1. Introduction
Polyethylene terephthalate (PET) is a material widely used in packaging [
1] including food packaging [
2], biomedical devices [
3], flexible electronics [
4] or microfluidic chips [
5] due to its transparency, chemical resistance, and mechanical durability. However, in some cases, there is a need for specific surface properties of the selected material. For example, PET’s moderate wettability (low surface energy ~42–46 mN/m [
6]) limits its performance in applications that require extreme wetting properties such as friction control [
7], self-cleaning [
8], anti-fogging [
9], anti-fouling surfaces [
10], or water harvesting [
11]. To address this, researchers have explored different surface modification techniques to induce superhydrophobicity (water contact angle > 150° and hysteresis < 10°) and superhydrophilicity (water contact angle < 5°) as well as other applications deriving from wetting control [
12,
13,
14,
15,
16].
Nowadays, there is also a demand to use “green” materials and processes for the fabrication of such surfaces and also to avoid per- and polyfluoroalkyl substances, which are commonly used in these applications [
17]. For example, Fu et al. [
18] fabricated ordered and periodic microstructures on PET substrates using patterned stamps on chromium plates fabricated with lasers using hot embossing. Using this environmentally friendly method, a water contact angle of 140° without modifying surface chemistry but with high contact angle hysteresis (23°) was reported. Vacuum oxygen plasma micro-nanotexturing is another dry “green” method that enables the micro-nanotexturing and chemical functionalization of polymeric substrates within a few minutes [
19], whereas atmospheric pressure plasma processing has also been used for PET surface functionalization [
20]. Even though plasma processing (depending on the mode) can modify surface chemistry and topography [
21], the treated surfaces often regain their initial properties over time due to hydrophobic recovery or environmental aging. Hydrophobic recovery happens because a high concentration of polar surface functional groups is against the rule of thermodynamics, so this effect cannot be permanent [
22].
The hydrophobic recovery of plasma-activated PET has been studied by several researchers. Gotoh et al. [
23] managed to enhance the hydrophilic nature of PET using two different dry processes, atmospheric pressure plasma (APP) exposure and 172 nm ultraviolet (UV) excimer light irradiation. With the APP method, the water contact angle decreased to 30° and with the UV light irradiation to 40°, but still, the advancing angle would increase due to water and aging time in air. In a later study [
24], the surface modification of PET was achieved by treating the surfaces with atmospheric pressure plasma jet and then adding a plasma-induced polymer coating with hexamethyldisiloxane (HMDSO) to fabricate superhydrophobic surfaces with a 158.8° contact angle. For the creation of superhydrophilic surfaces with an 8° contact angle, treatment with plasma oxidation with nitrogen gas and oxidation by excimer UV light irradiation followed. Both the superhydrophobic and superhydrophilic surfaces exhibit stable wettability for two weeks; after this time, hydrophobic recovery occurs.
Tarrade et al. [
13] used oxygen plasma to treat PET surfaces; then, the plasma-treated polymeric surfaces were hydrophobized with a tetrafluorocarbon plasma, allowing them to obtain a water contact angle of 145°, which was further enhanced with the addition of a thin polypyrrole layer before hydrophobization to achieve a contact angle of 157° but with high hysteresis (65°). If the protective polypyrrole layer is not added, degradation of the surfaces by the CF
4 plasma is observed. Apart from oxygen plasma, other gas mixtures have been used to study the creation of roughness on polymeric PET surfaces, like Rezaei et al. [
25], who used helium as the main seed gas (the carrier), and small fractions (0.5–1.1%) of oxygen (O
2) and/or nitrogen (N
2) were added to explore reactivity of plasmas generated from different gas mixtures. Plasma-treated samples became hydrophilic and the water contact angle was reduced from 87° (untreated sample) to 25° for the plasma-treated sample with the He/N
2 0.5% gas mixture. In a similar way, Prakash et al. [
26] performed two low-temperature plasma processes, an atmospheric pressure plasma discharge (APP) treatment and a low-pressure plasma (LPP) treatment to increase the hydrophilicity of PET films. After comparison of the two different types of plasma sources, APP is considered to create defect-free and smoother surface morphology on the PET films. In another work, PET films have been structured using oxygen plasma, but again, hydrophobic recovery was observed for low etching durations and hydrophilic stability was reported for durations longer than 20 min for a period of 25 days [
27]. Zanini et al. [
28] fabricated superhydrophobic PET surfaces after depositing a hydrophobic top coating through a hexamethyldisiloxane (HMDSO) plasma step. Atmospheric-pressure plasma has also been reported for the fabrication of hydrophilic coatings, which can exhibit high stability in water over 14 days [
29]. In another interesting approach, plasma is used to fabricate superhydrophobic PET fabric for oil–water separation, taking into account PET’s inherent hydrophobic properties [
30]. Finally, in other existing works, plasma processing is just used to activate PET surfaces and the wetting properties are caused by the addition of extra materials (i.e., particles, polymers) [
31], or plasma processing is performed on the coatings deposited on PET to make them superhydrophilic and not on the PET itself [
32].
It is, therefore, evident that achieving long-term stability of superhydrophobic and superhydrophilic PET surfaces remains a critical challenge, as surface chemistry and topography often degrade over time. Additionally, the modification of the material can usually lead to the degradation of other material properties (i.e., optical), and a few works have studied both functionalities (i.e., wettability and transparency or reflectivity) [
33,
34]. To address these drawbacks, a plasma-based micro-nanotexturing approach to create durable superhydrophilic and superhydrophobic PET surfaces is presented. In particular, PET films are first etched with oxygen plasma to create random micro-nanotextured topography and then coated with a hydrophilic or a hydrophobic coating to stabilize the acquired properties. For the superhydrophilic surfaces, a hydrophilic coating (PEG) is deposited and UV-cured (253.7 nm), whereas, for the superhydrophobic surfaces, an ultra-thin hydrophobic coating using C
4F
8 plasma deposition is applied. The wetting properties, surface morphology, and chemical and optical properties of both the superhydrophilic and the superhydrophobic surfaces fabricated are studied in detail to evaluate their performance stability over time. The 12 min superhydrophilic surfaces exhibit a water contact angle ~0° and remain stable for at least 60 days of storage, and the superhydrophobic surfaces exhibit a highly stable water contact angle of 160° and low hysteresis (<5°) even after a year of storage. The superhydrophobic fabricated surfaces are also tested regarding their oleophobicity using other liquids with different surface tension (glycerine and silicone oil) and exhibit a high contact angle against glycerine even after one year of storage. It also demonstrated that, by choosing an intermediate etching time (4–6 min), a superhydrophobic PET film with almost identical optical properties with the untreated one can be realized, enabling the use of the plasma-textured PET films in applications in which both wetting and optical property control are required.
2. Materials and Methods
2.1. Substrate Materials
The materials that were used as substrates are films of polyethylene terephthalate (PET) (50 μm thickness, acquired by i-transfer). PET was selected for its low gas permeability, low glass transition temperature (69–85 °C), and high melting point (260 °C), making it ideal for high-temperature applications. Water (high surface tension, 72 mN/m), silicone oil (low surface tension, 21 mN/m), and glycerine (intermediate surface tension, 63 mN/m) were used to measure static contact angles across liquids with varying surface properties. Acetone and isopropanol were used for surface cleaning. Polyethylene glycol (PEG) 8000, and PEG 1000 BioUltra purchased by Sigma-Aldrich (Louis, MI, USA) were used as hydrophilic coatings.
2.2. Plasma Micro-Nanotexturing
The PET surfaces were cleaned prior to their plasma treatment with acetone and isopropanol and then rinsed with deionized water and dried with dry nitrogen stream. They were also thermally treated at 150 °C for 30 min to ensure humidity was removed.
Plasma etching is performed in a custom-built Inductively Coupled Plasma (ICP) reactor equipped with a double helical source (operating at 13.56 MHz). The reactor has an anodized aluminum clamping ring in contact bearing a central opening for sample positioning. The clamping ring is in contact with the electrode and the sample and acts as a sputtering target that includes a variable ion flux shield. Three different process durations were studied (4, 6, and 12 min) with plasma processing conditions, 300 Watt power, 250 Volt bias voltage, 6 mTorr oxygen gas pressure, and 100 sccm gas flow rate, in order to choose the minimum time required to induce the appropriate micro-nanotexture for superhydrophilicity and superhydrophobicity on PET substrates. More details, including the schematic representation of this custom-built reactor and how roughness control can be achieved using a specially designed electrostatic shield, can be found in the work by Zeniou et al. [
35].
To extract the plasma micro-nanotexturing step duration, we performed experiments with lower duration (2 min). For low etching durations (i.e., 2 min), the roughness created was low and, therefore, the lowest etching time was set to 4 min; 6 min represents an intermediate treatment duration, while 12 min corresponds to the upper limit in which a high water contact angle is observed and there was no reason to further increase the plasma duration. Processing was performed with intermediate breaks every 2 min to ensure that the substrate did not overheat, since PET material is susceptible to temperature. In this type of reactor, these conditions enable highly anisotropic reactive ion etching, and the creation of micro-nanotexturing is the combinational result of this process and the small amounts (usually less than <1%) of unetchable material coming from the reactor wall and substrate acting as nano-inhibitors. More details about the process can be found in previous works. The micro- and nanotexturing method for polymers is highly reproducible and it has been used by several research groups worldwide to tune the surface topography and chemistry on demand [
36,
37,
38]. It has, therefore, been implemented for the fabrication of micro-nanotextured surfaces focused on a plethora of applications (i.e., atmospheric water collection [
11,
39] antibacterial surfaces [
40,
41], functional microdevices [
42,
43], passive fungal proliferation control [
44], electronic device cooling [
45], etc.). The oxygen plasma step, except for the creation of the micro- and nanotopography, simultaneously modifies the surface chemistry [
46] by introducing hydrophilic groups which can be used as binding sites for efficient and homogeneous coating deposition. The fabrication of superhydrophilic and superhydrophobic PET surfaces is described below, and plasma-etched PET surfaces for 4, 6, and 12 min are used in both cases.
2.3. Fabrication of the Superhydrophilic Surfaces
To develop durable superhydrophilic surfaces, a hydrophilic PEG coating was deposited on the first batch of the fabricated micro- and nanotextured surfaces of all different treatment durations (4, 6, and 12 min). The PEG coating was spin-coated for 30 s at 5000 rpm to ensure thin and uniform deposition [
39]. To investigate the influence of PEG’s molecular weight and concentration, two PEG solutions were prepared and tested, 1% PEG 8000 and 1% PEG 1000 in water. After deposition, the surfaces were UV-cured (253.7 nm) for 1 h to stabilize the coatings.
2.4. Fabrication of the Superhydrophobic Surfaces
For the creation of robust superhydrophobic surfaces, the micro- and nanotextured PET surfaces of the second batch after plasma etching for 4, 6, and 12 min were inserted again inside the reactor in order to deposit a hydrophobic coating on them. The thin hydrophobic film (40 nm thickness) was deposited using C4F8 plasma deposition with the following conditions: power 500 W, bias voltage 0 V, gas pressure 40 mTorr, gas flow rate 25 sccm, 60 s.
2.5. Surface Morphology, Wetting, and Optical and Chemical Composition Property Characterization
The characterization of the morphology induced on the surfaces was completed by scanning electron microscopy using the JEOL JSM-7401F FEG at 2 kV beam voltage and an Optical Profilometer (Profilm 3D, Filmetrics). The topography roughness characteristics were extracted using Profilm software and ISO 4287:1997 (Surface texture: Profile method). Three-dimensional roughness profiles and line roughness profiles were also extracted to evaluate topography height, width, and spacing.
For the wetting property characterization, both the water static contact angle and the contact angle hysteresis were measured using Data Physics OCA11 Goniometer system, Hailsham, UK. Our instrument has an accuracy of ±1° and a resolution of ±0.01°. For the water static contact angle measurements, 5 μL of deionized water drops were dispensed on the surfaces; the average value, as well as the standard deviation of 3 measurements, is calculated using the dpiMAX software. In cases where the water is fully absorbed, the static contact angle is approximately zero.
For the contact angle hysteresis measurement, again, a 5 μL drop of deionized water was placed on the surface and the volume of the droplet was increased up to 25 μL. During the increase in droplet volume, the advancing contact angle was measured; likewise, the receding contact angle was measured during the decrease in droplet volume. Additionally, some of the samples were studied before and after water immersion tests to determine their performance. Contact angle measurements were also performed using other liquids with lower surface tension such as silicone oil (low surface tension, 21 mN/m) and glycerine (intermediate surface tension, 63 mN/m). both acquired from Sigma-Alldrich (Shanghai, China).
The chemical compositions of the surfaces and the coatings applied in this work were analyzed using Fourier transform infrared spectroscopy (FTIR) on a PerkinElmer Spectrum 100 spectrometer. FTIR measurements were performed on samples both before and after the coatings were added to assess any chemical changes.
The optical properties of untreated and plasma-etched PET films were also studied using White Light Reflectance Spectroscopy (WLRS) [
47] and measurements of the reflectance (total and diffusive) using ThetaMetrisis FR-Reflection kit, Peristeri, Greece, which includes an Ocean Optics ISP-50-GT Integrating Sphere coupled to an Ocean Optics QE65000-ABSUV NIR Spectrometer. A combination of a deuterium and a tungsten lamp is used as a light source covering the spectra range between 200 and 1000 nm. The light excitation in the integrating sphere is angled at 8°.
3. Results and Discussion
3.1. Surface Morphology Characterization
In
Figure 1, SEM images of the surface topography before and after the oxygen plasma micro-nanotexturing step for 4, 6, and 12 min are presented. The mechanism responsible for the roughness formation is discussed in more detail in
Section 2.2 and has been previously explained [
48]. In short, micro- and nanotexturing is mainly formed due to the anisotropic etching conditions used and the simultaneous deposition of a really small amount of alumina molecules sputtered from the electrode of the reactor. Alumina is unetchable from the oxygen plasma, and these particles create masking effects that enable the creation of nano-grass—which depends on the material properties and the process duration—that grows to larger micro or hierarchical micro-nano topography features.
It is clear that the plasma micro-nanotexturing step induces significant morphological changes on the PET substrates as there is a really big difference between untreated (flat) and all treated surfaces. The 4 and 6 min plasma-etched surfaces exhibit random and dense hierarchical micro- and nanostructured features that increase in height and form larger structures and, as the process duration increases further to 12 min, wider micro-scale aggregates are formed. Small nanofilaments are also observed on top of these bigger formations for all plasma micro-nanotexturing durations. It is also evident that, after 12 min of etching time, the roughness evolves by exhibiting larger aggregates (i.e., wider valleys and wider peaks) with hierarchical features.
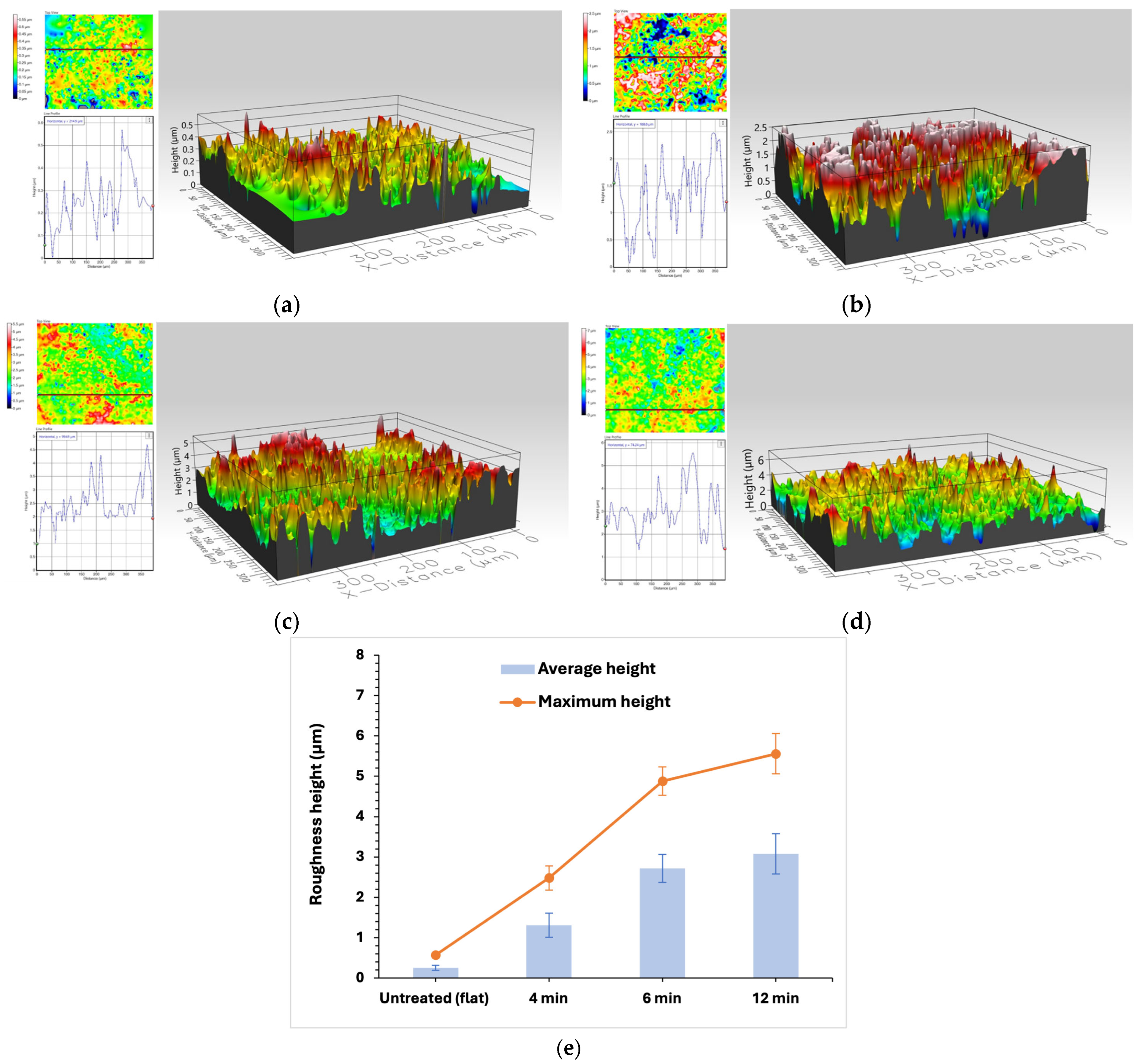
To further, examine the effect of the plasma micro-nanotexturing step in the PET surface topography, the surface morphology of all surfaces was analyzed using the Optical Profilometer (Profilm 3D, Filmetrics). In
Figure 2, 3D and 2D surface profiles as well as roughness line profiles are presented for each surface.
The untreated PET films are intrinsically hydrophobic (WSCA = 90°) and small-scale roughness is observed on their surface (
Figure 2a), with an average height of 0.3 μm along with the material’s non-polar groups. As plasma etching is performed, the roughness increases and, for 4 min samples, the roughness average height is 1.3 μm. After further increase in plasma etching duration, the average surface roughness increases to 2.7 μm for the 6 min surface and above 3 μm for 12 min surface, creating air pockets for hydrophobicity enhancement.
The mean and maximum height differences of the structures for the three plasma durations are presented in a separate graph in
Figure 2e. As treatment time increases, so does the roughness created, especially from flat to 6 min. When plasma treatment is 12 min, the height of the roughness increases to a maximum of 5.5 μm but not higher, and larger and wider topography features are created. In order to also study the width and the overall roughness shape and distribution of each surface, the skewness and kurtosis parameters from the profilometer were used to gain further insight. Skewness quantifies the asymmetry of the height distribution, where negative values are associated with wider structures, while positive values indicate profiles dominated by peaks. Kurtosis describes the sharpness of the profile features; values greater than three are indicative of sharp pronounced peaks or valleys, while values close to or below three correspond to broader more plateau-like features. Comparing these parameters across different treatment durations provides a more detailed understanding of the evolution of surface topography, highlighting changes in the lateral geometry and roughness feature. The untreated PET surfaces exhibit positive skewness and kurtosis slightly higher than three, which means that the small roughness present is relatively sharp. After 4 min of plasma treatment, the skewness becomes negative and kurtosis decreases, suggesting the development of larger and wider structures. As treatment time increases to 6 min, both skewness and kurtosis increase and result in very sharp peaks, as observed in
Figure 2c. For the 12 min treatment, the induced roughness features tend to be wider and less sharp, a finding that is also confirmed by the SEM images in
Figure 1d that show the presence of larger aggregates.
It is, therefore, evident that, for durations up to 6 min, roughness height increases almost linearly with process duration and, for higher etching durations, the structures tend to form wider formations, and roughness height still increases but at a slower rate.
3.2. Chemical Composition of Untreated, Superhydrophilic, and Superhydrophobic PET Surfaces
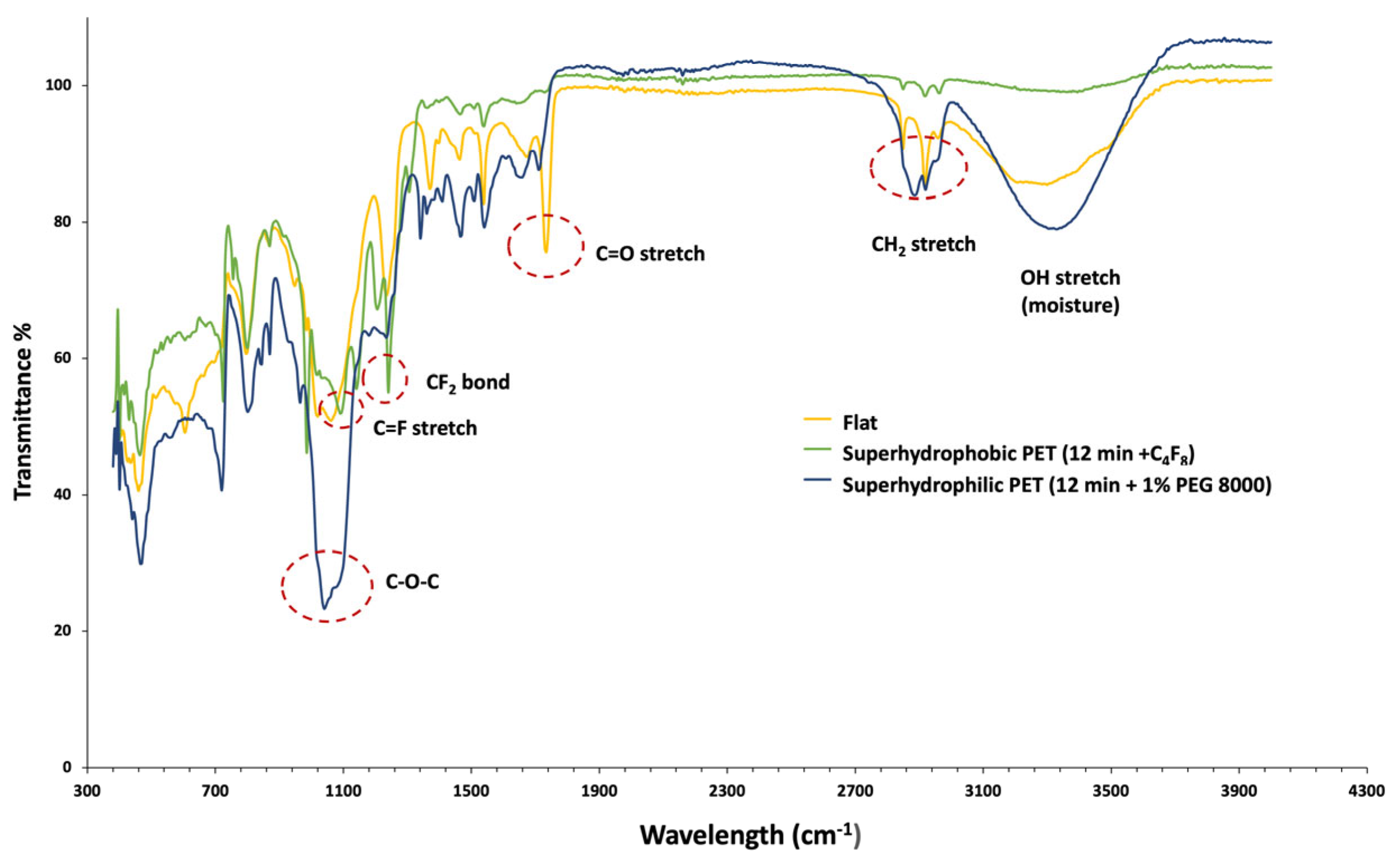
In
Figure 3, the chemical characterization via FTIR of the untreated (flat), the superhydrophilic plasma micro-nanotextured PET after coating with PEG (1% 8000) (blue line), and the superhydrophobic plasma micro-nanotextured PET after the plasma deposition with C
4F
8 (green line) are presented.
The comparative analysis of the samples’ surface chemistry shows that the untreated and flat PET sample spectrum (yellow line) exhibits two absorption bands around 3400 cm−1 (–OH) and 2900–2950 cm−1 (–CH). More importantly, one shows a carbonyl characteristic peak (C=O stretch, ~1725 cm−1), which is caused because untreated PET strongly absorbs the radiation due to ester carbonyl groups and, therefore, transmits only around 75–80%, resulting in this characteristic peak. On the contrary (as analyzed below), in both plasma-textured surfaces, superhydrophobic (C4F8-coated) and superhydrophilic (PEG-coated), this carbonyl characteristic peak (C=O stretch, ~1725 cm−1) cannot be detected due to a. the plasma micro-nanotexturing and the disruption of the native chain packing and b. the two coatings used, which reduce dipole–dipole coupling between carbonyl groups.
Finally, the absence of a strong C–F signal suggests that no fluorinated compound exists on the untreated PET surface, as expected.
Moving to the superhydrophobic PET surface etched for 12 min after C4F8 deposition (green line), the spectrum shows a significant increase in the C–F stretch and CF2 bond peaks compared to the untreated sample. The intensity of these peaks in the 1100–1300 cm−1 region is a clear signature of the fluorinated compound C4F8, which is used for the deposition of the hydrophobic coating. This indicates that the C4F8 was successfully deposited on the surface. The C=O and C-H stretch peaks are significantly less pronounced and a second characteristic peak that indicates the presence of fluorine is also observed for wavelengths around 500 cm−1 (C-F bending). In addition, the suppression of a PET-specific peak (C=O stretch, 1725 cm−1) reflects the masking of the PET surface by the hydrophobic coating and the disruption of the native chain packing after the plasma micro-nanotexturing step.
On the other hand, the spectrum of the superhydrophilic PET (blue line) shows distinct features related to the PEG 8000 addition. Most notably, the C–O–C ether stretch around 1100 cm−1 is a key indicator of the polyethylene glycol. In addition, a prominent peak around 3400 cm−1 is attributed to the O–H stretching of the hydroxyl groups, while peaks in the region of 2900–2950 cm−1 are associated with the C-H stretching of methylene groups (CH2). Similarly, the PET-specific peak (C=O stretch, 1725 cm−1) cannot be detected due to the PEG coating and the plasma micro-nanotexturing step.
3.3. Optical Property Characterization
In addition, we measured the total reflectance on the PET films using White Light Reflectance Spectroscopy (WLRS) to evaluate the effect of surface topography in the optical properties of PET. The analysis was completed using an integrating sphere as described in
Section 2.5.
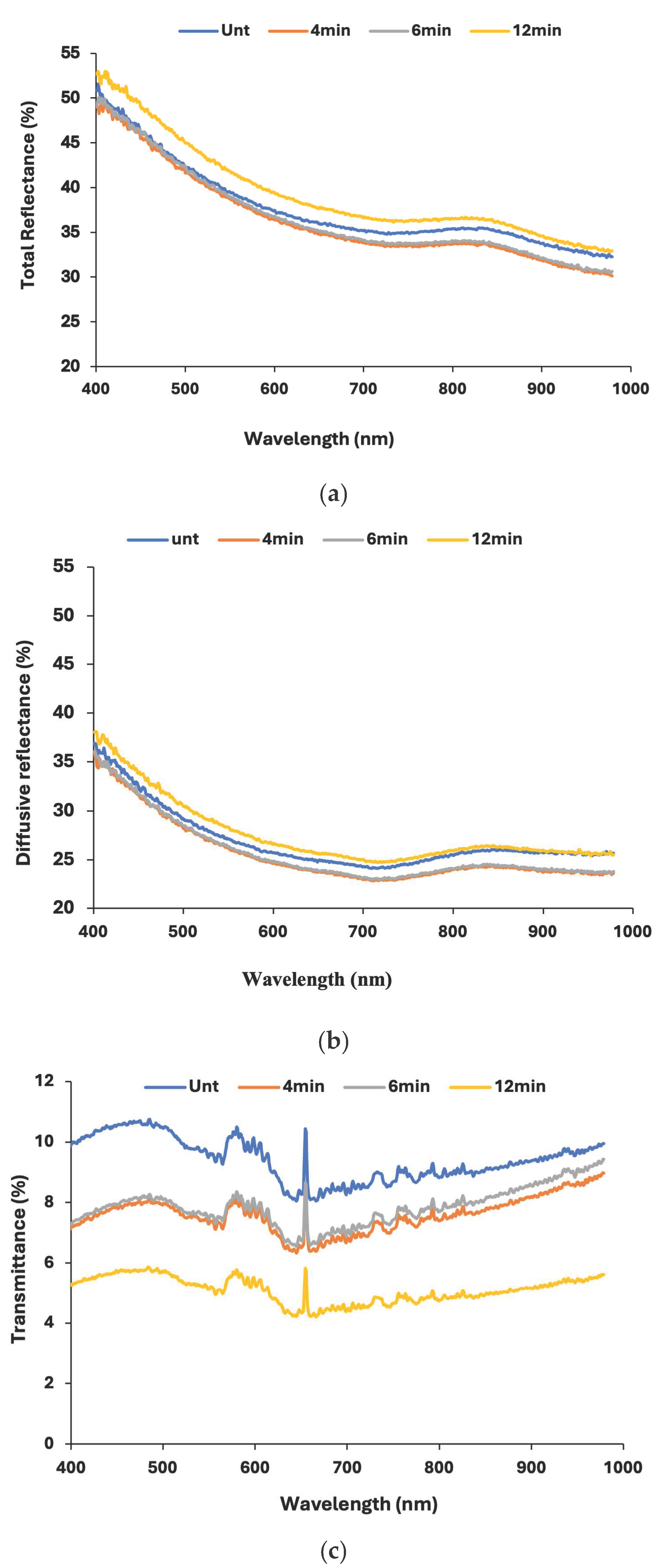
In
Figure 4, the reflectance of the flat and plasma-treated PET films is measured over the 400–900 nm wavelength range to assess the optical impact of surface modification. Across all samples, the total reflectance is around 50% at 400 nm and gradually decreases to 35% in the UV-visible region (up to 700 nm). A small increase in the reflectance is observed around 800 nm in the near-infrared region, which also degrades to almost 30% near 900 nm. This behavior is characteristic for the flat and for the treated PET films. Minimal variation is observed between the untreated (flat) and plasma-treated samples, indicating that plasma exposure up to 6 min does not significantly alter the optical reflectance of PET. Diffusive reflectance, as seen in
Figure 4b, has lower values than total reflectance as expected, since total reflectance captures all reflected light, while diffusive reflectance focuses on the scattered light. So, as surface roughness increases, more light is scattered, and the 12 min treated films have the highest values of diffusive reflectance.
UV-Vis transmittance (
Figure 4c) decreases with treatment time, with the untreated sample showing the highest transmittance and the 12 min treated sample the lowest. This is expected since larger topography features, due to prolonged plasma exposure, affect the transparency of the films, making them “blurry”. So, transmittance decreases when treatment time increases, even though the 4 min and the 6 min treated films show really similar results due to the small difference in treatment duration. The overall decrease in UV-Vis transmittance is around 2% and is practically not visual in the naked eye, so the method can effectively change the wetting properties of the PET films without significantly affecting the optical performance, as also reported in previous works [
49]. Moreover, it is also demonstrated that 12 min of plasma treatment effectively modifies the optical properties of PET, enhancing its light absorption capabilities, which could be beneficial for optoelectronic or photothermal applications.
3.4. Wetting Property Characterization and Ageing and Durability Evaluation
PET samples were characterized for their wetting properties by measuring the water contact angle and contact angle hysteresis. In
Figure 5, the water static contact angle and contact angle hysteresis are presented for the hydrophilic and superhydrophilic surfaces (
Figure 5a) and for the hydrophobic and superhydrophobic surfaces (
Figure 5b).
For the PET surfaces that were treated with plasma etching for different durations, we observe that, as the treatment time increases, the water static contact angle significantly decreases. This is due to the simultaneous increase in surface roughness and the surface chemistry modification happening during the plasma micro-nanotexturing step. Flat PET has a contact angle of 90°; after only 4 min it drops to 54° and eventually, after 12 min, it is 0°. Since the surfaces with the 12 min treatment have a remarkably low contact angle, a hydrophilic coating is added in order to improve their stability and durability since the plasma-treated surfaces tend to degrade over time. Two PEG coatings with different molecular weights but of the same concentration (1% PEG 8000, 1% PEG 1000) were used on the 12 min treated surfaces.
In order to render the plasma-treated surfaces superhydrophobic, a C4F8 layer is plasma deposited for all the etching durations and for the flat PET. The contact angle for the flat sample with the coating immediately rises by ten degrees (WSCA = 100°) with a relatively high contact angle hysteresis and, of course, when roughness is induced, the contact angle increases with treatment time. The 4 min and 6 min samples exhibit contact angle values of 116° and 154°, respectively, while the 12 min samples have the highest contact angle (160°) with almost zero contact angle hysteresis. So, we conclude that, after plasma etching for 6 min and deposition of a hydrophobic coating, the PET surfaces can be turned into superhydrophobic.
3.5. Durability of the Superhydrophilic and Superhydrophobic PET Surfaces
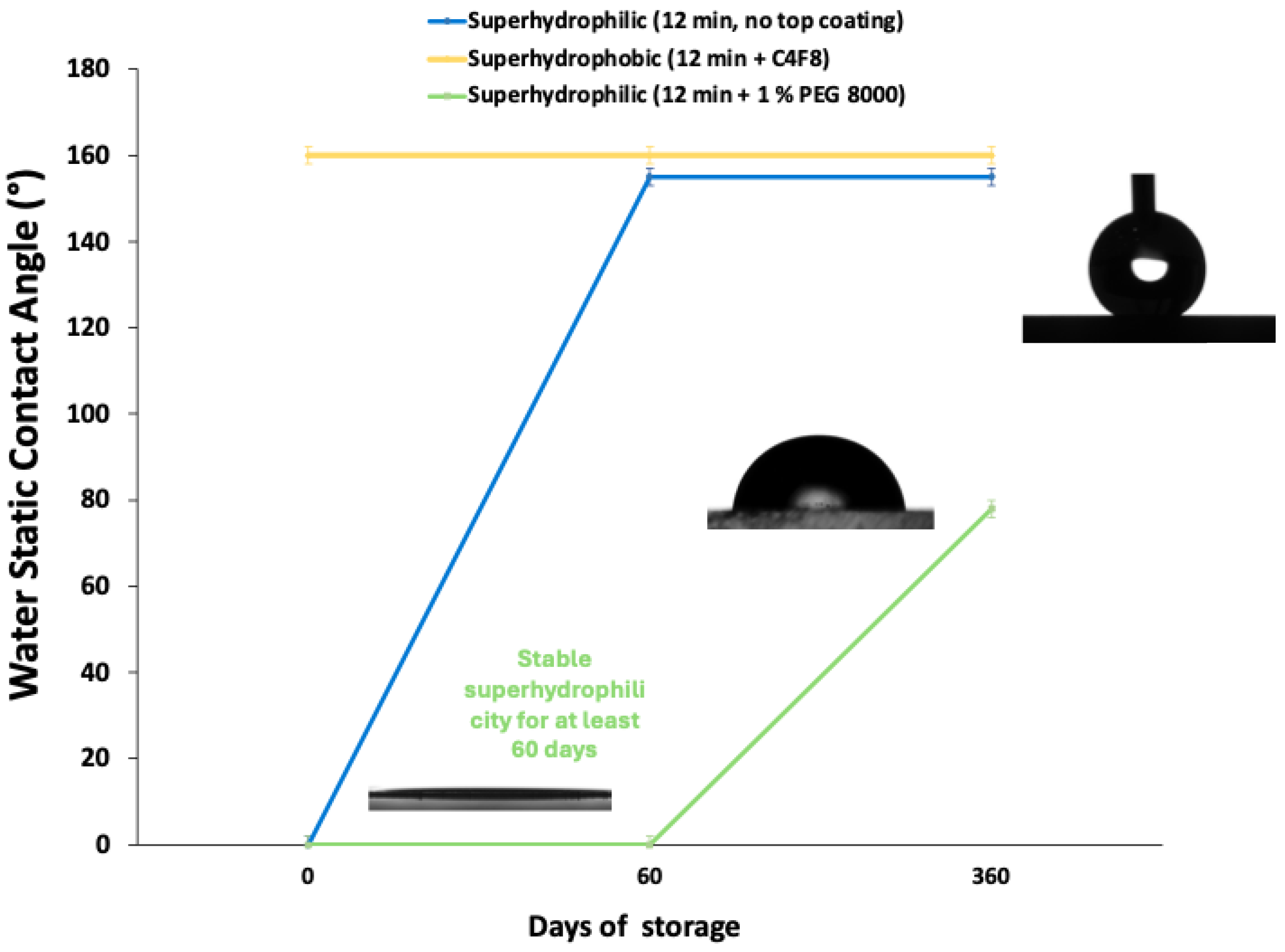
After determining the water static contact angle and contact angle hysteresis of the PET surfaces, the need to test the durability of our samples arises. Towards this direction, the samples are stored in ambient laboratory conditions (ambient temperature 20−30 °C, relative humidity 30−50%) and are evaluated again for their wetting properties after 60 and 360 days of storage. In
Figure 6, the graph for the aging tests of the surfaces with the 12 min treatment is presented and shows how the wetting properties of the surfaces change over time.
Although both superhydrophilic surfaces (with and without PEG coating) seem to be affected by aging, one can clearly observe that the 12 min surface without PEG exhibits strong hydrophobic properties after 60 days of storage, indicating that superhydrophobicity can be achieved without adding a top fluorinated coating. This is observed because, during the oxygen plasma micro- and nanotexturing step, polar functional groups are formed on the surface, such as hydroxyl, carbonyl, and carboxyl groups. These functional groups are prone to bond degradation and rearrangement into the bulk of the material and, simultaneously, they can react with the atmosphere, reducing their overall concentration over time. Increased plasma treatment duration enhances the number of functional groups formed and improves their long-term hydrophilic nature [
39], but not for long enough.
Thus, in order to realize a stable hydrophilic/superhydrophilic surface, PEG 8000 was deposited (a well-defined polymer with a stable ether backbone), which can prevent degradation and achieve a stable superhydrophilic state. We believe that PEG coating, especially when applied on the plasma-treated surface, homogenously covers all the functional groups created during the plasma processing and its large hydroxyl chains offer steric hindrance, protecting the underlying surface and promoting stability over time. This is the reason that, for the first 60 days of storage, we observe that the water contact angle remains at 0°. After one year, the water contact angle is 78°, but even so, the surface is still considered as hydrophilic (WSCA < 90°) and is much lower compared to the plasma-textured surface with no PEG coating.
On the other hand, for the 12 min surfaces with the C
4F
8 coating, superhydrophobicity is retained and stability against time is demonstrated, showing no or minimal degradation even after one year of storge. Plasma-deposited C
4F
8 coating creates a dense crosslinked network [
50] forming strong C-F bonds, making it an ideal candidate for long-term repelling applications. The stability of such films has also been studied in other works in the literature [
51].
3.6. Wetting Properties of the Superhydrophobic PET Surfaces Against Liquids with Lower Surface Tension
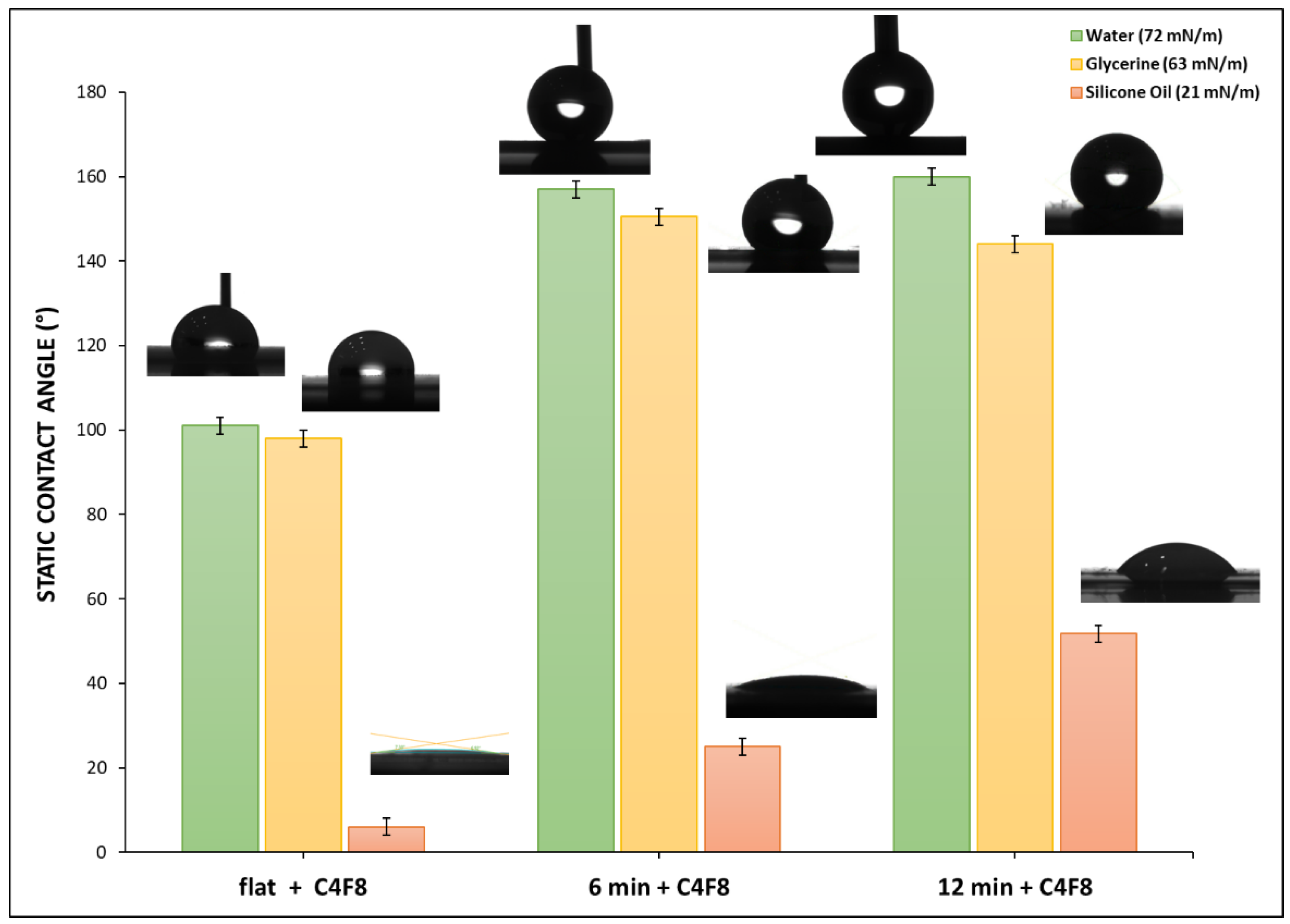
Contact angle measurements are indicators of the surface properties of the samples that we examine. Apart from water, we use two other liquids with low surface tension, such as glycerine (63 mN/m) and silicone oil (21 mN/m), to test the oleophobicity of the surfaces, as presented in
Figure 7.
In
Figure 7, it is observed that the measured contact angle exhibits a strong inverse dependence on liquid surface tension for the hydrophobic surfaces of all treatment durations. For the contact angle measurements, we used water, which has a surface tension (72 mN/m), glycerine, with surface tension (61 mN/m),and silicone oil, with a significantly lower tension (21 mN/m).
After applying the C4F8 coating contact angle against all liquids tested, increases as expected.As the treatment time increases, the contact angle for all three liquids also rises. Glycerine shows an intermediate behavior compared to the other two liquids, which aligns with its surface tension value being close to that of PET. In untreated surfaces, glycerine partially wets the surface with a contact angle of approximately 95°. With longer treatment times, the contact angle can increase to approximately 150°. As plasma treatment duration increases, glycerine’s contact angle begins to approach that of water, indicating that the enhanced surface roughness and chemical modification from prolonged treatment improve its repellency from PET surfaces. Interestingly, silicone oil, on the surfaces treated with plasma for 12 min, reaches a contact angle of 50°, whereas, for lower treatment durations or for the untreated surfaces, the oil drop spreads immediately across the surface. This is particularly promising for further investigations, as silicone oil is a non-polar liquid and such liquids typically wet most untreated polymers.
4. Conclusions
This study demonstrates a plasma-based micro-nanotexturing approach for creating durable superhydrophilic (WSCA 0°) and superhydrophobic (WSCA 160°) PET surfaces. Hierarchical roughness is created on the surfaces efficiently via oxygen plasma etching, a “green” method that is scalable and creates surfaces suitable for applications requiring extreme wettability (e.g., lab-on-chip devices, anti-fogging films). Different plasma etching durations (4–12 min) in combination with C4F8 (hydrophobic) or PEG (hydrophilic) coatings enable the fabrication of surfaces with extreme wettability that retain their functionality from 60 days (superhydrophilic) to 1 year (superhydrophobic). While the wetting properties of the PET films are altered, their optical properties remain unaffected for up to 6 min of treatment. Longer plasma treatment durations increase surface roughness, leading to higher reflectance. Notably, the 12 min treated surfaces not only exhibit a high degree of superhydrophobicity, but also show oleophobic tendencies, with contact angles of 150° for glycerine and 50° for silicone oil. So, this environmentally friendly method offers durable wettability control without compromising optical properties (at moderate treatment times), making it highly promising for several applications such as advanced interfaces for atmospheric water collection, lab-on-chip devices, anti-fogging films, and antibacterial packaging.