Cordura Fabric with Subtle Thin-Film Modifications Used for the Enhancement of Barrier Properties Against Toxic Gases (Part I)
Abstract
1. Introduction
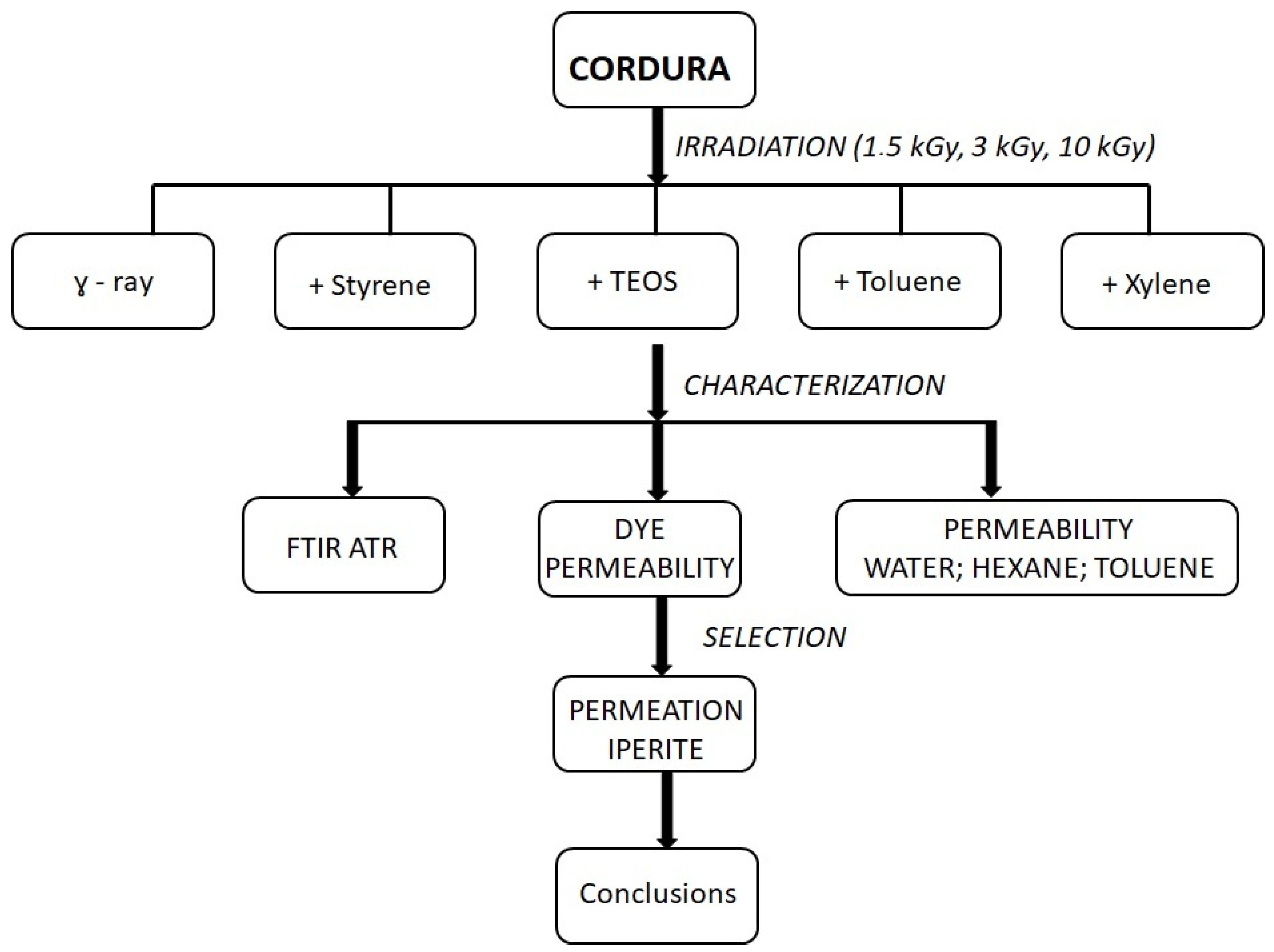
2. Materials and Methods
3. Results
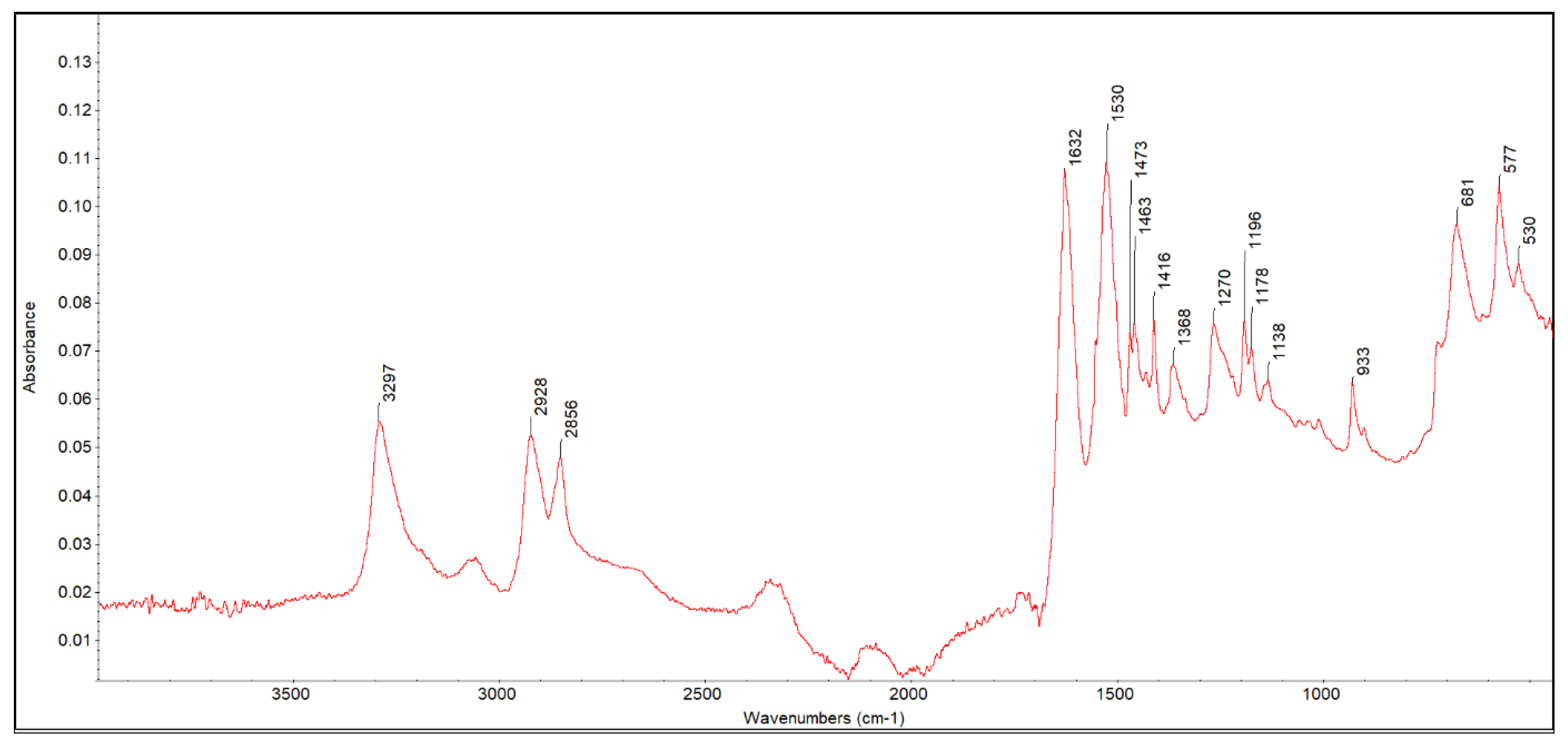
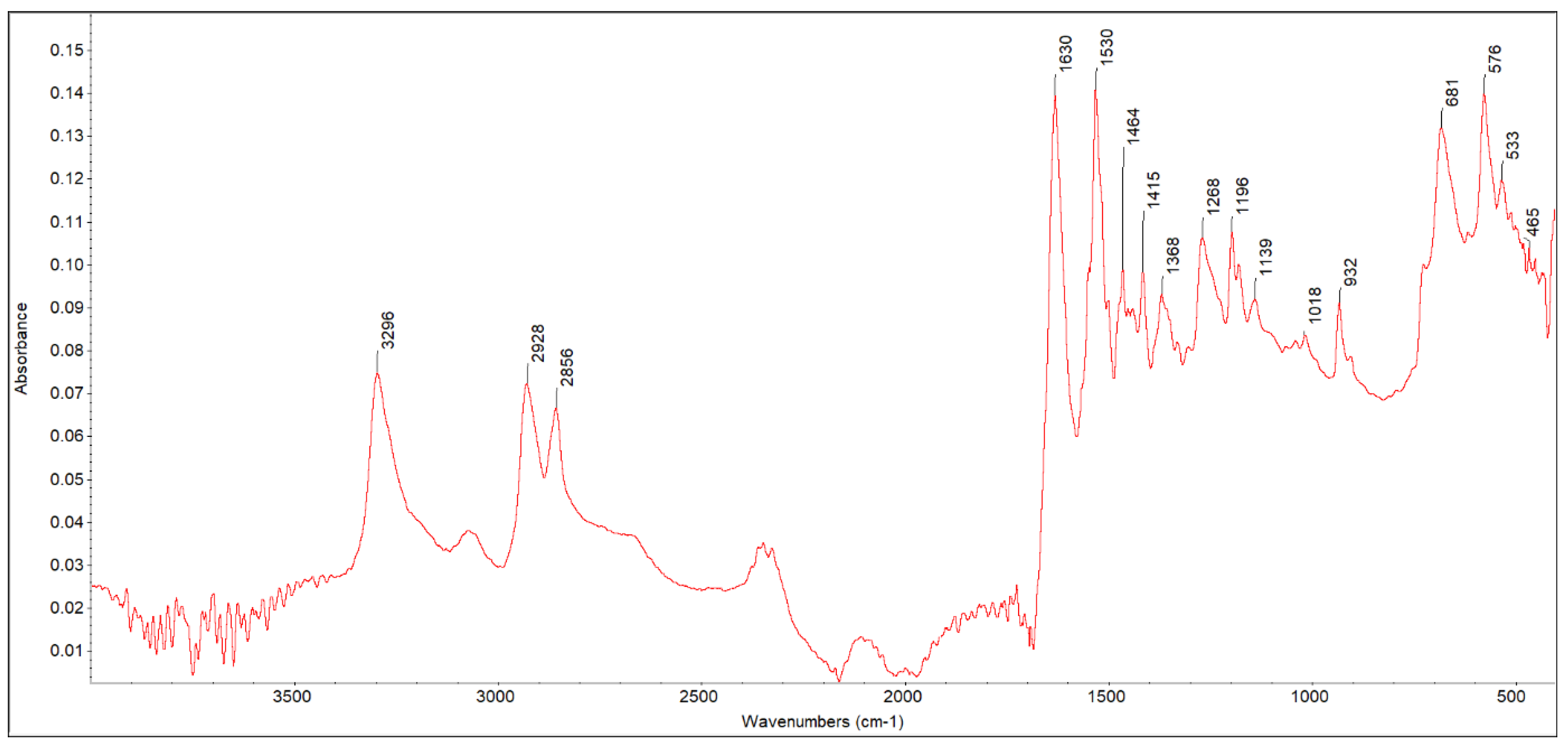
3.1. FTIR ATR Analysis
3.2. Dye Permeability
3.3. Water Vapor Permeability
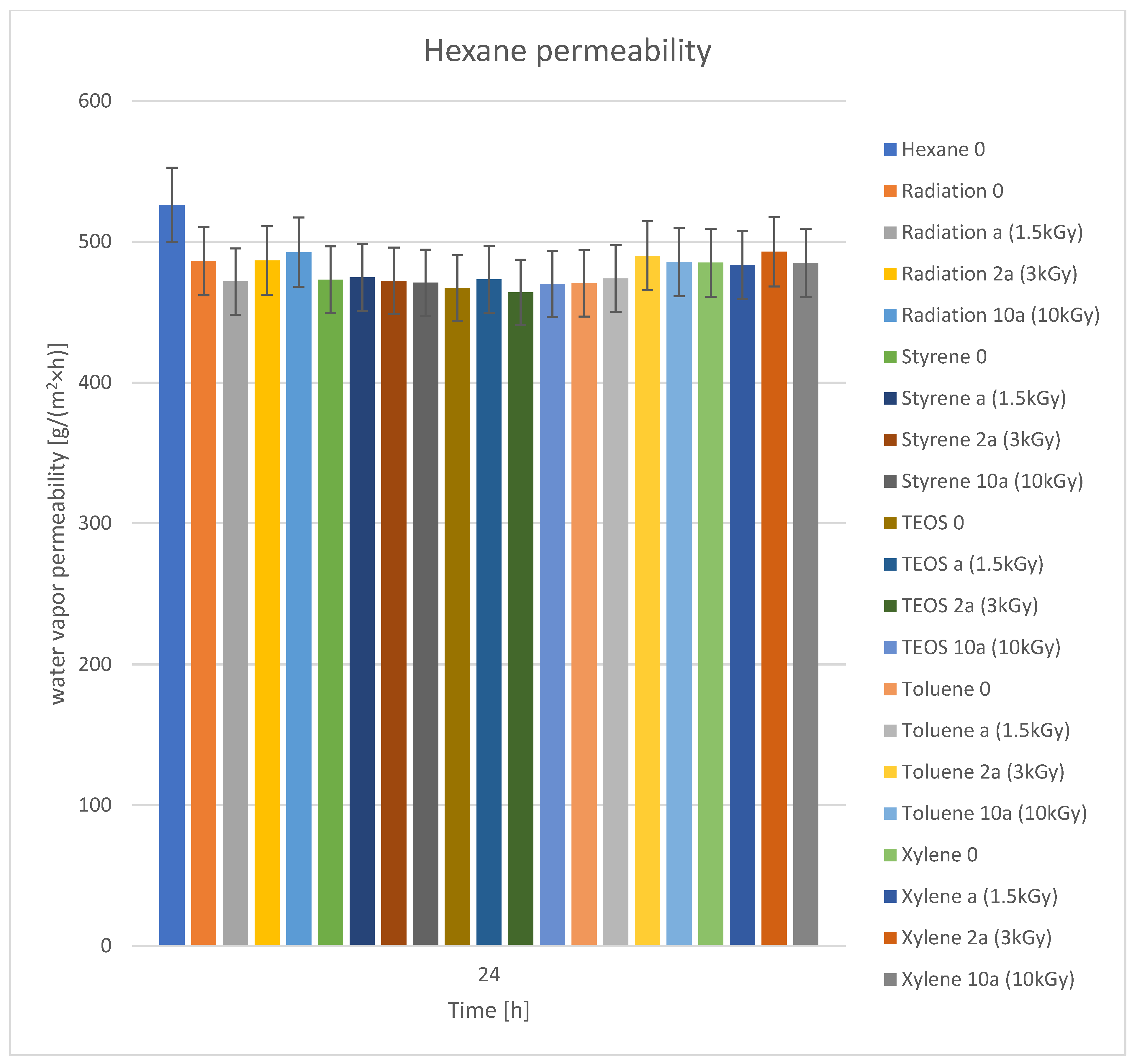
3.4. Hexane Vapor Permeability
3.5. Toluene Vapor Permeability
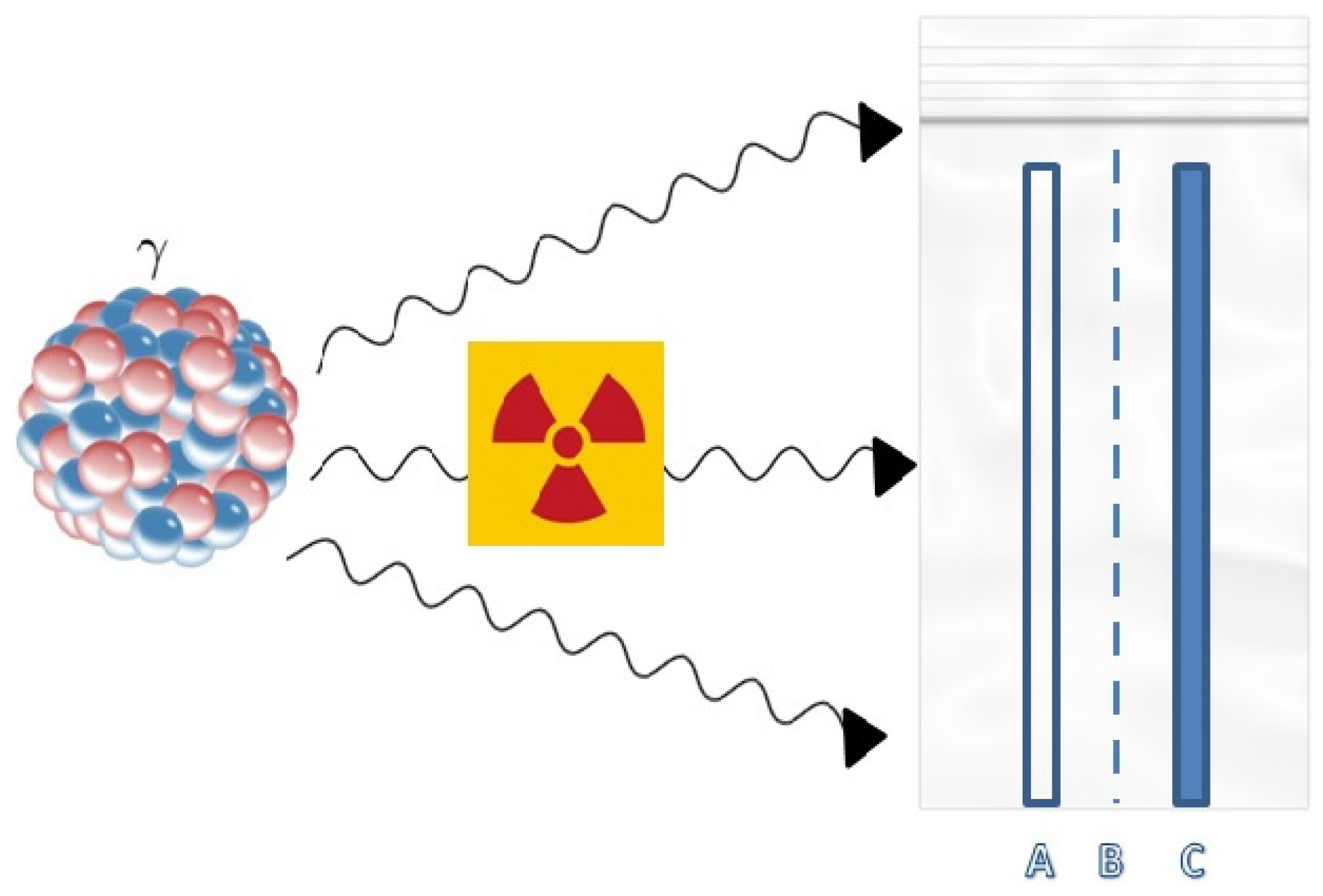
3.6. Toxic Sulfur Mustard Gas Permeability
- No irradiation, no chemical solvent vapor O-1; O-2; O-3;
- An amount of 3 kGy irradiation, no chemical solvent vapor R-1; R-2; R-3;
- An amount of 3 kGy irradiation, Toluene vapors T-1; T-2; T-3;
- An amount of 3 kGy irradiation, Xylene vapors X-1; X-2; X-3;
- An amount of 3 kGy irradiation, Tetraethoxysilane vapors D-1; D-2; D-3.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irzmańska, E.; Boczkowska, A.; Żyłka, E.; Jurczyk-Kowalska, M.; Strycharz, K.; Szroeder, P. Preliminary studies on the assessment of mechanical parameters of protective textile materials coated with polyurethane paste with the addition of graphite and carbon nanotubes. Przem. Chem. 2024, 1, 119–125. [Google Scholar] [CrossRef]
- Fabianowski, W.; Coyle, L.; Weber, B.; Granata, R.; Castner, D.; Sadownik, A.; Regen, S. Spontaneous assembly of phosphatidylcholine monolayers via chemisorption onto gold. Langmuir 1989, 5, 35. [Google Scholar] [CrossRef]
- Fabianowski, W.; Jaccodine, R.; Kodnani, R.; Pearson, R.; Smektala, P. Coupling monolayers for protection of microelectronic circuits. Adv. Mat. Opt. Electr. 1995, 5, 199–213. [Google Scholar] [CrossRef]
- Fabianowski, W.; Jachowicz, R.; Karpińska, A.; Ażgin, Z. Integrated circuits protection with the Langmuir-Blodgett Films. Bioelectrochemistry 2005, 66, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Rodger, D.C.; Li, W.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Flexible Circuit Technologies for Biomedical Applications. In Advances in Micro/Nano Electromechanical Systems and Fabrication Technologies; IntechOpen: London, UK, 2013; Chapter 1. [Google Scholar] [CrossRef][Green Version]
- SCS Parylene Properties. Available online: https://www.google.com/aclk?sa=L&ai=DChsSEwjq67Lyka6PAxWraJEFHXjROp4YACICCAEQABoCbHI&co=1&ase=2&gclid=EAIaIQobChMI6uuy8pGujwMVq2iRBR140TqeEAAYASAAEgKX4PD_BwE&cce=2&category=acrcp_v1_32&sig=AOD64_12McF6IfcnCcuY_HCve00C-1IeKg&q&nis=4&adurl&ved=2ahUKEwjS8q3yka6PAxV-JRAIHZYsBBAQ0Qx6BAgYEAE (accessed on 29 August 2025).[Green Version]
- Herman, M.J.; Blair, M.W. Determination of chemical decay mechanisms of Parylene-C during X-ray irradiation using two-dimensional correlation FTIR. Polym. Degrad. Stab. 2020, 171, 109024. [Google Scholar] [CrossRef]
- Military Standard NO-42-A500:2018; Clothing and Protective Equipment—Determination of Time Protection Against Drops and Vapors—Sulfur Mustard and Lewisite Under Static Conditions. 2018. Available online: https://wcnjik.wp.mil.pl/o-nas-2017-01-16-r/normalizacja/ (accessed on 12 July 2025).[Green Version]
- Wu, T.; Li, X.; Xue, J.; Xia, Y. Rational Fabrication of Functionally-Graded Surfaces for Biological and Biomedical Applications. Acc. Mater. Res. 2024, 5, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Martínez, V.M.; Ruzafa-Silvestre, C.; Hernández-Fernández, C.; Juan-Fernández, B.; Bañón-Gil, E.; Arán-Ais, F.; Orgilés-Calpena, E. Improving Cotton Fabric Dyeability by Oxygen Plasma Surface Activation. Surfaces 2024, 7, 1079–1095. [Google Scholar] [CrossRef]
- Bhattacharyyaa, K.G.; Guptab, S.S.; Sarma, G.K. Kinetics, equilibrium isotherms and thermodynamics of adsorption of Congo red onto natural and acid-treated kaolinite and montmorillonite. Desalination Water Treat. 2015, 53, 530–542. [Google Scholar] [CrossRef]
- Teepakakorn, A.; Hayakawa, T.; Ogawa, M. Remarkable stability of dye in polymer-clay nanocomposite film. App. Clay Sci. 2022, 218, 106405. [Google Scholar] [CrossRef]


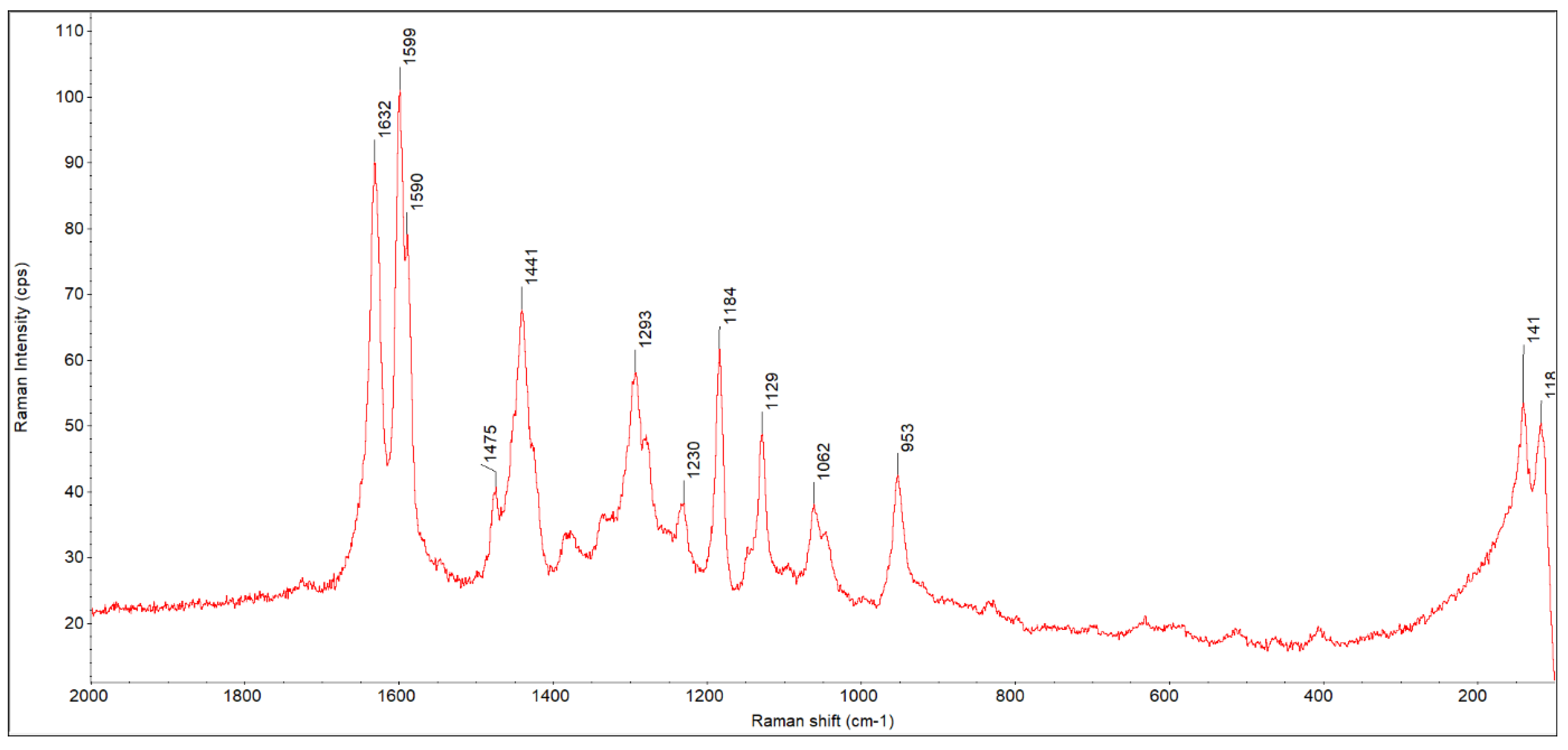
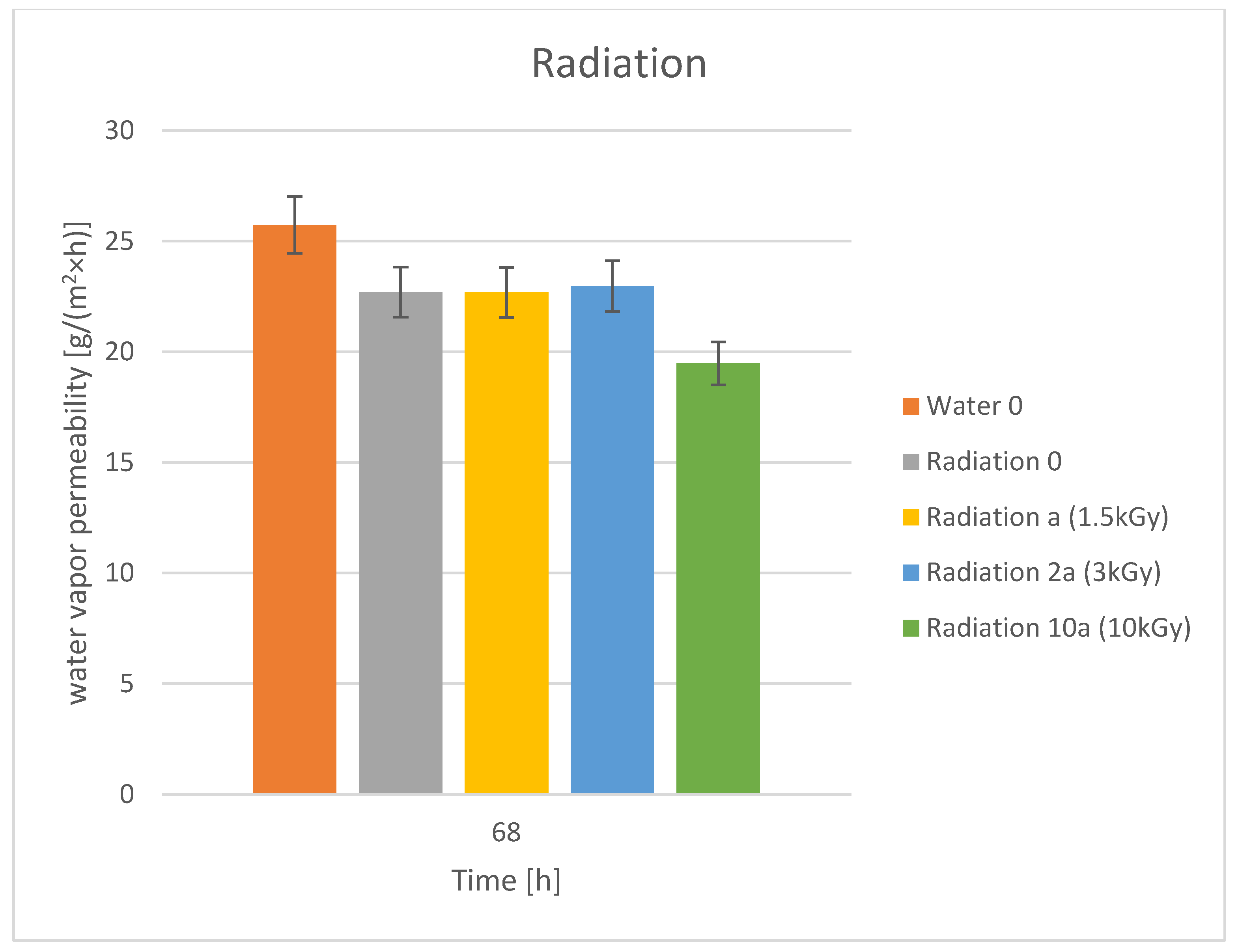
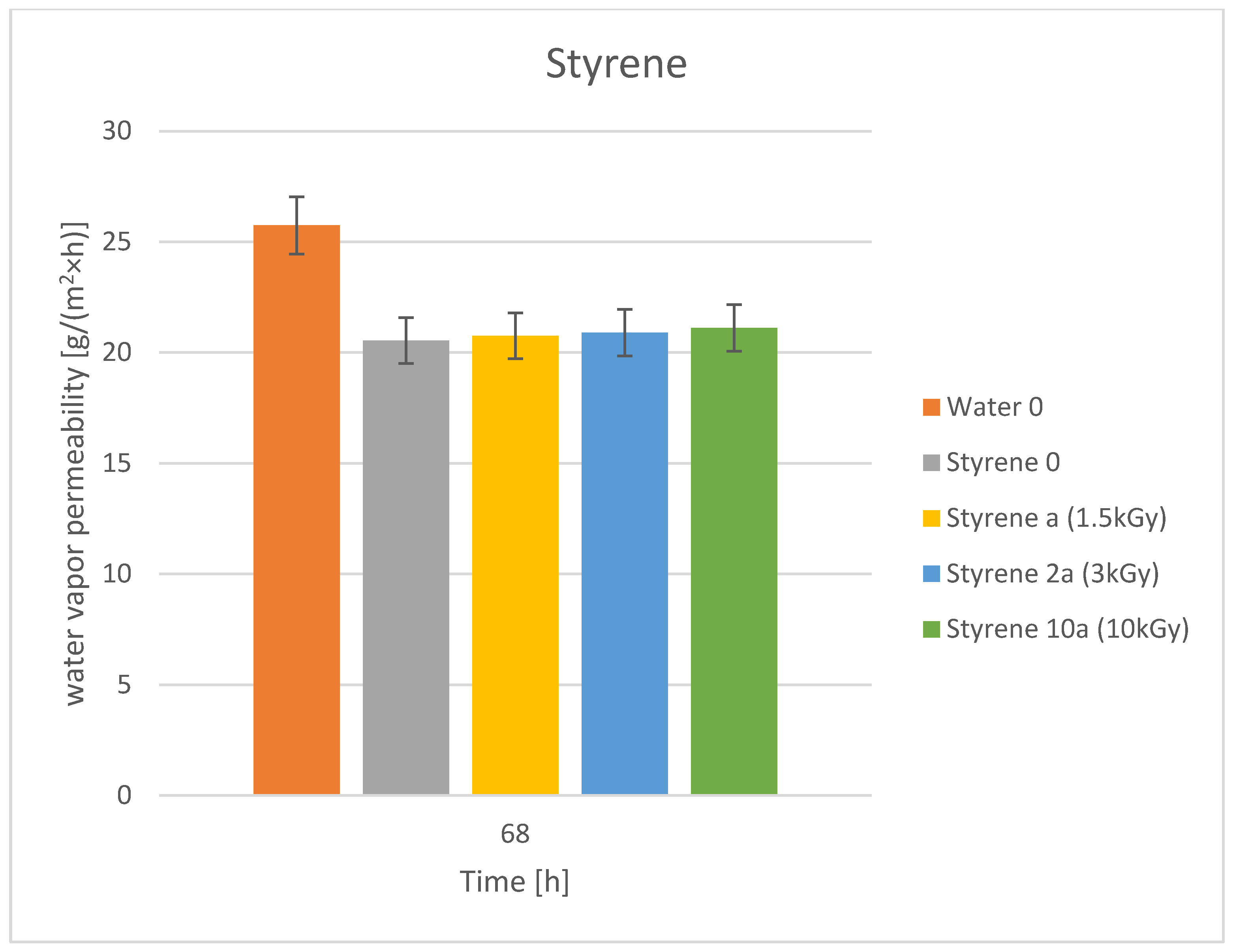


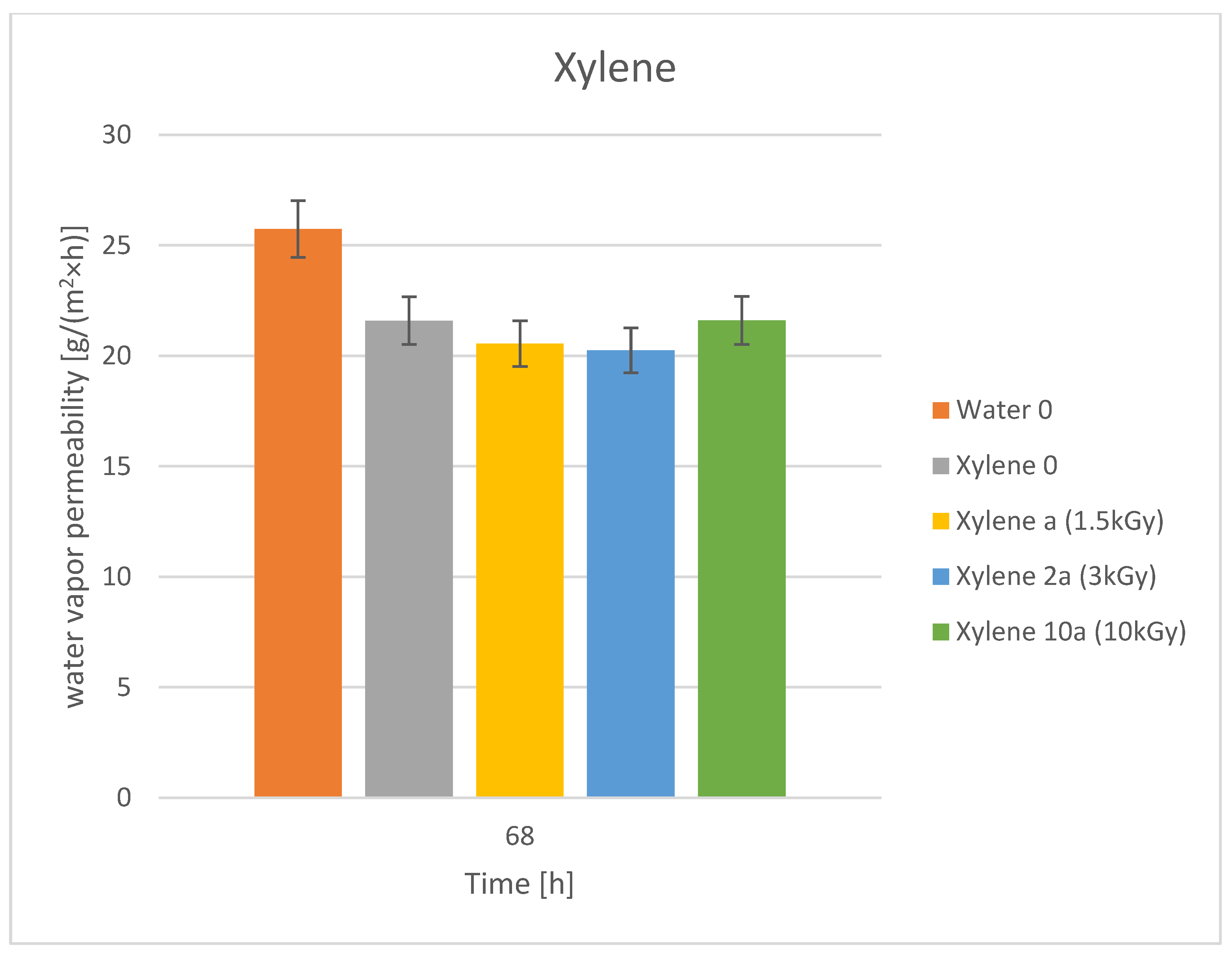




| No | Dye | Molecular Weight | Molar Concentration | Weight Concentration | Solvent |
|---|---|---|---|---|---|
| g/mol | mmol/dm3 | % w/w | |||
| 1 | Orasol Black X51 | Toluene | |||
| 2 | Alkali Blue 6B | 595.72 | 4.19 | 0.25 | Water |
| 3 | Eriochrome Black T | 461.40 | 4.34 | 0.2 | Water |
| 4 | Orasol Black X55 | deep reddish-black chromium compound | 0.1 | Toluene | |
| 5 | Naphtol Blue Black | 616.49 | 4.05 | 0.25 | Water |
| 6 | 1-(2-Pirydylo-azo)-2-Naftol | 249.29 | 4.02 | 0.1 | Ethanol |
| 7 | Xylenol Orange | 700.61 | 4.28 | 0.3 | Water |
| 8 | Beetle Juice (homemade) | 5 | Ethanol |
| Dye | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Paper | ++ | ++ | ++ | +++ | ++ | +++ | +++ | +++ |
| Cordura | ++ | + | + | +++ | + | +++ | + | + |
| Cordura and Radiation | ++ | + | + | +++ | + | +++ | + | + |
| Cordura + TEOS | ++ | + | + | +++ | + | +++ | + | + |
| Cordura + Toluene | ++ | + | + | +++ | + | +++ | + | + |
| Cordura + Styrene | ++ | + | + | +++ | + | +++ | + | + |
| Cordura + Xylene | ++ | + | + | +++ | + | +++ | + | + |
| Cordura Fabric | Dye 4 | Dye 6 | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose [kGy] | 0 | 1.5 | 3.0 | 10.0 | 0 | 1.5 | 3.0 | 10.0 |
| Cordura | + | ++ | + | ++ | ++ | + | ||
| Cordura/Styrene | ++ | ++ | + | ++ | ++ | ++ | ||
| Cordura/TEOS | + | ++ | ++ | + | ++ | |||
| Cordura/Toluene | + | ++ | + | |||||
| Cordura/Xylene | + | +++ | + | +++ | ||||
| Sample | Radiation Dose | Monomer | Retention Time | Sulfur Mustard Gas Peak Area | Sulfur Mustard Gas Peak Area Average |
|---|---|---|---|---|---|
| kGy | min | pAsec | pAsec | ||
| 0-1 | 0 | 1.766 | 114.38 | ||
| 0-1 | 0 | 1.763 | 82.19 | 79.8 ± 35.9 | |
| 0-3 | 0 | 1.770 | 42.70 | ||
| Rad-1 | 3.0 | 1.765 | 77.64 | ||
| Rad-2 | 3.0 | 1.769 | 258.81 | 165.7 ± 90.7 | |
| Rad-3 | 3.0 | 1.765 | 160.75 | ||
| D-1 | 3.0 | TEOS | 1.769 | 17 314 | |
| D-2 | 3.0 | TEOS | 1.761 | 15 529 | 23 106 ± 11 613 |
| D-3 | 3.0 | TEOS | 1.764 | 36 476 | |
| T-1 | 3.0 | Toluene | 1.765 | 61.59 | |
| T-2 | 3.0 | Toluene | 1.766 | 105.22 | 93.9 ± 28.4 |
| T-3 | 3.0 | Toluene | 1.768 | 114.91 | |
| X-1 | 3.0 | Xylene | 1.767 | 63.10 | |
| X-2 | 3.0 | Xylene | 1.763 | 146.19 | 94.5 ± 45.1 |
| X-3 | 3.0 | Xylene | 1.764 | 74.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchowski, J.K.; Fabianowski, W.; Kolacz, A.M.; Kot, P.; Natora, M.; Puchala, A.; Weiter, L.; Wiktorko, M. Cordura Fabric with Subtle Thin-Film Modifications Used for the Enhancement of Barrier Properties Against Toxic Gases (Part I). Coatings 2025, 15, 1025. https://doi.org/10.3390/coatings15091025
Duchowski JK, Fabianowski W, Kolacz AM, Kot P, Natora M, Puchala A, Weiter L, Wiktorko M. Cordura Fabric with Subtle Thin-Film Modifications Used for the Enhancement of Barrier Properties Against Toxic Gases (Part I). Coatings. 2025; 15(9):1025. https://doi.org/10.3390/coatings15091025
Chicago/Turabian StyleDuchowski, John K., Wojciech Fabianowski, Angelika Monika Kolacz, Piotr Kot, Marek Natora, Anna Puchala, Laura Weiter, and Michal Wiktorko. 2025. "Cordura Fabric with Subtle Thin-Film Modifications Used for the Enhancement of Barrier Properties Against Toxic Gases (Part I)" Coatings 15, no. 9: 1025. https://doi.org/10.3390/coatings15091025
APA StyleDuchowski, J. K., Fabianowski, W., Kolacz, A. M., Kot, P., Natora, M., Puchala, A., Weiter, L., & Wiktorko, M. (2025). Cordura Fabric with Subtle Thin-Film Modifications Used for the Enhancement of Barrier Properties Against Toxic Gases (Part I). Coatings, 15(9), 1025. https://doi.org/10.3390/coatings15091025









