1. Introduction
Austenitic stainless steel shows excellent corrosion resistance and is widely employed in several areas, including industrial and medical applications. However, its low mechanical resistance to wear and fatigue prevents its use in certain applications [
1,
2,
3]. Plasma nitriding is a technique to tackle these limitations by improving the surface mechanical properties. In addition, when the treatment is carried out at low temperatures, it keeps or even enhances the corrosion resistance [
4,
5,
6,
7].
Several studies have been conducted to better understand the phenomena involved in the stainless-steel low-temperature plasma nitriding process and the development of innovative and improved treatments [
8,
9,
10]. These treatments include the introduction of nitrogen in solid solution, originating a nitrogen supersaturated metastable phase, which is called expanded austenite or “S” phase [
2,
11]. This microstructure is capable of accommodating up to 38%at nitrogen in solid solution, leading to hardness values as high as 1500 HV [
12].
Understanding the phenomena that occurred during the treatment is highly relevant due to the problems that might result from improper treatment parameters, such as surface brittleness and sensitization. Reports of cracks in the nitride layer resulting from high residual tensions imposed by the high nitrogen content [
13,
14] show the formation of sliding bands, cracks, and even layer delamination [
15]. Furthermore, such weakening might impair the treatment of surfaces originating from power metallurgy, in which porosity is the tension relief point and results in cracks [
16].
Another undesirable effect for some applications is the ferromagnetic behavior that the expanded austenite layer might present, which limits applications in biomedical materials. This behavior change might be associated with the nitrogen amount in the layer, which motivates the interest in controlling nitrogen concentration in expanded austenite [
7,
9,
17,
18].
Previous investigations [
19,
20] have introduced pulsed nitrogen-flow nitriding to mitigate the problem of nitrogen oversaturation in nitride layers. It was demonstrated that alternating periods of nitrogen introduction with nitrogen-free intervals within the reactor chamber affords superior control over the resulting nitride layer’s growth, composition, and thickness.
dos Reis et al. [
19] reported that applying pulsed nitrogen atmospheres during nitriding at 450 °C, despite failing to suppress undesirable nitride precipitation entirely, enabled precise control over critical characteristics of the treated layer, including its thickness and hardness. These effects were attributed to variations in nitrogen uptake arising from differing ratios of nitrogen-on to nitrogen-off intervals.
Vianna et al. [
20] conducted a comparable study at 425 °C. Their hardness measurements and microstructural analyses of the nitrided layers revealed that pulsed nitrogen flow enabled the formation of the expanded austenite phase with reduced residual stresses within the layer, and diminished the quantity of precipitates. Nitrogen concentration in the layer was estimated using calculations based on the lattice expansion. Despite the pulsed regimen, cracking of the nitrided layers was observed under the applied treatment conditions. A comparison of the results reported by dos Reis et al. [
19] and Vianna et al. [
20] indicates that reducing the nitriding temperature from 450 °C to 425 °C led to differing amounts of chromium-based precipitates within the treated layers.
Since both nitride precipitation and crack formation are undesirable, and although pulsed nitrogen-flow nitriding has been shown to mitigate—but not eliminate—these deleterious effects, the present study investigates low-temperature plasma nitriding with intermittent nitrogen flow at 400 °C. Under these conditions, nitride layers with reduced nitrogen content can be produced, which are expected to exhibit diminished brittleness and lower susceptibility to sensitization while preserving their magnetic properties.
2. Materials and Methods
The material investigated in this study was the ISO 5832-1 [
21] steel, supplied by Villares Metals S.A. (São Paulo, Brazil), whose composition is shown in
Table 1. Cylindrical samples were used, measuring 15.8 mm in diameter and 7 mm in thickness. The samples were sanded up to a 1200 mesh granulometry and polished in 1 µm alumina in suspension. Before nitriding, the samples were cleaned using ultrasound and immersed in alcohol.
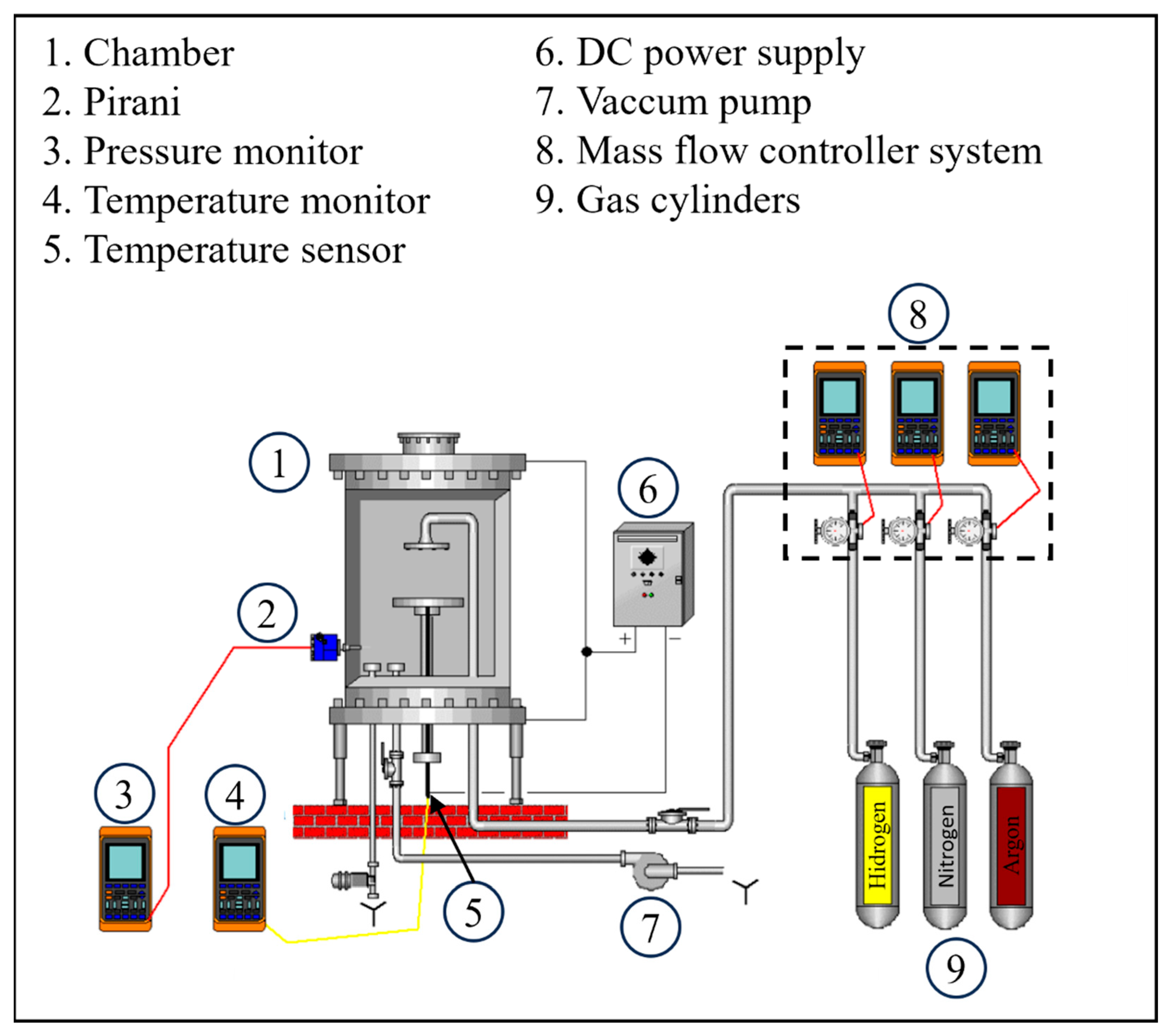
The nitriding treatment was carried out in a plasma reactor fed by a pulsed source, whose scheme is shown below (
Figure 1). The treatment thermal cycle had two phases, the first corresponded to sample cleaning by sputtering, while the second phase consisted of nitriding. The sputtering cleaning aimed to remove the native oxide layer, characteristic of stainless steels, and was conducted using hydrogen (50 sccm) and argon (100 sccm) plasma for 30 min, at 2 Torr pressure and 300 °C.
The nitriding process was carried out in 4 conditions, as shown in
Table 2, keeping the following fixed parameters: 400 °C temperature; 2 h nitriding plateau time; 200 sccm gas total flow, 3 Torr pressure, biased at 500 V. The variation observed between the experimental conditions was promoted by nitrogen pulses. For comparison purposes, one of the nitriding processes occurred under continuous gas flow (2C condition). The offer of nitrogen active species in the discharge was monitored via optical emission spectroscopy, using a Jobin-Yvon iHR550 (Horiba, Kyoto, Japan) spectrometer. Monitoring the nitrogen feed evolution enabled the appropriate selection of gas-pulsing parameters, considering the specific geometric and operational characteristics of the plasma treatment system used in this work. Based on these measurements, on-time durations were defined to ensure a steady-state flux of nitriding species. Similarly, off-time durations were chosen to guarantee the effective purge of residual nitrogen from the reactor.
After nitriding, the samples were cooled to 100 °C in the presence of hydrogen plasma and then cooled to room temperature (25 °C) under vacuum.
The metallographic preparation started with a longitudinal section mounted in high-hardness bakelite.
After cutting, the samples were prepared via a metallographic process until polishing with 1 µm diamond paste. The nitrided layer morphology was analyzed using scanning electron microscopy (SEM: Zeiss, model EVO MA 15, Carl Zeiss Ltd., Cambridge, UK). Nitrogen concentration was determined by employing the WDS technique (Wavelength Dispersive Spectroscopy) coupled to SEM.
The nitrided layer thickness was determined by Optical Microscopy (OM: Olympus BX51RF, Olympus Corporation Shinjuku Monolith, Tokyo, Japan) (software AnalySIS 5.1 to analyze the images). Before the microscopy analysis, the samples were metallographically etched with Marble reagent (1 g CuSO4, 5 mL HCl, 5 mL H2O).
The nano-hardness technique, with the quasi continuous stiffness measurement method, was used in the layer mechanical characterization. Measurements were carried out using the equipment Zwick-Roell ZNH nanoindenter (GmbH & Co. KG, Ulm, Germany). The loads applied ranged between 0.76 mN and 400 mN, with a Berkovich-type diamond indenter. Mean hardness results were obtained from measuring fourteen points in each sample.
In the X-ray diffraction tests to detect phases, the equipment Shimadzu, model XRD-6100 (Shimadzu Corporation, Tokyo, Japan) was employed, with copper radiation (λ = 1.5418 Ȧ), 20 mA current, and 40 kV voltage. The scanning of samples was carried out in the 2θ band between 30° and 100°, in the Bragg–Brentano setting, at 1°/min speed, and the results obtained had the intensity normalized for comparison purposes. The diffractogram analyses were conducted using the integrated software (version 7.00) and the ICDD PDF-2 database, released in 2012.
3. Results and Discussion
The results of the optical emissions spectroscopy analyses to evaluate the offer of nitriding species during the N
2 gas pulse are shown below (
Figure 2). We also observed N
2+ nitrogen molecular ion evolutions, referring to the first negative system (N
2+: B
2Σ
u+ → X
2Σ
g+) to 391.44 nm, and the nitrogen second positive system (N
2: C
3Π
u → B
3Π
g) to 357.69 nm.
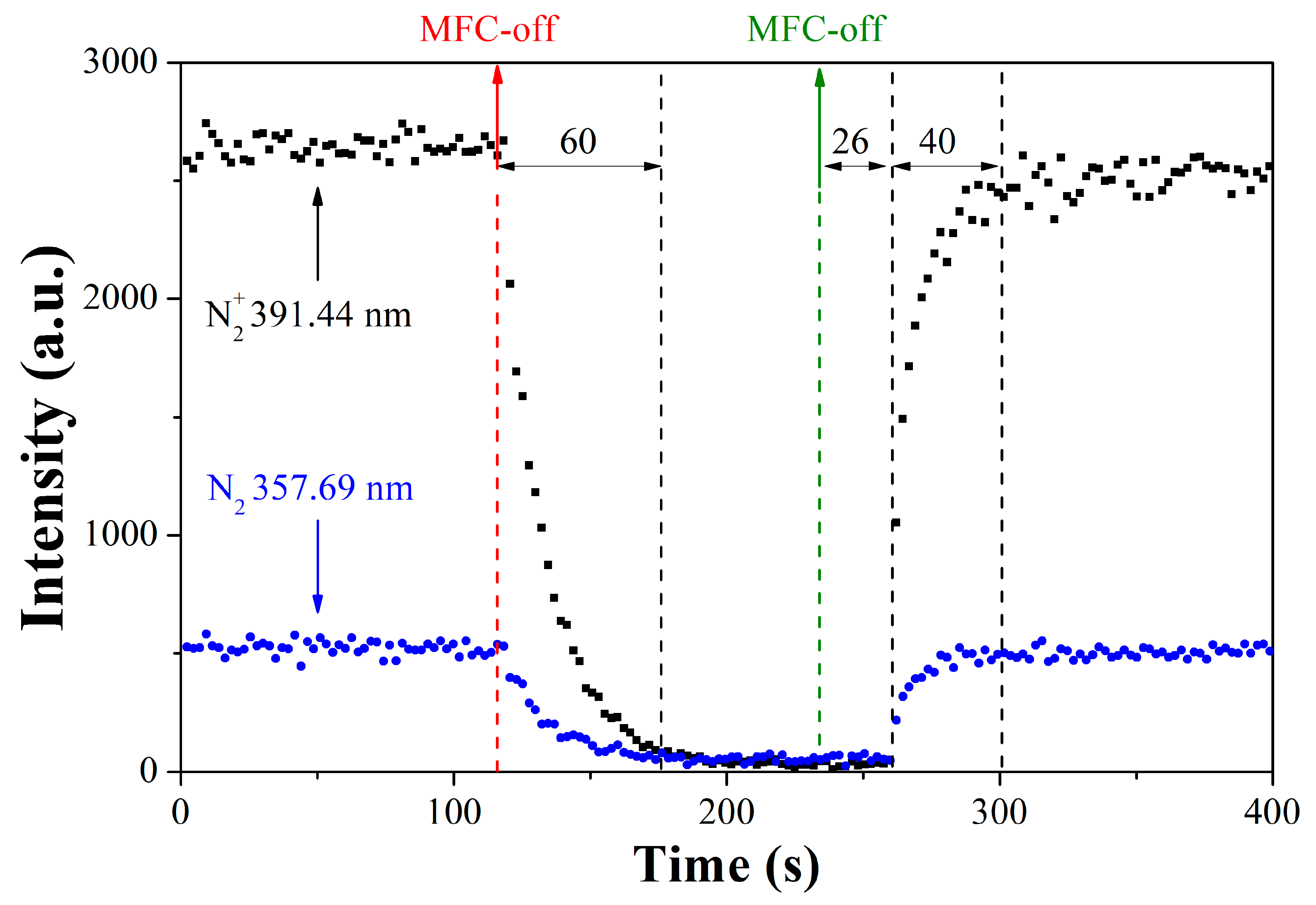
When the mass flow controller (MCF) was off (MFC off, red line in
Figure 2), the nitrogen emissions started to fall sharply. However, considerable nitrogen residence time is observed after the closing command due to the chamber volume, work pressure, and vacuum pump capacity. After 37 s, N
2+ emission decreased to 10% of the level observed before the interruption, but background values were only reached after 60 s.
The response to the flow return command was slower. Due to the characteristics of flowmeters and gas lines, emissions only leave the background level around 26 s after the mass flow control opening command (MFC on, green line in
Figure 2). Then, the nitrogen emissions are detected again, and the time to return to the levels observed before the flow interruption was around 40 s.
The result observed indicates that the periods in which the nitrogen flow was off, which ranged between 10 and 18 min, are effective in studying the nitrogen offer interruption effect during nitriding.
Figure 3 presents the micrographs of the samples nitrided using continuous and intermittent nitrogen gas flow. Layer thickness in each sample is remarkably uniform. All conditions studied presented double-layer formation, with an upper layer rich in nitrogen (γ
n) and a lower layer rich in carbon (γ
c) [
19,
20]. A distinct boundary apparent between the γ
n and γ
c layers can be observed. Under all conditions investigated, the nitrided layers are dense, exhibiting no evidence of porosity, cracking, or precipitate formation that could compromise material integrity. Compared with other low-temperature plasma nitriding studies on austenitic stainless steels [
6,
22], including those employing pulsed nitrogen flow [
19,
20], these findings demonstrate that, under the present treatment conditions, a continuous nitrided layer can be achieved across the entire surface.
Unlike sample 2P50, whose layer thickness was similar to the nitrided sample under nitrogen continuous flow, samples 2P15 and 2P10, nitrided with shorter nitrogen offer time, showed significant thickness reduction. This result could be expected since no nitrogen was supplied for the majority of the treatment duration of these two samples (85% and 90% of the cycle, respectively). During the nitrogen-off intervals, the plasma atmosphere consisted of argon and hydrogen. The reactive species generated within the plasma volume can even remove nitrogen atoms from the nitrided surface, which can be an important consideration for future pulsed nitrogen-flow plasma nitriding investigations.
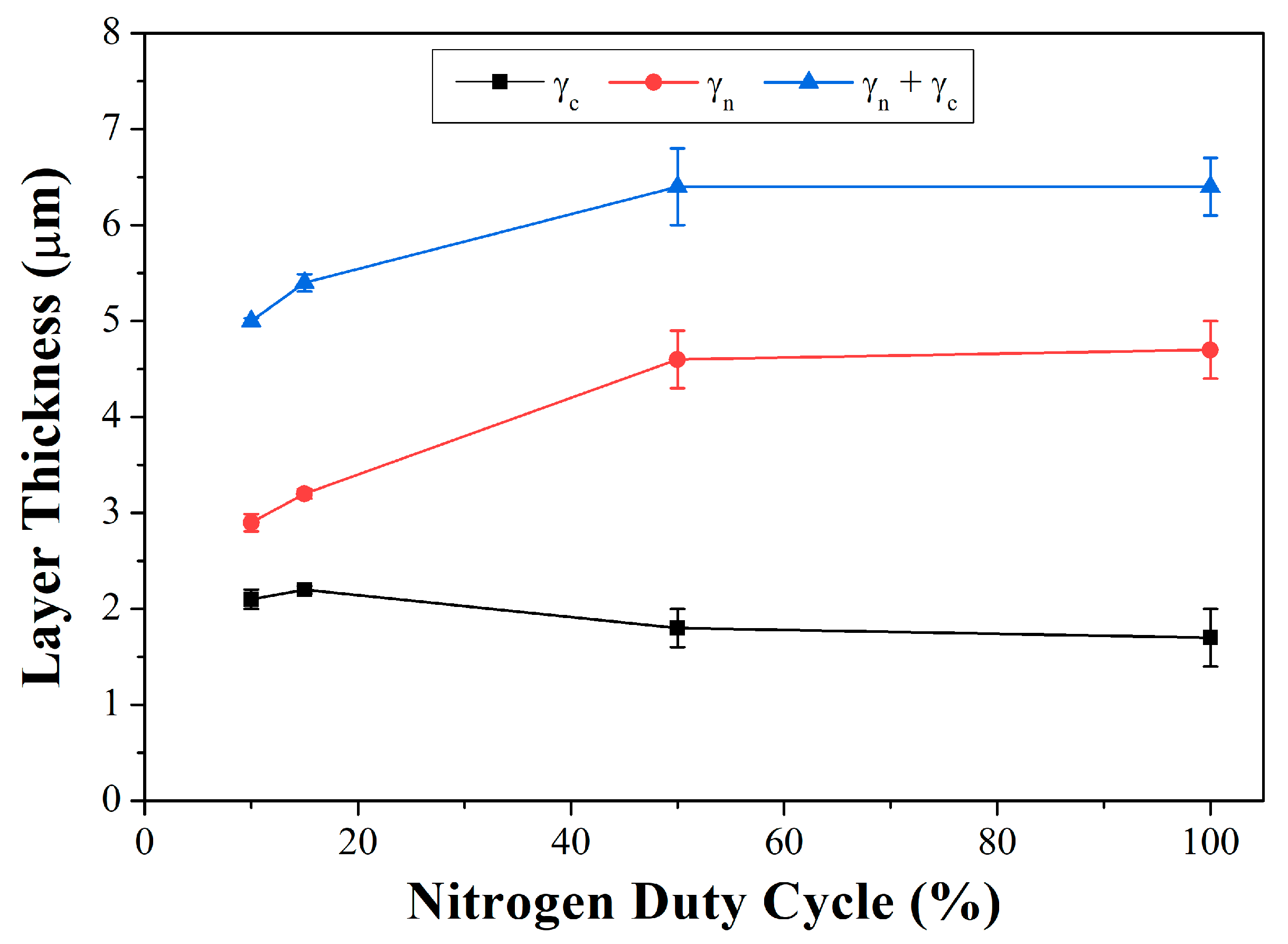
Thickness values for the inner (γ
c), outer (γ
n), and total (γ
c + γ
n) layers are represented as a function of the duty cycle in
Figure 4. In sample 2P50, the presence of nitrogen in the gaseous mixture occurred for a period corresponding to half the time in relation to that of sample 2C. Even so, layer thickness measurements for this sample were statistically identical to those obtained for the continuously nitrogen-flow-treated specimen (2C). It can be inferred that during the 10 min nitrogen-on interval, the surface incorporated a substantial amount of nitrogen. Accordingly, even during the interval when the nitrogen was not supplied to the atmosphere and then, to the surface, there was a considerable amount of nitrogen in the layer, and the element diffusion into the metallic matrix continued to occur.
In line with expectations, compared with other pulsed nitrogen-flow plasma nitriding studies conducted at higher temperatures [
19,
20], the layers obtained in the present work are thinner, reflecting the intrinsically diffusion-controlled nature of the process. A table comparing the layer-thickness values for pulsed nitrogen-flow plasma nitriding treatments is provided in Vianna et al. [
20].
Christiansen et al. (2008) [
23] demonstrated that the nitrogen diffusivity in expanded austenite depends on nitrogen concentration in the material’s crystalline structure, which might vary by a factor of eight times. The highest diffusion rate occurs in a concentration of around 30%at. However, expanded austenite can present a nitrogen content of approximately 38%at. In this band, diffusivity is reduced due to the lower mobility of nitrogen atoms, caused by the lack of free interstices in the crystalline lattice [
17].
Considering that, we propose that in treatments carried out with intermittent nitrogen flow, the expanded austenite layer reaches high nitrogen concentrations fast, approaching saturation. This effect might lead to a reduction in the diffusion rate. However, when the nitrogen flow ceases, the diffusion continues inside the material, thus reducing the surface concentration to values that favor greater diffusivity. As explained below, this hypothesis is supported by the austenite expansion observed in the X-ray diffractograms (
Figure 5) and hardness measurements (
Figure 6).
When analyzing treatments with shorter duty cycles, with 2 and 3 min (2P10 and 2P15) nitrogen pulses, a thinner layer was formed, while the sample 2P15 layer was slightly thicker. However, the γ
c layer showed different behavior, with noticeable growth in treatments with shorter duty cycles. This behavior was also reported by [
19,
20].
According to Czerwiec and collaborators [
24], in addition to the carbon found in the material, the sputtering carried out with argon and hydrogen dissociates the carbon bonds found on the reactor walls, enabling them to reach the sample surface and diffuse inside it, thus favoring the γ
c layer formation in the first nitrogen off periods. When introducing nitrogen in the gaseous mixture, preferential bonds occur between the nitrogen and carbon found in the treatment atmosphere, forming CN species that are eliminated by the vacuum pump, and the γ
c layer formation is less noticeable in the presence of nitrogen. Also, the nitrogen inserted in the material surface “pushes” the carbon found in the material to its interior, since nitrogen shows stronger bonds with Fe and Cr, causing carbon accumulation at the end of the γ
n layer [
25]. Therefore, the γ
c layer growth is smaller in the nitrogen continuous flow treatment than in pulsed treatments.
When observing nitriding conditions with intermittent nitrogen flow, the γ
c layer growth might occur longer, since the nitrogen supply time at the beginning of the nitriding is around 2 or 3 min, for the 2P10 or 2P15 conditions, respectively. Also, 5 to 10 min are needed for the expanded austenite detection [
11], and this time is longer than that of the nitrogen supply in pulses in those conditions. Therefore, some nitrogen supply cycles are needed until a nitrogen-saturated layer is formed to prevent the passage of carbon originating from the treatment atmosphere. This hypothesis is reinforced when observing the behavior in the 2P50 condition, where the γ
c layer thickness was similar to that found in the 2C condition.
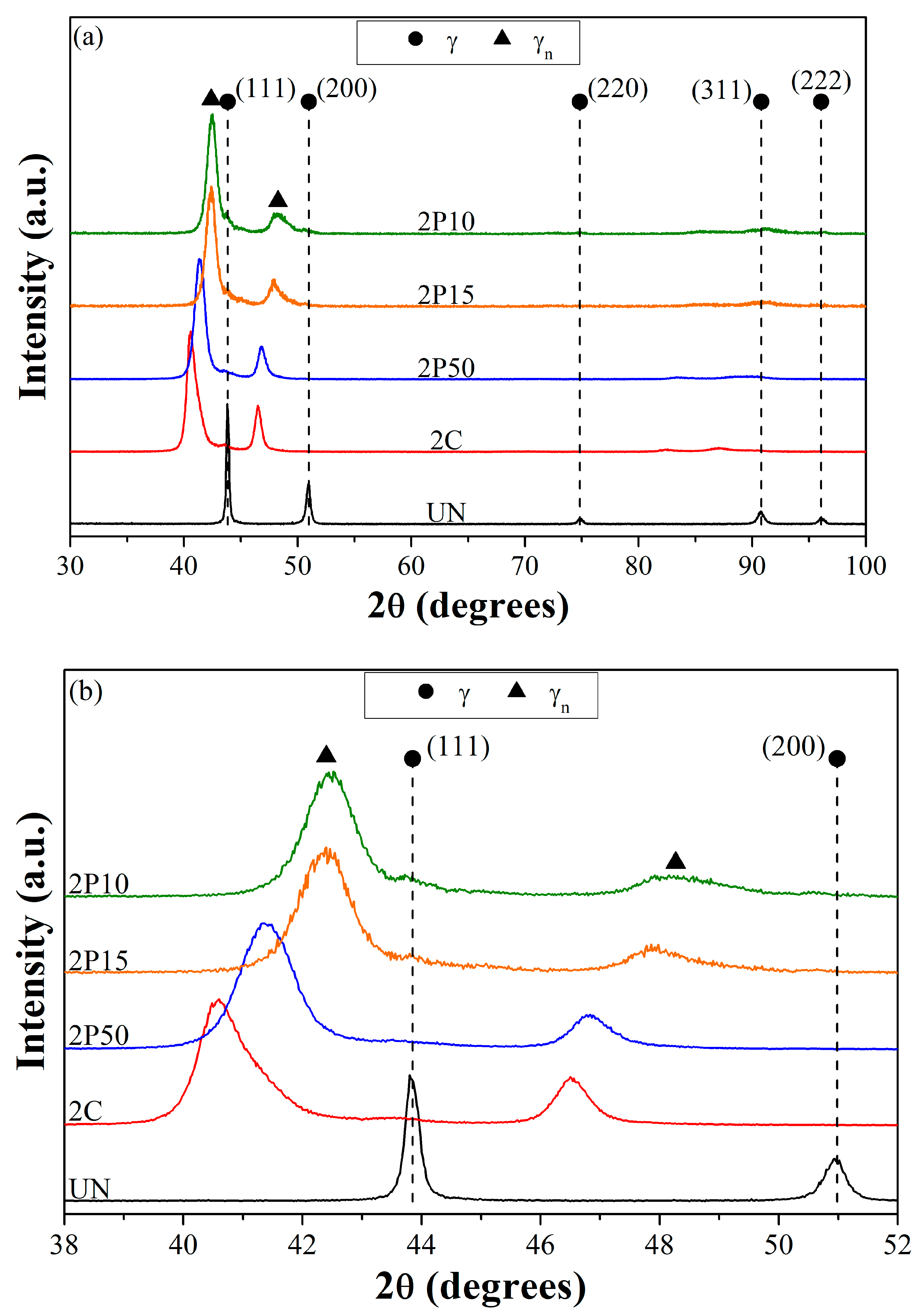
In
Figure 5, the X-ray diffraction results are presented as follows: graph (a) covers the 2θ band from 30° to 100°, while graph (b) shows, in detail, the 2θ band cut from 39° to 52°, emphasizing the evolution of austenite peaks of greater intensity according to the treatment parameters. For that incidence angle scanning band, the X-ray penetration depth ranged from 2.2 µm (2θ = 35°) to 5.7 µm (2θ = 100°), according to the results obtained from the Equation proposed by [
26], considering the diffracted intensity fraction of 95%.
The formation of expanded austenite was observed under all nitriding conditions, as evidenced by the shift in diffraction peaks toward lower angles compared to the untreated material (UN). This shift is attributed to an increase in the lattice parameter of the original austenite phase, resulting from nitrogen incorporation into the crystal structure [
14]. In addition, intensity reduction or disappearance of peaks in higher diffraction angles was also observed (
Figure 5a). Peak enlargement might be ascribed to the non-uniform deformation of the crystal lattice, resulting from the nitrogen gradient concentration. This variation occurs between grains due to the different crystallographic orientation, but it also changes with depth relative to the surface [
27]. The peak disappearance might be related to changes in grain orientation, which occurred during nitriding [
12].
Low-intensity, broad peaks that can be attributed to the substrate austenite (γ) (111) and (311) planes are detected. The (111) peak is shifted slightly toward lower 2θ angles, possibly signaling lattice expansion, which is consistent with carbon-expanded austenite (γc). An alternative interpretation is the presence of austenite under residual stress. Given the X-ray penetration depths, a stressed austenite phase would produce a weaker but detectable signal. Both interpretations align with the applied nitriding treatment, since nitrogen uptake induces significant lattice distortion and concomitant residual stresses that alter characteristic diffraction peaks.
No diffraction peaks corresponding to chromium or iron nitrides were detected in the analyzed samples. Given the selected treatment temperature of 400 °C and the short duration of 2 h, the formation of nitride-free layers is feasible, as previously reported by Landek et al. [
14].
Table 3 shows interplanar distances (d), lattice parameters (a), and the reticulate expansion percentages (
Δγ
N/γ %) for planes (111) and (200).
The 2C condition showed the highest reticulate expansion values compared to the nitrogen intermittent flow treatment, for both crystalline planes, with 7.65% for (111) and 8.90% for (200). These values agree with those reported by Czerwiec et al. (2019) [
28], who observed approximately 6.30% expansion for the (111) plane and 9.62% for the (200) plane.
When analyzing the nitrogen pulsed flow nitrided conditions, we observed that the reticulate expansion reduced with the duty cycle reduction, which might be attributed to the lower nitrogen concentration in the crystalline lattice.
Comparing the crystalline reticulate expansion results obtained for the planes (111) and (200) for all nitrided samples, both in continuous and pulsed flow, greater reticulate expansion was observed for plane (200), which confirms reports by other researchers, showing this crystallographic plane has greater diffusivity [
20,
29].
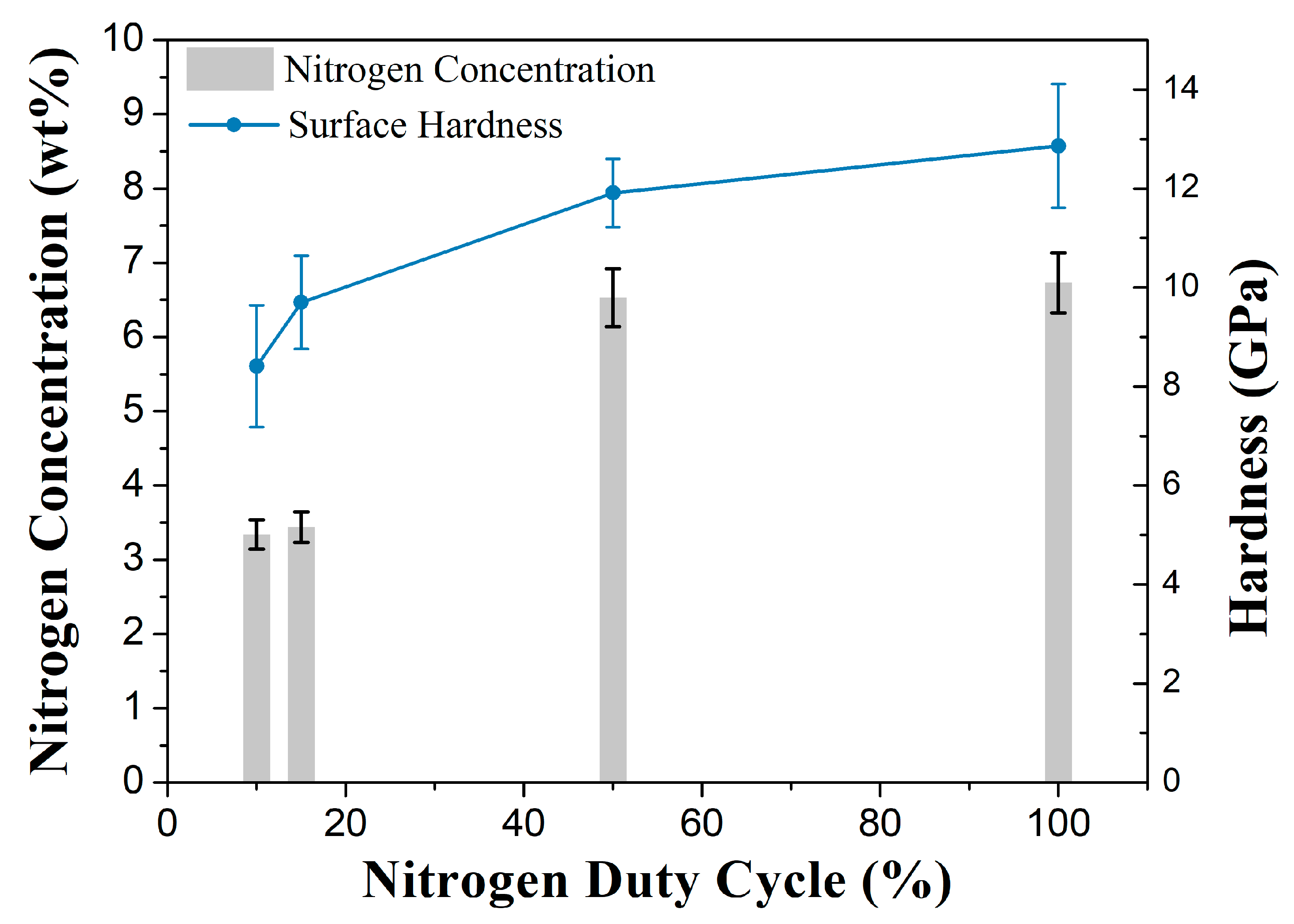
Figure 6 shows the nitrogen concentration results, obtained through WDS (wavelength dispersive spectroscopy) and hardness obtained using the nanoindentation technique. Each sample’s surface hardness was considered the highest value found in the profiles.
The highest nitrogen concentration, 6.73 wt%, was observed in the nitrogen continuous flow nitrided sample. Such value is in agreement with values found in the literature [
25,
28]. Although the 2P50 condition remained exposed to a nitrogen-rich atmosphere for only half of the total treatment time, it presented a very similar value: 6.53 wt%, which is statistically equivalent.
However, when the duty cycle was reduced, a progressive reduction was observed in the nitrogen concentration, thus confirming the XRD results previously discussed. This effect is related to the lower nitrogen availability in the plasma atmosphere when the nitrogen flow is interrupted. In those periods, in addition to the nitrogen migration to inner regions of the material, part of the nitrogen was released to the treatment atmosphere. It occurs because the samples remain exposed to an Ar-H
2 plasma atmosphere during the nitrogen off periods. Therefore, due to the argon–nitrogen plasma reactivity and the nitrogen concentration difference between the sample surface and the discharge atmosphere, nitrogen is lost by the surface. For the samples processed with nitrogen flow duty cycles of 10% and 15%, the nitrogen concentration was approximately 3.4%, with no statistically significant difference between them. This observation can be attributed to the brief nitrogen exposure time relative to the substantially more extended Ar-H
2; plasma treatment period, leading to convergent outcomes. Under such conditions, the nitrogen concentration may drop to levels at which its diffusivity within expanded austenite is markedly reduced [
23], thereby retarding diffusion kinetics and resulting in both decreased nitrogen incorporation and a concomitant thinning of the nitrided layer.
The highest surface hardness obtained was 12.6 GPa for the sample in the nitrogen continuous flow condition. The sample treated with a 50% duty cycle gas pulse showed a similar hardness value (11.9 GPa). As observed for the nitrogen concentration results, the hardness of these samples does not exhibit a statistically significant difference. The proximity of hardness values of these two samples, resulting from a slightly lower nitrogen concentration, draws attention to the effectiveness of controlling the nitrogen amount inserted into the expanded austenite by the gas pulse action. Such results also suggest that the surface enrichment with nitrogen must be fast, reaching a concentration limit in the expanded austenite. The nitriding phenomenon is then limited by the diffusion in the solid state, as indicated by [
23], and in the periods when the nitrogen input into the reactor is interrupted, the distribution of the nitrogen already inserted into the expanded austenite inside the sample should occur. Even with the considerable pulse off time, 10 min, hardness did not show a sharp reduction, thus indicating that the nitrogen amount already in the material was enough to keep layer growth.
The sample in the 2P10 condition showed the lowest hardness among the samples treated (8.4 GPa), which resulted from the 3.34 wt% nitrogen concentration. It was a much higher value than that of the untreated material. The nitrogen content reached in samples under nitrogen pulsed flow showed that it is possible to control the properties of the treated layer in a wide band of values.
According to [
8], the hardness value of 1479 HV
,0036 (around 14 Gpa) for samples treated for 4 h exposed to a 20% N
2 + 80% H
2 atmosphere, at 400 °C and a 3 Torr pressure. This hardness value, slightly higher than the one reported in this study, also results from the nitride precipitation.
Therefore, the interest in controlling the nitrogen added to the surface goes beyond the control of the layer’s hardness and frailty. Controlling the nitrogen concentration might reduce nitride precipitation and thus prevent sensitization.
Short gas pulses, around 10% to 15% of the period, resulted in smaller nitrogen concentrations. Therefore, it seems reasonable to state that the nitrogen pulse enables the control of the level of tension in the surface layer. Thus, inconveniences such as the formation of cracks observed in the nitriding of stainless steels might be controlled by nitriding under nitrogen pulsed flow. In addition, ferromagnetism problems resulting from excess nitrogen can be tackled by means of nitrogen pulsed flow treatments.