Abstract
Immune cells play a pivotal role in orchestrating tissue repair, executing functions such as debris clearance, extracellular matrix remodeling, and modulation of cytokine secretion profiles. However, when their activity is dysregulated or inadequately directed, these same processes can give rise to chronic inflammation and foreign body reactions (FBR), ultimately leading to fibrosis and compromised biomaterial performance. The immunological landscape following injury or biomaterial implantation is profoundly influenced by the physicochemical properties of material surfaces. By strategically tailoring these surface characteristics, it becomes possible to modulate immune cell responses—governing their adhesion, recruitment, proliferation, polarization, and cytokine expression patterns. This review elucidates the multifaceted roles of immune cells in tissue repair and their dynamic interactions with implanted biomaterials. It then explores how specific surface attributes—such as topography, chemistry, stiffness, and wettability—influence immune behavior. Particular emphasis is placed on recent advances in surface modification techniques aimed at engineering next-generation biomaterials that mitigate adverse immune responses while actively promoting regenerative healing. The review concludes by offering critical insights into the future of immunomodulatory biomaterial design, highlighting both emerging opportunities and persisting challenges in the field.
1. Introduction
The rapid advancement of tissue engineering and regenerative medicine has revolutionized approaches to restoring damaged tissues, enabling more efficient and targeted repair and replacement strategies [1]. At the core of this progress lies the integration of chemical and biological principles to engineer materials that not only serve as structural scaffolds but also mimic the native tissue environment and biological functions with high fidelity [2]. Early developments in the field favored the use of bioinert materials—such as those employed in contact lenses, orthopedic devices, and dental implants—primarily due to their chemical stability and minimal adverse biological responses. However, the limitations of these materials soon became apparent, particularly their inability to support the regeneration of parenchymal tissue after implantation [3]. This shortcoming is especially problematic in mechanically demanding environments like bone, where insufficient regenerative integration can severely compromise implant performance [4]. As a result, research in biomaterials has shifted focus from passive bioinert substances to bioactive materials capable of interacting with the biological microenvironment. Emerging evidence suggests that the physicochemical properties of implant surfaces—such as topography, roughness, and chemical composition—are instrumental in modulating host responses and promoting bone regeneration, particularly around titanium dental implants [5]. The host response to implanted materials mirrors the canonical phases of wound healing: hemostasis, inflammation, proliferation, and remodeling [6]. However, the implantation of bioactive materials elicits a particularly rapid and sensitive immune response [7]. This orchestrated reaction, termed the foreign body reaction (FBR), reflects the body’s attempt to isolate and respond to the non-native material [8]. Immediately following implantation, a complex array of host proteins adsorbs onto the biomaterial surface, initiating complement activation and triggering the recruitment and activation of neutrophils [9]. In subsequent stages, circulating monocytes and tissue-resident macrophages accumulate at the material–tissue interface, where immune signaling intensifies. Mast cells and T helper 2 (TH2) cells secrete cytokines such as interleukin (IL)-4 and IL-13, which stimulate macrophage fusion and lead to the formation of multinucleated foreign body giant cells (FBGCs) [10]. In addition to FBGC formation, macrophages mediate several downstream processes that significantly influence implant integration. These include the recruitment and activation of fibroblasts, the deposition of collagen-rich extracellular matrix, and ultimately, the formation of a fibrous capsule surrounding the implant—a hallmark of chronic inflammation and implant isolation [11]. Crucially, the long-term success of implants depends on a timely and effective transition from the inflammatory phase to the proliferative phase of healing [12]. When this transition fails, fibrous encapsulation prevails over tissue regeneration, diminishing both the functionality and biocompatibility of the implant [13]. Despite their association with chronic inflammation and osteolysis, macrophages and FBGCs also perform reparative functions. These include facilitating angiogenesis, phagocytosing cellular debris and pathogens, and remodeling the extracellular matrix—all of which are essential for regeneration under appropriate conditions [14,15]. Recent advances in the molecular design of biomaterials have sought to leverage this dual functionality by encouraging a phenotypic shift in macrophages from a pro-inflammatory (M1) state to an anti-inflammatory, pro-regenerative (M2) phenotype [16]. To this end, modulating the interactions between immune cells—particularly macrophages—and the biomaterial surface has emerged as a critical strategy for optimizing the host response and promoting constructive tissue integration. Tailoring surface properties such as roughness, wettability, chemical functionality, and stiffness has shown significant promise in tempering inflammatory responses while enhancing regenerative potential [17].
This review synthesizes recent findings on how material surface properties influence innate immune responses following biomaterial implantation. Particular emphasis is placed on the role of surface modifications in guiding immune cell behavior to favor tissue regeneration over chronic inflammation. The discussion concludes with future perspectives and the key challenges in the rational design of next-generation implantable biomaterials that elicit controlled and therapeutically beneficial immune responses.
2. Immunological Modulation in Tissue Regeneration and Functional Repair
The immune system is a complex and dynamic biological network essential for maintaining physiological homeostasis and defending the host against exogenous threats, including pathogenic microorganisms [18]. Broadly categorized into the innate and adaptive branches, the immune system orchestrates a highly coordinated response to injury and infection [19]. The innate immune system serves as the body’s first line of defense, initiating rapid, non-specific inflammatory responses upon recognizing foreign agents [20]. This arm includes various cell types such as innate lymphoid cells, γδ T-cells, and natural killer (NK) cells, as well as mononuclear phagocytes like monocytes, macrophages, and dendritic cells, and polymorphonuclear leukocytes [21]. In contrast, the adaptive immune system comprises T and B lymphocytes that mount highly specific responses and establish immunological memory for long-term protection [22]. Tissue repair is closely governed by immune processes, beginning with protein adsorption on the damaged or implanted surface, followed by immune cell infiltration and the release of signaling molecules that coordinate healing [23]. The regenerative cascade unfolds through four well-defined stages: hemostasis, inflammation, proliferation (or granulation), and remodeling [24]. The sequential and timely progression through these phases is critical to achieving functional restoration of the tissue [25]. Dysregulation of any phase may lead to pathological outcomes such as fibrosis and, in severe cases, organ failure [26]. Immune cells play a central role throughout these phases, particularly during the inflammatory stage, which sets the tone for downstream processes including tissue repair and extracellular matrix (ECM) remodeling [27]. Inflammation is a multifaceted biological process involving the early recruitment of neutrophils, the infiltration of monocytes, and their differentiation into macrophages at the injury site [28]. These cells are instrumental in amplifying immune signaling cascades and recruiting adaptive immune cells to the area of damage. As the tissue enters the repair phase, hallmarks such as angiogenesis, collagen synthesis, and ECM deposition emerge [29], while the remodeling phase is defined by the maturation of newly formed vasculature and tissue architecture [30]. Immune cells continue to exert influence during the remodeling phase, where they participate in clearing apoptotic cells and debris and promoting the proliferation and differentiation of stem and progenitor cells—especially in the context of skeletal tissue repair [31]. Among these, macrophages are indispensable due to their phagocytic capabilities and plasticity in function [32]. Depending on environmental cues, macrophages polarize into either pro-inflammatory (M1) or anti-inflammatory, pro-regenerative (M2) phenotypes [33]. M1 macrophages secrete cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and IL-6, contributing to the inflammatory milieu and facilitating clearance of pathogens and damaged tissue [34]. Conversely, M2 macrophages release anti-inflammatory cytokines and support processes essential for wound healing and tissue regeneration [35]. Neutrophils, the most abundant leukocytes in the circulation, are the first responders at sites of injury. They are critical not only for pathogen clearance but also for the degradation and removal of necrotic tissue through the secretion of proteolytic enzymes and antimicrobial agents [36,37]. During biomaterial implantation, neutrophils contribute to wound sanitation by engulfing bacteria and other microbes [38]. They also deploy neutrophil extracellular traps (NETs), comprising histones, DNA, and granular proteins, to immobilize invading pathogens [39]. Furthermore, activated neutrophils can produce immunomodulatory chemokines such as CCL2, CXCL8, and CCL4, which coordinate further immune cell recruitment [40]. Importantly, the resolution of inflammation is closely tied to neutrophil apoptosis and subsequent efferocytosis by macrophages, a process that promotes the shift from M1 to M2 macrophage phenotypes and facilitates healing [41,42,43]. However, excessive or prolonged neutrophil activation can sustain inflammation and lead to deleterious effects at the site of implantation [44]. Natural killer (NK) cells also play a regulatory role during the inflammatory phase of tissue repair [45]. With the capacity to recognize virally infected or stressed cells, NK cells induce cytolysis via the release of cytotoxic granules [46]. Emerging evidence suggests a role for NK cells in bone regeneration, where they contribute to tissue remodeling by eliminating damaged cells and promoting the recruitment of mesenchymal progenitors during the later stages of fracture repair [47,48]. Other immune cells, including innate lymphoid cells (ILCs), dendritic cells (DCs), and adaptive lymphocytes (T and B cells), are also implicated in tissue regeneration. T and B cells, in particular, have been shown to influence bone repair, with studies demonstrating that their absence can impair fracture healing [49,50]. T cells contribute to bone mineralization and structural integrity; deficiencies in these cells are associated with increased susceptibility to fractures [51]. At the end of the inflammatory phase and the onset of mineralization, T cells facilitate the recruitment, differentiation, and activation of osteoclasts by producing receptor activator of nuclear factor kappa-B ligand (RANKL), which aids in removing the fibrin clot to allow for cartilaginous callus formation [50]. Additionally, T cells secrete IL-17, a cytokine with both anti-inflammatory properties and a stimulatory effect on osteogenic differentiation and activity [49,52,53]. B cells also support bone healing by suppressing pro-inflammatory cytokines such as TNF-α and interferon-gamma (IFN-γ), while modulating osteoclast differentiation and activity [54,55]. These multifaceted immune interactions governing tissue repair and regeneration are schematically summarized in Figure 1. While much attention has been devoted to the immunological underpinnings of tissue regeneration, particularly the role of immune cells, this review extends beyond those discussions previously explored by Shanley et al. [56], focusing instead on the material–immune interface and how biomaterial design can be leveraged to direct immune responses toward constructive regeneration.
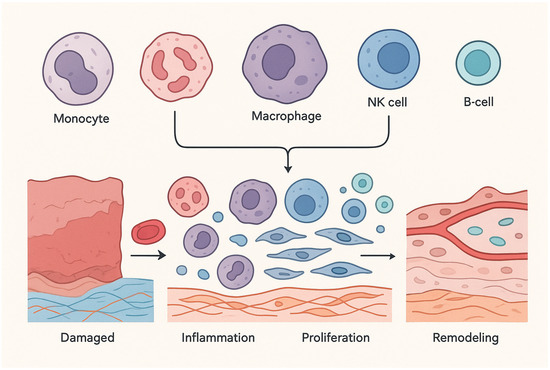
Figure 1.
Immune System and tissue recovery and restoration features.
3. Immunological Responses to Biomaterial Interfaces
The host immune response elicited by implanted biomaterials is widely acknowledged as a critical determinant of their biological performance and long-term integration. This immunological interaction plays a pivotal role in the success of biomedical devices, orthopedic implants, and scaffolds following implantation [57]. Immediately upon contact with host tissue, the biomaterial surface is rapidly coated by a layer of adsorbed proteins and complement factors, initiating a cascade of events that leads to the formation of a provisional blood clot. This clot is rich in matrix metalloproteinases (MMPs), cytokines, and growth factors, which together act to recruit neutrophils to the site of injury and activate the early innate immune response [58]. Platelet activation, a concurrent event, facilitates the migration of monocytes to the site of implantation. Once recruited, these monocytes differentiate into macrophages that recognize and adhere to the biomaterial surface as a foreign entity. In doing so, they establish a fibrinous matrix that aids in phagocytosis and the clearance of foreign matter. However, when the implanted material cannot be effectively internalized or degraded by macrophages, the immune system may transition into a chronic inflammatory state. This prolonged activation often culminates in the formation of a dense fibrotic capsule, a hallmark of the foreign body reaction (FBR), which physically separates the implant from the surrounding tissue [59]. The development of FBR and its associated fibrotic encapsulation poses a significant threat to implant functionality. Not only can it obstruct integration with host tissue, but it also compromises the performance of the biomaterial over time [60,61]. Central to this process is the initial phase of protein adsorption—its kinetics, composition, and molecular organization—which is intimately governed by the physicochemical properties of the biomaterial surface. These properties, in turn, dictate the recruitment, adhesion, and activation patterns of immune cells such as neutrophils, macrophages, and monocytes [62]. The following section delves into the specific surface characteristics of biomaterials—such as topography, chemistry, stiffness, and wettability—and their influence on immune cell behavior within the context of tissue engineering and regenerative medicine.
4. Surface-Driven Modulation of Host Immune Responses
4.1. Modulatory Effects of Surface Chemistry and Charge on Immune Cell Responses
The incorporation of organic functional groups into the surface chemistry of biomaterials—such as amine (–NH2), carboxyl (–COOH), sulfhydryl (–SH), hydroxyl (–OH), and phosphoryl (–PO3) moieties—plays a fundamental role in mediating specific cell–matrix interactions, which are essential for ensuring long-term biocompatibility and functional integration following implantation [63,64]. Considerable research has been dedicated to the strategic utilization of hydroxyl (–OH) groups to modulate immune responses, given that –OH can act as a reactive oxygen species (ROS) capable of activating innate immune pathways [65]. Notably, –OH groups have been implicated in initiating immune activation via several signaling mechanisms, including interleukin-3 (IL-3) signaling, oncostatin M signaling, antigen presentation pathways, and the migration inhibitory factor (MIF)–JAB1 (Jun activation domain-binding protein 1) axis [66]. Similarly, surfaces functionalized with amine groups (–NH3) have demonstrated immunomodulatory potential by reducing leukocyte adhesion and mitigating immune activation [67]. In contrast, modifications involving –OH and –CH3 groups have been associated with heightened inflammatory responses, whereas –COOH-functionalized surfaces tend to elicit diminished immune cell activation [68,69]. These findings underscore the potential of surface chemical tailoring as a strategy to mitigate foreign body reactions (FBR) and associated inflammatory responses following biomaterial implantation [70]. In an influential study by Kamath et al., polypropylene microparticle implants functionalized with –OH and –NH2 groups produced the thickest fibrotic capsules and most pronounced cellular infiltration, whereas –CFx and –COOH surface chemistries were associated with reduced fibrotic responses and lower immune cell accumulation [71]. Expanding upon these findings, Lopez-Silva et al. developed supramolecular hydrogels composed of multidomain peptides (MDPs) containing amine, guanidinium, and carboxylate groups to examine how distinct chemical functionalities affect immune responses [72]. Their data revealed that deprotonated carboxylic acids (as in aspartate- and glutamate-based MDPs D2 and E2) induced only minimal macrophage infiltration and cytokine secretion. Conversely, the lysine-based MDP K2, featuring protonated amine groups, prompted acute immune infiltration dominated by inflammatory monocytes, which transitioned into macrophages by day 7 post-implantation. Surface charge, another key parameter of biomaterial design, has also emerged as a significant modulator of immune cell and stem cell behavior, influencing both osteoimmune interactions and tissue regeneration outcomes [73,74]. The adsorption of biologically active molecules—including proteins that initiate immune signaling—is strongly affected by surface charge [75]. Additionally, since immune cell membranes, including those of macrophages, carry a net negative charge, the electrostatic characteristics of biomaterial surfaces can modulate immune cell adhesion, activation, and phenotype, particularly in ways that favor pro-regenerative behavior [76]. Dendritic cells (DCs) have been shown to respond sensitively to surface charge density: highly charged surfaces enhance DC maturation and cytokine release, whereas low-charge-density surfaces tend to provoke muted immune responses [77]. In general, positively charged (cationic) surfaces provoke more robust inflammatory responses than neutral or negatively charged (anionic) ones [78]. This effect is thought to result from the neutralization of the cell membrane’s negative charge by the biomaterial’s cationic surface, which can alter protein conformation, disrupt membrane integrity, and initiate inflammatory cascades [79]. However, these electrostatic interactions can be modulated in vivo due to surface contamination from host fluids and proteins upon implantation. Crucially, the impact of surface charge on immune responses is often interdependent with other surface characteristics, such as surface roughness and chemical composition. Therefore, investigations into the immunological effects of charge must consider these covariates. For instance, biomaterials presenting –OH and –NH2 groups (typically neutral or positively charged) have been shown to exacerbate immune cell recruitment and fibrotic responses in vivo, while –COOH-functionalized surfaces (anionic) exhibit the opposite effect [71,80,81,82].
A comparative study in 2002 examined poly (dimethylamino propyl acrylamide) (a cationic polymer) against poly(acrylic acid) (an anionic polymer) and found that the cationic material suppressed anti-inflammatory cytokines IL-10 and IL-1RA, both of which are important for osteoblast maturation. In contrast, the anionic polymer facilitated IL-10 expression, supporting a pro-healing immune environment [83]. Interestingly, conflicting findings suggest that surface modifications with divalent cations may foster macrophage polarization toward a tissue-remodeling phenotype—possibly due to increased hydrophilicity. For example, Lee et al. demonstrated that calcium (Ca2+) and strontium (Sr2+) ion-modified titanium surfaces enhanced prostaglandin E2 (PGE2) production by macrophages and stimulated osteogenic processes [84]. An emerging immunomodulatory strategy involves engineering biomaterial surfaces to sequester pro-inflammatory cytokines at the site of injury through electrostatic interactions. In one such example, Shin et al. designed a negatively charged phosphate-crosslinked polylactic acid (PLA) mesh to attenuate acute inflammation following implantation into the peritoneum [85]. The negatively charged scaffold effectively bound positively charged pro-inflammatory cytokines—including TNF-α, IL-1β, and IL-6—thereby reducing the local inflammatory burden, as confirmed through a bead-based bioassay. Additionally, zwitterionic biomaterials, which contain both positive and negative charges within the same functional group, have garnered increasing attention for their capacity to resist non-specific protein adsorption and cellular attachment [62]. This dual-charge property endows zwitterionic hydrogels with exceptional bio-inertness. Notably, research by Zhang et al. revealed that such hydrogels promote angiogenesis and wound healing while simultaneously reducing fibrotic encapsulation. When implanted for periods exceeding three months, these hydrogels resisted biofilm formation, inflammatory infiltration, and foreign body capsule development [62]. The collective influence of surface chemistry and surface charge on immune cell behavior, and their implications for tissue regeneration and biomaterial integration, are summarized in Table 1.

Table 1.
Influence of surface chemistry and charge on immune cell behavior.
4.2. The Impact of Surface Wettability on Immune Cell Behavior
Surface wettability is recognized as one of the critical determinants shaping the early host immune response to implanted biomaterials [86]. Generally, hydrophobic surfaces are associated with the recruitment of immune cells, the polarization of macrophages toward the pro-inflammatory M1 phenotype, and the initiation of acute inflammatory responses. In contrast, neutral and hydrophilic surfaces tend to favor immunomodulatory outcomes that support tissue regeneration by promoting anti-inflammatory signaling and M2 macrophage activation [87,88]. As such, fine-tuning surface wettability has emerged as a powerful strategy to modulate serum protein adsorption patterns on biomaterial surfaces, thereby directing the innate immune response toward more favorable, pro-healing trajectories [89].
The hydrophilic nature of a surface plays a pivotal role in determining the conformational state of adsorbed proteins such as fibrinogen and fibronectin—two extracellular matrix components crucial for immune recognition and cell adhesion [90]. A notable investigation by Lv et al. explored the immunological implications of surface hydrophilicity and hydrophobicity using two model systems: UV-activated hydrophilic TiO2 surfaces and micropatterned OTS/UV–TiO2 substrates that combined hydrophobic and hydrophilic regions [91]. Through atomic layer deposition (ALD) and ultraviolet (UV) treatment, the researchers fabricated anatase-phase TiO2 thin films enriched with –OH groups on silicon substrates. Subsequently, polydimethylsiloxane (PDMS)-assisted microcontact printing was used to generate self-assembled monolayers (SAMs) of octadecyltrichlorosilane (OTS), yielding micropatterned surfaces that juxtaposed hydrophilic and hydrophobic regions. The behavior of RAW 264.7 macrophages cultured on these surfaces revealed striking morphological and functional differences. On hydrophobic OTS regions, cells exhibited a rounded, less spread morphology, indicative of limited attachment and heightened inflammatory activation. In contrast, cells on hydrophilic UV–TiO2 surfaces displayed enhanced spreading and adopted phenotypes consistent with the anti-inflammatory, tissue-reparative M2 state. Mechanistic analyses further revealed that this shift in macrophage behavior was mediated by the adsorption and conformational modulation of fibronectin and fibrinogen, which in turn influenced integrin β1 and β2 expression. These integrin-mediated cues activated downstream signaling pathways including phosphoinositide 3-kinase (PI3K) and nuclear factor kappa B (NF-κB), ultimately fostering a microenvironment conducive to osteogenesis. However, the authors emphasize that hydrophilicity alone is insufficient as a sole predictor of macrophage polarization toward an M2 phenotype [91]. Instead, it is the complex interplay between wettability and other surface characteristics—such as chemical composition, surface energy, and topography—that collectively governs immune cell behavior at the biomaterial interface [87]. Thus, while hydrophilicity is an important modulator of immune response, it should be considered within a multifactorial context for the rational design of immunomodulatory biomaterials. The influence of surface wettability on immune cell responses, particularly macrophage polarization and inflammatory signaling, is illustrated in Figure 2.
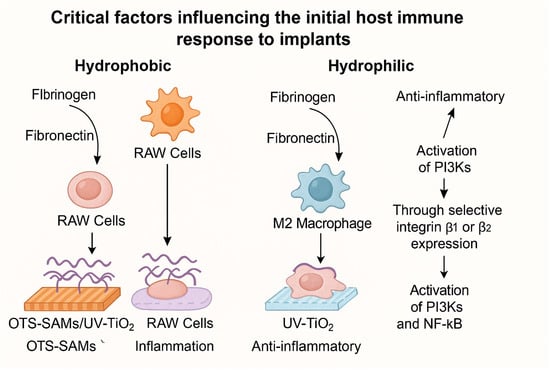
Figure 2.
Impact of surface wettability on immune cell behavior, adapted from [91].
4.3. Influence of Surface Topographical Features on Host Immune Reactions
The topographical architecture of biomaterial surfaces plays a pivotal role in shaping immune cell behavior and holds significant potential for advancing the design of implants and scaffolds aimed at optimizing tissue repair and regeneration [92,93,94]. Increasingly, researchers have harnessed surface topographical modifications as a strategy to guide immune cell phenotypes toward favorable, pro-healing states [95,96,97]. These structural alterations can influence the secretion of key pro-inflammatory mediators, including cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), as well as chemokines like monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α) [98]. Notably, surfaces with enhanced roughness have been linked to elevated in vitro production of MIP-1α by macrophages, suggesting a direct correlation between physical texture and inflammatory signaling [99]. Beyond pro-inflammatory responses, surface topography also modulates regenerative markers. For instance, rough titanium surfaces have been shown to increase the expression of bone morphogenetic protein-2 (BMP-2) in macrophages, hinting at their potential to promote osteogenic processes. Furthermore, surface texture significantly influences the adhesion and spreading of macrophages, though outcomes can vary depending on the biomaterial type and tissue environment. In some contexts, macrophages demonstrate a preference for smoother surfaces, while in others, rougher surfaces enhance cellular attachment and activation. Supporting this, studies on pure titanium have revealed increased macrophage adhesion and spreading in vitro on surfaces with greater roughness [100,101]. To achieve precise control over surface topography, a wide array of nano- and microfabrication techniques has been employed. These include chemical etching, sandblasting, microcontact printing, lithography, electrospinning, micromachining, replica molding, and self-assembly based systems [94,102,103]. Surface topographies are typically categorized by scale—macro, micro, and nano—each eliciting distinct cellular responses [104]. Microscale features are known to influence cytoskeletal dynamics and direct cell proliferation, whereas nanoscale textures enhance protein adsorption and cellular interactions by increasing surface energy, thus promoting cell recruitment, spreading, and differentiation [105]. Designing biomaterials that integrate both micro- and nanotopographical features has been proposed as an especially effective approach to modulate immune responses in favor of tissue regeneration [106]. For example, Liu et al. demonstrated that macrophage morphology and function can be directed by nanoscale geometries: nanoflakes and nanowires were essential for the formation of lamellipodia and filopodia, respectively—key structures involved in cell motility and environmental sensing [107,108,109]. Moreover, titanium surfaces incorporating a hybrid of nanoflakes and nanowires elicited stronger anti-inflammatory and osteo-immunomodulatory responses compared to their microstructured counterparts by skewing RAW 264.7 macrophages toward the M2 phenotype, which is associated with tissue repair and resolution of inflammation. Titanium-based implant surfaces engineered with a combination of nanowires and nanoflakes have demonstrated enhanced anti-inflammatory and osteoimmunomodulatory properties when compared to microstructured counterparts. These nanoscale features effectively promote the polarization of murine-derived RAW 264.7 macrophages toward the M2 phenotype, associated with tissue repair and pro-healing functions [110]. Among various topographical features, surface roughness is widely regarded as one of the most influential aspects of implant surface design [110]. The quantification of surface roughness involves measuring the vertical deviations—depressions and protrusions—across a material’s surface profile [111]. Notably, both smooth and rough surfaces can influence immune cell adhesion, though outcomes are highly context-dependent [112]. Surface roughness plays a critical role in facilitating osseointegration, particularly in dental and orthopedic implants, by enhancing bone-to-implant contact and interfacial mechanical interlocking [5]. However, accumulating evidence suggests that only a specific range of roughness effectively directs immune cell behavior toward regenerative outcomes [113]. For instance, moderate roughness values—typically characterized by an average roughness (Ra) between 1–2 μm—have been shown to optimize tissue regeneration and bone-implant integration more effectively than surfaces with excessive roughness (Ra > 2 μm), which may provoke undesirable immune responses or impede healing [114]. Beyond roughness alone, the interplay between surface texture and surface chemistry significantly shapes immune cell responses. In a study by Christo et al., the synergistic influence of both parameters was evident: rough surfaces combined with specific chemical modifications led to increased secretion of matrix metalloproteinase-9 (MMP-9) by primary neutrophils, while simultaneously reducing pro-inflammatory cytokine production by macrophages [115]. These findings underscore the complex, multifactorial nature of immune cell modulation, where surface attributes operate in tandem to direct cellular behavior. Importantly, the effects of surface roughness are mediated not only by direct mechanical cues but also by alterations in protein adsorption. Surface roughness influences both the type and conformation of adsorbed proteins, thereby modulating subsequent cell signaling. This partially explains the discrepancies frequently observed between in vitro and in vivo studies, where the biological environment may alter protein-surface interactions differently. To contextualize these effects, the concept of surface free energy becomes instrumental. Surface free energy can be described as half the energy required to split a bulk material into two surfaces; this excess energy influences surface chemistry and biological interactions. High surface energy correlates with polar, hydrophilic surfaces, which generally enhance protein adsorption and cellular adhesion compared to low-energy hydrophobic surfaces. Although surface roughness is distinct from surface chemistry, its effects often overlap—especially when roughness increases the available surface area of a hydrophilic material, thereby enhancing its wettability and bioactivity. A more refined approach to modifying surface topography involves the deliberate creation of patterned surfaces. These topographical patterns fall into two broad categories: isotropic and anisotropic. Anisotropic surfaces feature directionally aligned elements such as grooves and ridges, which guide cell orientation and migration. In contrast, isotropic surfaces contain non-directional features, including pits, posts, fibers, and randomly arranged protrusions [116]. Recent studies have demonstrated that parameters such as pillar diameter and micropillar density on these patterned surfaces can significantly influence macrophage polarization. Specifically, appropriately designed topographies can induce a shift from the pro-inflammatory M1 phenotype toward the regenerative M2 phenotype, thereby initiating an immune environment conducive to tissue regeneration [117]. In 2015, Luu et al. employed deep etching techniques to engineer titanium surfaces with micro- and nanoscale grooves, aiming to elucidate how specific topographical features influence macrophage polarization and morphology [118]. Their study revealed that while grooves measuring 400–500 nm in width did not induce inflammatory activation, they did promote the polarization of macrophages toward the anti-inflammatory, tissue-reparative M2 phenotype. Notably, intermediate groove widths significantly enhanced the secretion of interleukin-10 (IL-10), an immunoregulatory cytokine, underscoring the potential of topographical customization as a powerful strategy to modulate immune behavior and improve tissue regeneration. Expanding upon this principle, Vassey et al. (2020) developed an extensive topographical library comprising 2176 unique micropatterns—derived through both in vitro and in vivo algorithms—to investigate the relationship between surface architecture and the phenotypic behavior of human monocyte-derived macrophages [117]. Their findings demonstrated that micropillars ranging from 5–10 μm in diameter were particularly effective in promoting macrophage adhesion. Moreover, the coordinated modulation of pillar size and spatial density fostered a phenotypic shift from pro-inflammatory (M1) to anti-inflammatory (M2) states. This transition is critical not only for mitigating inflammation but also for maintaining bone homeostasis and stimulating osteogenic differentiation in mesenchymal stem cells (MSCs) [119]. Macrophage polarization also plays a central role in the early stages of fracture healing, orchestrating the recruitment of vascular progenitor cells, MSCs, and osteoprogenitors to the injury site [120]. Therefore, understanding how surface topography guides immune cell fate is pivotal for the rational design of osteoinductive biomaterials that expedite bone repair through immunomodulation [121]. Supporting this concept, Zhu et al. (2021) fabricated four honeycomb-like titanium dioxide (TiO2) surface structures—HC-90, HC-500, HC-1000, and HC-5000—on titanium substrates, with pore diameters ranging from nanometer to micrometer scale, to evaluate their impact on RAW 264.7 macrophage behavior and immunomodulatory potential in osteogenesis [122]. The smallest structure, HC-90 (90 nm), induced the strongest anti-inflammatory response, characterized by elevated expression of IL-10, IL-4, CD206, and bone morphogenetic protein-2 (BMP-2), all markers indicative of M2 macrophage polarization and pro-osteogenic signaling. Similarly, Wang et al. explored the effect of hydroxyapatite (HA) ceramics with varying nano-to-submicron grain sizes—S1 (448.19 ± 129.12 nm), S2 (221.84 ± 36.25 nm), and S3 (106.42 ± 19.82 nm)—on macrophage phenotype and function during osteogenesis [123]. These HA disks, fabricated through three distinct sintering methods (conventional muffle furnace, microwave, and two-step microwave), exhibited surface roughness values between ~25 and 65 nm. Results indicated that the nano-grained S3 disk not only suppressed macrophage spreading but also increased the proportion of CD206+ M2 macrophages while downregulating pro-inflammatory cytokine production. In a mouse intramuscular implantation model, the nano-topography significantly enhanced the expression of M2-associated markers such as ARG1, confirming its potential to mitigate local inflammation and support regenerative healing. In an effort to further dissect the immunomodulatory influence of hierarchical topographies, Wang et al. developed an amorphous metallic glass (Pt57.5Cu14.7Ni5.3P22.5, or Pt-BMG) featuring dual micro- and nanoscale patterns [124]. Compared to smooth and purely microstructured surfaces, nanotopographical features were markedly more effective at suppressing macrophage fusion into foreign body giant cells (FBGCs), a hallmark of chronic foreign body response. These results emphasize the critical role of nanoscale architecture in modulating innate immune behavior and reducing fibrotic encapsulation. Further innovation was demonstrated by Song et al., who introduced quasi-periodic, pimple-like TiO2−x nanostructures onto Ti6Al4V alloy surfaces via an alkalinity-activated solid-state dewetting (AAD) process to enhance osseointegration [125]. This modification resulted in a surface with improved hydrophilicity and higher surface energy. When RAW 264.7 macrophages were cultured on these modified surfaces, early M1-associated cytokines (TNF-α, IL-1β) peaked at day 3 but significantly declined by day 7. Concurrently, secretion of M2-associated cytokines (Arg-1, IL-10, TGF-β) increased, facilitating the transition toward long-term tissue repair. These findings illustrate a time-dependent polarization trajectory that mirrors the natural course of wound healing and highlights the potential of nanoscale TiO2−x surfaces to orchestrate a controlled immune response that fosters osseointegration. Collectively, the presence of nanopimple-like nanostructures on Ti6Al4V surfaces has been shown to intricately modulate the crosstalk between macrophages and osteoblasts, thereby significantly enhancing bone-implant integration in vivo. In a notable study by Nouri-Goushki et al., a cutting-edge 3D nanoprinting approach utilizing two-photon polymerization was harnessed to fabricate submicron 3D printed pillars with varying heights (250–1000 nm) and interspacing (700 nm, 1000 nm), aiming to decipher how these architectural parameters influence the immune response [126]. Their findings revealed that the tallest pillars with the highest density effectively induced the phenotypic shift in macrophages from the pro-inflammatory M1 state to the reparative M2 phenotype within three days, notably in the absence of inflammatory cytokines. Simultaneously, these topographies enhanced the osteogenic activity of preosteoblast cells, highlighting the dual immunomodulatory and regenerative potential of finely tuned surface architectures. Expanding the paradigm, the design of implants and scaffolds with dynamic, stimuli-responsive surface topographies has emerged as a promising strategy for non-invasive immunomodulation to promote tissue repair. For instance, Zheng et al. engineered a shape-memory film (SMP) integrating polyethylene glycol (PEG)-modified gold nanorods (AuNRs) with branched polycaprolactone macromonomers (4b- and 2b-PCLm), capable of transforming from a flat to a micro-grooved surface under near-infrared (NIR) irradiation [127]. This transformation dynamically influenced macrophage morphology, shifting cells from a rounded to an elongated phenotype, a morphological change correlated with polarization from the pro-inflammatory M1 to the anti-inflammatory, pro-healing M2 state. Correspondingly, increased expression of arginase-1 (Arg-1) and IL-10 was observed in vitro, alongside enhanced tissue healing in vivo following NIR exposure, demonstrating the power of responsive surface topographies in directing immune cell behavior and accelerating regeneration. Surface alignment—the orientation of predominant topographical features—also constitutes a critical parameter governing immune responses [128]. Fiber alignment, in particular, has been recognized as a pivotal factor in modulating macrophage function and polarization post-implantation [129]. In an effort to mitigate foreign body response (FBR)-induced capsular contracture, Choi et al. fabricated micro-textured polydimethylsiloxane (PDMS) substrates embedded with either aligned or randomly oriented polyvinylpyrrolidone (PVP) fibers using a facile, cost-effective surface modification technique [130]. Their results showed that while both aligned and random microtextures reduced fibrous capsule thickness and collagen density compared to bare PDMS, only the aligned microtextures promoted macrophage polarization towards the anti-inflammatory M2 phenotype, underscoring the immunomodulatory advantage of directional topographical cues. In a complementary investigation, Gao et al. examined the effects of Tanshinone IIA (Tan IIA)-loaded aligned and random microfibers on macrophage polarization, stem cell recruitment, and neovascularization [131]. Their data demonstrated that aligned microfibers, especially those incorporating 1 and 10 μm doses of Tan IIA, more effectively polarized RAW 264.7 macrophages towards the M2 phenotype and enhanced endogenous stem cell recruitment post-implantation compared to their randomly oriented counterparts. Adding further depth to the role of three-dimensional topographical cues, Ryma et al. pioneered a novel melt electrofibrillation technique to fabricate highly organized, anisotropic collagen-mimetic nanofibrillar microbundles, probing their influence on macrophage behavior at the tissue-implant interface [132]. These fibrillar scaffolds markedly elevated secretion of IL-10, a canonical M2 anti-inflammatory cytokine, compared to traditional fiber scaffolds. Phalloidin staining revealed that macrophages cultured on aligned fibril bundles adopted morphologies closely resembling chemically induced M2a macrophages. The authors concluded that the three-dimensional topographical guidance provided by aligned fibril bundles suppressed aberrant apical-basal polarization and efficiently steered macrophages toward a reparative M2-like phenotype, mimicking the effects of classical IL-4-driven biochemical induction. A generalized scheme of the degree of influence, strength, and nature of the immune response of modern biomaterials, including metals, ceramics, and polymers was summarized in Table 2.

Table 2.
Generalized scheme of the degree of influence, strength, and nature of the immune response of modern biomaterials, including metals, ceramics, and polymers.
5. Engineering Strategies for Surface Modification of Biomaterials
As outlined earlier, immune cells are pivotal architects of tissue repair, and the strategic modulation of their behavior via surface modification of biomaterials emerges as a powerful approach to attenuate the foreign body response (FBR) while simultaneously promoting tissue regeneration [133,134]. Surface modification techniques provide a versatile toolkit to finely tune the local immune milieu at the injury site, thereby orchestrating a more favorable environment for healing. It is well-established that such modifications regulate immune cell trafficking and activation both immediately following injury and after biomaterial implantation [135]. Central to this process are the physicochemical attributes of the biomaterial—particularly surface chemistry and charge—which critically influence the initial adsorption of proteins [136]. This adsorption event triggers activation of the complement cascade, facilitating the recruitment and adhesion of additional immune effectors. The acute inflammatory phase is characterized by the adherence of neutrophils and macrophages to the biomaterial surface, marking the onset of the immune response. However, precise surface engineering can significantly mitigate FBR and enable immune evasion. Biomaterials are often designed to minimize protein adsorption, thereby limiting subsequent cell adhesion and activation [10,137]. Protein adsorption functions as the crucial molecular dialogue at the biomaterial–tissue interface, igniting immune recognition and the downstream FBR. Although monocytes and macrophages preferentially adhere to implant surfaces, tailoring the chemical composition of the adsorbed protein layer can modulate both the quantity and quality of this interaction [138]. For instance, surface modification with polyethylene glycol (PEG) creates a hydrophilic, sterically hindering barrier that diminishes protein adsorption and cellular interactions, ultimately resulting in a significantly reduced fibrotic capsule enveloping the implant [139]. The following sections will delve deeper into both chemical and physical surface modification strategies, elucidating how these approaches manipulate immune responses to enhance tissue regeneration and curb FBR post-implantation.
5.1. Covalent Surface Modification Strategies for Immune Regulation and Mitigation of Foreign Body Reactions
5.1.1. Amide Bond Formation Strategies for Biomaterial Surface Functionalization
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) in combination with N-hydroxysuccinimide (NHS) chemistry has become a cornerstone technique for tailoring the surface chemistry of biomaterials to enhance their biocompatibility and biodegradability [140]. In this reaction, EDC serves as a water-soluble zero-length cross-linker, catalyzing the formation of stable amide bonds between carboxyl and amine functional groups. However, EDC’s inherent instability in aqueous environments—where nucleophilic attack by oxygen atoms leads to its rapid inactivation—poses a significant limitation. This challenge is effectively addressed by the addition of NHS, which stabilizes the reactive intermediate and prolongs cross-linking efficacy [141]. The EDC/NHS coupling system offers multiple advantages for biomaterial functionalization, including mild reaction conditions that preserve biomolecule bioactivity, high coupling efficiency, water-soluble byproducts that simplify purification, and extended surface presentation of bioactive molecules [142,143]. Moreover, this coupling chemistry has been leveraged to create superhydrophilic coatings that improve biomaterial–tissue interactions [144]. For instance, Park et al. engineered multilayered films composed of carboxymethylcellulose (CMC) and chitosan (CHI) crosslinked via EDC/NHS on silicon tube surfaces to reduce inflammation and bacterial colonization in nasolacrimal duct stenosis treatments [145]. Their approach not only enhanced chemical stability but also increased surface roughness and porosity, thereby significantly improving hydrophilicity and the biological interface. A promising avenue for attenuating foreign body response (FBR) and promoting regeneration post-implantation lies in scavenging inflammatory chemokines at the injury site. Glycosaminoglycans (GAGs) such as heparan sulfate and heparin, naturally abundant in the extracellular matrix, bind chemokines through electrostatic interactions between their negatively charged sulfate groups and the positively charged residues of chemokines [146,147]. Building upon this principle, Lohmann et al. synthesized hydrogels combining star-shaped polyethylene glycol (starPEG) and GAGs via EDC/NHS coupling to efficiently capture pro-inflammatory chemokine gradients at wound sites [74]. These hydrogels markedly diminished the sustained pro-inflammatory milieu by sequestering chemokines including macrophage inflammatory proteins (MIP-1α and MIP-1β), monocyte chemoattractant protein-1 (MCP-1), and interleukin-8 (IL-8). Addressing the challenge of FBR, Birajdar et al. functionalized polydimethylsiloxane (PDMS) surfaces with itaconic acid (IA) monomers or IA-conjugated gelatin (IA-GT) polymers via EDC/NHS chemistry [148]. Following O2 plasma treatment and silanization with (3-aminopropyl) triethoxysilane (APTES), IA or IA-GT was covalently anchored to the PDMS surface. This modification drastically reduced the contact angle (from 106.54 ± 0.78° to 31.59 ± 0.28° with IA-GTpoly-0.50 wt%), attributed to newly introduced surface –OH, –NH2, and –COOH groups. Correspondingly, protein adsorption in vitro was significantly suppressed, and in vivo, implants coated with IA-GTpoly exhibited the lowest collagen deposition and thinnest fibrotic capsules after eight weeks. Despite the clinical potential of xenogeneic grafts, their use is often hampered by immune rejection. Surface functionalization with stealth proteins can circumvent this issue by reducing protein adsorption and inflammatory activation. In this context, Tao et al. coated hyaline cartilage grafts with albumin modified by dopamine via EDC/NHS coupling [149]. The dopamine-functionalized albumin (BCD) formed a robust coating that remained stable for over 21 days and substantially attenuated inflammatory responses relative to unmodified grafts. The covalent immobilization of biomolecules, growth factors, or therapeutic agents onto biomaterial surfaces generally outperforms non-covalent methods in mitigating chronic inflammation due to improved stability and controlled release. Al-Maawi et al. examined this by comparing covalent versus non-covalent attachment of high-sulfated hyaluronan (sHA3) to collagen scaffolds [150]. Non-covalent modification relied on electrostatic interactions between sHA3 and positively charged collagen fibrils, whereas covalent conjugation utilized EDC/NHS chemistry in a MES buffer to activate carboxyl groups. The covalently modified scaffolds (SHA3+EDC/NHS) demonstrated enhanced structural stability, superior integration in vivo after 30 days, sustained growth factor release, and a marked reduction in multinucleated giant cell formation—a key driver of chronic inflammation. Nonetheless, despite the widespread adoption of EDC/NHS chemistry for amide bond formation, several limitations persist. The reactive intermediates are highly susceptible to hydrolysis in aqueous media, reducing coupling yields. Additionally, urea byproducts complicate purification, and nonspecific cross-linking may occur when substrates contain multiple reactive sites, leading to undesirable side products [142,151,152,153,154]. These drawbacks have spurred the exploration of alternative reagents and additives to enhance reaction specificity and efficiency. Notably, the catalytic system of EDC with 1-hydroxybenzotriazole (HOBt) effectively minimizes byproduct formation. For example, grafting chlorogenic acid onto chitosan using EDC/HOBt preserved the compound’s antioxidant and antibacterial activities, underscoring the system’s utility [154].
5.1.2. The Avidin–Biotin Interaction: A High-Affinity System for Biofunctionalization and Targeting
The avidin-biotin complex emerges as a compelling strategy for immobilizing biomolecules and particles onto biomaterial surfaces, owing to its unparalleled stability, specificity, and binding affinity—attributes that profoundly impact surface hydrophilicity, mechanical integrity, and ultimately cellular responses [155,156]. This interaction is recognized as one of the strongest noncovalent biological bonds known, with an extraordinarily low dissociation constant (~10−15 M) [157]. Comparative studies suggest that avidin-based surface modification surpasses traditional EDC/NHS coupling by better preserving the bioactivity of terminal molecules and enabling a higher density of immobilized biomolecules [158]. This superior immobilization capability is largely attributed to avidin’s tetrameric structure, allowing simultaneous binding of up to four biotin molecules [159]. Despite these advantages, the in vivo application of the avidin/biotin system faces notable challenges, including unintended binding of biotinylated molecules to endogenous biotin-binding proteins, such as those present in bacteria, as well as potential cross-reactivity with lectins or endogenous biotin [160]. Streptavidin, a bacterial analog derived from Streptomyces avidinii, has been introduced as a viable alternative, demonstrating strong biotin affinity while exhibiting markedly reduced nonspecific interactions [161,162]. The avidin-biotin linking approach has been effectively harnessed to functionalize biomaterial surfaces, mitigating inflammatory cell adhesion and activation, and thereby diminishing the incidence of fibrotic capsular contracture post-implantation [163,164]. This method further enables the design of scaffolds for controlled release of therapeutic biomolecules or anti-inflammatory agents, a critical feature for localized immunomodulation during tissue regeneration [165]. Macrophage phenotypes dynamically evolve throughout healing, with pro-inflammatory M1 macrophages predominating during early stages (1–5 days) to initiate angiogenesis but potentially exacerbating fibrotic encapsulation if sustained. Conversely, M2 macrophages emerge later, fostering extracellular matrix (ECM) synthesis, vascular maturation, and cellular proliferation, essential for constructive tissue repair. Building on this paradigm, Spiller et al. employed both simple physical adsorption and avidin-biotin complexation to functionalize decellularized bone scaffolds with immunomodulatory cues designed to temporally direct macrophage polarization during bone regeneration. Interferon-gamma (IFN-γ) was physically adsorbed onto the scaffold surface to prompt an early M1 response, while interleukin-4 (IL-4) was covalently tethered via biotin-streptavidin interactions to enable a sustained release that promoted M2 polarization during later stages (4–10 days). This sequential delivery strategy effectively optimized angiogenesis and enhanced new bone formation, with the authors concluding that the avidin-biotin complex facilitated controlled IL-4 release without compromising scaffold biofunctionality [166]. The efficacy of the avidin-biotin system as an immunomodulatory platform was further substantiated in Lurier et al.’s investigation, where porous gelatin scaffolds biotinylated to varying degrees were infused with streptavidin and biotinylated IL-4 to evaluate effects on primary human macrophage polarization in vitro [167]. The extent of scaffold biotinylation was positively correlated with streptavidin and CaptAvidin binding capacity, and higher biotinylation levels drove macrophage polarization toward the anti-inflammatory, pro-healing M2 phenotype. These findings underscore the promising potential of the avidin-biotin complex as a versatile and potent tool for endowing biomaterials with targeted immunomodulatory capabilities.
5.1.3. Click Chemistry as a Bioorthogonal Strategy for Surface Functionalization and Bioconjugation
In 2001, Sharpless and colleagues pioneered click chemistry as a transformative technique for polymer functionalization, highlighting its remarkable advantages: operational simplicity, high yields, robust product stability, insensitivity to oxygen and moisture, rapid reaction kinetics at ambient temperature using water as a solvent, generation of non-toxic byproducts, and facile purification without chromatographic intervention [168,169]. Since then, click chemistry has been extensively harnessed to fine-tune biomaterial surface properties, notably improving surface wettability and thereby mitigating nonspecific protein adsorption [170]. A diverse repertoire of click reactions—including thiol-X reactions, Diels–Alder (DA) cycloadditions, copper(I)-catalyzed alkyne–azide cycloaddition (CuAAC), oxime ligations, and strain-promoted alkyne–azide cycloaddition (SPAAC)—enables the fabrication of sophisticated and precisely defined substrates tailored for tissue engineering applications [171,172]. Among these, CuAAC click chemistry has emerged as a premier approach for the controlled conjugation of complex biomolecules onto biomaterial surfaces via the highly specific cycloaddition between azides and terminal alkynes [173,174]. This reaction yields hydrolytically and thermally stable 1,2,3-triazole linkages, structurally reminiscent of peptide bonds yet exhibiting superior resistance to enzymatic hydrolysis. The process critically depends on the catalytic action of Cu(I), which lowers the activation energy from approximately 24 to 11 kcal mol−1 through the formation of copper acetylide intermediates. This enhanced stability facilitates durable covalent immobilization of biomolecules while preserving their bioactivity. Several studies underscore the utility of CuAAC for modulating immune responses following implantation. Sánchez-Bodón et al. demonstrated the covalent immobilization of an indomethacin derivative—an anti-inflammatory agent—onto poly(L-lactic acid) (PLLA) surfaces via CuAAC to enhance bone regeneration by augmenting the local anti-inflammatory milieu [175]. Initial hydrolysis of the PLLA surface introduced polar carboxyl groups, improving hydrophilicity (contact angle reduced from 110° to 78°), while subsequent conjugation of the indomethacin derivative increased hydrophobicity (contact angle shifted to 88°) due to triazole ring formation and aromatic moieties. Importantly, the pharmacophore of the drug remained unaltered, confirming the biocompatibility of this surface modification strategy. In a related study, Kang et al. integrated CuAAC chemistry with thermal processing to engineer PLLA scaffolds functionalized with vascular endothelial growth factor (VEGF), combining topographical and biochemical cues to promote endothelialization and improve biocompatibility post-implantation [176]. Through electrospinning, aligned PLLA nanofibers imparted microtopographical guidance, while aminolysis introduced surface amines that were converted to alkyne groups via NHS ester chemistry. VEGF was then immobilized covalently by CuAAC, resulting in significantly reduced inflammation scores and thinner collagenous fibrotic capsules compared to unmodified scaffolds. To circumvent the cytotoxicity and oxidative stress linked to Cu(I) catalysts, metal-free click reactions such as SPAAC have gained traction for bio-orthogonal surface functionalization [177,178]. SPAAC proceeds via strain-promoted cycloaddition without requiring a catalyst, enhancing biocompatibility at the cost of slower reaction kinetics, which can be mitigated through reaction condition optimization. Yang et al. illustrated the potential of SPAAC by covalently decorating azide-functionalized silk fibroin (SF) on electrospun polystyrene (PS) microfibers with clickable liposomes, assembled via layer-by-layer (LbL) deposition driven by silk-silk hydrophobic interactions, to attenuate foreign body response (FBR) and enhance scaffold integration [179]. In vivo imaging revealed that liposomes covalently bound through SPAAC retained significantly greater fluorescence intensity (36.75% retention at 7 days) than those adsorbed physically (4.96%), demonstrating superior stability and prolonged retention. Both strategies reduced early inflammatory infiltration and fibrotic capsule thickness, yet the covalent SPAAC group uniquely promoted macrophage polarization towards the pro-regenerative CD206-positive M2 phenotype, attributable to the enhanced durability of the covalent linkage. Thiol–X click chemistries, especially thiol-ene reactions, have advanced as versatile methods for polymer surface modification, enabling the design of crosslinked and linear polymer networks with tunable properties relevant to controlling foreign body response [180,181,182,183,184]. Their attributes include rapid reaction kinetics, ambient reaction conditions, high yields, insensitivity to water and oxygen, and non-toxic byproduct formation [185,186]. Two principal pathways exist: radical-mediated thiol addition to electron-rich/poor alkenes and catalyzed thiol-Michael addition to electron-deficient double bonds [187]. Radical thiol-ene reactions predominate in tissue engineering due to their metal-free nature but often require UV initiation, posing risks of oxidative cell damage [188].
Thiol-ene chemistry has proven effective for mitigating nonspecific protein adsorption, an early step in FBR. Korogiannaki et al. demonstrated that applying a biomimetic hyaluronic acid layer onto contact lenses via thiol-ene chemistry enhanced hydrophilicity and reduced nonspecific protein binding [189]. Similarly, Zhang et al. synthesized a UV-free thiol-ene clickable hydrogel composed of methacrylated hyaluronic acid (HAGMA) and multi-thiol zwitterionic poly(CBMA-co-AC), exhibiting self-healing properties, biocompatibility, and resistance to protein fouling—critical for cell encapsulation applications [190]. This hydrogel reduced inflammatory cytokines TNF-α and IL-6 in macrophages, attributable to its zwitterionic components. The DA reaction, a metal-free click approach involving cycloaddition between dienes and dienophiles such as furyl and maleimide groups, offers advantages including catalyst-free conditions, physiological compatibility, and generation of non-toxic byproducts [191,192,193]. DA chemistry enables the controlled and sustained release of therapeutic agents like dexamethasone, crucial for modulating inflammation and promoting bone regeneration [194,195]. Chemical functionalization of nanomaterials using DA reactions mitigates cytotoxicity and acute inflammatory responses. For example, Mata et al. functionalized carbon nanotube (CNT) membranes via DA cycloaddition and mild oxidation to enhance biodegradability and biocompatibility, observing diminished inflammatory encapsulation in subcutaneous rat models compared to unmodified CNTs [196]. Similarly, Bi et al. engineered an in situ-forming thermosensitive hydroxypropyl chitin hydrogel crosslinked by DA click chemistry with maleimide-terminated PEG, which exhibited reduced macrophage spreading and pro-inflammatory cytokine secretion [197]. Further innovations include injectable composite hydrogels combining copper-doped bioactive glass-ceramic microspheres (CuBGM), furan-modified sodium alginate, and bis-maleimide PEG to promote bone defect repair while eradicating residual tumor cells via photothermal effects triggered by NIR irradiation [198]. The CuBGM-mediated photothermal acceleration of DA crosslinking facilitated tumor cell ablation and enhanced osteogenic differentiation, while the implanted hydrogels modulated the immune milieu towards a reparative M2 macrophage phenotype, as evidenced by increased Arg1 and CD206 expression with concomitant reduction in pro-inflammatory markers.
5.1.4. Surface-Initiated Graft Polymerization Techniques for Biomaterial Modification
Surface hydrophobicity remains a significant obstacle when employing synthetic polymers for tissue engineering applications, often compromising their biological integration and functionality [199]. To address this challenge, surface graft polymerization has emerged as a powerful strategy to functionalize material interfaces by introducing a variety of reactive groups such as hydroxyl (–OH), carbonyl (=O), and amino (–NH2) moieties [200]. This approach offers several distinct advantages: it ensures durable chemical stability through the covalent anchoring of bioactive molecules, is cost-effective and straightforward to implement, enhances surface hydrophilicity, and enables precise tuning of surface chemistry without perturbing the intrinsic properties of the underlying bulk material [201,202]. Critically, surface graft polymerization serves as an effective tool to mitigate foreign body response (FBR) and the consequent risk of capsular contracture by modulating the physicochemical cues that govern immune cell behavior—most notably surface topography and chemistry. In a compelling demonstration of this principle, Lee et al. utilized surface-initiated photopolymerization to graft poly(acrylic acid) (PAA) layers onto polydimethylsiloxane (PDMS) substrates, crafting both chemical and topographical microenvironments through precisely engineered hole- or stripe-shaped micropatterns [203]. Leveraging the versatility of photolithography, the authors established stable PAA micropatterns without delamination, capitalizing on the photo-induced polymerization process. The modification protocol entailed a two-step UV irradiation: initially, benzophenone was covalently immobilized onto the PDMS surface to act as a photoinitiator, followed by UV-triggered polymerization of acrylic acid, which generated grafted PAA layers on the substrate. This surface engineering markedly increased the hydrophilicity of PDMS relative to its unmodified counterpart. Biologically, these PAA micropatterns exerted profound effects on cellular behavior; notably, they suppressed the proliferation of NIH 3T3 fibroblasts and RAW 264.7 macrophages compared to both pristine PDMS and uniformly coated PAA-PDMS surfaces. Moreover, the patterned grafting inhibited fibroblast aggregation and impeded their differentiation into myofibroblasts, a key driver of fibrotic capsule formation. Collectively, these findings suggest that the synergistic interplay of chemical functionality and microscale topography imparted by PAA grafting may significantly attenuate collagen deposition and thereby diminish capsular contracture associated with FBR.
5.1.5. Plasma-Based Surface Modification Techniques Using Ionized Gas Treatments
Plasma, often described as the fourth state of matter, is an ionized gas composed of a complex mixture of charged particles, including positive and negative ions, neutral and excited atoms, electrons, radicals, and ultraviolet photons [204]. This highly reactive state can be generated by various energy sources such as radiofrequency oscillations, microwave irradiation, or hot filament discharges, which impart sufficient energy to electrons to surpass the ionization threshold [205]. Plasma treatment is widely employed to tailor surface properties—such as wettability, topography, and adhesion—by precisely modifying the chemical composition of polymeric surfaces [206,207]. This versatile technique enables the covalent grafting of therapeutic agents and bioactive molecules through the introduction of diverse functional moieties onto biomaterial surfaces. Plasma treatments are generally classified into two broad categories: thermal plasma and nonthermal (cold) plasma [208]. Thermal plasma spraying is extensively utilized to deposit bioactive particles like hydroxyapatite, silver, and titanium onto dental and orthopedic implants, thereby enhancing their biocompatibility and osseointegration [209]. In contrast, cold plasma treatment offers a gentler alternative, facilitating the incorporation of oxygen- and nitrogen-containing functional groups onto biomaterial surfaces using reactive gases such as O2, N2, NH3, and ambient air. This approach is particularly advantageous for modifying heat-sensitive biopolymers without compromising their structural integrity [210]. Compared to conventional wet chemical methods, plasma treatment presents notable benefits, including preservation of the polymer’s bulk properties and elimination of chemical solvent use, thus minimizing contamination risks [211]. A growing body of research confirms that plasma treatment improves implant wettability and biocompatibility, while effectively mitigating fibrous capsule formation associated with foreign body response (FBR) [212,213]. Moreover, plasma surface modification can modulate macrophage polarization dynamics, favoring a shift from the pro-inflammatory M1 phenotype to the pro-regenerative M2 phenotype [214]. For instance, Park et al. applied a sputtering-based plasma immersion ion implantation (S-PIII) technique to engineer nanoscale topographies on silicone surfaces, yielding implants with enhanced biocompatibility and markedly reduced fibrous capsule formation relative to untreated silicone [215]. The S-PIII method offers uniform, conformal ion implantation even on geometrically complex surfaces. Atomic force microscopy (AFM) revealed that the nano-textured Ta-implanted silicone exhibited a wrinkled, textured morphology compared to smooth bare silicone. Furthermore, contact angle measurements demonstrated a substantial increase in hydrophilicity for the nano-engineered surface (~75°), contrasting sharply with the hydrophobic bare silicone (~110°) and the smooth Ta-implanted surface (<90°). Functional assays showed that Nano/Ta silicone implants significantly inhibited macrophage adhesion and aggregation, reduced myofibroblast differentiation and activation, and attenuated the release of pro-fibrotic cytokines, culminating in diminished fibrous capsule formation. These outcomes were attributed to a synergistic effect of the chemical signaling from the tantalum ions and the physical cues provided by the nano-textured surface. Similarly, Chudinov et al. demonstrated that polyurethane implants subjected to plasma immersion ion implantation (PIII) with nitrogen ions at 20 keV exhibited minimal immune infiltration and formed only a thin, non-fibrotic connective tissue layer after five months in a rat model, compared to untreated controls [216]. Beyond enhancing surface wettability and biocompatibility, nonthermal plasma also shows promise for the immobilization of bioactive and anti-inflammatory molecules, such as hyaluronic acid, while maintaining surface integrity. Kudryavtseva et al. utilized atmospheric pressure plasma to covalently attach hyaluronic acid onto PLA scaffold surfaces, thereby modulating scaffold hydrophilicity and immunomodulatory properties [207]. The treated scaffolds exhibited an initial contact angle of 0°, which modestly increased to 17.95° ± 2.88° after ten months, reflecting durable surface chemical modifications. Functional studies revealed that these modified PLA scaffolds enhanced pro-angiogenic activity through elevated IL-8 secretion by primary human monocyte-derived macrophages. The duration of plasma treatment further influences immune responses and tissue regeneration. Bolbasov et al. reported that reactive magnetron sputtering of titanium in an N2 atmosphere onto electrospun PLLA scaffolds reduced inflammation post-implantation, resulting in the formation of only thin connective tissue capsules dominated by lymphocytes [217]. Moreover, prolonged plasma exposure correlated with increased scaffold thinning in the central regions and accelerated replacement by host tissue, likely due to improved wettability facilitating biological fluid diffusion into the scaffold interior. In a recent advancement, Ninan et al. fabricated polycaprolactone (PCL) scaffolds via 3D printing and subsequently applied plasma treatment with allylamine vapor to introduce amine-rich plasma polymer coatings. This modification promoted the electrostatic immobilization of silver nanoparticles (AgNPs) through interactions with protonated amine groups, enhancing antimicrobial potential [218]. The plasma-modified scaffolds displayed a significant reduction in TNF-α expression, a key pro-inflammatory cytokine, relative to untreated controls. Importantly, no adverse inflammatory responses such as fibrous capsule formation were detected at 21 days post-implantation, underscoring the biocompatibility of this surface engineering approach.
5.2. Non-Covalent Strategies for Immunomodulation and Mitigation of Foreign Body Responses
Physical adsorption, or physisorption, stands as one of the most accessible and non-invasive strategies for the surface modification of biomaterials and implants, aiming to bolster their functional longevity following implantation [219]. This technique involves the adherence of biomolecules, growth factors, therapeutic agents, and bioactive compounds onto substrate surfaces predominantly through a spectrum of electrochemical interactions. Unlike covalent bonding, these interactions induce minimal perturbations to the electronic configurations of the adsorbent [220]. The underlying forces governing physical adsorption encompass electrostatic attraction, hydrogen bonding, van der Waals forces, and hydrophobic interactions [221]. While the simplicity, biocompatibility, and economic viability of physisorption render it appealing, its dependency on relatively weak non-covalent bonds curtails its effectiveness in applications demanding tightly controlled and sustained release profiles, due to the propensity for premature desorption [222]. Indeed, this mode of attachment often results in a rapid, uncontrolled release of adsorbed agents at the implant interface, limiting its efficacy in long-term therapeutic delivery [223]. Consequently, physical adsorption is frequently integrated synergistically with complementary surface engineering methods to achieve tailored release kinetics [224,225]. Extensive research efforts have focused on leveraging physical adsorption to enhance implant and scaffold bioactivity through modulation of immune cell responses, thereby promoting tissue regeneration [226,227]. For example, Joseph et al. pioneered a surface modification approach wherein a 3D-printed polycaprolactone (PCL) scaffold underwent air plasma treatment followed by physical adsorption of gold nanoparticles (GNPs) functionalized with mercaptosuccinic acid (MSA) to impart negatively charged carboxyl groups [228]. The plasma treatment (50 W) effectively removed surface contaminants and conferred positive charges, enabling the electrostatic binding of MSA-GNPs. Subsequent in vitro assays revealed a marked attenuation of TNF-α secretion from lipopolysaccharide (LPS)-activated human monocyte-derived macrophages on the GNP-functionalized PCL compared to unmodified or plasma-only treated scaffolds. Complementing this, the same group demonstrated that positively charged amine groups introduced via plasma treatment on PCL surfaces facilitated concentration-dependent electrostatic immobilization of negatively charged silver nanoparticles (AgNPs), endowing the scaffolds with anti-inflammatory properties [218]. These pPCL–Ag composites exhibited enhanced wettability and superior thermal and mechanical characteristics relative to controls. Cellular studies indicated that specific AgNP loadings (pPCL–Ag6 and pPCL–Ag24) significantly suppressed pro-inflammatory cytokines TNF-α and IL-8 secretion by THP-1 derived macrophages. In vivo, pPCL–Ag6 scaffolds promoted favorable collagen deposition and vascular maturation with minimal inflammation by day 21, whereas elevated AgNP concentrations (pPCL–Ag24) elicited heightened foreign body reactions, underscoring the importance of dosage optimization. Expanding the repertoire of bioactive coatings, Kang et al. engineered magnesium–organic framework (Mg-MOF)-based scaffolds coated with exosomes derived from human adipose-derived stem cells (hADSCs-Exos) to mitigate inflammation and support osteogenesis [229]. Electrospun PLGA/Mg-gallic acid MOF composites served as substrates onto which negatively charged exosomes self-assembled via electrostatic attraction to the positively charged Mg-GA MOF surfaces. Cultures of RAW264.7 macrophages on PLGA/Exo-Mg-GA2 scaffolds exhibited the lowest expression levels of inflammatory enzymes cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), relative to controls. In vivo, these scaffolds fostered a bone immune milieu conducive to interfacial osteogenesis in a rat calvarial defect model. In a parallel vein, Zhang et al. immobilized aspirin-loaded nanoparticles composed of sodium hyaluronate and self-assembled type I collagen onto positively charged titanium discs via electrostatic interactions to potentiate osteoimmunomodulatory effects [230]. This surface modification significantly enhanced hydrophilicity, as evidenced by a reduced contact angle from 94.9° ± 1.5° to 38.4° ± 1.3°, facilitating improved biological interactions. Mussel-inspired chemistry, particularly dopamine self-polymerization, has emerged as an elegant non-covalent strategy for biomaterial surface functionalization, capitalizing on catechol-mediated adhesion akin to the natural adhesive proteins in mussel byssus [231,232,233]. Since the seminal work by Messersmith et al. in 2007, which elucidated the formation of thin, adherent polydopamine (PDA) films across diverse substrates [234], the field has expanded dramatically. Synthetic peptides rich in 3,4-dihydroxy-l-phenylalanine (DOPA), a key catecholic residue, replicate the adhesive prowess of mussel foot proteins and enable robust substrate adherence [235,236]. A significant advantage of this approach lies in the facile coating process achievable under mild alkaline conditions without organic solvents, preserving the intrinsic properties of polymer backbones [237]. Notably, PDA-modified scaffolds can restore the electrochemical microenvironment within bone defects, enhancing cellular electrical signaling and Ca2+ influx, while also conferring anti-inflammatory and reactive oxygen species (ROS)-scavenging functions [238]. Substantial evidence indicates that PDA coatings mitigate non-specific serum protein adsorption and temper inflammatory responses, thus enhancing the paracrine functionality of mesenchymal stem cells (MSCs) and modulating macrophage phenotypes [239,240,241,242]. Li et al. demonstrated that adipose-derived MSCs cultured on PDA-modified bacterial cellulose scaffolds secreted conditioned media enriched with immunomodulatory and pro-angiogenic factors, which, upon application in a diabetic rat skin defect model, promoted macrophage polarization from pro-inflammatory M1 to anti-inflammatory M2 phenotypes, thereby accelerating healing [243]. A complementary and biocompatible surface modification technique involves the application of 2-aminomethyl-naphthalene (AMN) coatings, which, akin to polydopamine (PDA), form conformal layers upon immersion in mildly basic buffers [244,245]. AMN, a trimer of hydrogen cyanide formed upon neutralization of p-toluenesulfonate, presents a facile route for coating diverse organic and inorganic substrates with bioactive molecules and metals, thus imparting anti-inflammatory and antimicrobial functionalities [246,247,248,249,250]. Chen et al. leveraged this property by synthesizing zwitterionic copolymers of sulfobetaine methacrylate (SB) and 2-aminoethyl methacrylate (AE) bearing primary amines, which were co-deposited with AMN onto polydimethylsiloxane (PDMS) and tissue culture polystyrene (TCPS) surfaces at pH 8.5 [251]. These coatings exhibited potent antifouling properties, evidenced by a significant reduction in L929 fibroblast adhesion and proliferation in vitro. Furthermore, in vivo implantation revealed markedly thinner fibrotic capsules around coated PDMS samples after two weeks, underscoring the promise of AMN-based coatings for immunomodulatory biomaterial surface engineering.
Peptide-Based Mussel-Inspired Functionalization: Immunomodulation, Osteointegration, and Multifunctional Therapeutic Platforms
Cell-adhesive peptides, such as Arg-Gly-Asp (RGD) sequences ubiquitous in extracellular matrix proteins, can be immobilized onto biomaterial surfaces through mussel-inspired catechol chemistry to attenuate chronic inflammation and foster tissue regeneration [252]. Guo et al. immobilized a tetravalent DOPA-RGD peptide onto titanium implants, achieving improved hydrophilicity and surface roughness via catechol-TiO2 coordination [253]. These RGD-functionalized implants facilitated macrophage polarization toward the pro-healing M2 phenotype by modulating integrin-α2β1 and integrin-αvβ3 signaling, reduced peri-implant inflammation, and enhanced osteoblast adhesion and bone formation under inflammatory conditions. Similarly, Bai et al. employed mussel-inspired chemistry to graft a bioactive PDA-based peptide (4-S5-YGFGG) onto titanium implants after oxygen plasma pretreatment, achieving stable peptide immobilization through strong catechol-TiO2 coordination [254]. The peptide-coated implants exhibited enhanced hydrophilicity and surface roughness, significantly attenuated M1 macrophage-driven inflammation, and improved osseointegration, establishing a more favorable environment for bone healing. Beyond tissue adhesion, mussel-inspired coatings serve as multifunctional platforms for drug delivery and metal ion chelation [255]. Wang et al. developed catechol-modified Poloxamer hydrogels (P-DA) via dihydrocaffeic acid conjugation, yielding thermosensitive, mucoadhesive systems that enhanced epidermal growth factor (EGF) stability and retention within inflamed colonic tissue, thereby amplifying therapeutic efficacy and promoting macrophage polarization toward M2 phenotypes [256]. Likewise, Peng et al. constructed a zinc-containing PDA film on Mg alloy via self-polymerization and Zn ion chelation, which supported sustained Zn2+ release over 14 days, polarized RAW264.7 macrophages toward an anti-inflammatory phenotype, and facilitated superior osteointegration in a rat femoral osteomyelitis model compared to bare alloy controls [257,258]. Recognizing that conventional DOPA-based conjugations can compromise biomolecule bioactivity through covalent binding, Sun et al. innovatively combined mussel-inspired chemistry with bio-orthogonal click chemistry to achieve multifunctional titanium implant surfaces [259]. A clickable DOPA-Azido peptide was first coordinated onto titanium, followed by the covalent attachment of RGD and BMP-2 peptides via strain-promoted azide-alkyne cycloaddition (SPAAC). This dual-functionalized surface not only enhanced macrophage polarization toward the reparative M2 phenotype but also improved mechanical stability and osseointegration in osteoporotic rats. Echoing this integrative strategy, Wang et al. fabricated titanium screws featuring a dual-effect coating that combined immunomodulatory Zn2+ ions coordinated via catechol residues with bio-orthogonally attached BMP-2 peptides to simultaneously modulate the osteoimmune microenvironment and stimulate bone regeneration [260]. This multifunctional coating synergistically promoted macrophage M2 polarization and accelerated bone formation and implant integration compared to single-modality or unmodified controls.
5.3. Physical Modification Strategies for Immunomodulation and Mitigation of Foreign Body Responses
5.3.1. Surface Modification via Layer-by-Layer Coating and Thin Film Deposition Techniques
The alteration of biomaterial surface properties via Layer-by-Layer (LbL) multilayer assembly fundamentally relies on the sequential deposition of oppositely charged polymers—polycations and polyanions—onto charged substrates, driven by electrostatic interactions [261]. This versatile LbL technique is distinguished for its precision in fabricating coatings with finely tunable thicknesses, ranging from mere nanometers to several micrometers [262]. The appeal of the LbL method in biomaterial surface modification stems from its cost-effectiveness, adaptability to diverse substrate geometries, and its capacity to produce uniform multilayers with controlled physicochemical properties, including the ability to enable the controlled release of therapeutic agents and bioactive molecules [263]. Nonetheless, a notable constraint of LbL assembly is the difficulty in incorporating insoluble or neutrally charged biomolecules or drugs within the multilayered constructs, limiting its application scope in certain contexts [264,265]. Despite this, the LbL approach has been increasingly harnessed to modulate immune responses after implantation, thereby promoting tissue regeneration while mitigating the formation of fibrotic capsules around implants [266,267]. This technique’s capacity to reduce platelet adhesion and subsequent activation contributes significantly to the attenuation of inflammatory cell recruitment and activation [268,269]. An illustrative example is provided by Zhang et al., who synthesized a pH-responsive antibacterial copolymer containing carboxyl groups and cationic quaternary ammonium salts via conventional free radical polymerization. They subsequently applied this copolymer onto titanium surfaces through LbL self-assembly, generating a multifunctional platform with antibacterial efficacy and enhanced osseointegration capabilities [270]. Their findings revealed that unmodified titanium nails attracted substantial inflammatory macrophage infiltration, whereas the LbL-coated implants notably diminished macrophage-mediated inflammation. Compared to traditional chemical cross-linkers such as glutaraldehyde—which pose risks of cytotoxicity, immune reactions, and calcification—the LbL method affords the fabrication of biofunctional scaffolds with tunable degradation rates and mechanical properties by adjusting the number of deposited layers, all without incurring adverse effects. Crucially, the choice of biomolecules embedded within polyelectrolyte multilayers (PEMs) profoundly influences inflammatory outcomes. For instance, Zhou et al. enhanced poly(ethylene imine) (PEI) surfaces via LbL assembly using chitosan as a polycation paired with glycosaminoglycans (GAGs) such as hyaluronic acid, chondroitin sulfate, and heparin as polyanions. Their work demonstrated that heparin-chitosan multilayers most effectively mitigated inflammatory responses relative to other GAG-chitosan combinations [264]. Moreover, LbL coatings have shown the capacity to preserve scaffold bioactivity, regulating macrophage polarization from pro-inflammatory M1 to reparative M2 phenotypes in vivo. Synergistic effects have been observed when LbL is combined with complementary surface engineering approaches to reduce capsular contracture, a common complication of silicone implants. Yoo et al. exemplified this by fabricating microtextured polydimethylsiloxane (PDMS) surfaces with concave hemisphere micropatterns (38, 70, and 100 μm), templated by polystyrene microparticles, followed by spin-assisted LbL deposition of poly-L-lysine hydrobromide (PLL) and hyaluronic acid to enhance wettability [271,272]. The dual modification strategy yielded marked reductions in inflammation, TGF-β expression, collagen deposition, and immune cell infiltration, surpassing the benefits achieved by microtexturing or multilayer coating alone. Similarly, Qian et al. utilized single-component LbL assembly to engineer silk fibroin (SF)-functionalized electrospun polycaprolactone (PCL) fibers, chemically modified with heparin disaccharide (HD) via diazonium coupling and click chemistry to facilitate the non-covalent immobilization of interleukin-4 (IL-4), an anti-inflammatory cytokine. The PCL/ASF/A-HD/IL-4 constructs demonstrated significant reductions in macrophage recruitment and fibrotic capsule formation in a murine subcutaneous implantation model, attributable to increased hydrophilicity and decreased nonspecific protein adsorption post functionalization [273]. In a further study, the same group functionalized SF-modified PCL nanofibrous scaffolds with mechano growth factor-1 (MGF-1) through click chemistry to promote anti-inflammatory macrophage polarization and mitigate scaffold rejection in situ [274]. Immunofluorescence analyses revealed elevated M2 macrophage markers (CD206 and Arg-1) on PCL/SF-MGF scaffolds compared to controls, and in vivo rat models showed minimal inflammatory signs post-implantation. Emerging evidence also suggests that combining LbL assembly with plasma surface treatment may yield synergistic benefits. Gao et al. treated pristine polyetheretherketone (PEEK) with oxygen plasma (150 W, 100 Pa, 2 min) before fabricating LbL films of poly(allylamine hydrochloride) (PAH) and poly(acrylic acid) (PAA) through alternate dipping [275]. Post-fabrication acidic treatments (pH 1.8 and 2.4) followed by thermal cross-linking stabilized porous film morphologies via amide bond formation. These modified surfaces significantly suppressed TNF-α secretion by THP-1 macrophages and fostered a pro-osteogenic microenvironment, enhancing human bone marrow mesenchymal stem cell (hBMSC) differentiation and improving trabecular bone metrics relative to controls. A complementary and biocompatible surface modification technique involves the application of 2-aminomethyl-naphthalene (AMN) coatings, which, akin to polydopamine (PDA), form conformal layers upon immersion in mildly basic buffers [244,245]. AMN, a trimer of hydrogen cyanide formed upon neutralization of p-toluenesulfonate, presents a facile route for coating diverse organic and inorganic substrates with bioactive molecules and metals, thus imparting anti-inflammatory and antimicrobial functionalities [246,247,248,249,250]. Chen et al. leveraged this property by synthesizing zwitterionic copolymers of sulfobetaine methacrylate (SB) and 2-aminoethyl methacrylate (AE) bearing primary amines, which were co-deposited with AMN onto polydimethylsiloxane (PDMS) and tissue culture polystyrene (TCPS) surfaces at pH 8.5 [251]. These coatings exhibited potent antifouling properties, evidenced by a significant reduction in L929 fibroblast adhesion and proliferation in vitro. Furthermore, in vivo implantation revealed markedly thinner fibrotic capsules around coated PDMS samples after two weeks, underscoring the promise of AMN-based coatings for immunomodulatory biomaterial surface engineering.
5.3.2. Coaxial Electrospinning Techniques for Fabrication of Multicomponent Nanofibers
Electrospinning is widely acknowledged as a facile and versatile technique for fabricating nanofibrous scaffolds from diverse polymeric materials, tailored to meet the stringent demands of tissue engineering applications [276]. Expanding upon this, co-electrospinning has emerged as a powerful strategy to functionalize the surface of electrospun nanofibers by incorporating biomolecules, growth factors, nanoparticles, and other therapeutic or bioactive agents. This surface enrichment aims to optimize cell–matrix interactions while simultaneously mitigating foreign body responses (FBR) post-implantation [277,278]. Accumulating evidence underscores that embedding bioactive components—such as magnesium ions (Mg2+)—within nanofibers enables their sustained, localized release at injury sites, thereby orchestrating immune cell behavior to favor tissue repair and regeneration [279]. Magnesium, in particular, plays a pivotal role in modulating lymphocyte development and attenuating the secretion of pro-inflammatory cytokines, positioning it as a promising immunomodulatory agent [280]. For instance, Adhikari et al. utilized co-electrospinning to fabricate biodegradable magnesium/polycaprolactone (Mg/PCL) composite nanofiber meshes designed to temper acute inflammatory reactions and promote tissue remodeling [281]. Their in vitro and in vivo studies demonstrated that Mg2+ incorporation into PCL matrices effectively dampened inflammatory processes and accelerated regenerative outcomes. Complementing these findings, Liu et al. integrated magnesium oxide (MgO) nanoparticles into electrospun PCL/gelatin membranes through co-electrospinning, achieving significant enhancement in diabetic wound healing in streptozotocin (STZ)-induced rat models [282]. The PCL/gelatin/MgO membranes exhibited robust immunomodulatory effects by downregulating pro-inflammatory gene expression (TNF-α, IL-1β) while upregulating genes associated with tissue repair and remodeling (α-SMA, TGF-β1), thereby facilitating accelerated wound closure. In a parallel advance, Ji et al. developed Janus nanofibers loaded with rose carbon sponge particles (RCSPs) and silver nanoparticles (Ag-NPs) via uniaxial electrospinning. These multifunctional fibers demonstrated pronounced wound healing efficacy in rat models through combined anti-inflammatory and antibacterial actions, primarily attributed to the potent antimicrobial properties of Ag-NPs [283]. Niu et al. harnessed coaxial electrospinning to engineer hyaluronic acid-functionalized collagen nanofibers, investigating their immunoregulatory influence on Raw 264.7 macrophages and urethral regeneration [284]. Compared to conventional collagen fibers, these modified scaffolds promoted macrophage elongation—a morphological hallmark linked to an anti-inflammatory phenotype—elevating the expression of M2-associated markers (IL-10, Arg-1) while suppressing inflammatory cytokine release (TNF-α). Ding et al. employed coaxial electrospinning to embed bone morphogenetic protein-2 (BMP-2) and basic fibroblast growth factor (bFGF) within poly(lactide-co-glycolide)/poly(lactic acid) (PLGA/PLLA) shell/core nanofibers, examining their immunomodulatory and angiogenic effects in a rat mandibular periodontal bone defect model [285]. Both in vitro and in vivo analyses revealed that these biomimetic substrates significantly enhanced neovascularization, stimulated osteogenic differentiation of periodontal ligament stem cells (PDLSCs), and promoted macrophage polarization toward the reparative M2 phenotype—key processes indicative of constructive bone remodeling.
6. Barriers and Catalysts in the Advancement of Translational Research
Immune cells have long been recognized as pivotal players in both tissue injury and the subsequent reparative cascade. Surface modification of biomaterials has emerged as a promising strategy to modulate immune cell behavior, steering it toward pro-healing phenotypes. Recent advances reveal that biomaterial surfaces can be deliberately engineered to orchestrate beneficial outcomes by finely tuning both innate and adaptive immune responses. Through the chemical or physical immobilization of biomolecules, anti-inflammatory agents, or growth factors on biomaterial interfaces, it is possible to precisely regulate the recruitment, activation, and polarization of immune cells—thereby promoting their transition into pro-regenerative states following tissue injury or implantation. Recent studies on chitosan–hydroxyapatite biocomposites and hydroxyapatite-based nanocomposites have highlighted their immunomodulatory potential and ability to foster favorable cell–biomaterial interactions, reinforcing the importance of rationally engineered surfaces for clinical translation [286,287,288]. Nonetheless, many biomaterials inherently suffer from limitations such as excessive hydrophobicity, nonspecific protein adsorption, and suboptimal surface properties. These shortcomings often provoke aberrant immune activation, ultimately compromising biomaterial performance and therapeutic efficacy. A comprehensive understanding of biomaterial surface characteristics and their nuanced influence on immune cell dynamics is thus indispensable for advancing the design of immunomodulatory implants. While numerous in vitro and preclinical animal studies have successfully demonstrated that tailored surface modifications can steer immune cells toward a reparative milieu, translating these findings to human clinical settings remains challenging. Notably, significant immunological discrepancies exist between humans and common murine models. For example, human macrophages lack expression of several canonical murine M1/M2 polarization markers—such as arginase and nitric oxide synthase—which complicates direct extrapolation [289,290]. Moreover, differences in macrophage morphology and tissue healing kinetics between species further underscore the limitations of rodent models for predicting human responses [109,291,292]. Consequently, the use of large animal models or ex vivo human tissue platforms is strongly advocated to bridge this translational gap and yield data more predictive of clinical outcomes. Chemical surface modification techniques frequently face practical barriers, including the risk of chemical contamination from solvents and harsh reaction conditions, which may jeopardize biocompatibility. Future research must prioritize the development of benign, efficient surface engineering approaches that not only ensure patient safety but also effectively harness immune modulation to enhance healing. A critical challenge arises from the inherent differences in immune cell activation stimuli in vitro versus in vivo, particularly regarding macrophage polarization dynamics. Deepening our mechanistic insight into the molecular cues governing immune cell plasticity and their switching between pro-inflammatory and anti-inflammatory phenotypes holds promise for refining strategies to mitigate Foreign Body Reaction (FBR) and promote tissue regeneration. Complementary advances in light-based immunotherapy, biosensing technologies for immune cell dynamics, and pharmacologically coated surfaces further exemplify translational strategies that bridge fundamental biomaterial research with clinical practice [293,294,295,296]. However, successful clinical translation demands overcoming several formidable hurdles. The complexity and heterogeneity of human immune responses often diverge markedly from animal models, rendering direct application of preclinical findings unpredictable. Furthermore, the regulatory landscape necessitates exhaustive safety, efficacy, and longitudinal studies before new biomaterials gain approval—a process that is both time-consuming and resource-intensive. Clinical trials face additional logistical and ethical complexities, including patient recruitment, control group design, and extended follow-up protocols. Ethical considerations are especially paramount given the profound interactions between biomaterials and the immune system. Interdisciplinary collaboration is indispensable for navigating these challenges. Integrating expertise from materials science, immunology, bioengineering, and clinical medicine demands effective communication and coordination—a difficult yet essential endeavor for driving biomaterial innovation forward. Adding further complexity, patient-specific factors such as age, comorbidities, and genetic variability critically influence immune responses to implants, highlighting the need for personalized therapeutic approaches that complicate development and regulatory approval pathways. In summary, while the prospect of clinically transformative biomaterial surface modifications that favorably modulate immune cell behavior to reduce FBR and enhance healing is highly promising, realizing this potential requires addressing substantial scientific, regulatory, and ethical challenges. Meeting these demands will depend on innovative research, significant investment, and collaborative synergy across multiple disciplines.
Author Contributions
Conceptualization, D.F.; methodology, D.F.; software, Ş.Ţ.; validation, D.F.; formal analysis, D.F.; investigation, D.F.; resources, Ş.Ţ.; data curation, D.F.; writing—original draft preparation, D.F. and Ş.Ţ.; writing—review and editing, D.F. and Ş.Ţ.; visualization, D.F.; supervision, Ş.Ţ.; project administration, Ş.Ţ.; funding acquisition, Ş.Ţ. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. Neither author has a financial or proprietary interest in any material or method mentioned.
References
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Subbiah, R.; Guldberg, R.E. Materials Science and Design Principles of Growth Factor Delivery Systems in Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2019, 8, 1801000. [Google Scholar] [CrossRef]
- Cha, G.D.; Kang, D.; Lee, J.; Kim, D.H. Bioresorbable Electronic Implants: History, Materials, Fabrication, Devices, and Clinical Applications. Adv. Healthc. Mater. 2019, 8, 1801660. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.S.; Booth, M.A.; Rifai, A.; Fox, K.; Gelmi, A. Diamond in the Rough: Toward Improved Materials for the Bone−Implant Interface. Adv. Healthc. Mater. 2021, 10, 2100007. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. Biomed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin Biomaterials in Dermal Repair. Trends Biotechnol. 2020, 38, 280. [Google Scholar] [CrossRef]
- Gammon, J.M.; Jewell, C.M. Engineering Immune Tolerance with Biomaterials. Adv. Healthc. Mater. 2019, 8, 1801419. [Google Scholar] [CrossRef]
- Anderson, J.M.; Gristina, A.G.; Hanson, S.R.; Harker, L.A.; Johnson, R.J.; Merritt, K.; Naylor, P.T.; Schoen, F.J. Chapter 4—Host Reactions to Biomaterials and Their Evaluation. In Biomaterials Science; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 165–214. [Google Scholar]
- Firkowska-Boden, I.; Zhang, X.; Jandt, K.D. Controlling Protein Adsorption through Nanostructured Polymeric Surfaces. Adv. Healthc. Mater. 2018, 7, 1700995. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef] [PubMed]
- Grainger, D.W. All charged up about implanted biomaterialse. Nat. Biotechnol. 2013, 31, 507. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 927. [Google Scholar] [CrossRef]
- Xia, Z.; Triffitt, J.T. T A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1. [Google Scholar] [CrossRef]
- Solheim, E.; Sudmann, B.; Bang, G.; Sudmann, E. Biocompatibility and effect on osteogenesis of poly(ortho ester) compared to poly(DL-lactic acid). J. Biomed. Mater. Res. 2000, 49, 257. [Google Scholar] [CrossRef]
- Kou, P.M.; Babensee, J.E. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J. Biomed. Mater. Res. Part A 2011, 96, 239. [Google Scholar] [CrossRef]
- Zaveri, T.D.; Dolgova, N.V.; Chu, B.H.; Lee, J.; Wong, J.; Lele, T.P.; Ren, F.; Keselowsky, B.G. Contributions of surface topography and cytotoxicity to the macrophage response to zinc oxide nanorods. Biomaterials 2010, 31, 2999. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat. Rev. Immunol. 2021, 21, 137. [Google Scholar] [CrossRef]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.; Schultze, J.L. Innate and Adaptive Immune Memory: An Evolutionary Continuum in the Host’s Response to Pathogens. Cell Host Microbe 2019, 25, 13. [Google Scholar] [CrossRef]
- Farber, D.L.; Netea, M.G.; Radbruch, A.; Rajewsky, K.; Zinkernagel, R.M. Immunological memory: Lessons from the past and a look to the future. Nat. Rev. Immunol. 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Huntoon, K.; Wang, Y.; Jiang, W.; Kim, B.Y. Harnessing Innate Immunity Using Biomaterials for Cancer Immunotherapy. Adv. Mater. 2021, 33, e2007576. [Google Scholar] [CrossRef]
- Jewell, C.M.; Collier, J.H. Biomaterial interactions with the immune system. Biomater. Sci. 2019, 7, 713. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.; Oreffo, R. Osteogenesis And Angiogenesis: The Potential For Engineering Bone. Eur. Cells Mater. 2008, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665. [Google Scholar] [CrossRef]
- Dalisson, B.; Barralet, J. Bioinorganics and Wound Healing. Adv. Healthc. Mater. 2019, 8, e1900764. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Chasing the recipe for a pro-regenerative immune system. Semin. Cell Dev. Biol. 2017, 61, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sindrilaru, A.; Scharffetter-Kochanek, K. Disclosure of the Culprits: Macrophages-Versatile Regulators of Wound Healing. Adv. Wound Care 2013, 2, 357. [Google Scholar] [CrossRef]
- Dhavalikar, P.; Robinson, A.; Lan, Z.; Jenkins, D.; Chwatko, M.; Salhadar, K.; Jose, A.; Kar, R.; Shoga, E.; Kannapiran, A.; et al. Review of Integrin-Targeting Biomaterials in Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 2000795. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426. [Google Scholar] [CrossRef]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z.; et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. 2017, 35, 2378. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Ghobrial, R.M.; Wosik, J.; Lewicka, A.; Lewicki, S.; Kubiak, J.Z. Macrophage functions in wound healing. J. Tissue Eng. Regener. Med. 2019, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159. [Google Scholar] [CrossRef]
- Smigiel, K.S.; Parks, W.C. Matrix Metalloproteinases and Leukocyte Activation. Progress Mol. Biol. Transl. Sci. 2017, 147, 167. [Google Scholar]
- Bartneck, M.; Tacke, F. Targeted Modulation of Macrophage Functionality by Nanotheranostics in Inflammatory Liver Disease and Cancer. In The Immune Response to Implanted Materials and Devices; Corradetti, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 213–223. [Google Scholar]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regener. Biomater. 2017, 4, 55. [Google Scholar] [CrossRef]
- Rungelrath, V.; Kobayashi, S.D.; DeLeo, F.R. Neutrophils in innate immunity and systems biology-level approaches. Wiley Interdiscipl. Rev. Syst. Biol. Med. 2020, 12, 1458. [Google Scholar] [CrossRef] [PubMed]
- Boni, B.O.O.; Lamboni, L.; Souho, T.; Gauthier, M.; Yang, G. Immunomodulation and cellular response to biomaterials: The overriding role of neutrophils in healing. Mater. Horiz. 2019, 6, 1122. [Google Scholar] [CrossRef]
- Hind, L.E.; Huttenlocher, A. Neutrophil Reverse Migration and a Chemokinetic Resolution. Dev. Cell 2018, 47, 404. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Aresta-DaSilva, S.; Tang, K.; Alvarez, D.; Webber, M.J.; Tang, B.C.; Lavin, D.M.; Veiseh, O.; Doloff, J.C.; Bose, S. Neutrophil Responses to Sterile Implant Materials. PLoS ONE 2015, 10, e0137550. [Google Scholar] [CrossRef]
- Barkhausen, T.; Frerker, C.; Pütz, C.; Pape, H.-C.; Krettek, C.; van Griensven, M. Depletion Of Nk Cells In A Murine Polytrauma Model Is Associated with Improved Outcome And A Modulation Of The Inflammatory Response. Shock 2008, 30, 401. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.J.; Joshi, P.; Jones, Q.; Zhou, X.; Livingston, D.H.; Lavery, R.F. Suppression of Natural Killer Cell Activity in Patients with Fracture/Soft Tissue Injury. Arch. Surg. 1997, 132, 1326. [Google Scholar] [CrossRef]
- Almeida, C.R.; Caires, H.R.; Vasconcelos, D.P.; Barbosa, M.A. NAP-2 Secreted by Human NK Cells Can Stimulate Mesenchymal Stem/Stromal Cell Recruitment. Stem Cell Rep. 2016, 6, 466. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.; Mau, E.; Wang, Y.; Wright, D.; Silkstone, D.; Whetstone, H.; Whyne, C.; Alman, B. T-Lymphocytes Enable Osteoblast Maturation via IL-17F during the Early Phase of Fracture Repair. PLoS ONE 2012, 7, e40044. [Google Scholar] [CrossRef]
- Könnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.-D.; Radbruch, A.; Duda, G.N. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155. [Google Scholar] [CrossRef]
- El Khassawna, T.; Serra, A.; Bucher, C.H.; Petersen, A.; Schlundt, C.; Könnecke, I.; Malhan, D.; Wendler, S.; Schell, H.; Volk, H.-D. T Lymphocytes Influence the Mineralization Process of Bone. Front. Immunol. 2017, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Q.; Lin, L.; Xu, C.; Zheng, C.; Chen, X.; Han, Y.; Li, M.; Cao, W.; Cao, K. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014, 21, 1758. [Google Scholar] [CrossRef]
- Huang, H.; Kim, H.; Chang, E.; Lee, Z.; Hwang, S.; Kim, H.; Lee, Y.; Kim, H. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: Implications for bone remodeling. Cell Death Differ. 2009, 16, 1332. [Google Scholar] [CrossRef]
- Sun, G.; Wang, Y.; Ti, Y.; Wang, J.; Zhao, J.; Qian, H. Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells. Clin. Exp. Pharmacol. Physiol. 2017, 44, 455. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292. [Google Scholar] [CrossRef]
- Shanley, L.C.; Mahon, O.R.; Kelly, D.J.; Dunne, A. Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages. Acta Biomater. 2021, 133, 208. [Google Scholar] [CrossRef]
- Zhou, G.; Groth, T. Host Responses to Biomaterials and Anti-Inflammatory Design—A Brief Review. Macromol. Biosci. 2018, 18, 1800112. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Bridges, A.W.; Burns, K.L.; Tate, C.C.; Babensee, J.E.; LaPlaca, M.C.; García, A.J. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials 2007, 28, 3626. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013, 9, 4948. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553. [Google Scholar] [CrossRef]
- Wronska, M.A.; O’Connor, I.B.; Tilbury, M.A.; Srivastava, A.; Wall, J.G. Adding Functions to Biomaterial Surfaces through Protein Incorporation. Adv. Mater. 2016, 28, 5485. [Google Scholar] [CrossRef]
- Rostam, H.M.; Fisher, L.E.; Hook, A.L.; Burroughs, L.; Luckett, J.C.; Figueredo, G.P.; Mbadugha, C.; Teo, A.C.; Latif, A.; Kämmerling, L. Immune-Instructive Polymers Control Macrophage Phenotype and Modulate the Foreign Body Response In Vivo. Matter 2020, 2, 1564. [Google Scholar] [CrossRef]
- Petersen, L.K.; Ramer-Tait, A.E.; Broderick, S.R.; Kong, C.-S.; Ulery, B.D.; Rajan, K.; Wannemuehler, M.J.; Narasimhan, B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials 2011, 32, 6815. [Google Scholar] [CrossRef]
- Briedé, J.J.; van Delft, J.M.; de Kok, T.M.; van Herwijnen, M.H.; Maas, L.M.; Gottschalk, R.W.; Kleinjans, J.C. Global Gene Expression Analysis Reveals Differences in Cellular Responses to Hydroxyl- and Superoxide Anion Radical–Induced Oxidative Stress in Caco-2 Cells. Toxicol. Sci. 2010, 114, 193. [Google Scholar] [CrossRef]
- Sperling, C.; Schweiss, R.B.; Streller, U.; Werner, C. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials 2005, 26, 6547. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, J.; Wang, B.; Li, L.; Kong, X.; Liao, J. The immune reaction and degradation fate of scaffold in cartilage/bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109927. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Thevenot, P.; Hu, W. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Hung, K.-C.; Hung, H.-S.; Hsu, S.-h. Modulation of Macrophage Phenotype by Biodegradable Polyurethane Nanoparticles: Possible Relation between Macrophage Polarization and Immune Response of Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19436. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Bhattacharyya, D.; Padukudru, C.; Timmons, R.B.; Tang, L. Surface chemistry influences implant-mediated host tissue responses. J. Biomed. Mater. Res. Part A 2008, 86, 617. [Google Scholar] [CrossRef]
- Lopez-Silva, T.L.; Leach, D.G.; Azares, A.; Li, I.-C.; Woodside, D.G.; Hartgerink, J.D. Chemical functionality of multidomain peptide hydrogels governs early host immune response. Biomaterials 2020, 231, 119667. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mou, X.; Qiu, J.; Wang, S.; Wang, D.; Sun, D.; Guo, W.; Li, D.; Kumar, A.; Yang, X. Surface Charge Regulation of Osteogenic Differentiation of Mesenchymal Stem Cell on Polarized Ferroelectric Crystal Substrate. Adv. Healthc. Mater. 2015, 4, 998. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 2017, 9, eaai9044. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: A too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C 2017, 70, 889. [Google Scholar] [CrossRef]
- Ma, Y.; Zhuang, Y.; Xie, X.; Wang, C.; Wang, F.; Zhou, D.; Zeng, J.; Cai, L. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale 2011, 3, 2307. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New perspectives: In-situ tissue engineering for bone repair scaffold. Compos. Part B 2020, 202, 108445. [Google Scholar] [CrossRef]
- Nair, A.; Zou, L.; Bhattacharyya, D.; Timmons, R.B.; Tang, L. Species and density of implant surface chemistry affect the extent of foreign body reactions. Langmuir 2008, 24, 2015. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Madureira, P.; Barbosa, M.A.; Aguas, A.P. The influence of functional groups of self-assembled monolayers on fibrous capsule formation and cell recruitment. J. Biomed. Mater. Res. Part A 2006, 76, 737. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Barbosa, M.A.; Aguas, A.P. Adhesion of human leukocytes to biomaterials: An in vitro study using alkanethiolate monolayers with different chemically functionalized surfaces. J. Biomed. Mater. Res. Part A 2003, 65, 429. [Google Scholar] [CrossRef]
- Brodbeck, W.; Nakayama, Y.; Matsuda, T.; Colton, E.; Ziats, N.; Anderson, J. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine 2002, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, Y.J.; Jang, J.H.; Park, J.W. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology 2016, 27, 085101. [Google Scholar] [CrossRef]
- Shin, C.S.; Cabrera, F.J.; Lee, R.; Kim, J.; Ammassam Veettil, R.; Zaheer, M.; Adumbumkulath, A.; Mhatre, K.; Ajayan, P.M.; Curley, S.A. 3D-Bioprinted Inflammation Modulating Polymer Scaffolds for Soft Tissue Repair. Adv. Mater. 2021, 33, 2003778. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Clark, N.M.; Olivares-Navarrete, R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials 2018, 182, 202. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Jiang, M.; Yin, X.; Luo, X.; Sun, H. Effect of the immune responses induced by implants in a integrated three-dimensional micro-nano topography on osseointegration. J. Biomed. Mater. Res. Part A 2021, 109, 1429. [Google Scholar] [CrossRef] [PubMed]
- Visalakshan, R.M.; MacGregor, M.N.; Sasidharan, S.; Ghazaryan, A.; Mierczynska-Vasilev, A.; Morsbach, S.; Mailänder, V.; Landfester, K.; Hayball, J.D.; Vasilev, K. Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses. ACS Appl. Mater. Interfaces 2019, 11, 27615. [Google Scholar] [CrossRef]
- Lu, D.R.; Park, K. Effect of surface hydrophobicity on the conformational changes of adsorbed fibrinogen. J. Colloid Interface Sci. 1991, 144, 271. [Google Scholar] [CrossRef]
- Lv, L.; Xie, Y.; Li, K.; Hu, T.; Lu, X.; Cao, Y.; Zheng, X. Unveiling the Mechanism of Surface Hydrophilicity-Modulated Macrophage Polarization. Adv. Healthc. Mater. 2018, 7, 1800675. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Ruzga, M.N.; Olivares-Navarrete, R. Surface characteristics on commercial dental implants differentially activate macrophages in vitro and in vivo. Clin. Oral Implant. Res. 2021, 32, 487. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58. [Google Scholar] [CrossRef]
- Harvey, A.G.; Hill, E.W.; Bayat, A. Designing implant surface topography for improved biocompatibility. Expert Rev. Med. Devices 2013, 10, 257. [Google Scholar] [CrossRef]
- Tan, K.S.; Qian, L.; Rosado, R.; Flood, P.M.; Cooper, L.F. The role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide production. Biomaterials 2006, 27, 5170. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Y.; Xi, K.; Gu, Y.; Tang, J.; Xin, T.; Yang, H.; Wang, L.; Cui, W.; Chen, L. Regulation of macrophage subtype via injectable micro/nano-structured porous microsphere for reprogramming osteoimmune microenvironment. Chem. Eng. J. 2022, 439, 135692. [Google Scholar] [CrossRef]
- Yu, D.; Guo, S.; Yu, M.; Liu, W.; Li, X.; Chen, D.; Li, B.; Guo, Z.; Han, Y. Immunomodulation and osseointegration activities of Na2TiO3 nanorods-arrayed coatings doped with different Sr content. Bioact. Mater. 2022, 10, 323. [Google Scholar] [CrossRef]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. Part A 2004, 70, 194. [Google Scholar] [CrossRef]
- Takebe, J.; Champagne, C.; Offenbacher, S.; Ishibashi, K.; Cooper, L. Titanium surface topography alters cell shape and modulates bone morphogenetic protein 2 expression in the J774A.1 macrophage cell line. J. Biomed. Mater. Res. Part A 2003, 64, 207. [Google Scholar] [CrossRef] [PubMed]
- Bota, P.C.; Collie, A.M.; Puolakkainen, P.; Vernon, R.B.; Sage, E.H.; Ratner, B.D.; Stayton, P.S. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J. Biomed. Mater. Res. Part A 2010, 95, 649. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Downer, M.A.; Berry, C.E.; Valencia, C.; Fazilat, A.Z.; Griffin, M. Investigating immunomodulatory biomaterials for preventing the foreign body response. Bioengineering 2023, 10, 1411. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Desai, T.A. Methods for Fabrication of Nanoscale Topography for Tissue Engineering Scaffolds. Ann. Biomed. Eng. 2006, 34, 89. [Google Scholar] [CrossRef]
- Neves, S.C.; Mota, C.; Longoni, A.; Barrias, C.C.; Granja, P.L.; Moroni, L. Additive manufactured polymeric 3D scaffolds with tailored surface topography influence mesenchymal stromal cells activity. Biofabrication 2016, 8, 025012. [Google Scholar] [CrossRef]
- Petrini, M.; Pierfelice, T.V.; D’Amico, E.; Di Pietro, N.; Pandolfi, A.; D’Arcangelo, C.; De Angelis, F.; Mandatori, D.; Schiavone, V.; Piattelli, A. Influence of Nano, Micro, and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts. Int. J. Mol. Sci. 2021, 22, 9871. [Google Scholar] [CrossRef]
- Genin, G.M.; Shenoy, V.B.; Peng, G.C.; Buehler, M.J. Integrated Multiscale Biomaterials Experiment and Modeling. ACS Biomater. Sci. Eng. 2017, 3, 2628. [Google Scholar] [CrossRef]
- Huang, Q.; Ouyang, Z.; Tan, Y.; Wu, H.; Liu, Y. Activating macrophages for enhanced osteogenic and bactericidal performance by Cu ion release from micro/nano-topographical coating on a titanium substrate. Acta Biomater. 2019, 100, 415. [Google Scholar] [CrossRef]
- Liu, W.; Liang, L.; Liu, B.; Zhao, D.; Tian, Y.; Huang, Q.; Wu, H. The response of macrophages and their osteogenic potential modulated by micro/nano-structured Ti surfaces. Colloids Surf. B 2021, 205, 111848. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.-Y.; Anderson, J.M.; Leong, K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, L.; Xie, W.; Camacho, L.C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface Roughness and Substrate Stiffness Synergize To Drive Cellular Mechanoresponse. Nano Lett. 2019, 20, 748. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, F.; Wang, S.; Ramakrishna, S. In vitro study of human vascular endothelial cell function on materials with various surface roughness. J. Biomed. Mater. Res. Part A 2004, 71, 154. [Google Scholar] [CrossRef]
- Roy, S.; Sharma, A.; Ghosh, S. Macrophage Polarization Profiling on Native and Regenerated Silk Biomaterials. ACS Biomater. Sci. Eng. 2022, 8, 659. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jansen, J.A.; Yang, F.; van den Beucken, J.J. Titanium surfaces characteristics modulate macrophage polarization. Mater. Sci. Eng. C 2019, 95, 143. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172. [Google Scholar] [CrossRef]
- Christo, S.N.; Bachhuka, A.; Diener, K.R.; Mierczynska, A.; Hayball, J.D.; Vasilev, K. Role of bioactive coatings in tissue engineering. Adv. Healthc. Mater. 2016, 5, 956. [Google Scholar] [CrossRef] [PubMed]
- Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses. ACS Biomater. Sci. Eng. 2016, 2, 142. [Google Scholar]
- Vassey, M.J.; Figueredo, G.P.; Scurr, D.J.; Vasilevich, A.S.; Vermeulen, S.; Carlier, A.; Luckett, J.; Beijer, N.R.; Williams, P.; Winkler, D.A. Immune Modulation by Design: Using Topography to Control Human Monocyte Attachment and Macrophage Differentiation. Adv. Sci. 2020, 7, 1903392. [Google Scholar] [CrossRef]
- Luu, T.U.; Gott, S.C.; Woo, B.W.; Rao, M.P.; Liu, W.F. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl. Mater. Interfaces 2015, 7, 28665. [Google Scholar] [CrossRef]
- Gong, L.; Zhao, Y.; Zhang, Y.; Ruan, Z. The Macrophage Polarization Regulates MSC Osteoblast Differentiation in vitro. Ann. Clin. Lab. Sci. 2016, 46, 65. [Google Scholar] [PubMed]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80. [Google Scholar] [CrossRef]
- Burroughs, L.; Amer, M.H.; Vassey, M.; Koch, B.; Figueredo, G.P.; Mukonoweshuro, B.; Mikulskis, P.; Vasilevich, A.; Vermeulen, S.; Dryden, I.L. Discovery of synergistic material-topography combinations to achieve immunomodulatory osteoinductive biomaterials using a novel in vitro screening method: The ChemoTopoChip. Biomaterials 2021, 271, 120740. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.; Cheung, K.M.; Wu, S.; Yeung, K.W. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021, 7, eabf6654. [Google Scholar] [CrossRef]
- Wang, M.; Chen, F.; Tang, Y.; Wang, J.; Chen, X.; Li, X.; Zhang, X. Regulation of macrophage polarization and functional status by modulating hydroxyapatite ceramic micro/nano-topography. Mater. Des. 2022, 213, 110302. [Google Scholar] [CrossRef]
- Wang, J.; Loye, A.M.; Ketkaew, J.; Schroers, J.; Kyriakides, T.R. Hierarchical Micro- and Nanopatterning of Metallic Glass to Engineer Cellular Responses. ACS Appl. Bio Mater. 2018, 1, 51. [Google Scholar] [CrossRef]
- Song, X.; Liu, F.; Qiu, C.; Coy, E.; Liu, H.; Aperador, W.; Załe˛ski, K.; Li, J.J.; Song, W.; Lu, Z. Nanosurfacing Ti alloy by weak alkalinity-activated solid-state dewetting (AAD) and its biointerfacial enhancement effect. Mater. Horiz. 2021, 8, 912. [Google Scholar] [CrossRef]
- Nouri-Goushki, M.; Isaakidou, A.; Eijkel, B.; Minneboo, M.; Liu, Q.; Boukany, P.; Mirzaali, M.; Fratila-Apachitei, L.; Zadpoor, A. 3D printed submicron patterns orchestrate the response of macrophages. Nanoscale 2021, 13, 14304. [Google Scholar] [CrossRef]
- Zheng, X.; Xin, L.; Luo, Y.; Yang, H.; Ye, X.; Mao, Z.; Zhang, S.; Ma, L.; Gao, C. Near-Infrared-Triggered Dynamic Surface Topography for Sequential Modulation of Macrophage Phenotypes. ACS Appl. Mater. Interfaces 2019, 11, 43689. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef]
- Dong, X.; Wu, P.; Yan, L.; Liu, K.; Wei, W.; Cheng, Q.; Liang, X.; Chen, Y.; Dai, H. Oriented nanofibrous P(MMD-co-LA)/Deferoxamine nerve scaffold facilitates peripheral nerve regeneration by regulating macrophage phenotype and revascularization. Biomaterials 2022, 280, 121288. [Google Scholar] [CrossRef]
- Choi, J.; Shin, B.H.; Kim, T.; Lee, J.S.; Kim, S.; Choy, Y.B.; Heo, C.Y.; Koh, W.-G. Micro-textured silicone-based implant fabrication using electrospun fibers as a sacrificial template to suppress fibrous capsule formation. Mater. Sci. Eng. C 2022, 112687. [Google Scholar] [CrossRef]
- Gao, S.; Wang, L.; Zhang, Y.; Li, L.; Zhang, Y.; Gao, X.; Mao, J.; Wang, L.; Wang, H. Tanshinone IIA-loaded aligned microfibers facilitate stem cell recruitment and capillary formation by inducing M2 macrophage polarization. Appl. Mater. Today 2020, 21, 100841. [Google Scholar] [CrossRef]
- Ryma, M.; Tylek, T.; Liebscher, J.; Blum, C.; Fernandez, R.; Böhm, C.; Kastenmüller, W.; Groll, J. Translation of Collagen Ultrastructure to Biomaterial Fabrication for Material-Independent but Highly Efficient Topographic Immunomodulation. Adv. Mater. 2021, 33, 2101228. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, L.; Song, Y.; Fang, Y.; Liu, J.; Chen, P.; Wang, S.; Xia, T.; Liu, W. MSC-derived immunomodulatory extracellular matrix functionalized electrospun fibers for mitigating foreign-body reaction and tendon adhesion. Acta Biomater. 2021, 133, 280. [Google Scholar] [CrossRef]
- Deng, M.; Tan, J.; Hu, C.; Hou, T.; Peng, W.; Liu, J.; Yu, B.; Dai, Q.; Zhou, J.; Yang, Y. Modification of PLGA Scaffold by MSC-Derived Extracellular Matrix Combats Macrophage Inflammation to Initiate Bone Regeneration via TGF-β-Induced Protein. Adv. Healthc. Mater. 2020, 9, e2000353. [Google Scholar] [CrossRef]
- Li, W.A.; Lu, B.Y.; Gu, L.; Choi, Y.; Kim, J.; Mooney, D.J. The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration. Biomaterials 2016, 83, 249. [Google Scholar] [CrossRef] [PubMed]
- Elalouf, A. Immune response against the biomaterials used in 3D bioprinting of organs. Transpl. Immunol. 2021, 69, 101446. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Su, Y.; Xi, Y.; Tian, L.; Xu, M.; Wang, Q.; Padidan, S.; Li, P.; Huang, W. Dual-Functional Polyethylene Glycol-b-polyhexanide Surface Coating with in Vitro and in Vivo Antimicrobial and Antifouling Activities. ACS Appl. Mater. Interfaces 2017, 9, 10383. [Google Scholar] [CrossRef] [PubMed]
- Kämmerling, L.; Fisher, L.E.; Antmen, E.; Simsek, G.M.; Rostam, H.M.; Vrana, N.E.; Ghaemmaghami, A.M. Mitigating the foreign body response through ‘immune-instructive’ biomaterials. J. Immunol. Regen. Med 2021, 12, 100040. [Google Scholar] [CrossRef]
- Kim, Y.; Wu, L.; Park, H.C.; Yang, H.-C. Reduction of fibrous encapsulation by polyethylene glycol-grafted liposomes containing phosphatidylserine. Biomed. Mater. 2020, 15, 065007. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Sampath, S.; Muthusamy, S.; John, M.A. Importance of crosslinking strategies in designing smart biomaterials for bone tissue engineering: A systematic review. Mater. Sci. Eng. C 2019, 96, 941. [Google Scholar] [CrossRef]
- Bartczak, D.; Kanaras, A.G. Preparation of peptide-functionalized gold nanoparticles using one pot EDC/sulfo-NHS coupling. Langmuir 2011, 27, 10119. [Google Scholar] [CrossRef]
- Wang, C.; Yan, Q.; Liu, H.-B.; Zhou, X.-H.; Xiao, S.-J. Different EDC/NHS activation mechanisms between PAA and PMAA brushes and the following amidation reactions. Langmuir 2011, 27, 12058. [Google Scholar] [CrossRef]
- Totaro, K.A.; Liao, X.; Bhattacharya, K.; Finneman, J.I.; Sperry, J.B.; Massa, M.A.; Thorn, J.; Ho, S.V.; Pentelute, B.L. Systematic Investigation of EDC/sNHS-Mediated Bioconjugation Reactions for Carboxylated Peptide Substrates. Bioconjugate Chem. 2016, 27, 994. [Google Scholar] [CrossRef] [PubMed]
- AlKhoury, H.; Hautmann, A.; Erdmann, F.; Zhou, G.; Stojanović, S.; Najman, S.; Groth, T. Study on the potential mechanism of anti-inflammatory activity of covalently immobilized hyaluronan and heparin. J. Biomed. Mater. Res. Part A 2020, 108, 1099. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, J.; Heo, J.; Lee, S.-E.; Shin, J.-W.; Chang, M.; Hong, J. Polysaccharide-based superhydrophilic coatings with antibacterial and anti-inflammatory agent-delivering capabilities for ophthalmic applications. J. Ind. Eng. Chem. 2018, 68, 229. [Google Scholar] [CrossRef]
- Handel, T.; Johnson, Z.; Crown, S.; Lau, E.; Sweeney, M.; Proudfoot, A. Regulation of protein function by glycosaminoglycans--as exemplified by chemokines. Annu. Rev. Biochem. 2005, 74, 385. [Google Scholar] [CrossRef]
- Johnson, Z.; Proudfoot, A.; Handel, T. Interaction of chemokines and glycosaminoglycans: A new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev. 2005, 16, 625. [Google Scholar] [CrossRef]
- Birajdar, M.S.; Kim, B.H.; Sutthiwanjampa, C.; Kang, S.H.; Heo, C.Y.; Park, H. Inhibition of Capsular Contracture of Poly (Dimethyl Siloxane) Medical Implants by Surface Modification with Itaconic Acid Conjugated Gelatin. J. Ind. Eng. Chem. 2020, 89, 128. [Google Scholar] [CrossRef]
- Tao, C.; Zhu, W.; Iqbal, J.; Xu, C.; Wang, D.-A. Stabilized albumin coatings on engineered xenografts for attenuation of acute immune and inflammatory responses. J. Mater. Chem. B 2020, 8, 6080. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Rother, S.; Halfter, N.; Fiebig, K.M.; Moritz, J.; Moeller, S.; Schnabelrauch, M.; Kirkpatrick, C.J.; Sader, R.; Wiesmann, H.-P. Covalent linkage of sulfated hyaluronan to the collagen scaffold Mucograft® enhances scaffold stability and reduces proinflammatory macrophage activation in vivo. Bioact. Mater. 2022, 8, 420. [Google Scholar] [CrossRef]
- Sam, S.; Touahir, L.; Andresa, J.S.; Allongue, P.; Chazalviel, J.-N.; Gouget-Laemmel, A.; Henry de Villeneuve, C.; Moraillon, A.; Ozanam, F.; Gabouze, N. Semiquantitative study of the EDC/NHS activation of acid terminal groups at modified porous silicon surfaces. Langmuir 2010, 26, 809. [Google Scholar] [CrossRef] [PubMed]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, Y. Evaluation of structural and functional properties of chitosan-chlorogenic acid complexes. Int. J. Biol. Macromol. 2016, 86, 376. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Shen, B.; Zheng, C.; Huang, B.; Zou, J.; Zhang, G.; Fei, P. Chlorogenic acid-chitosan copolymers: Synthesis, characterization and application in O/W emulsions for enhanced β-carotene stability. Int. J. Biol. Macromol. 2024, 254, 127839. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-f.; Liu, N.-h.; Shu, L.-y.; Sun, H. Application of avidin-biotin technology to improve cell adhesion on nanofibrous matrices. J. Nanobiotechnol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Chen, L.; Mou, S.; Li, F.; Zeng, Y.; Sun, Y.; Horch, R.E.; Wei, W.; Wang, Z.; Sun, J. Self-Assembled Human Adipose-Derived Stem Cell-Derived Extracellular Vesicle-Functionalized Biotin-Doped Polypyrrole Titanium with Long-Term Stability and Potential Osteoinductive Ability. ACS Appl. Mater. Interfaces 2019, 11, 46183. [Google Scholar] [CrossRef]
- Bearden, S.; Wang, F.; Hall, A.R. Simple and Efficient Room-Temperature Release of Biotinylated Nucleic Acids from Streptavidin and Its Application to Selective Molecular Detection. Anal. Chem. 2019, 91, 7996. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.; Oxburgh, L.; Kaplan, D.L.; Coburn, J.M. Avidin Adsorption to Silk Fibroin Films as a Facile Method for Functionalization. Biomacromolecules 2018, 19, 3705. [Google Scholar] [CrossRef]
- Green, N. Thermodynamics of the binding of biotin and some analogues by avidin. Biochem. J. 1966, 101, 774. [Google Scholar] [CrossRef]
- Jain, A.; Cheng, K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J. Control. Release 2017, 245, 27. [Google Scholar] [CrossRef]
- Meyer, S.C.; Gaj, T.; Ghosh, I. Highly selective cyclic peptide ligands for NeutrAvidin and avidin identified by phage display. Chem. Biol. Drug Des. 2006, 68, 3. [Google Scholar] [CrossRef]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.-K.V.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347. [Google Scholar] [CrossRef] [PubMed]
- Stachelek, S.J.; Finley, M.J.; Alferiev, I.S.; Wang, F.; Tsai, R.K.; Eckells, E.C.; Tomczyk, N.; Connolly, J.M.; Discher, D.E.; Eckmann, D.M. The effect of CD47 modified polymer surfaces on inflammatory cell attachment and activation. Biomaterials 2011, 32, 4317. [Google Scholar] [CrossRef]
- Khang, M.K.; Zhou, J.; Co, C.M.; Li, S.; Tang, L. A pretargeting nanoplatform for imaging and enhancing anti-inflammatory drug delivery. Bioact. Mater. 2020, 5, 1102. [Google Scholar] [CrossRef]
- Dumont, C.M.; Park, J.; Shea, L.D. Controlled release strategies for modulating immune responses to promote tissue regeneration. J. Control. Release 2015, 219, 155. [Google Scholar] [CrossRef]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194. [Google Scholar] [CrossRef] [PubMed]
- Lurier, E.B.; Nash, V.A.; Abee, H.S.; Wissing, T.B.; Bouten, C.V.; Smits, A.I.; Spiller, K.L. Imparting Immunomodulatory Activity to Scaffolds via Biotin-Avidin Interactions. ACS Biomater. Sci. Eng. 2021, 7, 5611. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Korogiannaki, M.; Rastegari, B.; Zhang, J.; Chen, M.; Fu, Q.; Sheardown, H.; Filipe, C.D.; Hoare, T. “Click” Chemistry-Tethered Hyaluronic Acid-Based Contact Lens Coatings Improve Lens Wettability and Lower Protein Adsorption. ACS Appl. Mater. Interfaces 2016, 8, 22064. [Google Scholar] [CrossRef]
- Battigelli, A.; Almeida, B.; Shukla, A. Recent Advances in Bioorthogonal Click Chemistry for Biomedical Applications. Bioconjugate Chem. 2022, 33, 263. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2004, 127, 210–216. [Google Scholar] [CrossRef]
- Chen, P.-J.; Chen, H.-Y.; Tsai, W.-B. Fabrication of Low-Fouling Surfaces on Alkyne-Functionalized Poly-(p-xylylenes) Using Click Chemistry. Polymers 2022, 14, 225. [Google Scholar]
- Sánchez-Bodón, J.; Ruiz-Rubio, L.; Hernáez-Laviña, E.; Vilas-Vilela, J.L.; Moreno-Benítez, M.I. Poly(l-lactide)-Based Anti-Inflammatory Responsive Surfaces for Surgical Implants. Polymers 2020, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Chi, Y.J.; Cho, K.; Lee, H.J.; Koh, W.-G. Dual surface modification of poly(L-lactide) scaffold achieved by thermal incorporation of aligned nanofiber and click immobilization of VEGF to enhance endothelialization and blood compatibility. Appl. Surf. Sci. 2022, 589, 152969. [Google Scholar] [CrossRef]
- Hong, T.; Liu, W.; Li, M.; Chen, C. Click chemistry at the microscale. Analyst 2019, 144, 1492. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In Vitro, In Vivo, and Ex Vivo. Chem. Sci. 2019, 10, 7835. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lin, X.; Zhou, J.; Hou, S.; Fang, Y.; Bi, X.; Yang, L.; Li, L.; Fan, Y. Cell membrane-biomimetic coating via click-mediated liposome fusion for mitigating the foreign-body reaction. Biomaterials 2021, 271, 120768. [Google Scholar] [CrossRef]
- Guindani, C.; Dozoretz, P.; Araujo, P.H.; Ferreira, S.R.; de Oliveira, D. N-acetylcysteine side-chain functionalization of poly(globalide-co-ε-caprolactone) through thiol-ene reaction. Mater. Sci. Eng. C 2019, 94, 477. [Google Scholar] [CrossRef]
- Resetco, C.; Hendriks, B.; Badi, N.; D’u Prez, F. Thiol–ene chemistry for polymer coatings and surface modification–building in sustainability and performance. Mater. Horiz. 2017, 4, 1041. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Wollenberg, A.L.; Kim, J.H.; Ao, Y.; Deming, T.J.; Sofroniew, M.V. Foreign body responses in mouse central nervous system mimic natural wound responses and alter biomaterial functions. Nat. Commun. 2020, 11, 6203. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.; Chen, Z.; Li, S.; Yan, C. Thiol-Ene Click Reaction Initiated Rapid Gelation of PEGDA/Silk Fibroin Hydrogels. Polymers 2019, 11, 2102. [Google Scholar]
- Ding, H.; Li, B.; Liu, Z.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. Decoupled pH- and Thermo-Responsive Injectable Chitosan/PNIPAM Hydrogel via Thiol-Ene Click Chemistry for Potential Applications in Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e2000454. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Rehmann, M.S.; Skeens, K.M.; Maverakis, E.; Kloxin, A.M. Thiol-ene click hydrogels for therapeutic delivery. ACS Biomater. Sci. Eng. 2016, 2, 165. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17. [Google Scholar] [CrossRef]
- Korogiannaki, M.; Zhang, J.; Sheardown, H. Surface modification of model hydrogel contact lenses with hyaluronic acid via thiol-ene “click” chemistry for enhancing surface characteristics. J. Biomater. Appl. 2017, 32, 446. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Li, T.; Zhang, L.; Azhar, U.; Ma, J.; Zhai, C.; Zong, C.; Zhang, S. Cytocompatible and non-fouling zwitterionic hyaluronic acid-based hydrogels using thiol-ene “click” chemistry for cell encapsulation. Carbohydr. Polym. 2020, 236, 116021. [Google Scholar] [CrossRef]
- Briou, B.; Ameduri, B.; Boutevin, B. Trends in the Diels-Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055. [Google Scholar] [CrossRef]
- Guaresti, O.; García-Astrain, C.; Aguirresarobe, R.; Eceiza, A.; Gabilondo, N. Synthesis of stimuli-responsive chitosan-based hydrogels by Diels-Alder cross-linking ‘click’ reaction as potential carriers for drug administration. Carbohydr. Polym. 2018, 183, 278. [Google Scholar] [CrossRef]
- Meng, X.; Edgar, K.J. “Click” reactions in polysaccharide modification. Prog. Polym. Sci. 2016, 53, 52. [Google Scholar] [CrossRef]
- Koehler, K.C.; Alge, D.L.; Anseth, K.S.; Bowman, C.N. A Diels-Alder modulated approach to control and sustain the release of dexamethasone and induce osteogenic differentiation of human mesenchymal stem cells. Biomaterials 2013, 34, 4150. [Google Scholar] [CrossRef]
- Koehler, K.C.; Anseth, K.S.; Bowman, C.N. Diels-Alder mediated controlled release from a poly(ethylene glycol) based hydroge. Biomacromolecules 2013, 14, 538. [Google Scholar] [CrossRef] [PubMed]
- Mata, D.; Amaral, M.; Fernandes, A.; Colaço, B.; Gama, A.; Paiva, M.; Gomes, P.; Silva, R.; Fernandes, M. Diels-Alder functionalized carbon nanotubes for bone tissue engineering: In Vitro/In Vivo biocompatibility and biodegradability. Nanoscale 2015, 7, 9238. [Google Scholar]
- Bi, B.; Ma, M.; Lv, S.; Zhuo, R.; Jiang, X. In-situ forming thermosensitive hydroxypropyl chitin-based hydrogel crosslinked by Diels-Alder reaction for three dimensional cell culture. Carbohydr. Polym. 2019, 212, 368. [Google Scholar] [PubMed]
- Yang, Z.; Zhao, F.; Zhang, W.; Yang, Z.; Luo, M.; Liu, L.; Cao, X.; Chen, D.; Chen, X. Degradable photothermal bioactive glass composite hydrogel for the sequential treatment of tumor-related bone defects: From anti-tumor to repairing bone defects. Chem. Eng. J. 2021, 419, 129520. [Google Scholar]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2020, 11, 21. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Borase, T.; Heise, A. Hybrid Nanomaterials by Surface Grafting of Synthetic Polypeptides Using N-Carboxyanhydride (NCA) Polymerization. Adv. Mater. 2016, 28, 5725. [Google Scholar]
- Ikada, Y. Surface modification of polymers for medical applications. Biomaterials 1994, 15, 725. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, B.H.; Yoo, B.Y.; Nam, S.Y.; Lee, M.; Choi, J.; Park, H.; Choy, Y.B.; Heo, C.Y.; Koh, W.G. Modulation of Foreign Body Reaction against PDMS Implant by Grafting Topographically Different Poly(acrylic acid) Micropatterns. Macromol. Biosci. 2019, 19, e1900206. [Google Scholar] [CrossRef]
- Kang, S.H.; Sutthiwanjampa, C.; Kim, H.S.; Heo, C.Y.; Kim, M.K.; Kim, H.K.; Bae, T.H.; Chang, S.H.; Kim, W.S.; Park, H. Optimization of oxygen plasma treatment of silicone implant surface for inhibition of capsular contracture. J. Ind. Eng. Chem. 2021, 97, 226. [Google Scholar] [CrossRef]
- Chim, H.; Ong, J.L.; Schantz, J.T.; Hutmacher, D.W.; Agrawal, C.M. Efficacy of glow discharge gas plasma treatment as a surface modification process for three-dimensional poly (D,L-lactide) scaffolds. J. Biomed. Mater. Res. Part A 2003, 65, 327. [Google Scholar]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Stankevich, K.; Gudima, A.; Kibler, E.; Zhukov, Y.; Bolbasov, E.; Malashicheva, A.; Zhuravlev, M.; Riabov, V.; Liu, T. Atmospheric pressure plasma assisted immobilization of hyaluronic acid on tissue engineering PLA-based scaffolds and its effect on primary human macrophages. Mater. Des. 2017, 127, 261. [Google Scholar] [CrossRef]
- Vajpayee, M.; Singh, M.; Ledwani, L. Non-thermal plasma treatment of cellulosic biopolymer to enhance its surface property for various applications: A review. Mater. Today Proc. 2021, 43, 3250. [Google Scholar]
- Moriguchi, Y.; Lee, D.-S.; Chijimatsu, R.; Thamina, K.; Masuda, K.; Itsuki, D.; Yoshikawa, H.; Hamaguchi, S.; Myoui, A. Impact of non-thermal plasma surface modification on porous calcium hydroxyapatite ceramics for bone regeneration. PLoS ONE 2018, 13, e0194303. [Google Scholar]
- Yanling, C.; Yingkuan, W.; Chen, P.; Deng, S.; Ruan, R. Non-thermal plasma assisted polymer surface modification and synthesis: A review. Int. J. Agric. Biol. Eng. 2014, 7, 1. [Google Scholar]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef]
- Damanik, F.F.; Rothuizen, T.C.; van Blitterswijk, C.; Rotmans, J.I.; Moroni, L. Towards an in vitro model mimicking the foreign body response: Tailoring the surface properties of biomaterials to modulate extracellular matrix. Sci. Rep. 2014, 4, 6325. [Google Scholar] [CrossRef]
- de Valence, S.; Tille, J.-C.; Chaabane, C.; Gurny, R.; Bochaton-Piallat, M.-L.; Walpoth, B.H.; Möller, M. Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur. J. Pharm. Biopharm. 2013, 85, 78. [Google Scholar] [CrossRef]
- Shao, D.; Lia, K.; You, M.; Liu, S.; Hu, T.; Huang, L.; Xie, Y.; Zheng, X. Macrophage polarization by plasma sprayed ceria coatings on titanium-based implants: Cerium valence state matters. Appl. Surf. Sci. 2020, 504, 144070. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.-W.; Kim, J.; Song, E.-H.; Jung, H.-D.; Park, J.-U.; Kim, H.-E.; Kim, S.; Jang, T.-S. Reduced fibrous capsule formation at nano-engineered silicone surfaces via tantalum ion implantation. Biomater. Sci. 2019, 7, 2907. [Google Scholar] [CrossRef]
- Chudinov, V.; Kondyurina, I.; Terpugov, V.; Kondyurin, A. Weakened foreign body response to medical polyureaurethane treated by plasma immersion ion implantation. Mater. Atoms 2019, 440, 163. [Google Scholar]
- Bolbasov, E.; Maryin, P.; Stankevich, K.; Kozelskaya, A.; Shesterikov, E.; Khodyrevskaya, Y.I.; Nasonova, M.; Shishkova, D.; Kudryavtseva, Y.A.; Anissimov, Y. Surface modification of electrospun poly-(l-lactic) acid scaffolds by reactive magnetron sputtering. Colloids Surf. B 2018, 162, 43. [Google Scholar] [CrossRef]
- Ninan, N.; Joseph, B.; Visalakshan, R.M.; Bright, R.; Denoual, C.; Zilm, P.; Dalvi, Y.B.; Priya, P.; Mathew, A.; Grohens, Y. Plasma assisted design of biocompatible 3D printed PCL/silver nanoparticle scaffolds: In Vitro and In Vivo analyses. Mater. Adv. 2021, 2, 6620. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef]
- King, W.J.; Krebsbach, P.H. Growth factor delivery: How surface interactions modulate release in vitro and in vivo. Adv. Drug Deliv. Rev. 2012, 64, 1239. [Google Scholar] [CrossRef]
- Tortorella, S.; Buratti, V.V.; Maturi, M.; Sambri, L.; Franchini, M.C.; Locatelli, E. Surface-Modified Nanocellulose for Application in Biomedical Engineering and Nanomedicine: A Review. Int. J. Nanomed. 2020, 15, 9909. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Tang, R. Electrospun Scaffold with Sustained Antibacterial and Tissue-Matched Mechanical Properties for Potential Application as Functional Mesh. Int. J. Nanomed. 2020, 15, 4991. [Google Scholar] [CrossRef]
- Li, J.; Liu, X. Chapter 5-Chemical surface modification of metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Nouri, A., Wen, C., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 159–183. [Google Scholar]
- Yang, X.; Roach, H.; Clarke, N.; Howdle, S.; Quirk, R.; Shakesheff, K.; Oreffo, R. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone 2001, 29, 523. [Google Scholar] [CrossRef]
- Liu, L.; Chen, G.; Chao, T.; Ratner, B.D.; Sage, E.H.; Jiang, S. Reduced foreign body reaction to implanted biomaterials by surface treatment with oriented osteopontin. J. Biomater. Polym. Ed. 2008, 19, 821. [Google Scholar] [CrossRef]
- Suliman, S.; Sun, Y.; Pedersen, T.O.; Xue, Y.; Nickel, J.; Waag, T.; Finne-Wistrand, A.; Steinmüller-Nethl, D.; Krueger, A.; Costea, D.E. In Vivo Host Response and Degradation of Copolymer Scaffolds Functionalized with Nanodiamonds and Bone Morphogenetic Protein 2. Adv. Healthc. Mater. 2016, 5, 730. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Ninan, N.; Visalakshan, R.M.; Denoual, C.; Bright, R.; Kalarikkal, N.; Grohens, Y.; Vasilev, K.; Thomas, S. Insights into the biomechanical properties of plasma treated 3D printed PCL scaffolds decorated with gold nanoparticles. Compos. Sci. Technol. 2021, 202, 108544. [Google Scholar] [CrossRef]
- Kang, Y.; Xu, C.; Dong, X.; Qi, M.; Jiang, D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, X.; Yuan, Z.; Shen, M.; Song, Y.; Liu, H.; Deng, J.; Zhong, X.; Zhang, X. Establishing an osteoimmunomodulatory coating loaded with aspirin on the surface of titanium primed with phase-transited lysozyme. Int. J. Nanomed. 2019, 14, 977. [Google Scholar] [CrossRef]
- Chen, X.; Gao, Y.; Wang, Y.; Pan, G. Mussel-inspired peptide mimicking: An emerging strategy for surface bioengineering of medical implants. Smart Mater. Med. 2021, 2, 26. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Chen, F.; Fu, Q. Mussel-inspired chemistry: A promising strategy for natural polysaccharides in biomedical applications. Prog. Polym. Sci. 2021, 123, 101472. [Google Scholar] [CrossRef]
- Wang, H.; Lin, C.; Zhang, X.; Lin, K.; Wang, X.; Shen, S.G. Mussel-Inspired Polydopamine Coating: A General Strategy To Enhance Osteogenic Differentiation and Osseointegration for Diverse Implants. ACS Appl. Mater. Interfaces 2019, 11, 7615. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182. [Google Scholar] [CrossRef]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J.; Zhao, Z.; Zhang, Q.; Zhu, X.; Li, B. Polydopamine-assisted surface modification for orthopaedic implants. J. Orthop. Transl. 2019, 17, 82. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Dopamine-Modified Cationic Conjugated Polymer as a New Platform for pH Sensing and Autophagy Imaging. Adv. Funct. Mater. 2013, 23, 764. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Hou, Y.; Zhang, Z.; Chen, M.; Wang, M.; Liu, J.; Wang, J.; Zhao, Z.; Xie, C. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact. Mater. 2022, 18, 213–217. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Liu, Y.; Xia, Q.; Yang, Y.; Chen, N.; Yang, M.; Luo, R.; Liu, G.; Wang, Y. A robust mussel-inspired zwitterionic coating on biodegradable poly(L-lactide) stent with enhanced anticoagulant, anti-inflammatory, and anti-hyperplasia properties. Chem. Eng. J. 2022, 427, 130910. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.; Wu, Q.; An, Y.; Wang, K.; Xuan, T.; Zhang, J.; Song, W.; He, H.; Song, L. Mussel-Inspired Surface Immobilization of Heparin on Magnetic Nanoparticles for Enhanced Wound Repair via Sustained Release of a Growth Factor and M2 Macrophage Polarization. ACS Appl. Mater. Interfaces 2021, 13, 2230. [Google Scholar] [CrossRef]
- Hong, S.; Kim, K.Y.; Wook, H.J.; Park, S.Y.; Lee, K.D.; Lee, D.Y.; Lee, H. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine 2011, 6, 793. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Chen, M.; Wang, M.; Shao, Z.; Jiang, X.; Wang, K.; Yao, Z.; Yang, S.; Zhang, X.; Gao, W. Engineering Bioactive M2 Macrophage-Polarized Anti-Inflammatory, Antioxidant, and Antibacterial Scaffolds for Rapid Angiogenesis and Diabetic Wound Repair. Adv. Funct. Mater. 2021, 31, 2100924. [Google Scholar] [CrossRef]
- Li, T.; Ma, H.; Ma, H.; Ma, Z.; Qiang, L.; Yang, Z.; Yang, X.; Zhou, X.; Dai, K.; Wang, J. Mussel-Inspired Nanostructures Potentiate the Immunomodulatory Properties and Angiogenesis of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2019, 11, 17134. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-Y.; Easton, C.D.; Thissen, H.; Tsai, W.-B. Aminomalononitrile-Assisted Multifunctional Antibacterial Coatings. ACS Biomater. Sci. Eng. 2020, 6, 3349. [Google Scholar] [CrossRef] [PubMed]
- Toh, R.J.; Evans, R.; Thissen, H.; Voelcker, N.H.; d’Ischia, M.; Ball, V. Deposition of Aminomalononitrile-Based Films: Kinetics, Chemistry, and Morphology. Langmuir 2019, 35, 9896. [Google Scholar] [CrossRef]
- Thissen, H.; Koegler, A.; Salwiczek, M.; Easton, C.D.; Qu, Y.; Lithgow, T.; Evans, R.A. Prebiotic-chemistry inspired polymer coatings for biomedical and material science applications. NPG Asia Mater. 2015, 7, e225. [Google Scholar] [CrossRef]
- Ball, V.; Toh, R.J.; Voelcker, N.H.; Thissen, H.; Evans, R.A. Electrochemical deposition of aminomalonitrile based films. Colloids Surf. A 2018, 552, 124. [Google Scholar] [CrossRef]
- Ball, V. Antioxidant activity of films inspired by prebiotic chemistry. Mater. Lett. 2021, 285, 129050. [Google Scholar] [CrossRef]
- Menzies, D.J.; Ang, A.; Thissen, H.; Evans, R.A. Adhesive Prebiotic Chemistry Inspired Coatings for Bone Contacting Applications. ACS Biomater. Sci. Eng. 2017, 3, 793. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chiang, Y.-L.; Chang, Y.-H.; Thissen, H.; Tsai, S.-W. An aqueous-based process to bioactivate poly(ε-caprolactone)/mesoporous bioglass composite surfaces by prebiotic chemistry-inspired polymer coatings for biomedical applications. Colloids Surf. B 2021, 205, 111913. [Google Scholar] [CrossRef]
- Chen, W.-H.; Liao, T.-Y.; Thissen, H.; Tsai, W.-B. One-Step Aminomalononitrile-Based Coatings Containing Zwitterionic Copolymers for the Reduction of Biofouling and the Foreign Body Response. ACS Biomater. Sci. Eng. 2019, 5, 6454. [Google Scholar] [CrossRef]
- Zaveri, T.D.; Lewis, J.S.; Dolgova, N.V.; Clare-Salzler, M.J.; Keselowsky, B.G. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials 2014, 35, 3504. [Google Scholar] [CrossRef]
- Guo, X.; Bai, J.; Ge, G.; Wang, Z.; Wang, Q.; Zheng, K.; Tao, H.; Zhang, L.; Zhang, H.; Wang, D. Bioinspired peptide adhesion on Ti implants alleviates wear particle-induced inflammation and improves interfacial osteogénesis. J. Colloid Interface Sci. 2022, 605, 410. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, H.; Chen, H.; Ge, G.; Wang, M.; Gao, A.; Tong, L.; Xu, Y.; Yang, H.; Pan, G. Biomimetic osteogenic peptide with mussel adhesion and osteoimmunomodulatory functions to ameliorate interfacial osseointegration under chronic inflammation. Biomaterials 2020, 255, 120197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhong, K.; Zong, Y.; Wang, S.; Yang, H.; Zhen, L.; Tao, S.; Sun, L.; Yang, J.; Li, J. A mussel-inspired wet-adhesion hydrogel with hemostasis and local anti-inflammation for managing the development of acute wounds. Mater. Des. 2022, 213, 110347. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Xue, P.; Liu, J.; Luo, L.; Zhuge, D.; Yao, Q.; Li, X.; Zhao, Y.; Xu, H. Thermo-sensitive hydrogel with mussel-inspired adhesion enhanced the non-fibrotic repair effect of EGF on colonic mucosa barrier of TNBS-induced ulcerative colitis rats through macrophage polarizing. Chem. Eng. J. 2021, 416, 129221. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. 2014, 28, 364. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Cheng, S.; Zhang, R.; Li, M.; Zhou, J.; Wang, D.; Zhang, Y. Zn-contained mussel-inspired film on Mg alloy for inhibiting bacterial infection and promoting bone regeneration. Regen. Biomater. 2021, 8, rbaa044. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Zhao, H.; Niu, J.; Ling, X.; Zhu, C.; Wang, L.; Yang, H.; Yang, Z.; Pan, G. Bio-clickable mussel-inspired peptides improve titanium-based material osseointegration synergistically with immunopolarization-regulation. Bioact. Mater. 2022, 9, 1. [Google Scholar] [CrossRef]
- Wang, T.; Bai, J.; Lu, M.; Huang, C.; Geng, D.; Chen, G.; Wang, L.; Qi, J.; Cui, W.; Deng, L. Engineering immunomodulatory and osteoinductive implant surfaces via mussel adhesion-mediated ion coordination and molecular clicking. Nat. Commun. 2022, 13, 160. [Google Scholar] [CrossRef]
- Michel, M.; Toniazzo, V.; Ruch, D.; Ball, V. Deposition Mechanisms in Layer-by-Layer or Step-by-Step Deposition Methods: From Elastic and Impermeable Films to Soft Membranes with Ion Exchange Properties. ISRN Mater. Sci. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Aravamudhan, A.; Lee, P.; Arul, M.R.; Yu, X.; Rudraiah, S.; Kumbar, S.G. Spiral Layer-by-Layer Micro-Nanostructured Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 2181. [Google Scholar] [CrossRef]
- Gentile, P.; Carmagnola, I.; Nardo, T.; Chiono, V. Layer-by-layer assembly for biomedical applications in the last decade. Nanotechnology 2015, 26, 422001. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Zhou, Y.; Ye, Z.; Tan, W.-S. Layer-by-layer assembled polyelectrolytes on honeycomb-like porous poly(ε-caprolactone) films modulate the spatial distribution of mesenchymal stem cells. Mater. Sci. Eng. C 2017, 78, 579. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Cui, J.; Bjornmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Mao, Z.; Liu, B.; Yang, L.; He, W.; Fan, Y.; Li, X. The effects of silk layer-by-layer surface modification on the mechanical and structural retention of extracellular matrix scaffolds. Biomater. Sci. 2020, 8, 4026. [Google Scholar] [CrossRef]
- He, Y.; Xu, K.; Li, K.; Yuan, Z.; Ding, Y.; Chen, M.; Lin, C.; Tao, B.; Li, X.; Zhang, G. Improved osteointegration by SEW2871-encapsulated multilayers on micro-structured titanium via macrophages recruitment and immunomodulation. Appl. Mater. Today 2020, 20, 100673. [Google Scholar] [CrossRef]
- Yu, D.-G.; Jou, C.-H.; Lin, W.-C.; Yang, M.-C. Surface modification of poly(tetramethylene adipate-co-terephthalate) membrane via layer-by-layer assembly of chitosan and dextran sulfate polyelectrolyte multiplayer. Colloids Surf. B 2007, 54, 222. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants-a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, Q.; Wei, Y.; Meng, W.; Wang, R.; Liu, J.; Nie, Y.; Luo, R.; Wang, Y.; Shen, B. Surface modification of titanium implants by pH-Responsive coating designed for Self-Adaptive antibacterial and promoted osseointegration. Chem. Eng. J. 2022, 435, 134802. [Google Scholar] [CrossRef]
- Zhou, G.; Niepel, M.S.; Saretia, S.; Groth, T. Reducing the inflammatory responses of biomaterials by surface modification with glycosaminoglycan multilayers. J. Biomed. Mater. Res. Part A 2016, 104, 493. [Google Scholar] [CrossRef]
- Yoo, B.Y.; Kim, B.H.; Lee, J.S.; Shin, B.H.; Kwon, H.; Koh, W.-G.; Heo, C.Y. Dual surface modification of PDMS-based silicone implants to suppress capsular contracture. Acta Biomater. 2018, 76, 56. [Google Scholar] [CrossRef]
- Qian, Y.; Li, L.; Song, Y.; Dong, L.; Chen, P.; Li, X.; Cai, K.; Germershaus, O.; Yang, L.; Fan, Y. Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials 2018, 164, 22. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, L.; Zhao, W.; Qian, Y.; Dong, L.; Fang, Y.; Yang, L.; Fan, Y. Surface modification of electrospun fibers with mechano-growth factor for mitigating the foreign-body reaction. Bioact. Mater. 2021, 6, 2983. [Google Scholar] [CrossRef]
- Gao, A.; Liao, Q.; Xie, L.; Wang, G.; Zhang, W.; Wu, Y.; Li, P.; Guan, M.; Pan, H.; Tong, L.; et al. Tuning the surface immunomodulatory functions of polyetheretherketone for enhanced osseointegration. Biomaterials 2020, 230, 119642. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- He, L.; Shi, Y.; Han, Q.; Zuo, Q.; Ramakrishna, S.; Xue, W.; Zhou, L. Surface modification of electrospun nanofibrous scaffolds viapolysaccharide–proteinassembly multilayer for neurite outgrowth. J. Mater. Chem. 2012, 22, 13187. [Google Scholar] [CrossRef]
- Park, H.K.; Joo, W.; Gu, B.K.; Ha, M.Y.; You, S.J.; Chun, H.J. Collagen/poly(d,l-lactic-co-glycolic acid) composite fibrous scaffold prepared by independent nozzle control multi-electrospinning apparatus for dura repair. J. Ind. Eng. Chem. 2018, 66, 430. [Google Scholar] [CrossRef]
- Zou, P.; Lee, W.-H.; Gao, Z.; Qin, D.; Wang, Y.; Liu, J.; Sun, T.; Gao, Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr. Polym. 2020, 232, 115786. [Google Scholar] [CrossRef]
- Brandao, K.; Deason-Towne, F.; Perraud, A.-L.; Schmitz, C. The role of Mg2+ in immune cells. Immunol. Res. 2013, 55, 261. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; An, X.; Rijal, N.; Hopkins, T.; Khanal, S.; Chavez, T.; Tatu, R.; Sankar, J.; Little, K.J.; Hom, D.B. Embedding magnesium metallic particles in polycaprolactone nanofiber mesh improves applicability for biomedical applications. Acta Biomater. 2019, 98, 215. [Google Scholar] [CrossRef]
- Liu, M.; Wang, R.; Liu, J.; Zhang, W.; Liu, Z.; Lou, X.; Nie, H.; Wang, H.; Mo, X. Incorporation of magnesium oxide nanoparticles into electrospun membranes improves pro-angiogenic activity and promotes diabetic wound healing. Mater. Sci. Eng. C 2021, 133, 112609. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, R.; Liu, G.; Jia, W.; Sun, M.; Liu, Y.; Luo, Y.; Cheng, Z. Phase separation-based electrospun Janus nanofibers loaded with Rana chensinensis skin peptides/silver nanoparticles for wound healing. Mater. Des. 2021, 207, 109864. [Google Scholar] [CrossRef]
- Niu, Y.; Stadler, F.J.; Yang, X.; Deng, F.; Liu, G.; Xia, H. HA-coated collagen nanofibers for urethral regeneration via in situ polarization of M2 macrophages. J. Nanobiotechnol. 2021, 19, 283. [Google Scholar] [CrossRef]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnol. 2021, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Frumento, D.; Țălu, Ș. Immunomodulatory Potential and Biocompatibility of Chitosan–Hydroxyapatite Biocomposites for Tissue Engineering. J. Compos. Sci. 2025, 9, 305. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Ciobanu, C.; Predoi, S.-A.; Țălu, Ș. Hydroxyapatite: Innovations in Synthesis, Properties, Nanocomposites, Biomedical Applications, and Technological Developments; Napoca Star Publishing House: Cluj-Napoca, Romania, 2024; pp. 9–14. ISBN 978-606-062-901-6. [Google Scholar]
- Ţălu, Ş.; Matos, R.S.; Da Fonseca Filho, H.D.; Predoi, D.; Iconaru, S.L.; Ciobanu, C.S.; Ghegoiu, L. Morphological and fractal features of cancer cells anchored on composite layers based on magnesium-doped hydroxyapatite loaded in chitosan matrix. Micron 2024, 176, 103548. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 2011, 89, 557. [Google Scholar] [CrossRef]
- Raes, G.; Van den Bergh, R.; De Baetselier, P.; Ghassabeh, G.H. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J. Immunol. 2005, 174, 6561. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695. [Google Scholar] [CrossRef]
- Galiano, R.D.; Michaels, V.; Joseph, M.D.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regener. 2004, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Ţălu, Ş. Micro and Nanoscale Characterization of Three Dimensional Surfaces. In Basics and Applications; Napoca Star Publishing House: Cluj-Napoca, Romania, 2015; ISBN 978-606-690-349-3. [Google Scholar]
- Frumento, D.; Țălu, Ș. Light-based technologies in immunotherapy: Advances, mechanisms and applications, mechanisms and applications. Immunotherapy 2025, 17, 123–131. [Google Scholar] [CrossRef]
- Frumento, D.; Țălu, Ș. Biosensors for Human Cell Analysis: Immune Cell Dynamics and Diagnostic Capabilities. Biomed. Mater. Devices 2025. [Google Scholar] [CrossRef]
- Frumento, D.; Țălu, Ș. Surface coating with pharmacological molecules. Indian J. Physiol. Pharmacol. 2025, in press. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).