S4-based photocatalysts for photocatalytic hydrogen evolution via water splitting. Coord. Chem. Rev. 2023, 475, 214898. [Google Scholar] [CrossRef]
- Dai, M.; Yu, H.; Chen, W.; Qu, K.-A.; Zhai, D.; Liu, C.; Zhao, S.; Wang, S.; He, Z. Boosting photocatalytic activity of CdLa2S4/ZnIn2S4 S-scheme heterojunctions with spatial separation of photoexcited carries. Chem. Eng. J. 2023, 470, 144240. [Google Scholar] [CrossRef]
- Zheng, X.; Song, Y.; Wang, C.; Gao, Q.; Shao, Z.; Lin, J.; Zhai, J.; Li, J.; Shi, X.; Wu, D.; et al. Properties, applications, and challenges of copper- and zinc-based multinary metal sulfide photocatalysts for photocatalytic hydrogen evolution. Chin. J. Catal. 2025, 74, 22–70. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.Y.; Yan, B.; Xia, C.X.; Yang, G.W. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Yadav, G.; Ahmaruzzaman, M. ZnIn2S4 and ZnIn2S4 based advanced hybrid materials: Structure, morphology and applications in environment and energy. Inorg. Chem. Commun. 2022, 138, 109288. [Google Scholar] [CrossRef]
- Zhong, Y.Q.; Li, M.Y.; Luan, X.; Gao, F.F.; Wu, H.X.; Zi, J.Z.; Lian, Z.C. Ultrathin ZnIn2S4/ZnSe heteronanosheets with modulated S-scheme enable high efficiency of visible-light-responsive photocatalytic hydrogen evolution. Appl. Catal. B Environ. Energy 2023, 335, 122859. [Google Scholar] [CrossRef]
- Yao, L.; Zeng, S.C.; Yang, S.X.; Zhang, H.H.; Ma, Y.; Zhou, G.Y.; Fang, J.Z. Zinc indium sulfide materials for photocatalytic hydrogen production via water splitting: A short review. Catalysts 2025, 15, 271. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, T.; Hu, Z.; Fang, K.; Zhang, X.; Xiao, W.; Jiang, F.; Wang, L.; Liu, D.; Zhang, Y. Rapid carrier extraction and d-band center regulation of Pd-ZnIn2S4 for efficient photocatalytic water splitting. J. Alloys Compd. 2025, 1027, 180652. [Google Scholar] [CrossRef]
- Wu, X.; Qian, Y.; Lv, G.; Long, L.; Zhou, Y.; Wang, D. Realizing efficient photoelectrochemical performance for well-designed CdS@ZnIn2S4 heterostructure photoanode with directional interfacial charge transfer dynamics. Coatings 2022, 12, 1210. [Google Scholar] [CrossRef]
- Wang, B.; Ding, Y.; Deng, Z.; Li, Z. Rational design of ternary NiS/CQDs/ZnIn2S4 nanocomposites as efficient noble-metal-free photocatalyst for hydrogen evolution under visible light. Chin. J. Catal. 2019, 40, 335–342. [Google Scholar] [CrossRef]
- Li, X.-J.; Qi, M.-Y.; Li, J.-Y.; Tan, C.L.; Tang, Z.-R.; Xu, Y.-J. Visible light-driven dehydrocoupling of thiols to disulfides and H2 evolution over PdS-decorated ZnIn2S4 composites. Chin. J. Catal. 2023, 51, 55–65. [Google Scholar] [CrossRef]
- Rahman, M.H.; Yang, J.; Sun, Y.; Mannodi-Kanakkithodi, A. Defect engineering in ZnIn2X4 (X = S, Se, Te) semiconductors for improved photocatalysis. Surf. Interf. 2023, 39, 102960. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Li, J.; Dong, B.; Zhao, L.; Wang, S. 1D/2D TiO2/ZnIn2S4 S-scheme heterojunction photocatalyst for efficient hydrogen evolution. Chin. J. Catal. 2022, 43, 339–349. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Chen, D.; Zhang, S.; Carabineiro, S.A.C.; Lv, K. A novel S-scheme 3D ZnIn2S4/WO3 heterostructure for improved hydrogen production under visible light irradiation. Chin. J. Catal. 2022, 43, 2615–2624. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Li, Y.; Zhang, P.; Li, H. Highly efficient photocathodic protection performance of ZIS@CNNs composites under visible light. Coatings 2023, 13, 1479. [Google Scholar] [CrossRef]
- Li, W.-Y.; Liu, J.; Li, Z.-R.; Shen, T.-T.; Chen, J.; Hu, Z.-Y.; Yu, W.-B.; Li, Y.; Su, B.-L. Probing the deactivation and regeneration of ZnIn2S4 in photocatalytic degradation of tetracycline. J. Colloid Interface Sci. 2025, 694, 137700. [Google Scholar] [CrossRef]
- Zhang, K.; Dan, M.; Yang, J.; Wu, F.; Wang, L.; Tang, H.; Liu, Z. Surface energy mediated sulfur vacancy of ZnIn2S4 atomic layers for photocatalytic H2O2 production. Adv. Funct. Mater. 2023, 33, 2302964. [Google Scholar] [CrossRef]
- Zou, P.; Wu, Z.; Ma, S.; Cao, G.; Jiang, X.; Wang, H. Crystal plane regulation and heterostructure construction of ZnIn2S4/h-BN for boosting photocatalytic hydrogen evolution. Appl. Surf. Sci. 2025, 697, 163017. [Google Scholar] [CrossRef]
- Tan, M.; Huang, C.; Yu, C.; Li, C.; Yin, R.; Liu, C.; Dong, W.; Meng, H.; Su, Y.; Qiao, L.; et al. Unexpected high-performance photocatalytic hydrogen evolution in Co@NCNT@ZnIn2S4 triggered by directional charge separation and transfer. Small 2022, 18, 2205266. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Z.; Liu, K.; Liu, W. Fabrication of 3D CuS@ZnIn2S4 hierarchical nanocages with 2D/2D nanosheet subunits p-n heterojunctions for improved photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 433, 134474. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Ho, W.; Han, S.; Wang, P.; Lee, S.; Zhang, Z. Modulation of sulfur vacancies at ZnIn2S4-δ/g-C3N4 heterojunction interface for successive C-H secession in photocatalytic gaseous formaldehyde complete oxidation. Appl. Catal. B Environ. 2023, 338, 123048. [Google Scholar] [CrossRef]
- Sun, R.; Liu, Y.; Yang, J.; Wuren, T.; Duan, H.; Tan, Z.; Yu, S. Mo-W18O49/ZnIn2S4 composites synthesized by metal doping for photocatalytic hydrogen evolution. Molecules 2025, 30, 1563. [Google Scholar] [CrossRef]
- Shi, W.L.; Chen, Z.Z.; Lu, J.L.; Sun, X.H.; Wang, Z.Y.; Yan, Y.J.; Guo, F.; Chen, L.Z.; Wang, G.Z. Construction of ZrC@ZnIn2S4 core-shell heterostructures for boosted near-infrared-light driven photothermal-assisted photocatalytic H2 evolution. Chem. Eng. J. 2023, 474, 145690. [Google Scholar] [CrossRef]
- Chen, X.; Liang, B.; Yan, L. Improvement in the photocatalytic hydrogen production of flower-shaped ZnIn2S4 by surface modification with amino silane. Catalysts 2024, 14, 607. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Y.; Huang, W.; Dou, M.; Shao, H.; Yao, M.; Ding, K.; Ye, T.; Zhou, R.; Li, S.; et al. The Zn vacancy-mediated de-accumulation based process for hydrogen production performance promotion of 1D Zn─Cd─S nanorods. Small 2023, 20, 2306447. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Pant, K.K.; Tripathi, K.; Yadav, G.; Ahmaruzzaman, M. Harnessing metal sulfides for efficient hydrogen production employing photocatalytic water splitting: Current status and future direction. Int. J. Hydrogen Energy 2025, 101, 1221–1253. [Google Scholar] [CrossRef]
- Zhang, N.; Xing, Z.; Li, Z.; Zhou, W. Sulfur vacancy engineering of metal sulfide photocatalysts for solar energy conversion. Chem. Catal. 2023, 3, 100375. [Google Scholar] [CrossRef]
- He, Z.; Li, L.; Pang, Y.; Cui, M.; Lin, Y.; Xie, T. Ag-modified sulfur vacancy-rich ZnIn2S4 ultrathin Nanosheets promote oxygen activation for efficient photocatalytic hydrogen peroxide synthesis. J. Colloid. Interface Sci. 2025, 700, 138353. [Google Scholar] [CrossRef]
- Jing, H.; Xu, G.; Yao, B.; Ren, J.; Wang, Y.; Fang, Z.; Liang, Q.; Wu, R.; Wei, S. Sulfur vacancy-enriched rhombohedral ZnIn2S4 nanosheets for highly efficient photocatalytic overall water splitting under visible light irradiation. ACS Appl. Energy Mater. 2022, 5, 10187–10195. [Google Scholar] [CrossRef]
- Jing, H.T.; Ren, J.; Yue, J.Y.; Liu, S.Y.; Liang, Q.F.; Wu, R.; Wang, Y.W.; Fang, Z.B.; Li, H.L.; Wei, S.H. ZnIn2S4 with oxygen atom doping and surface sulfur vacancies for overall water splitting under visible light irradiation. Catal. Sci. Technol. 2023, 13, 226–232. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.H.; Wen, Z.; Sun, C.N.; Wang, G.Y.; Wang, M.-S.; Yang, C.C.; Jiang, Q. ZnIn2S4 with a hybrid reaction mechanism and sulfur vacancies for sustainable sodium storage. Carbon. Energy 2025, 7, e654. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, H.; Xu, M.; Chen, W.-J.; Pan, X.; Liang, S. Controllable engineering of ZnIn2S4 with sulfur vacancy as an efficient piezocatalyst toward H2 production. J. Alloys Compd. 2025, 1016, 179005. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Wang, Z.; Gu, L.; Qiu, J.; Ran, J. Sulfur-vacancy-enriched ZnIn2S4 mediates efficient charge transfer for hydrogen evolution from lignocellulose photoreforming. Chem. Eng. J. 2025, 503, 158433. [Google Scholar] [CrossRef]
- Tang, J.-P.; Chen, Y.; Wang, Z.-Y.; Hu, Y.-H.; Wang, J.-H.; Bao, L.; Zhao, Z.-Y.; Yuan, Y.-J. Sustainable H2 production from lignocellulosic biomass over MoS2 modified sulfur vacancy enriched ZnIn2S4 photocatalyst. ACS Catal. 2025, 15, 265–274. [Google Scholar] [CrossRef]
- Xu, M.H.; Ruan, X.W.; Meng, D.P.; Fang, G.Z.; Jiao, D.X.; Zhao, S.L.; Liu, Z.Y.; Jiang, Z.F.; Ba, K.K.; Xie, T.F.; et al. Modulation of sulfur vacancies in ZnIn2S4/MXene schottky heterojunction photocatalyst promotes hydrogen evolution. Adv. Funct. Mater. 2024, 34, 2402330. [Google Scholar] [CrossRef]
- Ren, S.; Wang, S.; Chen, H.; Li, M.; Li, Z.; Liao, L.; Liang, Z.; Zhou, W. S vacancies engineering ZnIn2S4 nanosheets for boosted photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 51, 1128–1135. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, X.H.; Huang, J.F.; Li, S.X.; Meng, A.; Li, Z.J. Interfacial chemical bond and internal electric field modulated Z-scheme SV-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution. Nat. Commun. 2021, 12, 4112. [Google Scholar] [CrossRef]
- Li, G.Q.; Liang, H.O.; Fan, X.Y.; Lv, X.L.; Sun, X.W.; Wang, H.G.; Bai, J. Modulating and optimizing 2D/2D Fe-Ni2P/ZnIn2S4 with S vacancy through surface engineering for efficient photocatalytic H2 evolution. J. Mater. Chem. A 2023, 11, 14809–14818. [Google Scholar] [CrossRef]
- Liu, S.; Man, Z.; Fang, F.; Li, P.; Chang, K. Controllable engineering of surface defects on ZnIn2S4 nanosheets: Implications to efficient hydrogen evolution activity under visible light. ACS Appl. Nano Mater. 2023, 6, 14876–14884. [Google Scholar] [CrossRef]
- Jiang, R.Q.; Cai, X.Y.; Gu, X.Q.; Yang, D.; Zhang, J.Y.; Zhao, Y.L.; Mao, L. Photocatalytic H2 evolution over sulfur vacancy-rich ZnIn2S4 hierarchical microspheres under visible light. J. Mater. Sci. 2021, 56, 19439–19451. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Cai, X.; Zhao, Y.; Gu, X.; Mao, L. Sulfur vacancy-rich ZnIn2S4 nanosheet arrays for visible-light-driven water splitting. Mater. Sci. Semicond. Process. 2022, 143, 106547. [Google Scholar] [CrossRef]
- Hsia, H.-H.; Maggay, I.V.B.; Muruganantham, R.; Liu, W.-R. ZnIn2S4: A promising anode material with high electrochemical performance for sodium-ion batteries. Ceram. Int. 2021, 47, 28634–28641. [Google Scholar] [CrossRef]
- Roda, D.; Trzciński, K.; Sawczak, M.; Ilnicka, A.; Łapiński, M.; Szkoda, M.; Nowak, A.P. ZnIn2S4 thin films grown by pulsed laser deposition: Effect of electrolyte and illumination conditions on photoanode performance. Appl. Surf. Sci. 2025, 708, 163694. [Google Scholar] [CrossRef]
- He, Y.; Rao, H.; Song, K.; Li, J.; Yu, Y.; Lou, Y.; Li, C.; Han, Y.; Shi, Z.; Feng, S. 3D Hierarchical ZnIn2S4 Nanosheets with Rich Zn Vacancies Boosting Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2019, 29, 1905153. [Google Scholar] [CrossRef]
- Long, L.; Lv, G.; Pan, F.; Li, Z.; Zhu, H.; Wang, D. Photocarrier dynamics regulation by constructing a 2D ZnIn2S4 nanosheet array with highly exposed Zn vacancies for efficient solar-driven CO2 conversion. J. Phys. Chem. C 2023, 127, 24077–24087. [Google Scholar] [CrossRef]
- Xu, B.; Li, H.; Chong, B.; Lin, B.; Yan, X.; Yang, G. Zn vacancy-tailoring mediated ZnIn2S4 nanosheets with accelerated orderly charge flow for boosting photocatalytic hydrogen evolution. Chem. Eng. Sci. 2023, 270, 118533. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Li, H.; Jiang, Q.; Tang, S.; Pang, H.; Ma, H. Synergizing Zinc vacancy engineering and electron-rich polyoxometalates in ZnIn2S4 for solar-light-driven hydrogen evolution with boosted charge dynamics. J. Alloys Compd. 2025, 1036, 182085. [Google Scholar] [CrossRef]
- Wu, W.D.; Luo, Z.C.; Liu, B.W.; Qiu, X.Q.; Lin, J.X.; Sun, S.R.; Wang, X.F.; Lin, X.L.; Qin, Y.L. Zinc vacancy promotes photo-reforming Ligninmodel to H2 evolution and value-added chemicals production. Small Methods 2023, 7, 2300462. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Quan, X.; Chen, Z.A.; Wang, K.; Gu, X.; Hong, Z.; Zhi, M. Intercalation- and vacancy-enhanced internal electric fields in ZnIn2S4 for highly efficient photocatalytic H2O2 production. J. Phys. Chem. C 2023, 127, 20683–20699. [Google Scholar] [CrossRef]
- He, Y.; Chen, C.; Liu, Y.; Yang, Y.; Li, C.; Shi, Z.; Han, Y.; Feng, S. Quantitative evaluation of carrier dynamics in full-spectrum responsive metallic ZnIn2S4 with indium vacancies for boosting photocatalytic CO2 reduction. Nano Lett. 2022, 22, 4970–4978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Liu, H.; Zhang, R.; Jia, W.; Zhang, J.; Sun, Y.; Peng, L. Understanding the role of dual zinc and indium vacancies in ZnIn2S4 for the visible-light-driven photocatalytic air-oxidation of 5-hydroxymethylfurfural. ACS Catal. 2025, 15, 3464–3474. [Google Scholar] [CrossRef]
- Zhong, W.J.; Hung, M.Y.; Kuo, Y.T.; Tian, H.K.; Tsai, C.N.; Wu, C.J.; Lin, Y.D.; Yu, H.C.; Lin, Y.G.; Wu, J.J. Dual-vacancy-engineered ZnIn2S4 nanosheets for harnessing low-frequency vibration induced piezoelectric polarization coupled with static dipole field to enhance photocatalytic H2 evolution. Adv. Mater. 2024, 36, 2403228. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, S.; Cui, H.; Chang, J.; Feng, Y.; Wang, S.; He, Z. Promoted photocatalytic performances over Ti3+-B co-doped TiO2/BN with high carrier transfer and absorption capabilities driven by SWCNT addition. Mater. Sci. Semicond. Process. 2024, 177, 108364. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Jiang, X.; Bai, Y. First-principles study of non-metallic doping and noble metal loading on ZnIn2S4 semiconductor photocatalyst. Inorg. Chem. Commun. 2025, 174, 114124. [Google Scholar] [CrossRef]
- Li, J.-Y.; Qi, M.-Y.; Xu, Y.-J. Efficient splitting of alcohols into hydrogen and C–C coupled products over ultrathin Ni-doped ZnIn2S4 nanosheet photocatalyst. Chin. J. Catal. 2022, 43, 1084–1091. [Google Scholar] [CrossRef]
- Xue, J.R.; Liu, H.H.; Zeng, S.Y.; Feng, Y.F.; Zhang, Y.Y.; Zhu, Y.; Cheng, M.Y.; Zhang, H.K.; Shi, L.; Zhang, G.Q. Bifunctional cobalt-doped ZnIn2S4 hierarchical nanotubes endow noble-metal cocatalyst-free photocatalytic H2 production coupled with benzyl alcohol oxidation. Sol. RRL 2022, 6, 2101042. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, M.; Guo, J.; Wang, G.; Wang, S.; He, Z. Stable Ti3+ in B-TiO2/BN based hybrids for efficient photocatalytic reduction. Chem. Eng. J. Adv. 2022, 11, 100333. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Xiao, Q.; Yang, Y.; Chen, H.; Qiu, X. Self-doped zinc-mediated ZnIn2S4 microflowers towards optimized visible-light photocatalytic hydrogen production. J. Environ. Chem. Eng. 2024, 12, 112844. [Google Scholar] [CrossRef]
- He, Z.; Xia, Y.; He, G.; Wang, F.; Su, J.; Fareed, H.; Fu, X.; Chen, G.; Yang, H.; Zhou, W. Insight into synergy of Mn active sites and spin polarization electrons in Mn-incorporated ZnIn2S4 for boosting photocatalytic hydrogen evolution coupled with benzyl alcohol oxidation. Chem. Eng. J. 2025, 506, 159957. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Wang, Z.; Qiu, J.; Ran, J. Photothermal catalysis enhances cellulose oxidation kinetics to promote the hydrogen evolution in alkaline solution over Pt/ZnIn2S4. Energy Convers. Manag. 2024, 314, 118634. [Google Scholar] [CrossRef]
- Yang, M.; Zhan, X.-Q.; Ou, D.-L.; Wang, L.; Zhao, L.-L.; Yang, H.-L.; Liao, Z.-Y.; Yang, W.-Y.; Ma, G.-Z.; Hou, H.-L. Efficient visible-light-driven hydrogen production with Ag-doped flower-like ZnIn2S4 microspheres. Rare Met. 2025, 44, 1024–1041. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, G.; Zhang, Q.; Liu, T.; Yang, J.; Guan, Z. Synergistic effect of Na doping and CoSe2 cocatalyst for enhanced photocatalytic hydrogen evolution performance of ZnIn2S4. J. Colloid Interface Sci. 2024, 676, 272–282. [Google Scholar] [CrossRef]
- Chong, W.-K.; Ng, B.-J.; Kong, X.Y.; Tan, L.-L.; Putri, L.K.; Chai, S.-P. Non-metal doping induced dual p-n charge properties in a single ZnIn2S4 crystal structure provoking charge transfer behaviors and boosting photocatalytic hydrogen generation. Appl. Catal. B Environ. 2023, 325, 122372. [Google Scholar] [CrossRef]
- Farhan, S.; Hassan Raza, A.; Yang, S.; Yu, Z.; Wu, Y. Boosted photocatalytic hydrogen evolution of S-scheme N-doped CeO2-δ@ZnIn2S4 heterostructure photocatalyst. J. Colloid Interface Sci. 2024, 669, 430–443. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Liu, R.; Gao, Z.; Yin, Z. Design of AgXAu1−X alloy/ZnIn2S4 system with tunable spectral response and Schottky barrier height for visible-light-driven hydrogen evolution. Chem. Eng. J. 2020, 382, 122953. [Google Scholar] [CrossRef]
- An, H.; Lv, Z.; Zhang, K.; Deng, C.; Wang, H.; Xu, Z.; Wang, M.; Yin, Z. Plasmonic coupling enhancement of core-shell Au@Pt assemblies on ZnIn2S4 nanosheets towards photocatalytic H2 production. Appl. Surf. Sci. 2021, 536, 147934. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Wang, X.; Zhao, L.; Guo, L. Microwave-assisted hydrothermal synthesis of transition-metal doped ZnIn2S4 and its photocatalytic activity for hydrogen evolution under visible light. J. Power Sources 2011, 196, 10112–10119. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Si, Y.; Li, B.; Deng, F.; Yang, L.; Liu, X.; Dai, W.; Luo, S. Gradient hydrogen migration modulated with self-adapting S vacancy in copper-doped ZnIn2S4 nanosheet for photocatalytic hydrogen evolution. ACS Nano 2021, 15, 15238–15248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Gu, S.; Zhang, C.; Gao, K.; Xia, J.; Cheng, C.; Wang, X. Regulating electron-transfer over ZnIn2S4 by Sn(II)/Sn(IV) co-doping for efficient photocatalytic hydrogen production. Catal. Lett. 2022, 153, 2245–2259. [Google Scholar] [CrossRef]
- Zeng, Z.; Mao, L.; Zhang, R.; Liu, Y.; Ling, Y.; Cai, X.; Zhang, J. A triple-benefit strategy of microstructure, electronic property and active site modulation on ZnIn2S4 photocatalyst by Sn atom doping. Chem. Eng. J. 2024, 496, 153769. [Google Scholar] [CrossRef]
- Feng, X.; Chen, H.; Yin, H.; Yuan, C.; Lv, H.; Fei, Q.; Zhang, Y.; Zhao, Q.; Zheng, M.; Zhang, Y. Facile synthesis of P-doped ZnIn2S4 with enhanced visible-light-driven photocatalytic hydrogen production. Molecules 2023, 28, 4520. [Google Scholar] [CrossRef]
- Goswami, T.; Yadav, D.K.; Bhatt, H.; Kaur, G.; Shukla, A.; Babu, K.J.; Ghosh, H.N. Defect-mediated slow carrier recombination and broad photoluminescence in non-metal-doped ZnIn2S4 nanosheets for enhanced photocatalytic activity. J. Phys. Chem. Lett. 2021, 12, 5000–5008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, M.; Ren, Y.; Zhu, Y.; Dong, Y.; Liao, J.; Chen, X.; Zheng, Q.; Yin, H. One-step synthesis N, O co-doped ZnIn2S4 for efficient photocatalytic H2 production and Cr(VI) reduction. J. Environ. Chem. Eng. 2024, 12, 113261. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, M.; He, Y.; Wang, X.; Su, W. Photochemical route for synthesizing Co–P alloy decorated ZnIn2S4 with enhanced photocatalytic H2 production activity under visible light irradiation. Nanoscale 2018, 10, 19100–19106. [Google Scholar] [CrossRef]
- Wu, M.; Sheng, Q.; Yang, L.; Lv, W.; Zhen, W.; Liu, H.; Yang, Y.; Yao, T.; Zhang, J.; Liu, L. Preparation of N,Ni co-doped ZnIn2S4 for simultaneously trapping electron and hole: Insight on photocatalytic H2 evolution coupled with Benzyl Alcohols oxidative dehydrogenation. Small 2025, 21, 2500443. [Google Scholar] [CrossRef]
- Yi, L.; Nkinahamira, F.; Jiang, H.; Huang, J.; Ma, Y.; Zhu, R. Mo2N decorated hierarchical ZnIn2S4 with sulfur-rich vacancy photocatalyst for efficient photocatalytic hydrogen production. J. Environ. Chem. Eng. 2023, 11, 110240. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Qu, L. Graphitic carbon nitride/nitrogen-rich carbon nanofibers: Highly efficient photocatalytic hydrogen evolution without cocatalysts. Angew. Chem. Int. Ed. 2016, 55, 10849–10853. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Yu, Z.; Jiang, R.; Hou, Y.; Huang, J.; Zhu, H.; Yang, F.; Li, M.; Li, F.; Sun, Q. A novel, noble-metal-free core-shell structure Ni–P@C cocatalyst modified sulfur vacancy-rich ZnIn2S4 2D ultrathin sheets for visible light-driven photocatalytic hydrogen evolution. J. Alloys Compd. 2021, 855, 157333. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Jiang, Y.; Du, Y.; Zhang, J.; Lin, K. In-situ construction of CdS@ZIS Z-scheme heterojunction with core-shell structure: Defect engineering, enhance photocatalytic hydrogen evolution and inhibit photo-corrosion. Int. J. Hydrogen Energy 2022, 47, 35241–35253. [Google Scholar] [CrossRef]
- Wang, S.D.; Huang, L.Y.; Xue, L.J.; Kang, Q.; Wen, L.L.; Lv, K.L. Sulfur-vacancy-modified ZnIn2S4/TpPa-1 S-scheme heterojunction with enhanced internal electric field for boosted photocatalytic hydrogen production. Appl. Catal. B Environ. Energy 2024, 358, 124366. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Wang, X.; Hu, J.; Zhang, J.; Zheng, L.; Mao, L.; Zheng, H.; Zhu, M. Protruding Pt single-sites on hexagonal ZnIn2S4 to accelerate photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 1287. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Pan, X.; Wang, T.; Li, Y. Ultrathin nickel-doped ZnIn2S4 nanosheets with sulfur vacancies for efficient photocatalytic hydrogen evolution. ChemCatChem 2021, 13, 5148–5155. [Google Scholar] [CrossRef]
- Zeng, D.; Shen, T.; Hu, Y.; Zhang, Z.; Liu, Z.; Xu, N.; Song, J.; Guan, R.; Zhou, C. Nitrogen-doped ZnIn2S4 and TPA multi-dimensional synergistically enhance photocatalytic hydrogen production. Fuel 2025, 380, 133151. [Google Scholar] [CrossRef]
- Tien, T.-M.; Chen, E.L. S-scheme system of MoS2/Co3O4 nanocomposites for enhanced photocatalytic hydrogen evolution and methyl violet dye removal under visible light irradiation. Coatings 2023, 13, 80. [Google Scholar] [CrossRef]
- Cheng, T.; Zhu, J.; Chen, C.; Hu, Y.; Wu, L.; Zhang, M.; Cui, L.; Dai, Y.; Zhang, X.; Tian, Y.; et al. Construction of advanced S-scheme heterojunction interface composites of bimetallic phosphate MnMgPO4 with C3N4 surface with remarkable performance in photocatalytic hydrogen production and pollutant degradation. Coatings 2025, 15, 103. [Google Scholar] [CrossRef]

| Catalysts | Light Source | Scavenger | HER Rate (μmol·g−1·h−1) | Ref. |
|---|---|---|---|---|
| VS-ZIS (with Pt/Cr cocatalysts) | 300W Xenon lamp | 10 vol% acetone | 3.40 | [55] |
| VS-ZIS/Mo2N | 300W Xenon lamp | 15 vol% TEOA | 10,280 | [102] |
| VS-ZIS/Cu | 300W Xenon lamp (λ > 420 nm) | 0.2 M ascorbic acid | 9864.7 | [103] |
| VS-ZIS/Ni-P@C | 300W Xenon lamp (λ > 400 nm) | 14 vol% TEOA | 11,064 | [104] |
| VS-ZIS/CdS | 300W Xenon lamp (λ > 420 nm) | 10 vol% lactic aqueous | 16,630 | [105] |
| VS-ZIS/TpPa-1 | 300W Xenon lamp (λ > 420 nm) | 0.05 M L-ascorbic acid | 2745 | [106] |
| VS-ZIS nanosheets | 300W Xenon lamp (λ > 420 nm) | 10 vol% TEOA | 5437 | [62] |
| VS-ZIS (plasma-etched ZIS) | 300W Xenon lamp | Na2S/Na2SO3 (0.35 M/0.25 M) | 261.9 | [65] |
| VS-ZIS-P (plasma-etched ZIS) | 300W Xenon lamp | Na2S/Na2SO3 (0.35 M/0.25 M) | 706 | [66] |
| VZn-ZIS nanoflowers | 300W Xenon lamp | 10 vol% TEOA | 21,430 | [73] |
| VZn-ZIS | 300W Xenon lamp | 10 vol% TEOA | 212 | [72] |
| Pt/VZn-ZIS | 300W Xenon lamp | 4-methylbenzyl alcohol | 6410 | [74] |
| Sn-ZIS | 300W Xenon lamp (λ > 400 nm) | 20 vol% TEOA | 62.18 | [96] |
| Pt-ZIS | simulated solar light (λ > 420 nm) | 10 vol% TEOA | 17,500 | [107] |
| Pt-ZIS | 300W Xenon lamp | 15 vol% TEOA | 577 | [86] |
| Mn-ZIS | 300W Xenon lamp | 1% benzyl alcohol | 32,750 | [85] |
| Ni-ZIS | 300W Xenon lamp (λ > 420 nm) | 14 vol% TEOA | 8910 | [108] |
| Cu-ZIS | 300W Xenon lamp | 0.2 M ascorbic acid | 9864.7 | [94] |
| Pd-ZIS | 300W Xenon lamp | 0.25 M Na2SO4 | 2310 | [34] |
| Na-ZIS/CoSe2 | 300W Xenon lamp | 20 vol% TEOA | 4525 | [88] |
| Au@Pt/ZIS | Xenon lamp (λ > 420 nm) | Na2S/Na2SO3 (0.35 M/0.25 M) | 41,747 | [92] |
| Ag0.6Au0.4/ZIS | 300W Xenon lamp | Na2S/Na2SO3 (0.35 M/0.25 M) | 54,007 | [91] |
| P-ZIS | 300W Xenon lamp (420 nm < λ < 760 nm) | Na2S/Na2SO3 (0.25 M/0.35 M) | 1566.6 | [97] |
| N-ZIS/(tungsten-based polyoxometalate) | 300W Xenon lamp (λ>320nm) | 15 vol% TEOA | 17,345.53 | [109] |
| N, O co-doped ZIS | 300W Xenon lamp | Na2S/Na2SO3 (0.35 M/0.25 M) | 2254 | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, F.; Jing, T.; Wang, S.; He, Z. Defect Engineering of ZnIn2S4 Photocatalysts for Enhanced Hydrogen Evolution Reaction. Coatings 2025, 15, 1061. https://doi.org/10.3390/coatings15091061
Hong F, Jing T, Wang S, He Z. Defect Engineering of ZnIn2S4 Photocatalysts for Enhanced Hydrogen Evolution Reaction. Coatings. 2025; 15(9):1061. https://doi.org/10.3390/coatings15091061
Chicago/Turabian StyleHong, Fangying, Tong Jing, Sen Wang, and Zuoli He. 2025. "Defect Engineering of ZnIn2S4 Photocatalysts for Enhanced Hydrogen Evolution Reaction" Coatings 15, no. 9: 1061. https://doi.org/10.3390/coatings15091061
APA StyleHong, F., Jing, T., Wang, S., & He, Z. (2025). Defect Engineering of ZnIn2S4 Photocatalysts for Enhanced Hydrogen Evolution Reaction. Coatings, 15(9), 1061. https://doi.org/10.3390/coatings15091061







