Abstract
This study determined the feasibility of using zinc oxide nanoparticles of various origins as an active compound for biopolymer packaging films. The study focused on the effects of green synthesis using passion fruit or tomato extracts and commercial zinc oxide nanoparticles on the physicochemical properties of pectin films, including thickness, microstructure, water content, optical properties, water vapour permeability, water contact angle, sorption properties, and thermal stability. Zinc oxide nanoparticles resulted in lower lightness, higher absorbance, especially in the UV light range, and increased transparency, from 1.55 to 2.18 a.u./mm. Films containing zinc oxide nanoparticles showed reduced water vapour adsorption but increased water vapour permeability, from 6.35 to 12.07 × 10−10 g/m·s·Pa. The initial water contact angles were in a similar range, from 57.3° to 59.2°, but a decrease in contact angle values was observed over 60 s. All films containing nanoparticles exhibited better thermal stability, particularly during the third stage of degradation above 200 °C. Developed composite active films, prepared from apple pectin and zinc oxide nanoparticles of different origins, showed their potential for practical use as UV-VIS light barrier packaging films or protective coatings for food applications.
1. Introduction
Food packaging plays a vital role in the food supply chain, as it is specially designed to protect products from external conditions and mechanical damage, effectively reducing food losses [1,2]. Thus, packaged foods retain nutritional and flavour value and remain free of chemical and microbiological contaminants longer [3,4]. Nevertheless, the massive use of non-organic plastics in the packaging industry negatively affects the environment, mainly due to their durability and resistance to degradation [5]. This unsustainable approach underscores the need to seek innovative solutions, including environmentally friendly packaging, such as biodegradable or compostable. Thus, using biopolymers is a promising alternative because it reduces the extraction of natural resources, which translates into lower greenhouse gas emissions [6,7]. In addition, the process of producing packaging from biopolymers can be more environmentally friendly by reducing the use of harmful chemicals. Due to its edible, health-safe properties and ability to form an effective barrier to oxygen and aromatic compounds, pectin is often used as a raw material for producing protective food packaging or food films [8,9].
Pectin is a readily available compound that can be extracted from by-products of the fruit and vegetable industry, such as apples and citrus [10,11]. The extraction process and the use of industrial waste contribute to the development of a circular economy and bioeconomy [12,13]. However, this plant-based biopolymer is hydrophilic, causing the films produced also to be hydrophilic and have low elasticity [14]. To offset this effect, it is necessary to use a plasticiser, such as glycerol, to increase the plasticity of the film structure [15,16]. For this reason, it is becoming more common to add reinforcements or other substances to improve the functional properties of films [17,18,19]. Recently, scientific research has explored new developments, including nanotechnology. Nanocomponents are increasingly used in the chemical and cosmetic industries, and there is growing interest in their application in food production and packaging. Reducing materials to the nanoscale alters their physicochemical properties, which can help reduce waste and increase product functionality [20].
Zinc oxide nanoparticles are the second most abundant metal oxide after iron and are among the most researched due to their applicability in various fields. The advantages of zinc oxide nanoparticles include their inexpensive, safe, and easy preparation. Their physical and chemical behaviours can be modified by changing the morphology using different synthesis routes, precursors, or materials to produce the nanomaterial [21,22,23]. These inorganic compounds are known for their unique properties, occurring in their most common and stable form as zinc atoms tetrahedrally surrounded by oxygen atoms. They are frequently used in various fields, including food packaging materials [24,25]. Incorporating additives into packaging to improve their functionality is becoming more common. Zinc oxide nanoparticles are considered harmless to human health and have been recognised as GRAS (Generally Recognised as Safe) [26]. They do not tend to accumulate in tissues for long periods and are mainly excreted from the body through faeces [27]. Studies suggest a beneficial effect of zinc oxide nanoparticles on extending packaged foods’ microbiological and chemical shelf life [16,28] while not affecting the product’s organoleptic characteristics [29]. Zinc oxide nanoparticles are widely used as sunscreen, antimicrobial agents, dietary supplements, food additives, and semiconductor material [27]. In a study conducted by Ngo et al. [30], pectin/alginate films enriched with zinc oxide nanoparticles in various proportions were developed. They found that adding 5% nanoparticles increased the film’s flexibility and tensile strength and reduced its permeability to water vapour, oxygen, UV radiation, and water absorption and solubility. In addition, it was found that the tested materials exhibited antibacterial properties. On the other hand, the work of Malik and Mitra [31], which studied hydroxy cellulose films containing zinc oxide nanoparticles and citric acid, found that despite their good antibacterial properties, the materials exhibited high hydrophilicity and a tendency to swell. There was also a problem with the uniform dispersion of the particles in the film, leading to the formation of clusters. The research shows that zinc oxide nanoparticles added to oxidised sodium alginate films were more effective than those not coated due to the larger surface area and smaller particle size resulting from additional nanostructure defect sites [30]. However, different trends were also observed, as in a study by Singh et al. [32], where films based on oxidised guar gum and zinc oxide nanoparticles showed antibacterial properties but with lower effectiveness than pure zinc oxide nanoparticles. Presumably, the raw material blocked access to the nanoparticles in the product. Souza et al. [33] also found that adding nanoparticles improved the barrier properties of pectin-based films.
Various methods can produce zinc oxide nanoparticles. The first is the physical approach, where mechanical forces, hot steam, or ultrasound are used [30]. The second method is chemical techniques, where reactions occur at lower temperatures than physical methods. With these, nanoparticles of various shapes and sizes can be obtained. Electrochemical methods, sonochemical methods, precipitation reactions, and sol-to-gel transformations are used here [32]. The third and final method is biological techniques, which are gaining popularity due to their many benefits. The process occurs naturally, using organisms such as microorganisms, plant extracts, or plant waste [34]. Their main advantage is that they are non-toxic and environmentally friendly, as they allow artificial chemicals and energetic, raw materials to be replaced with natural ones and operate at lower pressures and temperatures than traditional methods, making them more environmentally friendly [35]. In addition, due to the natural properties of the bioresources, the nanoparticles obtained are less toxic and more reproducible in production, with minimal expense [33]. Various phytochemicals present in these sources play an essential role in reducing, stabilizing, and controlling the size of nanoparticles during their synthesis [36]. In our previous work, we investigated zinc oxide nanoparticles using extracts of passion fruit and tomato that were compared to particles obtained by the chemical method as active compounds in pectin-based films. All analysed films used as primary bio-based packaging for poultry meat showed that the deterioration rate, measured both by microbiological growth and total volatile essential nitrogen content, was reduced, and the extension of their shelf-life time was achieved [37]. Therefore, the present study aims to evaluate the physicochemical characteristics of pectin nanocomposites containing synthesised zinc oxide nanoparticles through a green approach using passion fruit and tomato extracts, compared to commercially available zinc oxide nanoparticles. Microstructural, optical, sorption, wetting, barrier, and thermal properties were investigated to understand the potential of these bio-based nanocomposites for food applications.
2. Materials and Methods
2.1. Materials and Reagents
The method to synthesise and characterise zinc oxide nanoparticles has been described in our previous work [37] using zinc nitrate (Zn(NO3)2·6H2O, purchased from Sigma-Aldrich, Schnelldorf, Germany) and potassium hydroxide (KOH, from Sigma-Aldrich, Schnelldorf, Germany), along with purple passion fruit (Passiflora edulis f. edulis) and red tomatoes (Solanum lycopersicum). Films were prepared from apple pectin (ZPOW PEKTOWIN S.A., Jasło, Poland) and glycerol (Avantor Performance Materials Poland S.A., Gliwice, Poland). Commercial nanoparticles Roti®nanoMETIC 25 nm were purchased from Carl Roth GmbH+ Co KG (Karlsruhe, Germany). All reagents used were of analytical grade and were used as procured.
2.2. Preparation of Pectin Films
Aqueous solutions for film formation were prepared by dissolving apple pectin at a concentration of 5% and zinc oxide nanoparticles at a concentration of 5% relative to pectin (0.25 g mixed with 5 g pectin powder), along with glycerol as a plasticiser at 30% relative to pectin (1.5 g per 100 g water). Film-forming solutions were then heated to 60 °C for 20 min at 250 rpm using an RCT basic IKAMAG magnetic stirrer (IKA Poland, Warsaw, Poland) to achieve a homogeneous film-forming solution. The final step involved casting the films onto sheets at a speed of 10 mm/s and a thickness of 1500 µm using a Zehntner ZAA 2300 automatic film applicator (Zehntner GmbH Testing Instruments, Sissach, Switzerland), followed by drying at 30 °C for approximately 24 h (SUP-65 WG, WAMED S.A., Warsaw, Poland). This method resulted in a consistent film thickness of 80 ± 10 µm for control films and 100 ± 10 µm for films containing zinc oxide nanoparticles, regardless of the particle source. The films were coded as controls without nanoparticles (AP), and films containing zinc oxide nanoparticles commercially available (AP-ZnO-C NPs) or those synthesised from passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
2.3. Film Thickness
The thickness measurements were conducted using a thickness tester (Thwing-Albert, ProGage Thickness Tester, West Berlin, NJ, USA) with a precision of 1 μm. Each experiment measured the film thickness in at least three replicates, and the results were used to evaluate water vapour permeability.
2.4. Film Microstructure
The microstructure analysis of the film was performed using a tabletop scanning electron microscope, model TM3000 (HITACHI High-Technologies Europe GmbH, Tokyo, Japan). Initially, the films were cut into 5 × 5 cm pieces and placed on a probe table using carbon double-sided paste double-sided tape PELCO with a diameter of 9 mm (Pik Instruments Sp. z o.o., Piaseczno, Poland). Surface observations were conducted at a magnification of 600×. To enhance the microscopic observations of the surface, the samples were coated with gold using an auto-Sputter Coater 108 (Cressington Scientific Instruments, Watford, UK).
2.5. Light Transmission
Samples were placed in the magnetic film adaptor of a UV-visible spectrophotometer EVOLUTION 220 UV (Thermo Electron Corporation, Waltham, MA, USA). An empty test cell was used as a reference. Thermo INSIGHT software recorded the UV-VIS absorption spectra in the 200 to 800 nm wavelength range.
2.6. Colour
A colorimeter (Konica Minolta, model CR-400, Tokyo, Japan) was employed for colour analysis. Before measurements, the instrument was calibrated using a white calibration plate. The parameters examined included L* ranging from black (0) to white (100), a* from green (−) to red (+), and b* from blue (−) to yellow (+). The total colour difference, ΔE, representing the colour of the films, was calculated using the equation [38]:
where ΔL*, Δa*, and Δb* represent the disparities between the parameter associated with the colour of the sample and the parameter related to the colour of the control films without the addition of zinc oxide nanoparticles (AP). These parameters were logged using SpectraMagic NK software. Ten repetitions were conducted for each film.
2.7. Film Transparency
The transparency was assessed using an EVOLUTION 220 UV–visible spectrometer (Thermo Electron Corporation, Waltham, MA, USA) equipped with a magnetic film adapter. Measurements were conducted at a wavelength of 600 nm, with eight repetitions performed for each type of film. Data were recorded using Thermo INSIGHT software, and the transparency (T600) was calculated as follows:
where A is absorbance at the wavelength of 600 nm, l is film thickness in mm.
2.8. Water Content
The water content was evaluated in three replicates. The film samples were dried at 105 °C to a constant weight using a laboratory dryer SUP-65 WG (WAMED S.A., Warsaw, Poland). It was computed based on the initial mass and the mass after drying. The results were used in the evaluation of water vapour sorption properties.
2.9. Water Vapour Sorption Kinetics
To maintain a constant ambient relative humidity of 75% at 25 °C, a saturated solution of sodium chloride (NaCl) was used [39]. Water vapour sorption kinetics were assessed at 0.5, 1, 3, 6, 9, 12, 24, 48, 72, 96, and 120 h. Samples weighing 250 ± 5 mg were sectioned into small pieces and periodically weighed. The measurements were conducted at 25 ± 1 °C, with a minimum of three replicates performed for each type of film [14]. The water vapour adsorption kinetics were evaluated using the classical diffusion equation based on Fick’s law through an L-thick membrane [40]:
where t is time (s), Mt/M∞ is the total amount of water vapour that was adsorbed by the sample at time t, D is diffusion coefficient (m2/s), and L is film thickness (m). The measurement was performed in 3 replicates.
2.10. Water Vapour Sorption Isotherms
The AQUADYNE DVS-2HT dynamic water vapour sorption analyser (Quantachrome Instruments by Anton Paar Sp. z o.o., Warsaw, Poland) was used to determine the isotherms of water adsorption by films. Samples weighing 20 ± 1 mg were placed in a glass pan and exposed to varying relative humidities (RH): 0, 10, 20, 30, 40, 50, 60, and 75% until equilibrium was attained at 25 °C. The equilibrium criterion at each relative humidity was a percentage rate of mass change over time (dm/dt) of ≤0.002% min−1 within a 10-min interval. Microsoft Excel 2019 with DVS Standard Analysis software was employed to analyse the experimental data points. Further data was processed using OriginPro 8.0 software (OriginLab Corporation, Northampton, MA, USA). The measurements were conducted in duplicate.
2.11. Water Contact Angle
Contact angle analysis was executed utilising the sessile drop technique with a goniometer (DataPhysics Instruments, OCA 25, Filderstadt, Germany). The contact angle was measured after dispensing a 10 μL drop of distilled water at a rate of 10 μL/s onto the surface of the films. Ten repetitions were conducted for each film. The outcomes were documented using SCA20_U software (Version 5.0.37).
2.12. Water Vapour Permeability
A gravimetric approach was employed, adhering to the ASTM E96 standard method adapted to packaging [41] using Mater Cup FX-3180 equipment (Textest AG, Schwerzenbach, Switzerland). Three samples were excised from each film, and their thickness was measured. Subsequently, the samples were interposed between two rubber-based cells filled with distilled water, establishing a relative humidity gradient ranging from 50 to 100%. The apparatus computed the water vapour transmission every 6 h, factoring in the mass alteration in the cup attributable to evapouration, the surface area of the films, and the duration of the measurement.
2.13. Fourier Transform Infrared Spectra of Films
The Fourier transform infrared (FT-IR) spectra were obtained employing the attenuated total reflection (ATR) technique, utilising the spectrometer model Cary-630 (Agilent Technologies, Cary, NC, USA). The spectra of the examined samples were captured within the absorption range of 4000–650 cm−1 at a resolution of 4 cm−1. Each spectrum was an average of 32 interferograms and was depicted as the relationship between absorbance and wavenumber.
2.14. Thermal Properties
Thermogravimetric analysis was conducted employing a TGA thermal analyser (Mettler Toledo, Warsaw, Poland) to assess the thermal stability and degradation of the samples. Each sample (5 mg) underwent heating at a rate of 5 °C/min, transitioning from 30 to 600 °C within a nitrogen atmosphere (N2 flow rate set at 50 mL/min). TGA and DTG curves were derived from the differential TGA values.
2.15. Statistical Analysis
Statistical analysis was performed using Statistica 13 software (StatSoft Inc., Tulsa, OK, USA). One-way analysis of variance (ANOVA) with Tukey’s post hoc test was performed to detect significant differences in the properties of the samples at the 0.05 significance level.
3. Results and Discussion
3.1. The Effect of Zinc Oxide Nanoparticles on Film Characterisation and Microstructure
Edible films based on apple pectin and zinc oxide nanoparticles, and those obtained by green synthesis using plant extracts from passion fruit and tomato extracts, were prepared and analysed. Control films did not contain nanoparticles. Pectin as a film matrix showed good gelling and thickening properties and a lack of nanoparticle reactivity, making it a suitable material for bionanocomposite preparation, which was also observed by others [42,43]. Film-forming solutions were characterised by similar density, which allowed the addition of nanoparticles and the production of edible films. During the film preparation, it was noted that the addition of zinc oxide nanoparticles, regardless of the origin, resulted in a slightly greyer colour and higher density of the mixture than the control sample (Figure 1). The differences between the solutions were probably because nanoparticles at higher concentrations can cause a decrease in the activity of peroxidase and catalase, which are responsible for oxidation reactions [44]. After heating, pouring and drying, all films had similar yellowish colour characteristics of apple pectin. The materials were smooth, without pores or cracks, gently shiny, evenly distributed and of good elasticity. However, the surface of the films in the control sample was smoother and more flexible than other samples. At this stage, it could be concluded that adding zinc oxide nanoparticles caused the films to become less shiny and transparent. Similar observations were made for alginate films with zinc oxide nanoparticles analysed by Motelica et al. [45].

Figure 1.
Views of film-forming solutions based on apple pectin (AP) without and with incorporation of zinc oxide commercial nanoparticles (AP-ZnO-C NPs).
The microstructure of the pectin films was analysed using surface images obtained with a scanning electron microscope, shown in Figure 2. The surface of the films has a few inhomogeneities and bubbles. In addition, each film has small cracks. The control film and the film with commercial zinc oxide nanoparticles are the smoothest and most compact in comparison to films containing nanoparticles synthesised using passion fruit or tomato extracts.
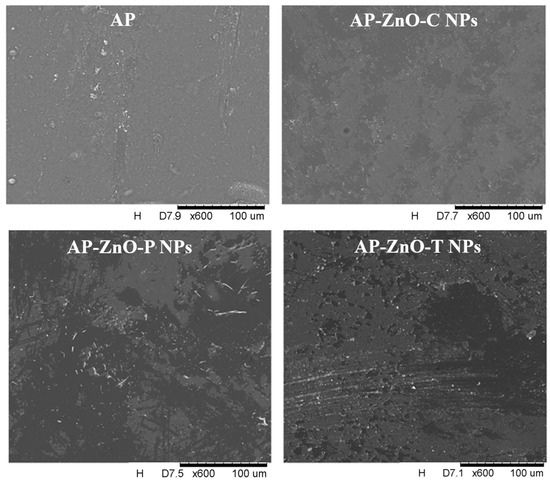
Figure 2.
Surface of active films based on apple pectin (AP) incorporated with zinc oxide commercial nanoparticles (AP-ZnO-C NPs) and zinc oxide nanoparticles obtained from passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs). Magnification 600×.
The unevenness present on the films with nanoparticles, and seen most prominently on the surface of films with nanoparticles from extracts, indicates agglomerates. Similar observations were made by Peighambardoust et al. [46], who examined the surface of starch films with various nanoparticles. The authors observed that films with nanoparticles were characterised by a rough and heterogeneous structure, in contrast to the control sample. In addition, the highest roughness and different thicknesses of the structure were observed in the samples with zinc oxide nanoparticles. Analogous conclusions were made by Mirjalili and Yassini Ardekani [47], who observed a much rougher and more heterogeneous surface when zinc oxide nanoparticles were added to cellulose-starch coatings. In addition, as the concentration increased, their number increased, indicating heterogeneity by the agglomerates present. This is due to their tendency to combine under the influence of Van der Waals forces, which are very difficult to break down during the manufacture of nanocomposites, consequently reducing the physical and mechanical properties of the films. It is also important to mention that the authors speculate that good distribution and breakdown of nanoparticle agglomerates in the coatings are important for their mechanical properties, since the microstructure directly affects most of the physical properties of the films.
3.2. The Effect of Zinc Oxide Nanoparticles on the UV Light Transmission of Pectin Films
Figure 3 shows that the light barrier properties of the analysed pectin films across the entire spectral range between 200 and 800 nm can significantly affect the quality of the packaged food. All films showed light protection in the range of 200–400 nm. All types of films had maximum absorbance between 200 nm and 230 nm, corresponding to the maximum protection range for these types of films. For control films (AP), the highest absorbance values were in this range. All analysed films showed a relatively similar spectrum across the studied wavelength range. These films had an average absorbency of 4.5 from 200 nm to 230 nm, indicating they provided the most effective UV protection.
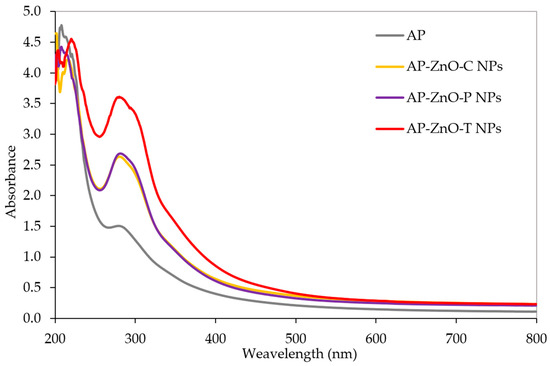
Figure 3.
UV-VIS spectra of active films based on apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
The second peak was observed between 280 and 300 nm, where nanofilms showed higher values and control films showed the lowest. Regarding nanofilms, those containing zinc oxide nanoparticles synthesised using tomato extracts showed the best light protection in the range 280–300 nm. For this range, the absorbance was 1.5 for control films, 2.5 for commercially available zinc oxide and those synthesised on passion fruit extract, and 3.5 for films containing nanoparticles synthesised from the tomato extract. Therefore, zinc oxide nanoparticles can improve light protection in this range. The differences in the efficiency of light protection can be due to the different compositions of the films and the presence of zinc oxide nanoparticles, their characteristics, and their origin. Khodaiyana et al. [48] observed that the UV absorbance of the nanocomposite polymers increased as the zinc oxide nanoparticle content increased, which was also presented by Ngo et al. [30], do Nascimento et al. [49]. Similar results were observed for biopolymer films incorporated with crystalline nanocellulose/zinc oxide nanoparticles [50], phenolic acids [51], and glucose/lysine mixture [52].
3.3. The Effect of Zinc Oxide Nanoparticles on the Colour of Pectin Films
The visual aspect is important in evaluating the film, as it is the first differentiator the consumer pays attention to when buying a product. Colour is the main component of this evaluation. Given this, the parameters L*, a*, and b* were measured, and the total colour difference (∆E) between the films containing zinc oxide nanoparticles and the control films was calculated. Table 1 shows the obtained colour measurement results for the analysed pectin films with zinc oxide nanoparticles and the transparency values. The parameter L* denotes lightness and covers the range from black to white. The a* parameter is measured from green to red, and the b* parameter covers the range from blue to yellow [53]. Calculating the total colour difference (∆E) can quantify the difference between a developed material and a specific standard or control material without modifications. The higher the value, the greater the colour discrepancy observed, while the lower the parameter, the closer the colour is to the specified standard [54]. The obtained films did not differ significantly in terms of the value of the L* parameter, which means that the films showed a similar degree of lightness. The values were between 86.30 and 89.03. Kim and Song [55] obtained the value of the L* parameter for films by adding zinc nanoparticles at 89.87, which is similar to the values obtained in the present work. Similar values were also obtained for active films based on citrus pectin by Hari et al. [56] for films containing zinc oxide nanoparticles and Mellinasa et al. [57] for films with cocoa bean shells and zinc oxide nanoparticles.

Table 1.
The colour parameters L*, a*, b*; the total colour difference (ΔE); and transparency (T600) of active films based on apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from the green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
The parameters a* showed values from −0.72 for control films to −0.33 for films containing commercially available zinc oxide nanoparticles. A similar tendency was observed for parameter b*. The lowest value is for control films (7.74), and the highest is for films with commercial zinc oxide nanoparticles (12.53). Parameter b* indicated that all films showed colour toward yellow, which is typical for pectin films [14].
The total colour difference for the tested films was 1.64–3.38 (Table 1), indicating the presence of significant colour differences between the film samples and the control films. When interpreting the obtained values of total colour difference, appropriate indications should be used: when ∆E is less than 1, it means that there are no visible differences in colour; ∆E falling in the range 1–2 informs about colour differences that are perceived by a person who is properly trained; while ∆E in the range 2–3.5 indicates the presence of a perceptible difference of colour by a person who does not have adequate training in this area. In contrast, ∆E above 5 indicates a significant colour difference. The highest value of ∆E was obtained for the film enriched with commercial zinc oxide nanoparticles (3.38). A lower value of total colour difference was obtained for the film using green biosynthesis from tomato extract (2.08). The lowest value was observed for the film using green biosynthesis from passion fruit extract (1.64). The addition of zinc oxide nanoparticles affected the colour change of the obtained films. Commercial zinc oxide nanoparticles caused the largest difference in colour, while the smallest difference was obtained when green biosynthesis from tomato extract was used. The colour differences between the samples were statistically significant (p < 0.05).
3.4. The Effect of Zinc Oxide Nanoparticles on the Transparency of Pectin Films
Transparency is a parameter describing the optical properties of the resulting films. Also, it is an important parameter for the film’s applicability as an edible packaging material for food products. Transparency describes the degree to which a given film retains sunlight [58]. The values were in a range from 1.55 to 2.18 a.u./mm (Table 1). The highest value was obtained for films with commercial zinc oxide nanoparticles (2.18 a.u./mm). The lower value was characterised by the film using green biosynthesis from tomato extract from tomato (1.89 a.u./mm). In contrast, the green biosynthesis film from passion fruit extract was characterised by a transparency of 1.62 a.u./mm. The lowest transparent films were made from apple pectin without and with the nanoparticles from passion fruit extract. This means that the addition of zinc oxide nanoparticles increases the transparency of the film, which is crucial, as lower transparency prevents the incidence of UV light and thus may prolong food shelf life [59]. In our study, the type of nanoparticles used significantly impacted the results. Both commercially available nanoparticles and those synthesised from tomato extracts showed notable differences compared to the control films. However, films containing zinc oxide synthesised using passion fruit extract showed comparable transparency to control films.
3.5. The Effect of Zinc Oxide Nanoparticles on Water Vapour Sorption Kinetics
An even better understanding of the interaction of films with water particles can be gained by studying their sorption properties [60,61]. The water vapour kinetics and water vapour sorption isotherms allow us to determine whether and at what moisture content the zinc oxide nanoparticles cause higher water absorption and, thus, barrier properties of the films. The kinetic curves for the tested films are shown in Figure 4. The largest increases in film water vapour were obtained within the first 20 h of measurement, a characteristic phenomenon during adsorption. This is because, initially, water adsorbs on the top layer of the film and then in the deeper layers of the film. The process decreases over time by increasing the film’s water content and reaching equilibrium (constant values of adsorbed water content). When thermodynamic equilibrium is reached, the kinetics (and also the water content) remain constant. Through water saturation, the spaces between polysaccharides in the film decrease and thus, the kinetics of water vapour adsorption decrease [62]. An analogous phenomenon is observed in the study by Rivera-Hernández et al. [63] for the water vapour sorption kinetics of pectin/gelate films and in the study by Ciurzyńska et al. [64] evaluating the sorption of freeze-dried strawberries.
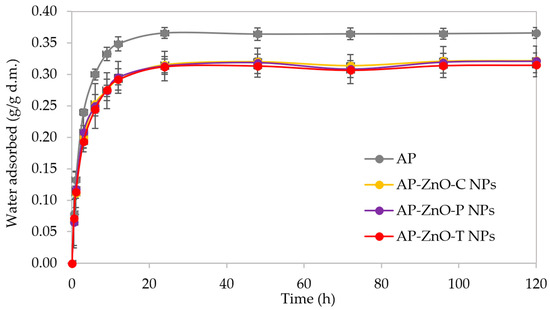
Figure 4.
Kinetics of water vapour sorption of active films based on apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from the green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
Analysing the kinetic curves (Figure 4), it can be seen that all the tested films showed very similar water vapour sorption kinetics, and the control film showed significantly higher adsorbed water values, indicating their higher hygroscopicity. It was characterised by higher kinetics results across the entire range, i.e., the film absorbed water more intensively. This could be a consequence of the replacement of pectin with the nanoparticles that fill the spaces between particles in the film so that access to the film structure was limited for water, resulting in weaker water vapour absorption by the film. Moreover, the fact that in the case of commercially available zinc oxide nanoparticles, the aggregates were formed in the films to facilitate water vapour migration but not water vapour absorption.
3.6. The Effect of Zinc Oxide Nanoparticles on the Water Vapour Sorption Isotherms
Figure 5 shows the results of water vapour sorption isotherms for pectin films, which show a similar pattern for all the variants tested. All curves showed a sigmoidal shape characteristic of most food products [65]. The initial water content was the same for all tested sample variants except for the film with zinc oxide nanoparticles created with passion fruit extract from passion fruit, which was slightly lower. Then, at the initial water activities of 0.15 to 0.5, the equilibrium water content was similar for each sample from the range 0–0.15 g/g d.m. and the values increased linearly up to 0.38 g/g d.m. at higher water activity of 0.5–0.75.
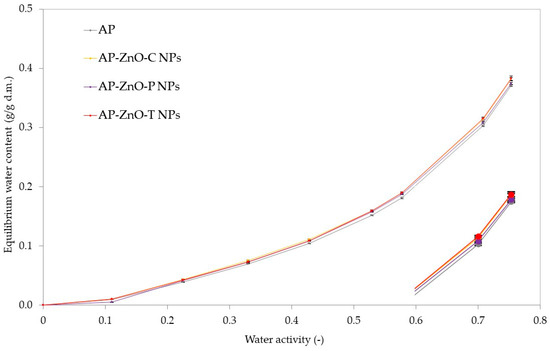
Figure 5.
Water vapour sorption isotherms of active films based on apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from the green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
The shape of the isotherms may indicate the high sugar content, characterised by absorbing a small amount of water at low and a large amount at high water activity. A similar shape of water vapour sorption isotherms was observed for other pectin-based films [14,66] or food products [67]. Pectin contains a lot of sugar and, in its structure, hydrophilic molecules, which cause greater binding to water molecules, resulting in increased hydrophilicity [65,68]. Another rationale for the shape of the water vapour sorption isotherm is due to galacturonic acid, the main component of pectin. It contains numerous -OH groups, which show a significant ability to bind to water molecules through hydrogen bonds. As a result, moisture, i.e., water, can adsorb intensively on the surface of the pectin membrane and then gradually penetrate through it to the other side. In addition, it has been observed that oxygen cannot adsorb on the pectin membrane due to unfavourable surface interactions. Therefore, oxygen diffusion occurs mainly through the pores or when there are microscopic defects in the membrane structure. Based on this, it can be concluded that the pectin film can be a good oxygen barrier. In addition, carbon dioxide may exhibit enhanced adsorption and diffusion capacity through the pectin membrane due to relatively favourable interactions, although this capacity depends on competitive interactions with water [69].
3.7. The Effect of Zinc Oxide Nanoparticles on the Water Contact Angle
The hydrophilicity or hydrophobicity of a packaging film, or its degree of wettability, can be determined by analysing the film’s water contact angle, which is defined as the angle between the surface under test and the tangent line at the point of contact of the applied liquid droplet. In general, the rule of thumb is that the smaller the water contact angle, the higher the level of hydrophilicity that characterises the film [24,70]. It is also assumed that a water contact angle greater than 90° on a hydrophobic surface. If, on the other hand, the angle is less than 90°, the surface can be considered hydrophilic [71]. The results of the water contact angle of the analysed films are presented in Table 2. The initial values of water contact angle (at 0 s) and at 15, 30, and 60 s were examined to analyse the surface of all film variants tested from the top and bottom sides. At the moment of contact between the water droplet and the film at time 0 s, the values of the water contact angle for each type of film were between 57.3 and 81.3° and were lower than 90°. This demonstrates the hydrophilic character of the films regardless of the side of the film being tested (air or support). The film adopted the highest water contact angle values for both the air and support sides with the addition of commercial zinc oxide nanoparticles. The initial values were 59.2° and 81.3°, respectively, and after 60 s were 31.4° and 34.1°, respectively. The water contact angle values for the air and support sides for the control sample were 57.3° and 68.9°. These values are similar and not statistically significantly different from those for films with zinc oxide nanoparticles prepared by biological synthesis, which showed significant droplet absorption. It follows that the addition of only commercial zinc oxide nanoparticles leads to an improvement in the hydrophobicity of the film surface. This observation is also attributed to the film structure and probably higher roughness (Figure 2). The water contact angle for each film variant changes significantly over the analysed time (60 s). The changes of the water droplets deposited on the tested films at the initial time (0 s) and after 30 s were presented in Figure 6. It can be observed that films modified with the nanoparticles showed lower changes in the shape of water droplets in comparison to control films.

Table 2.
The water contact angle (Θ) of active films based on apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
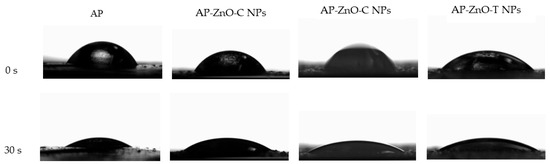
Figure 6.
The shape of the initial water droplet (0 s) and after 30 s deposited on active films based on apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from the green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
The largest change is observed for the control film (AP), whose angles took decreasing values over the measurement time (0–60 s) from 57.3° to 14.6°. In contrast, the smallest change over time was observed for the film with commercial nanoparticles, whose values oscillate from 81.3° to 34.1°. In the case of films with nanoparticles formed by green synthesis, it was not possible to read the value of the water contact angle after 60 s due to too much absorption of the droplets and, thus, too much distortion of the film, which caused a high risk of disturbing the result. These changes also confirm the earlier finding that films with commercial nanoparticles show the best hydrophobicity at longer times. The water contact angle is also related to the roughness of the film surface because on the surface side shaped by the drying process of film-forming solutions, i.e., the rougher side, the water contact angle in each variant and throughout the duration was smaller; i.e., the water droplet was absorbed much faster and wetting capacity was greater. Ngo et al. [30] also noted such a relationship when studying pectin and chitosan films. A study conducted by Al.-Naamani et al. [24] showed that chitosan coatings with zinc oxide nanoparticles had a significantly higher water contact angle (95°) compared to control samples, whose angle was 62°. Ngo et al. [30] noted that adding zinc oxide nanoparticles to alginate/pectin films in the amount of 0.5 to 5 g/100 g of polymer did not significantly change the water contact angle value.
3.8. The Effect of Zinc Oxide Nanoparticles on the Water Vapour Permeability of Pectin Films
Table 3 presents the water vapour permeability of the analysed active films. Values ranged from 6.35 ± 0.34 to 12.07 ± 0.54 × 10−10 g/m·s·Pa. Control films (AP) exhibited the lowest water vapour permeability, whereas films containing zinc oxide nanoparticles showed significantly higher values. Furthermore, films prepared with nanoparticles from green synthesis displayed higher water vapour permeability (11.99–12.07 × 10−10 g/m·s·Pa) compared to films containing commercial zinc oxide nanoparticles (9.85 ± 0.26 × 10−10 g/m·s·Pa). This can be attributed to the nature of the nanoparticles and their synthesis method. However, these observations may also be due to the film structure, which probably resulted in greater migration of water molecules. As seen in the scanning electron photographs of the films (Figure 2), the control films were smoother and more compact, whereas films containing zinc oxide nanoparticles resulted in an uneven surface. In this context, the film thickness of these structures was also higher, as was indicated in Section 2.2.

Table 3.
Water vapour permeability (WVP) of active films based on apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
The water vapour permeability of composite packaging films is expected to decrease with the addition of functional compounds [47]. Hari et al. [56] observed this phenomenon in citrus pectin films enhanced with zinc oxide nanoparticles, but the decrease was not significant. On the other hand, Sharaby et al. [50] noted a significant reduction in water vapour permeability for pectin films incorporated with crystalline nanocellulose and zinc oxide nanoparticles, similar to Ngo et al. [30] for sodium alginate films enhanced with zinc oxide nanoparticles. However, the opposite effect was observed in our study. Dos Santos et al. [72] observed a similar tendency of increased water vapour permeability in active films made from citrus pectin and zinc oxide nanoparticles; however, the differences between the values were not statistically significant. The authors indicated that water vapour permeability is a function of both solubility and diffusivity. Thus, the lack of significant variation in the obtained results of water vapour permeability may be attributed to a simultaneous increase in water molecule diffusivity, which is a result of the formation of a discontinuous phase between the nanoparticles and the biopolymer matrix of the film. Nevertheless, for biopolymer-based packaging films, many conditions affect the final film properties, especially the interaction between components, their compatibility and integrity, the type of plasticiser, and film formation during the drying process. When films are formed through solvent evapouration (mostly water for edible films), different mechanisms, such as increased porosity, aggregation, or phase separation, may occur, affecting film characteristics [73].
Mirjalili and Ardekani [47] observed that the water vapour permeability of starch films containing a constant amount of oil decreased significantly as a consequence of the addition of increasing amounts of nano-sized zinc oxide particles. Thus, different concentrations may result in various effects due to the internal interactions in the film structure. The authors highlighted that the important reduction in barrier property against water vapour after adding metal nanoparticles could result from the greater resistance to water permeability. It can probably be attributed to the presence of more hydrogen bonds between the zinc oxide nanoparticles and the film components. For this purpose, free water molecules did not interact as powerfully as with biopolymer films. However, these interactions are dependent on the biopolymer’s nature and film structure. In addition to material structure, environmental conditions also influence water vapour permeability. Reinas et al. [74] observed that increasing temperature at constant relative humidity leads to increased film permeability. This is due to the movement of molecules, which creates voids that allow water vapour to pass through. The ability to transmit water vapour depends on the relative humidity. The greater the humidity differences across barriers, the greater the amount of water vapour that can migrate through the film.
3.9. The Effect of Zinc Oxide Nanoparticles on the Chemical Structure of Pectin Films
Fourier transform infrared spectroscopy (FT-IR) is a technique that is used to identify the chemical composition and internal molecular bonds. It allows the assessment of interactions between the components of the material under study [75]. Fourier transform infrared spectroscopy spectra of pectin films are shown in Figure 7. The spectroscopy of the various pectin film variants shown in the graph takes spectra in the wave number range from 648 to 4003 cm−1. All spectra show the main characteristic bands associated with apple pectin. The first wave number band in the range of 3570–3050 cm−1 indicates the presence of -OH groups, as vibrations in this area are caused by the presence of water molecules. A characteristic spectrum in the absorbance limit of 2900 cm−1 provides information about the presence of groups -CH2 and -CH3. Vibrations spread over the 1490–1800 cm−1 range indicate the presence of C=O bonds. Regions of the spectrum in the range 1170–1480 cm−1, on the other hand, indicate the presence of -CH groups and bonds characteristic of the methyl group. The most intense spectrum for all samples studied is in the range of 850–1100 cm−1 and indicates the presence of C-O bonds, and a peak of about 1130 cm−1, indicating the presence of C-O-C bonds [71]. Absorbance values are highest for the control sample, which is probably due to the absence of additives, that is, the presence of only functional groups characteristic of apple pectin. The second-highest absorbance values were recorded for the sample with commercial zinc oxide nanoparticles. This is likely due to the lowest contamination and agglomeration of nanoparticles, which consequently translates into the result in the final film. The lowest, but also comparable, absorbance values were obtained by the samples with nanoparticles made with passion fruit and tomato extract. This means that the method of formation influences the composition of the mixture of nanoparticles, their degree of agglomeration, as well as the subsequent composition of the film. Absorbance peaks in the range of 400–500 cm−1 are attributed to the presence of zinc oxide nanoparticles [76,77], which were observed to a small extent in this work, probably due to their small amount in the structure of the films studied, as well as the low sensitivity of the apparatus in these absorbance ranges. Similar results for pectin films were obtained by Zhang et al. [78] and Souza et al. [33], whose results for pectin films took absorbance peaks at 1697, 1221, and 1606 cm−1. The authors also found that the addition of cellulose nanocrystals and sodium montmorillonite nanoparticles did not cause differences in the FT-IR spectra of the films, which was explained by their small amount [33].
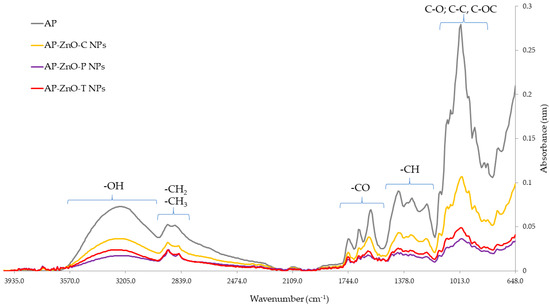
Figure 7.
Fourier transform infrared (FT-IR) spectra of apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
3.10. The Effect of Zinc Oxide Nanoparticles on the Thermal Properties of Pectin Films
The thermogravimetric analysis (TGA) curves and their first derivatives (dTG) of the analysed active pectin films containing zinc oxide nanoparticles are presented in Figure 8. These were investigated to assess the thermal stability of the films (Table 4). dTG curves have been shifted vertically for a clear comparison of the results. All analysed films showed similar behaviour with three main stages of weight loss (Figure 8). The initial stage of thermal decomposition, up to 90 °C, is primarily characterised by the loss of adsorbed and bound water from the material. In the case of the analysed films, it is attributed to the desorption of water linked to hydrophilic groups in the pectin structure. This stage is often followed by a broader range, from 25 to 200 °C, where evapouration of water and lighter volatile compounds occurs [57,79]. Film composition affected this stage. Films containing zinc oxide nanoparticles from passion fruit extract showed the highest temperature (65.44 °C), whereas other films showed lower temperatures (59.01–60.41 °C), similar to control films (60.73 °C). However, the lowest weight loss (3.32%) at the first stage was observed for control films in comparison to similar values for those containing zinc oxide nanoparticles (3.53–3.54%), indicating lower thermal stability of nanofilms in this stage. The temperatures and the weight losses were lower than those obtained by Homthawornchoo et al. [80] for rice starch–gelatin composite films with zinc oxide nanoparticles.
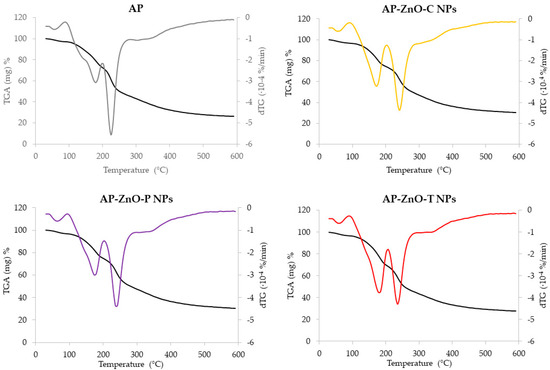
Figure 8.
Thermogravimetric analysis (TGA) (black) and derivative thermogravimetry (dTG) (coloured) curves of films based on apple pectin (AP) enriched with commercial zinc oxide nanoparticles (AP-ZnO-C NPs) and those obtained from green biosynthesis using passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).

Table 4.
Temperature and weight losses related to stages of TG/DTG curves of apple pectin films (AP) incorporated with zinc oxide commercial nanoparticles (AP-ZnO-C NPs) and zinc oxide nanoparticles obtained from passion fruit extract (AP-ZnO-P NPs) and tomato extract (AP-ZnO-T NPs).
The second stage of degradation temperature appeared between 90 and 210 °C, resulting in a weight loss in the range 21.67–28.34%. The highest temperature observed for the film containing nanoparticles from tomato extracts, whereas the lowest was for those films with commercial and passion fruit extracts. The addition of zinc oxide nanoparticles decreased this initial stage, except for tomato extract, resulting in lower stability. Regarding weight losses, it can be noted that at the second stage, the lowest values were observed for films containing zinc oxide nanoparticles commercially synthesised and from the passion fruit extract (21.67–21.92%), while the highest degradation (28.34%) was for films containing nanoparticles obtained from tomato extract. Generally, the second stage, mainly from 200 to 350 °C, is attributed to the thermal decomposition of the components in the analysed films, which is confirmed by the largest peaks (Figure 8). In the third stage, from 210 to 600 °C, more thermal changes were seen, responsible for a weight loss of 40.23–46.73% at the temperatures of 225.23–241.63 °C. The melting process ends at approximately 240 °C, with no evidence of post-melting transitions such as crystallisation. In this stage, all films containing nanoparticles presented a similar higher degradation temperature (236.50–241.63 °C), in comparison to control films (225.23 °C), followed by the highest weight loss (46.73%). Thus, the addition of zinc oxide nanoparticles resulted in better thermal stability at this stage, affecting lower weight losses. This phenomenon was the most significant for films containing zinc oxide nanoparticles obtained with the use of tomato extracts. In the third stage, above 350 °C, no changes were observed because the degradation of carbonaceous residues, formed during the second stage, combined with their complex oxidation, was complete [81]. Therefore, the thermogravimetric analysis of prepared films demonstrated that blending apple pectin with zinc oxide nanoparticles of different origins increased the stability of pectin films, as observed by higher heat resistance and intra/intermolecular interactions between the components of analysed films. It was better observed for the third stage when degradation occurred at higher temperatures, and greater weight losses were observed. It can be attributed to the film composition and structure.
Similar results have been presented by others for different biopolymer-based films containing zinc oxide nanoparticles [80,82,83,84,85]. This phenomenon might be connected to the properties of the zinc oxide nanoparticles, such as heat insulation, the enhancement of biopolymer chain interactions, and the escape of volatile-compound blocking [80]. However, some reports indicate that the inclusion of zinc oxide nanoparticles can lower the thermal stability of certain biopolymer films. While zinc oxide nanoparticles can enhance properties such as mechanical strength, moisture content, and antibacterial activity, their presence can reduce a film’s resistance to high temperatures. This is often attributed to the catalytic behaviour of zinc nanoparticles, which can accelerate biopolymer decomposition [80,82,83].
4. Conclusions
The study investigated the effect of zinc oxide nanoparticles of various origins on selected physical properties of pectin packaging films. The addition of zinc oxide nanoparticles, both commercial and produced with tomato and passion fruit extracts, allowed for the creation of active pectin films with distinct physical properties. Control films, without the addition of zinc oxide nanoparticles, were characterised by greater smoothness, flexibility, and higher gloss compared to the nanofilms, which were rougher and thicker. Colour parameters indicated similar lightness and green/yellow colouration across all sample variants. However, a significant total colour difference existed between the white standard and the analysed films, with the greatest differences observed in films containing commercial zinc oxide nanoparticles. Additionally, the zinc oxide nanoparticles increased film transparency. The presence of zinc oxide nanoparticles also increased the water vapour permeability of the tested films, consistent with their wetting properties. Water contact angle measurements indicated the hydrophilicity of active films since all values were below 90°. Water vapour sorption kinetic curves showed a lower water absorption capacity for films with zinc oxide nanoparticles, while water vapour sorption isotherms were similar across all active films. Fourier transform infrared spectroscopy identified pectin’s typical functional groups, with the highest absorbances observed in the control film. Thermogravimetric analysis confirmed the enhanced thermal stability of films containing zinc oxide nanoparticles, particularly at temperatures exceeding 200 °C. Therefore, the composite active films prepared from apple pectin and zinc oxide nanoparticles of different origins can be used as UV-VIS light barrier films and bioactive coatings for food packaging applications.
Author Contributions
Conceptualisation, S.G., M.M.A., I.C. and A.L.F.; Data curation, S.G. and A.P.; Formal analysis, S.G. and A.P.; Funding acquisition, S.G.; Investigation, S.G.; Methodology, S.G., C.H.B., C.R., V.G.L.S., M.M.A. and C.F.S.; Project administration, S.G.; Resources, S.G. and A.P.; Software, S.G.; Supervision, S.G., I.C. and A.L.F.; Validation, S.G., C.H.B., C.R., V.G.L.S., M.M.A. and C.F.S.; Visualisation, S.G.; Writing—original draft, S.G. and Adrianna Przybyszewska; Writing—review and editing, S.G., A.P., C.H.B., C.R., V.G.L.S., M.M.A., C.F.S., I.C. and A.L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the MEtRICs unit, which is financed by national funds from FCT/MECI (UID/4077: Mechanical Engineering and Resources Sustainability Center). This work was also supported by the Associate Laboratory for Green Chemistry—LAQV, and by the CQE unit, which is financed by national funds from FCT/MECI (UIDB/50006/2020 and UIDP/50006/2020), and from UIDB/00100/2020 and UIDP/00100/2020, respectively. The authors also acknowledge FCT/MECI for funding Carolina Rodrigues’s Ph.D. fellowship (https://doi.org/10.54499/2020.04441.BD), Cássia H. Barbosa’s Ph.D. fellowship (2021.08154.BD) and Victor Souza’s individual contract (https://doi.org/10.54499/2023.09446.CEECIND/CP2836/CT0011). This research was also supported by the Associate Laboratory for Green Chemistry—LAQV—which received financial support from the FCT/MCTES (LA/P/0008/2020 DOI 10.54499/LA/P/0008/2020, UIDP/50006/2020 DOI 10.54499/UIDP/50006/2020 and UIDB/50006/2020 DOI 10.54499/UIDB/50006/2020) using national funds. Research equipment for film properties was purchased as part of the “Food and Nutrition Centre—modernisation of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mahmud, M.Z.A.; Mobarak, M.H.; Hossain, N. Emerging trends in biomaterials for sustainable food packaging: A comprehensive review. Heliyon 2024, 10, e24122. [Google Scholar] [CrossRef]
- Li, D.; Xue, R. Nanostructured materials for smart food packaging: Integrating preservation and antimicrobial properties. Alex. Eng. J. 2025, 124, 446–461. [Google Scholar] [CrossRef]
- Nian, L.; Wang, M.; Sun, X.; Zeng, Y.; Xie, Y.; Cheng, S.; Cao, C. Biodegradable active packaging: Components, preparation, and applications in the preservation of postharvest perishable fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2024, 64, 2304–2339. [Google Scholar] [CrossRef] [PubMed]
- Karwacka, M.; Ciurzyńska, A.; Galus, S.; Janowicz, M. The Effect of Storage Time and Temperature on Quality Changes in Freeze-Dried Snacks Obtained with Fruit Pomace and Pectin Powders as a Sustainable Approach for New Product Development. Sustainability 2024, 16, 4736. [Google Scholar] [CrossRef]
- Ribeiro, I.S.; Maciel, G.M.; Bortolini, D.G.; Fernandes, I.d.A.A.; Maroldi, W.V.; Pedro, A.C.; Rubio, F.T.V.; Haminiuk, C.W.I. Sustainable innovations in edible films and coatings: An overview. Trends Food Sci. Technol. 2024, 143, 104272. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.; García, M. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.; Junior, J.; Ramos, A.; Martinazzo, A.; Habert, A.; Carvalho, C.; Soares, A.; Tonon, R.; Cabral, L. Chitosan-based nanocomposite films with carnauba wax, rosin resin, and zinc oxide nanoparticles. Food Res. Int. 2024, 188, 114475. [Google Scholar] [CrossRef]
- Alasalvar, H.; Yildirim, Z.; Yildirim, M. Development and characterization of sustainable active pectin films: The role of choline chloride/glycerol-based natural deep eutectic solvent and lavender extracts. Heliyon 2023, 9, e21756. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Muflih, M.H.; Izzah, N.; Fadillah, U.; Ainani, A.F.; Dirpan, A. Pectin-based edible films and coatings: From extraction to application on food packaging towards circular economy—A review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100680. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Z.; Hu, L.; Li, G.; Yao, Q.; Hu, Y. Pectin-based active packaging: A critical review on preparation, physical properties and novel application in food preservation. Trends Food Sci. Technol. 2021, 118, 167–178. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Pakulska, A.; Bartosiewicz, E.; Galus, S. The Potential of Apple and Blackcurrant Pomace Powders as the Components of Pectin Packaging Films. Coatings 2023, 13, 1409. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and Functionalized Films/Coatings—Performances and Perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Mikus, M.; Galus, S. Biopolymer active materials for food. Food Sci. Technol. Qual. 2023, 30, 18–32. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S. The Effect of Phenolic Acids on the Sorption and Wetting Properties of Apple Pectin-Based Packaging Films. Molecules 2025, 30, 1960. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.-P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Alves, M.M.; Santos, C.F.; Ribeiro, I.A.C.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L. Biodegradable Chitosan Films with ZnO Nanoparticles Synthesized Using Food Industry By-Products—Production and Characterization. Coatings 2021, 11, 646. [Google Scholar] [CrossRef]
- Mousazadeh, S.; Ehsani, A.; Moghaddas Kia, E.; Ghasempour, Z. Zinc oxide nanoparticles and periodate oxidation in developing pH-sensitive packaging film based on modified gelatin. Food Packag. Shelf Life 2021, 28, 100654. [Google Scholar] [CrossRef]
- El Habbasha, E.S.; Abouzeid, R.; Ibrahim, F.M.; Youssef, A.M.; Mahdy, S.Z.A.; El-Liethy, M.A. Developing a novel, low-cost, antimicrobial, and biodegradable pectin/HEC/ZnO biofilm for edible food packaging applications. Biomass Convers. Biorefinery 2024, 15, 6377–6388. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Souza, V.; Alves, M.; Santos, C.; Fernando, A.; Coelhoso, I. Polymer–nano-ZnO composites for food packaging. In Micro and Nano Technologies, Nanostructured Materials for Food Packaging Applications; Jissy, J.I.C., Sabu, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 263–293. [Google Scholar]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Mo, X.; Jiang, Y.; Wang, H. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Fujihara, J.; Nishimoto, N. Review of Zinc Oxide Nanoparticles: Toxicokinetics, Tissue Distribution for Various Exposure Routes, Toxicological Effects, Toxicity Mechanism in Mammals, and an Approach for Toxicity Reduction. Biol. Trace Elem. Res. 2024, 202, 9–23. [Google Scholar] [CrossRef]
- Dwivedi, L.M.; Baranwal, K.; Gupta, S.; Mishra, M.; Sundaram, S.; Singh, V. Antibacterial nanostructures derived from oxidized sodium alginate-ZnO. Int. J. Biol. Macromol. 2020, 149, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Alrumman, S.A. Influence of nanoparticles on food: An analytical assessment. J. King Saud Univ. Sci. 2021, 33, 101530. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Effects of Zinc Oxide Nanoparticles on the Properties of Pectin/Alginate Edible Films. Int. J. Polym. Sci. 2018, 2018, 5645797. [Google Scholar] [CrossRef]
- Malik, G.; Mitra, J. Zinc Oxide Nanoparticle Synthesis, Characterization, and Their Effect on Mechanical, Barrier, and Optical Properties of HPMC-Based Edible Film. Food Bioprocess Technol. 2021, 14, 441–456. [Google Scholar] [CrossRef]
- Singh, V.; Dwivedi, L.; Baranwal, K.; Asthana, S.; Sundaram, S. Oxidized guar gum–ZnO hybrid nanostructures: Synthesis, characterization and antibacterial activity. Appl. Nanosci. 2018, 8, 1149–1160. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Mello, I.P.; Khalid, O.; Pires, J.R.A.; Rodrigues, C.; Alves, M.M.; Santos, C.; Fernando, A.L.; Coelhoso, I. Strategies to Improve the Barrier and Mechanical Properties of Pectin Films for Food Packaging: Comparing Nanocomposites with Bilayers. Coatings 2022, 12, 108. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewska, A.; Galus, S. Green Approach for Biopolymer-Based Food Packaging Films Enhanced by Zinc Oxide Nanoparticles. In Biopolymeric Nanoparticles for Agricultural Applications; Abd-Elsalam, K.A., Hashim, A.F., Ahmed, F.K., Thomas, S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 319–342. [Google Scholar]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Przybyszewska, A.; Barbosa, C.H.; Pires, F.; Pires, J.R.A.; Rodrigues, C.; Galus, S.; Souza, V.G.L.; Alves, M.M.; Santos, C.F.; Coelhoso, I.; et al. Packaging of Fresh Poultry Meat with Innovative and Sustainable ZnO/Pectin Bionanocomposite Films—A Contribution to the Bio and Circular Economy. Coatings 2023, 13, 1208. [Google Scholar] [CrossRef]
- Sobral, P.; Santos, J.; García, F. Effect of protein and plasticizer concentrations in film forming solutions on physical properties of edible films based on muscle proteins of a Thai Tilapia. J. Food Eng. 2005, 70, 93–100. [Google Scholar] [CrossRef]
- Janowicz, M.; Rybak, K.; Ciurzyńska, A.; Galus, S. Effect of interactions of locust bean gum and rosehip juice on the physical properties of gum tragacanth composite films. J. Food Process. Preserv. 2022, 46, e16898. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975. [Google Scholar]
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity Homogeneity and Structure Affect Water Vapor Permeability of Model Edible Films. J. Food Sci. 1993, 58, 426–429. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, R.; Ding, C.; Gong, T.; Sun, J.J.; Li, F.; Zhang, C.; Wang, X.Y.; Guo, Y.; Zhong, T.; et al. Fabraction of edible bio-nanocomposite coatings from pectin-containing lignocellulosic nanofibers isolated from apple pomace. Int. J. Biol. Macromol. 2024, 279, 135030. [Google Scholar] [CrossRef]
- Nesic, A.; Meseldzija, S.; Cabrera-Barjas, G.; Onjia, A. Novel Biocomposite Films Based on High Methoxyl Pectin Reinforced with Zeolite Y for Food Packaging Applications. Foods 2022, 11, 360. [Google Scholar] [CrossRef]
- Knijnenburg, J.T.N.; Kasemsiri, P.; Amornrantanaworn, K.; Suwanree, S.; Iamamornphan, W.; Chindaprasirt, P.; Jetsrisuparb, K. Entrapment of nano-ZnO into alginate/polyvinyl alcohol beads with different crosslinking ions for fertilizer applications. Int. J. Biol. Macromol. 2021, 181, 349–356. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.-D.; Andronescu, E.; Holban, A.M. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh Pakdel, P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Mirjalili, F.; Yassini Ardekani, A. Preparation and characterization of starch film accompanied with ZnO nanoparticles. J. Food Process Eng. 2017, 40, e12561. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Preparation of UV-protective kefiran/nano-ZnO nanocomposites: Physical and mechanical properties. Int. J. Biol. Macromol. 2015, 72, 41–46. [Google Scholar] [CrossRef]
- do Nascimento, W.J.; da Costa, J.C.M.; Alves, E.S.; de Oliveira, M.C.; Monteiro, J.P.; Souza, P.R.; Martins, A.F.; Bonafe, E.G. Zinc oxide nanoparticle-reinforced pectin/starch functionalized films: A sustainable solution for biodegradable packaging. Int. J. Biol. Macromol. 2024, 257, 128461. [Google Scholar] [CrossRef] [PubMed]
- Sharaby, M.R.; Soliman, E.A.; Abdel-Rahman, A.B.; Osman, A.; Khalil, R. Novel pectin-based nanocomposite film for active food packaging applications. Sci. Rep. 2022, 12, 20673. [Google Scholar] [CrossRef]
- Kurek, M.; Ščetar, M.; Nuskol, M.; Janči, T.; Tanksoić, M.; Klepac, D.; Čakić Semenčić, M.; Galić, K. Assessment of Chitosan/Gelatin Blend Enriched with Natural Antioxidants for Antioxidant Packaging of Fish Oil. Antioxidants 2024, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, H.; Jridi, M.; Nasri, M.; Debeaufort, F. Design of Gelatin Pouches for the Preservation of Flaxseed Oil during Storage. Coatings 2020, 10, 150. [Google Scholar] [CrossRef]
- Pirsa, S.; Shamusi, T. Intelligent and active packaging of chicken thigh meat by conducting nano structure cellulose-polypyrrole-ZnO film. Mater. Sci. Eng. C 2019, 102, 798–809. [Google Scholar] [CrossRef]
- Salem, A.; Jridi, M.; Abdelhedi, O.; Fakhfakh, N.; Nasri, M.; Debeaufort, F.; Zouari, N. Development and characterization of fish gelatin-based biodegradable film enriched with Lepidium sativum extract as active packaging for cheese preservation. Heliyon 2021, 7, e08099. [Google Scholar] [CrossRef]
- Kim, S.; Song, K.B. Antimicrobial activity of buckwheat starch films containing zinc oxide nanoparticles against Listeria monocytogenes on mushrooms. Int. J. Food Sci. Technol. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Hari, K.D.; Garcia, C.V.; Shin, G.-H.; Kim, J.-T. Improvement of the UV Barrier and Antibacterial Properties of Crosslinked Pectin/Zinc Oxide Bionanocomposite Films. Polymers 2021, 13, 2403. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods 2020, 9, 1572. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Lenart, A. Effect of fat emulsion on the optical properties of whey films. Acta Agrophysica 2012, 19, 29–36. [Google Scholar]
- Li, X.; Ren, Z.; Wang, R.; Liu, L.; Zhang, J.; Ma, F.; Khan, M.Z.; Zhao, D.; Liu, X.-H. Characterization and antibacterial activity of edible films based on carboxymethyl cellulose, Dioscorea opposita mucilage, glycerol and ZnO nanoparticles. Food Chem. 2021, 349, 129208. [Google Scholar] [CrossRef]
- Gökkaya Erdem, B.; Dıblan, S.; Kaya, S. A Comprehensive Study on Sorption, Water Barrier, and Physicochemical Properties of Some Protein- and Carbohydrate-Based Edible Films. Food Bioprocess Technol. 2021, 14, 2161–2179. [Google Scholar] [CrossRef]
- Nazreen, A.Z.; Jai, J.; Ali, S.A.; Manshor, N.M. Moisture Adsorption Isotherm Model for Edible Food Film Packaging—A review. Sci. Res. J. 2020, 17, 221–245. [Google Scholar] [CrossRef]
- Galus, S. Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocoll. 2018, 85, 233–241. [Google Scholar] [CrossRef]
- Rivera-Hernández, L.; Chavarría-Hernández, N.; López Cuellar, M.D.R.; Martínez-Juárez, V.M.; Rodríguez-Hernández, A.I. Pectin-gellan films intended for active food packaging: Release kinetics of nisin and physico-mechanical characterization. J. Food Sci. Technol. 2021, 58, 2973–2981. [Google Scholar] [CrossRef]
- Agnieszka, C.; Andrzej, L. Rehydration and sorption properties of osmotically pretreated freeze-dried strawberries. J. Food Eng. 2010, 97, 267–274, Erratum in J. Food Eng. 2012, 113, 361. [Google Scholar] [CrossRef]
- Ouaabou, R.; Ennahli, S.; Hssaini, L.; Nabil, B.; Idlimam, A.; Lamharrar, A.; Mahrouz, M.; Hanine, H.; Bozkurt, H. Moisture Sorption Isotherms of Sweet Cherry (Prunus Avium L.): Comparative Study of Kinetics and Thermodynamic Modeling of Five Varieties. Int. J. Food Sci. 2022, 2022, 6786590. [Google Scholar] [CrossRef]
- Galus, S.; Turska, A.; Lenart, A. Sorption and wetting properties of pectin edible films. Czech J. Food Sci. 2012, 30, 446–455. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Galus, S.; Karwacka, M.; Janowicz, M. The sorption properties, structure and shrinkage of freeze-dried multi-vegetable snack bars in the aspect of the environmental water activity. LWT 2022, 171, 114090. [Google Scholar] [CrossRef]
- Castel, A.P.D.; Kaufmann, A.I.; Endres, C.M.; Robazza, W.D.S.; Paulino, A.T. Water sorption isotherms on lyophilized jabuticaba (Myrciaria cauliflora) peel: Potential byproduct for the production of dehydrated foods. J. Food Sci. Technol. 2023, 60, 419–428. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Mehraj, S. Molecular Simulations to Understand the Moisture, Carbon Dioxide, and Oxygen Barrier Properties of Pectin Films. J. Mol. Model. 2022, 28, 83. [Google Scholar] [CrossRef]
- Norizan, M.N.; Shazleen, S.S.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Zainudin, E.S.; Abdullah, N.; Samsudin, M.S.; Kamarudin, S.H.; Norrrahim, M.N.F. Nanocellulose-Based Nanocomposites for Sustainable Applications: A Review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef]
- Wang, P.; Fei, P.; Zhou, C.; Hong, P. Stearic acid esterified pectin: Preparation, characterization, and application in edible hydrophobic pectin/chitosan composite films. Int. J. Biol. Macromol. 2021, 186, 528–534. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.S.; Cagnin, C.; de Freitas, B.S.M.; da Silva, R.M.; de Jesus, G.B.L.; Belisário, C.M.; Egea, M.B.; de Oliveira Filho, J.G.; Plácido, G.R. Nanocomposite Coatings of Pectin and Oxide Zinc Nanoparticles to Increase Papaya Shelf Life. Coatings 2024, 14, 990. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Reinas, I.; Oliveira, J.; Pereira, J.; Mahajan, P.; Poças, F. A quantitative approach to assess the contribution of seals to the permeability of water vapour and oxygen in thermosealed packages. Food Packag. Shelf Life 2016, 7, 34–40. [Google Scholar] [CrossRef]
- Pola, C.C.; Medeiros, E.A.A.; Pereira, O.L.; Souza, V.G.L.; Otoni, C.G.; Camilloto, G.P.; Soares, N.F.F. Cellulose acetate active films incorporated with oregano (Origanum vulgare) essential oil and organophilic montmorillonite clay control the growth of phytopathogenic fungi. Food Packag. Shelf Life 2016, 9, 69–78. [Google Scholar] [CrossRef]
- Altuner, E.E.; Gulbagca, F.; Tiri, R.N.E.; Aygun, A.; Sen, F. Highly efficient palladium-zinc oxide nanoparticles synthesized by biogenic methods: Characterization, hydrogen production and photocatalytic activities. Chem. Eng. J. Adv. 2023, 14, 100465. [Google Scholar] [CrossRef]
- Kaningini, A.; Azizi, S.; Sintwa, N.; Mokalane, K.; Mohale, K.; Mudau, F.; Maaza, M. Effect of Optimized Precursor Concentration, Temperature, and Doping on Optical Properties of ZnO Nanoparticles Synthesized via a Green Route Using Bush Tea (Athrixia phylicoides DC.) Leaf Extracts. ACS Omega 2022, 7, 31658–31666. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.; Wang, D.; Ma, Y. Novel multilayer chitosan/emulsion-loaded syringic acid grafted apple pectin film with sustained control release for active food packaging. Food Hydrocoll. 2023, 142, 108823. [Google Scholar] [CrossRef]
- Andrade Martins, Y.A.; Ferreira, S.V.; Silva, N.M.; Sandre, M.F.B.; Filho, J.G.O.; Leão, P.V.T.; Leão, K.M.; Nicolau, E.S.; Plácido, G.R.; Egea, M.B.; et al. Edible Films of Whey and Cassava Starch: Physical, Thermal, and Microstructural Characterization. Coatings 2020, 10, 1059. [Google Scholar] [CrossRef]
- Homthawornchoo, W.; Kaewprachu, P.; Pinijsuwan, S.; Romruen, O.; Rawdkuen, S. Enhancing the UV-Light Barrier, Thermal Stability, Tensile Strength, and Antimicrobial Properties of Rice Starch–Gelatin Composite Films through the Incorporation of Zinc Oxide Nanoparticles. Polymers 2022, 14, 2505. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, V.M.; Borges, S.V.; Marconcini, J.M.; Yoshida, M.I.; Neto, A.R.S.; Pereira, T.C.; Pereira, C.F.G. Effect of replacement of corn starch by whey protein isolate in biodegradable film blends obtained by extrusion. Carbohydr. Polym. 2017, 157, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, E.; Hasani, M.; Salehi, N.; Sabbagh, F.; Hasani, S. Enhancement of Biopolymer Film Properties Using Spermidine, Zinc Oxide, and Graphene Oxide Nanoparticles: A Study of Physical, Thermal, and Mechanical Characteristics. Materials 2025, 18, 225. [Google Scholar] [CrossRef]
- da Silva Bruni, A.R.; de Souza Alves Friedrichsen, J.; de Jesus, G.A.M.; da Silva Alves, E.; da Costa, J.C.M.; Souza, P.R.; de Oliveira Santos Junior, O.; Bonafe, E.G. Characterization and application of active films based on commercial polysaccharides incorporating ZnONPs. Int. J. Biol. Macromol. 2023, 224, 1322–1336. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Carrageenan-based hydrogels and films: Effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties. Food Hydrocoll. 2017, 67, 45–53. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).