Synergistic Disinfection of Photocatalytic Nanomaterials Exposed to UVC, Electricity and Magnetic Fields Against Candida albicans
Abstract
1. Introduction
2. Materials and Methods
2.1. Cuprite and Silver Nanoparticles Synthesis and Analysis
2.2. SnO2 Films Synthesis and Characterization
2.3. Ferrite Nanoparticle Synthesis and Characterization
2.4. WO3 Thin Films Growth by Sputtering
2.5. Viability Assays of C.albicans Exposed to Physical Treatments
2.5.1. SnO2 Antimicrobial Assays on Dry Surface
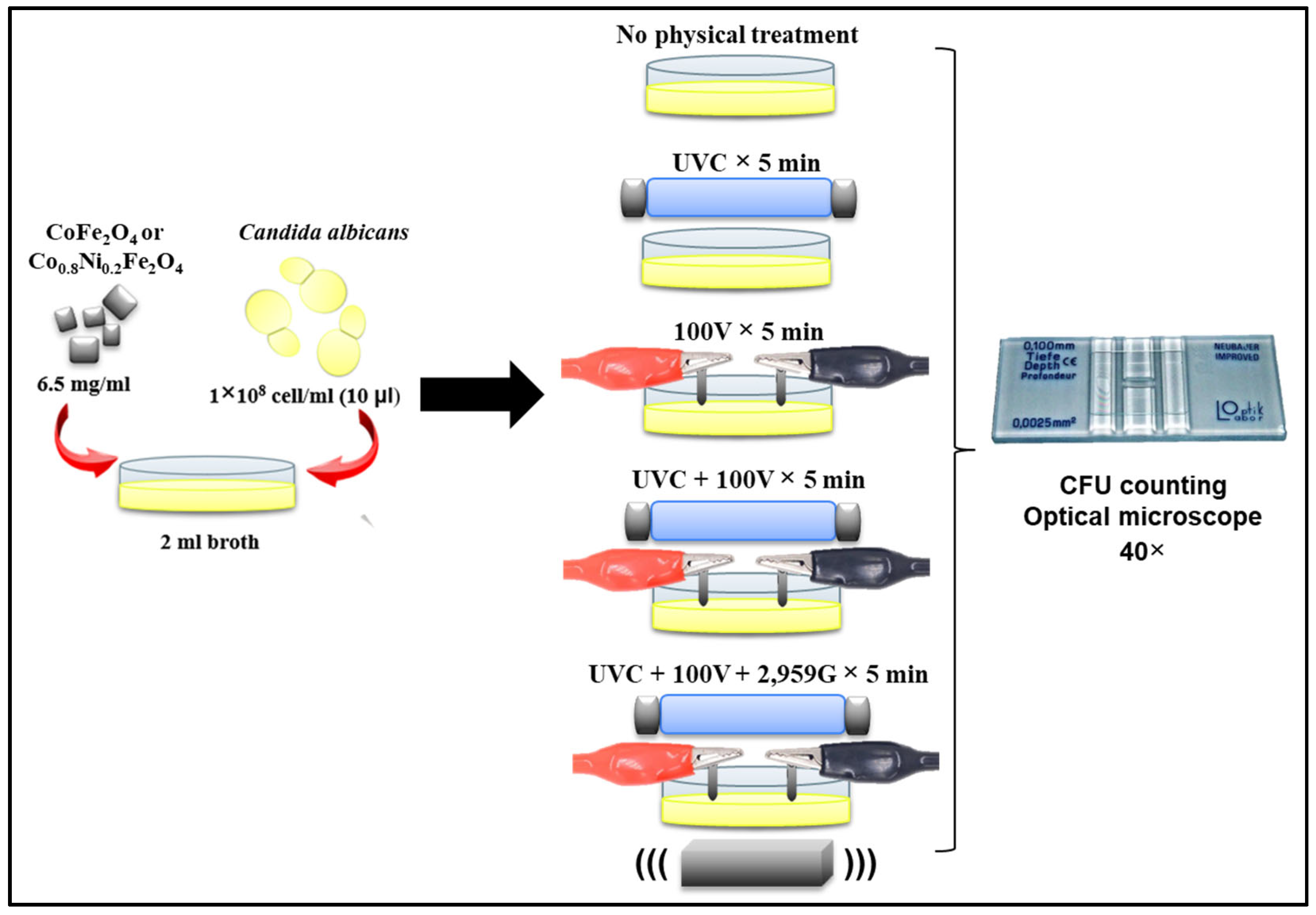
2.5.2. Ferrite Nanoparticles Antimicrobial Assays in Aqueous Media
- Treatment 1: Nanoparticles only.
- Treatment 2: Nanoparticles + 5 min of UVC radiation.
- Treatment 3: Nanoparticles + 5 min exposure to 100 V electric current.
- Treatment 4: Nanoparticles + 5 min of UVC + 100 V electric current.
- Treatment 5: Nanoparticles + 5 min of UVC, 100 V electric current, and a magnetic field.
- Positive control (untreated): Broth inoculated with C. albicans but without nanoparticles or physical treatments, representing 100% viability.
- Physical treatment controls: Each physical treatment was also applied in the absence of nanoparticles to evaluate the specific contribution of the ferrite materials.
2.5.3. WO3 Antimicrobial Assays in Dry Surfaces
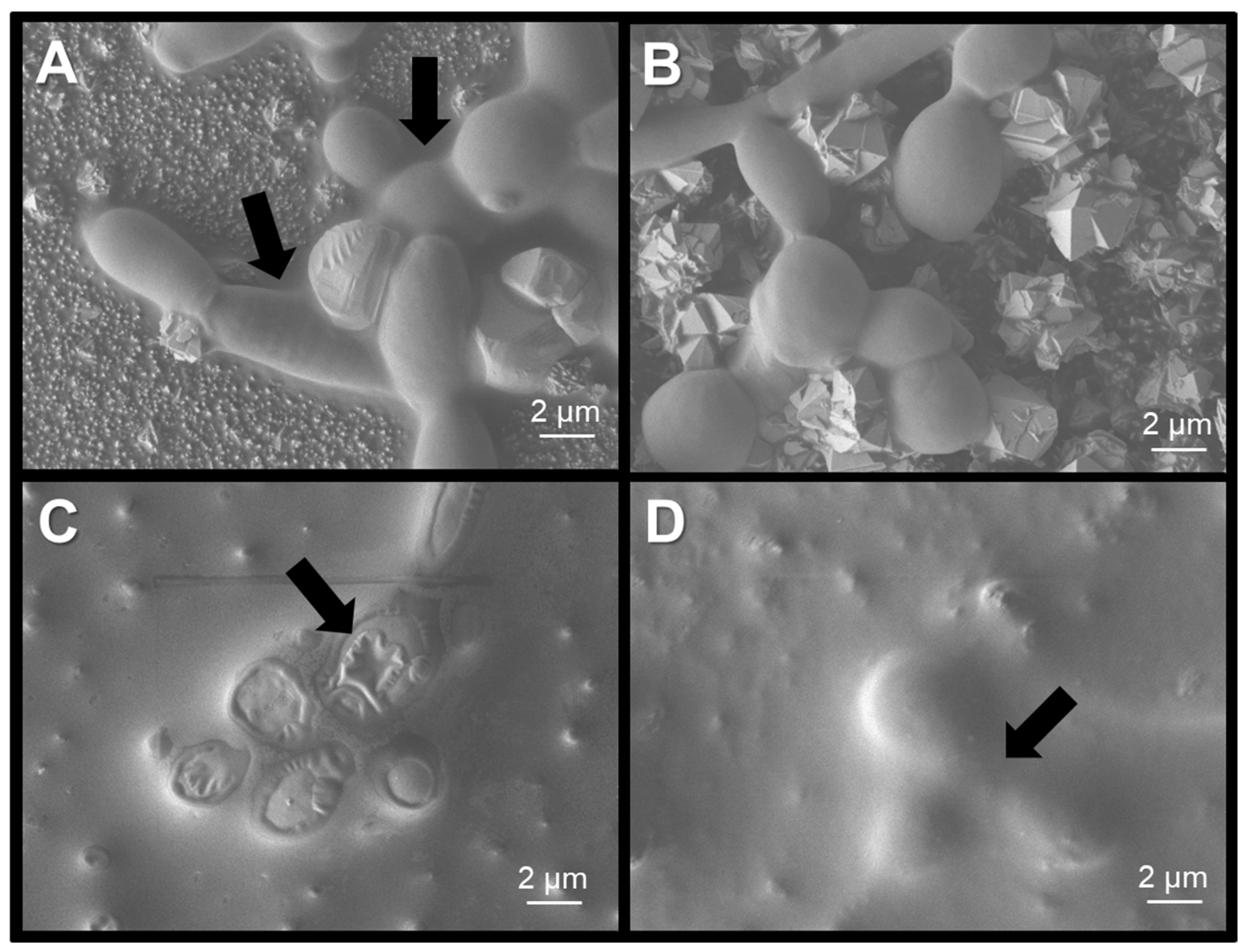
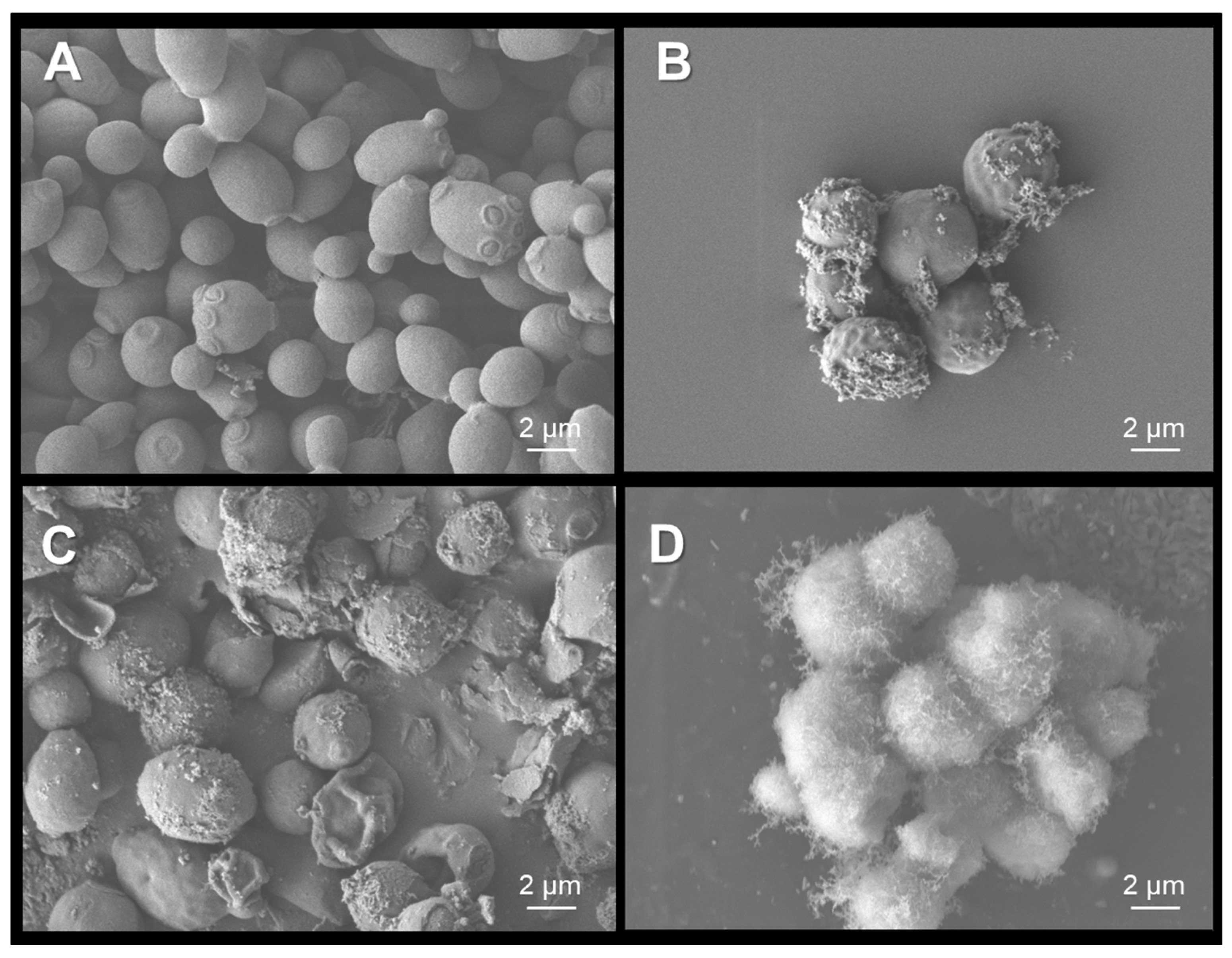
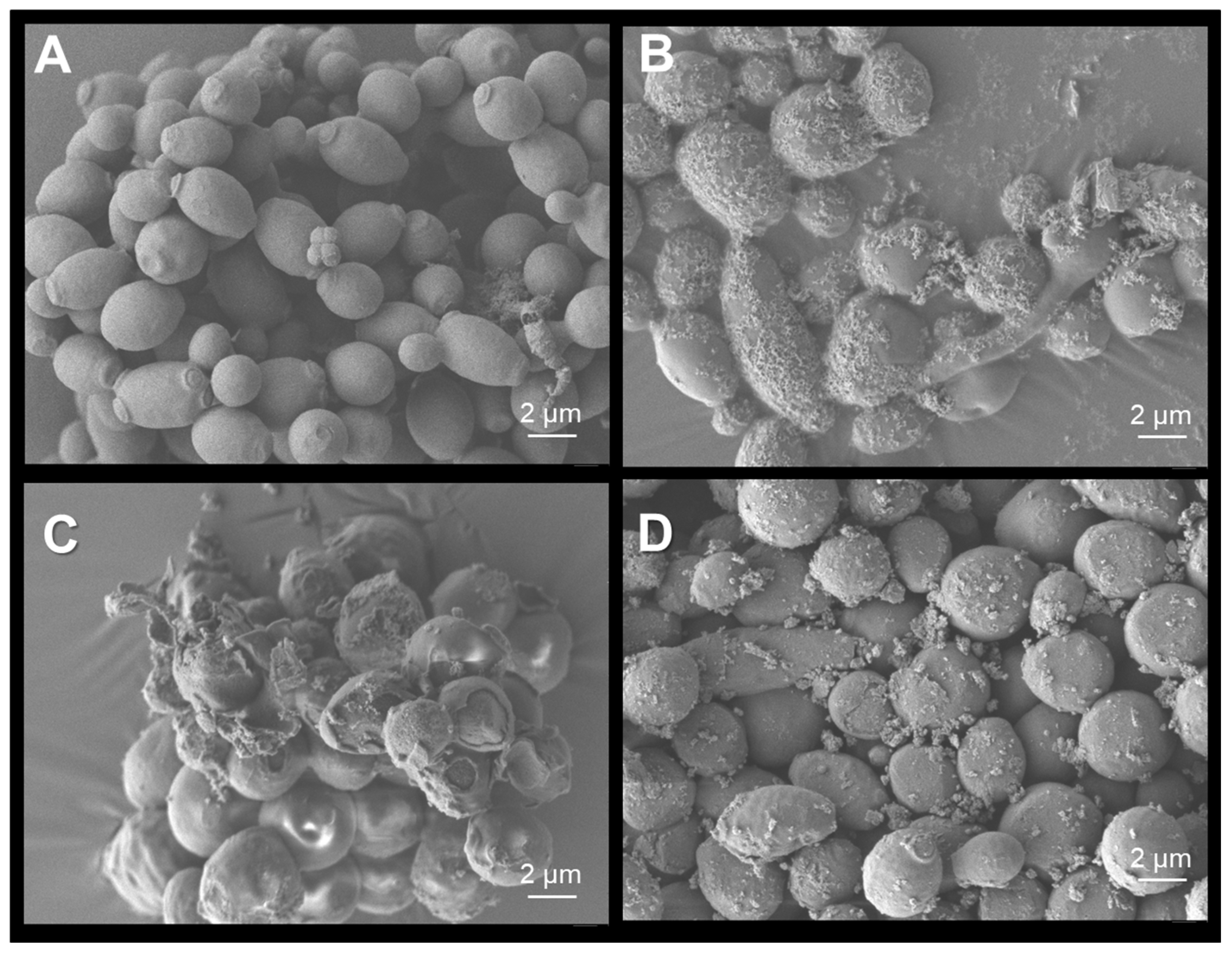
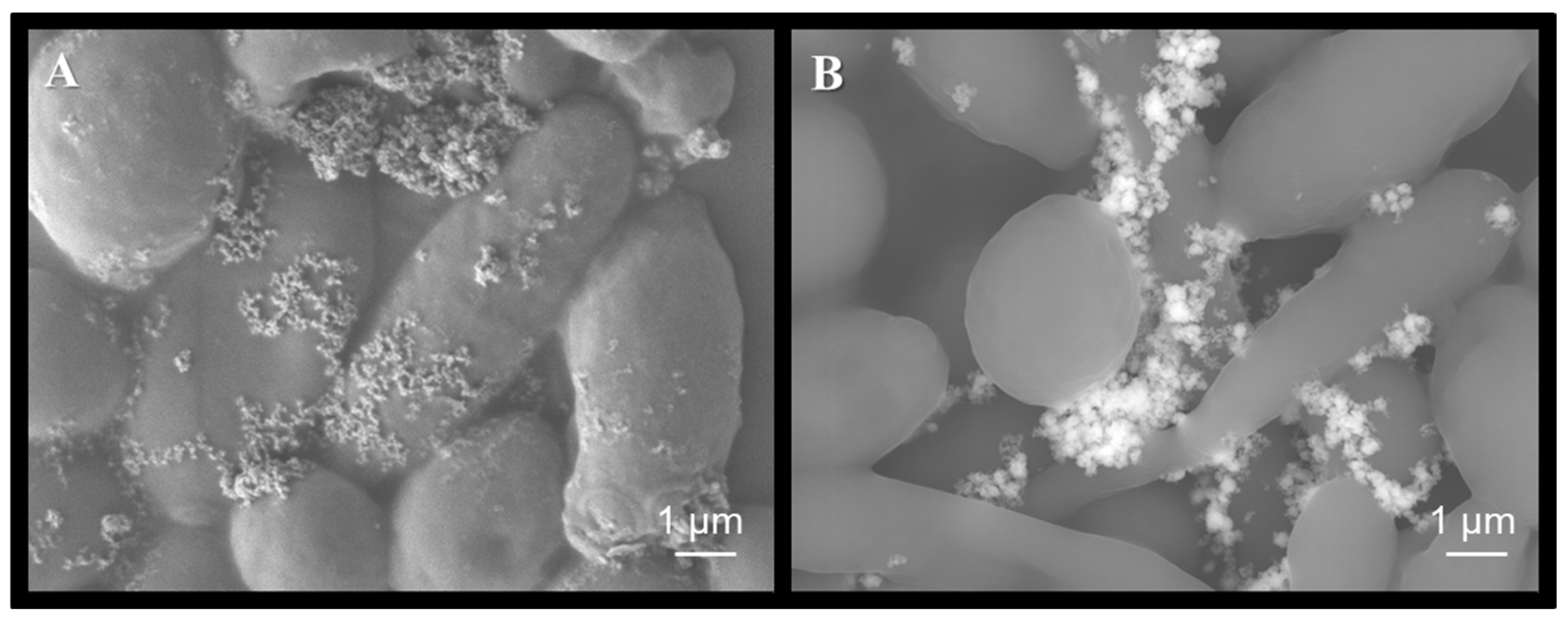
2.6. Scanning Electron Microscopy of C.albicans Exposed to Treatments
3. Results
3.1. Cuprite and Silver Nanoparticle Characterization
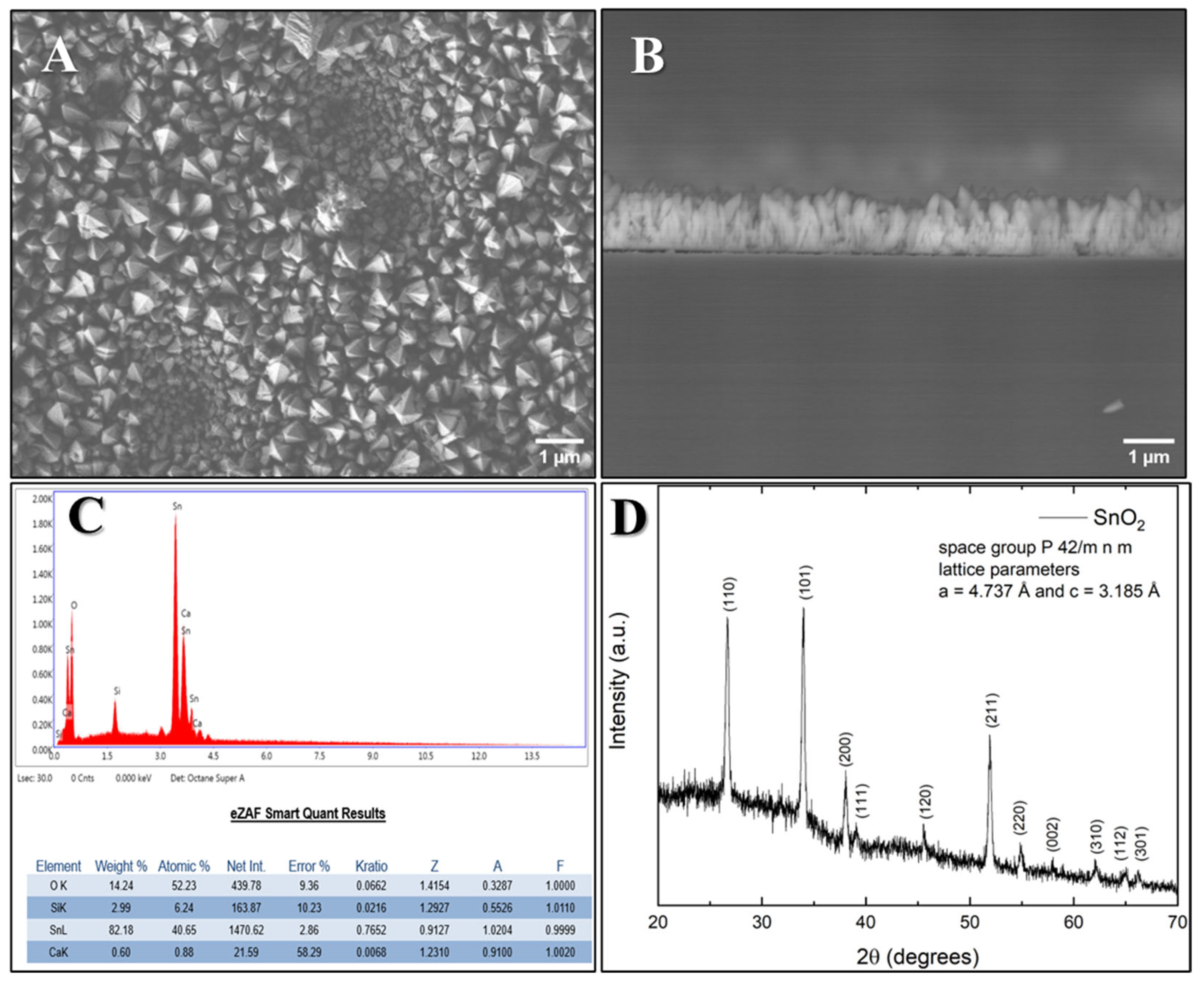
3.2. Characterization of SnO2 Films
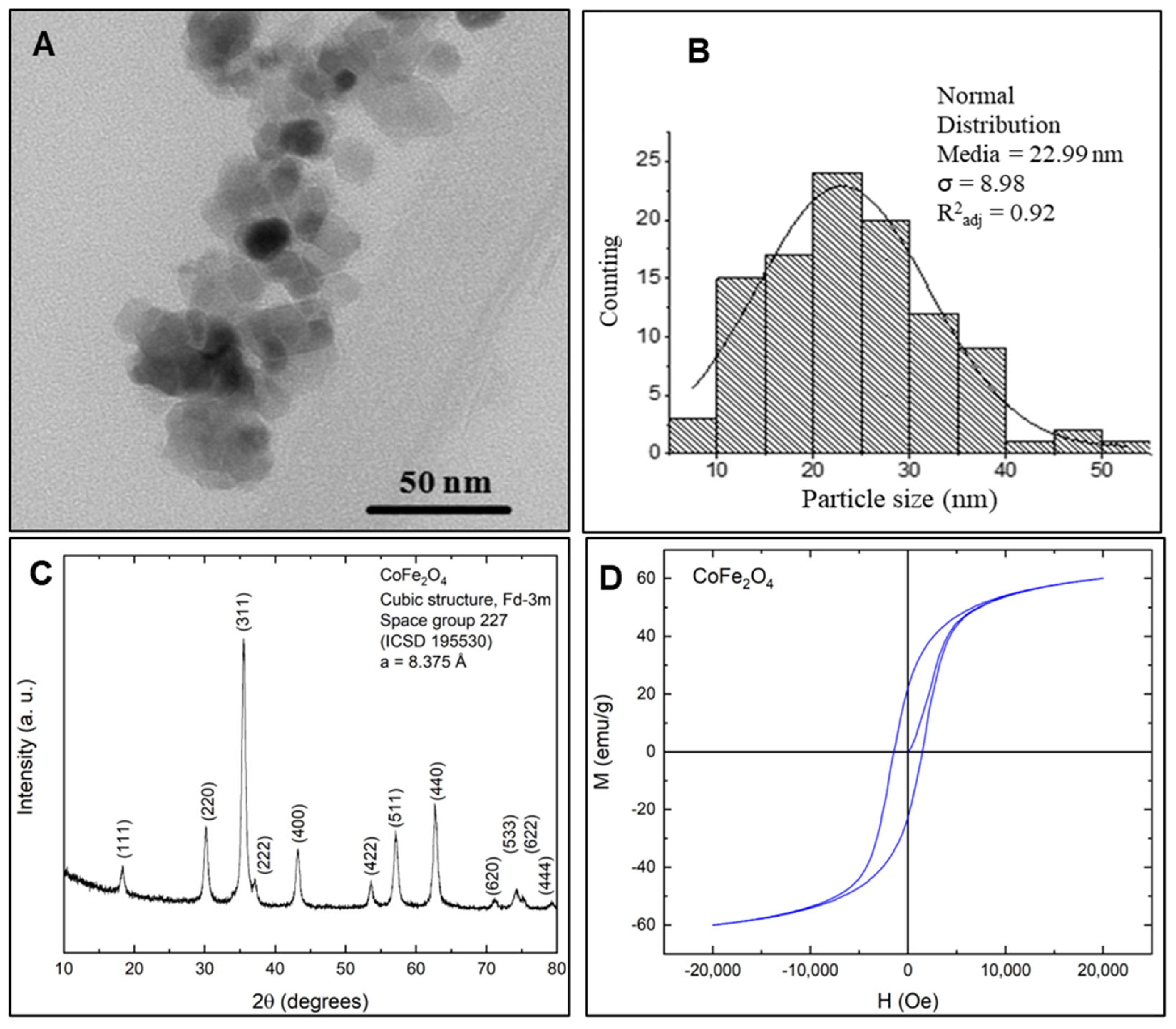
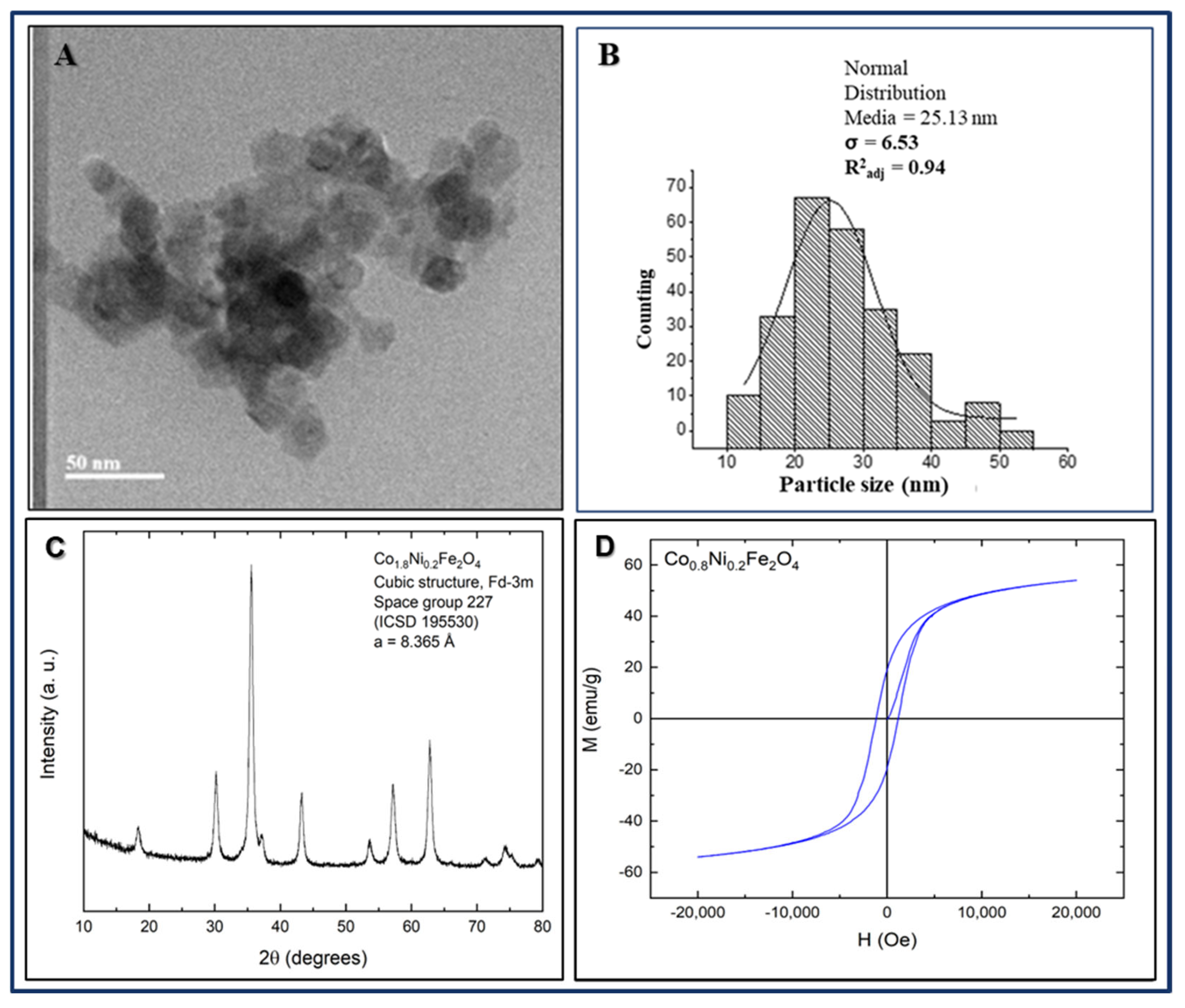
3.3. Characterization of Ferrite Nanoparticles
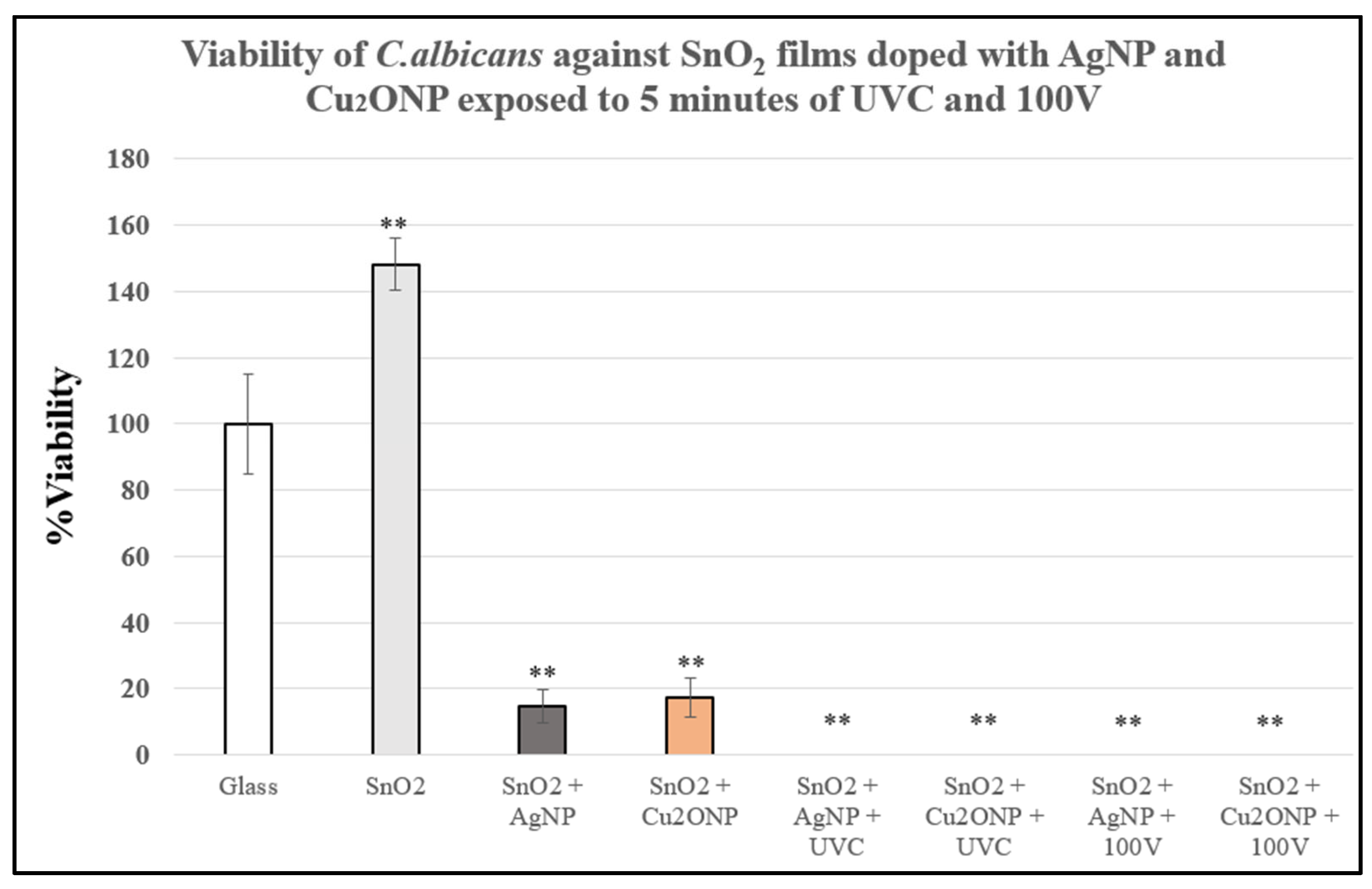
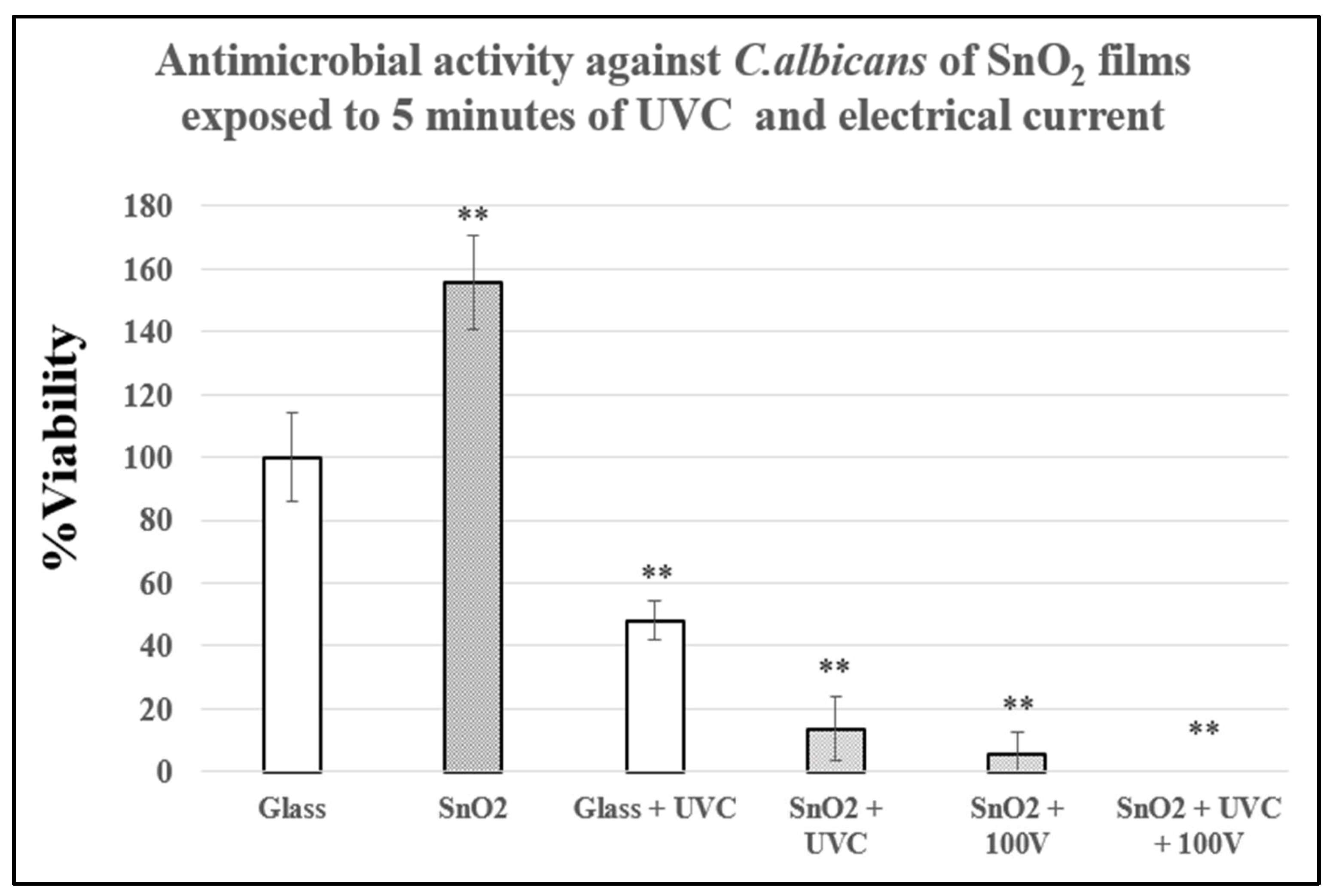
3.4. Antimicrobial Activity of SnO2 Films Against C.albicans Exposed to Physical Treatments and Metallic Nanoparticles on Dry Surfaces
3.5. Antimicrobial Assays of Ferrite Nanoparticles Exposed to Physical Treatments
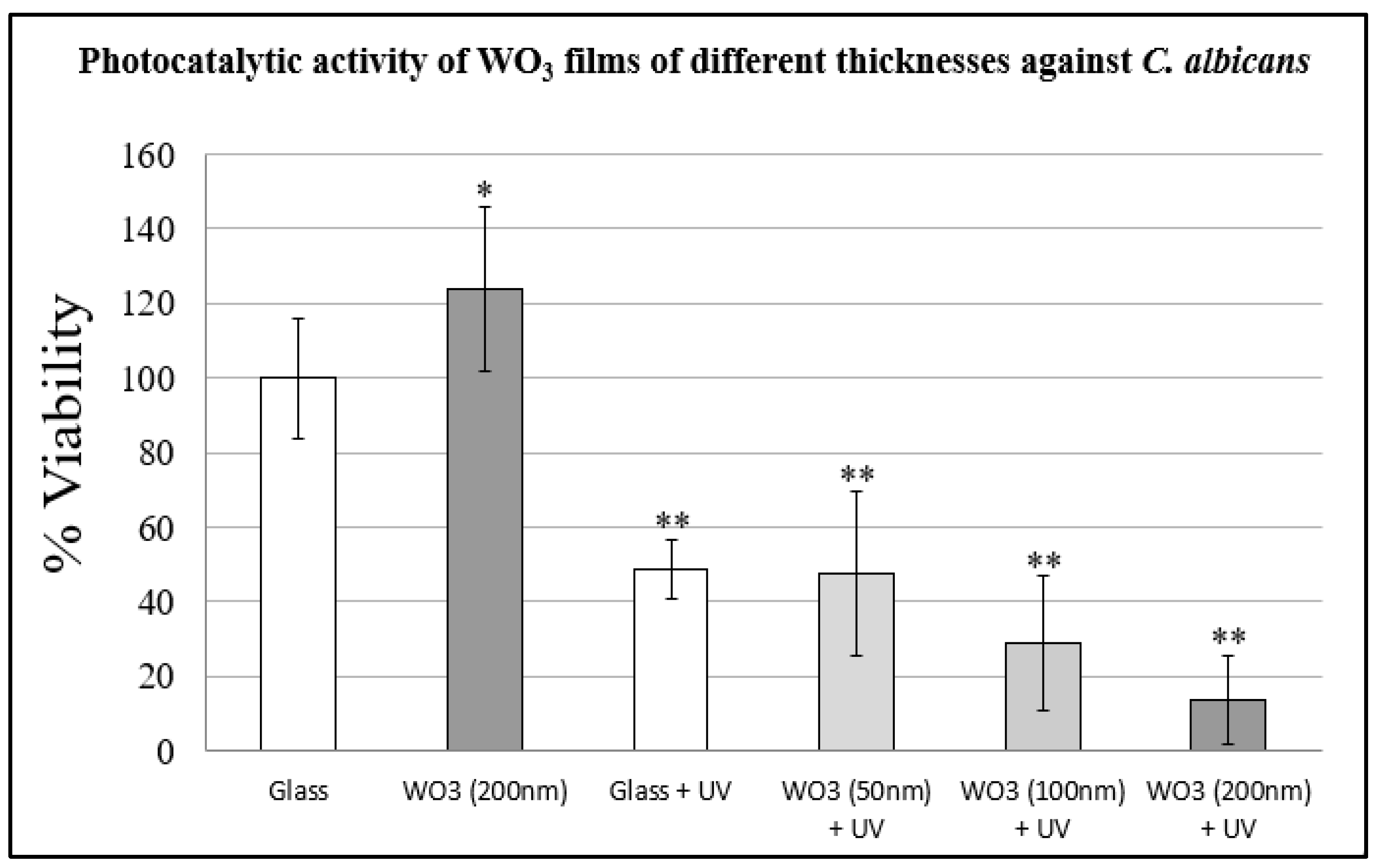
3.6. Photocatalytic Activity of WO3 Films on Dry Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warris, A.; Pana, Z.-D.; Oletto, A.; Lundin, R.; Castagnola, E.; Lehrnbecher, T.; Groll, A.H.; Roilides, E. Etiology and Outcome of Candidemia in Neonates and Children in Europe: An 11-year Multinational Retrospective Study. Pediatr. Infect. Dis. J. 2020, 39, 114–120. [Google Scholar] [CrossRef]
- Özenen, G.G.; Bal, Z.S.; Avcu, G.; Yazici, P.O.; Karakoyun, M.; Metin, D.Y.; Polat, S.H. Evaluation of candidemia in children at a university hospital: A retrospective cohort. Mycoses 2023, 66, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Román-Aguirre, M.; Barraza-Jimenez, D.; Leyva-Porras, C.; Peinado-Villalobos, R.; Molina-Jáquez, D.; Olivas-Espino, J.A.; Castillo-González, A.R.; Camarillo-Cisneros, J.; Favila-Pérez, M.A.; Quiñonez-Flores, C.M.; et al. Mechanisms of Integration and Release of AgNO3 in Chitosan Films and Their Interaction with Nosocomial Pathogens. Coatings 2024, 14, 1453. [Google Scholar] [CrossRef]
- Jeck, J.; Jakobs, F.; Kurte, M.S.; Cornely, O.A.; Kron, F. Health-economic modelling of cost savings due to the use of rezafungin based on a German cost-of-illness study of candidiasis. JAC Antimicrob. Resist. 2023, 5, dlad079. [Google Scholar] [CrossRef] [PubMed]
- Faudoa-Arzate, A.; Camarillo-Cisneros, J.; Castillo-González, A.R.; Favila-Pérez, M.A.; Sáenz-Hernández, R.J.; Realyvazquez-Guevara, P.R.; Arzate-Quintana, C. Disinfection mechanism of the photocatalytic activity of SnO2 thin films against Candida albicans, proposed from experimental and simulated perspectives. Can. J. Microbiol. 2021, 67, 667–676. [Google Scholar] [CrossRef]
- Micelly-Moreno, J.; Barreto-Santamaría, A.; Arévalo-Pinzón, G.; Firacative, C.; Gómez, B.L.; Escandón, P.; Patarroyo, M.A.; Muñoz, J.E. Therapeutic Use of the Antimicrobial Peptide PNR20 to Resolve Disseminated Candidiasis in a Murine Model. J. Fungi 2023, 9, 1149. [Google Scholar] [CrossRef]
- Jothi, R.; Hong, S.T.; Enkhtsatsral, M.; Pandian, S.K.; Gowrishankar, S. ROS mediated anticandidal efficacy of 3-Bromopyruvate prevents vulvovaginal candidiasis in mice model. PLoS ONE 2023, 18, e0295922. [Google Scholar] [CrossRef]
- Widodo, T.T.; Siswomiharjo, W.; Sunarintyas, S.; Yulianto, D.K. Effect of method and concentration of titanium dioxide addition on anti-biofilm ability in extraoral maxillofacial prosthetic fungus. Int. J. Adv. Med. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Binns, R.; Li, W.; Wu, C.D.; Campbell, S.; Knoernschild, K.; Yang, B. Effect of Ultraviolet Radiation on Candida albicans Biofilm on Poly(methylmethacrylate) Resin. J. Prosthodont. 2020, 29, 686–692. [Google Scholar] [CrossRef]
- Ghosh, S.; Mukherjee, R.; Basak, D.; Haldar, J. One-Step Curable, Covalently Immobilized Coating for Clinically Relevant Surfaces That Can Kill Bacteria, Fungi, and Influenza Virus. ACS Appl. Mater. Interfaces 2020, 12, 27853–27865. [Google Scholar] [CrossRef]
- Dumpati, S.; Naroo, S.A.; Shah, S.; Dutta, D. Antimicrobial Efficacy of an Ultraviolet-C Device against Microorganisms Related to Contact Lens Adverse Events. Antibiotics 2022, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Politano, G.G. Optical Properties of Thick TiO2-P25 Films. Nanomaterials 2025, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Sukriti, C.P. Effect of pH Values on the Structural, Optical and Electrical Properties of SnO2 Nanostructures. Optik 2019, 181, 768–778. [Google Scholar] [CrossRef]
- Kim, S.; Chang, H.-K.; Kim, K.B.; Kim, H.-J.; Lee, H.-N.; Park, T.J.; Park, Y.M. Highly Porous SnO2/TiO2 Heterojunction Thin-Film Photocatalyst Using Gas-Flow Thermal Evaporation and Atomic Layer Deposition. Catalysts 2021, 11, 1144. [Google Scholar] [CrossRef]
- Kuang, Y.; Zardetto, V.; van Gils, R.; Karwal, S.; Koushik, D.; Verheijen, M.A.; Black, L.E.; Weijtens, C.; Veenstra, S.; Andriessen, R.; et al. Low-Temperature Plasma-Assisted Atomic-Layer-Deposited SnO2 as an Electron Transport Layer in Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 30367–30378. [Google Scholar] [CrossRef]
- Akyildiz, H.I.; Diler, S.; Islam, S. Evaluation of TiO2 and ZnO atomic layer deposition coated polyamide 66 fabrics for photocatalytic activity and antibacterial applications. J. Vac. Sci. Technol 2021, 39, 022405. [Google Scholar] [CrossRef]
- Mwathe, P.; Musembi, R.; Munji, M.; Odari, V.; Munguti, L.; Ntilakigwa, A.; Nguu, J.; Muthoka, B. Effect of Surface Passivation on Electrical Properties of Pd-F:SnO2 Thin Films Prepared by Spray Pyrolysis Technique. Coatings 2014, 4, 747–755. [Google Scholar] [CrossRef]
- Sáenz-Hernández, R.J.; Herrera-Pérez, G.M.; Uribe-Chavira, J.S.; Grijalva-Castillo, M.C.; Elizalde-Galindo, J.T.; Matutes-Aquino, J.A. Correlation between Thickness and Optical Properties in Nanocrystalline γ-Monoclinic WO3 Thin Films. Coatings 2022, 12, 1727. [Google Scholar] [CrossRef]
- Burgain, A.; Tebbji, F.; Khemiri, I.; Sellam, A. Metabolic Reprogramming in the Opportunistic Yeast Candida albicans in Response to Hypoxia. mSphere 2020, 5, e00913–e00919. [Google Scholar] [CrossRef]
- Dunker, C.; Polke, M.; Schulze-Richter, B.; Schubert, K.; Rudolphi, S.; Gressler, A.E.; Pawlik, T.; Prada-Salcedo, J.P.; Niemiec, M.J.; Slesiona-Künzel, S.; et al. Rapid proliferation due to better metabolic adaptation results in full virulence of a filament deficient Candida albicans strain. Nat. Commun. 2021, 12, 3899. [Google Scholar] [CrossRef]
- Liboro, K.; Yu, S.R.; Lim, J.; So, Y.S.; Bahn, Y.S.; Eoh, H.; Park, H. Transcriptomic and Metabolomic Analysis Revealed Roles of Yck2 in Carbon Metabolism and Morphogenesis of Candida albicans. Front. Cell. Infect. Microbiol. 2021, 11, 636834. [Google Scholar] [CrossRef] [PubMed]
- Gheni, M.R.; Odaa, N.H. The antimicrobial activity of melanin-mediated synthesis of silver nanoparticles. Egypt. J. Hosp. Med. 2023, 90, 3383–3394. [Google Scholar] [CrossRef]
- Nam, S.; Easson, M.W.; Jordan, J.H.; He, Z.; Zhang, H.; Cintrón, M.S.; Chang, S. Unveiling the hidden value of cotton gin waste: Natural synthesis and hosting of silver nanoparticles. ACS Omega 2023, 8, 31281–31292. [Google Scholar] [CrossRef] [PubMed]
- SadrHaghighi, A.; Sarvari, R.; Fakhri, E.; Poortahmasebi, V.; Sedighnia, N.; Torabi, M.; Mohammadzadeh, M.; Azhiri, A.H.; Eskandarinezhad, M.; Moharamzadeh, K.; et al. Copper-nanoparticle-coated melt-blown facemask filter with antibacterial and sars-cov-2 antiviral ability. ACS Appl. Nano Mater. 2023, 6, 12849–12861. [Google Scholar] [CrossRef]
- Raja, F.N.S.; Worthington, T.; Martin, R.A. The antimicrobial efficacy of copper, cobalt, zinc and silver nanoparticles: Alone and in combination. Biomed. Mater. 2023, 18, 045003. [Google Scholar] [CrossRef]
- Arzate-Quintana, C.; Sánchez-Ramírez, B.; Infante-Ramírez, R.; Piñón-Castillo, H.A.; Montes-Fonseca, S.L.; Duarte-Moller, A.; Luna-Velasco, A.; Solís-Martínez, F.J.; Orrantia-Borunda, E. Toxicity effects in pathogen microorganisms exposed to silver nanoparticles. Nanosci. Nanotechnol. Lett. 2017, 9, 165–173. [Google Scholar] [CrossRef]
- Rezić, I.; Škoc, M.S.; Majdak, M.; Jurić, S.; Stracenski, K.S.; Vinceković, M. Functionalization of polymer surface with antimicrobial microcapsules. Polymers 2022, 14, 1961. [Google Scholar] [CrossRef]
- Burdușel, A.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Santoro, A.M.; Monaco, I.; Attanasio, F.; Lanza, V.; Pappalardo, G.; Tomasello, M.F.; Cunsolo, A.; Rizzarelli, E.; De Luigi, A.; Salmona, M.; et al. Copper(II) ions affect the gating dynamics of the 20S proteasome: A molecular and in cell study. Sci Rep. 2016, 16, 33444. [Google Scholar] [CrossRef]
- Aihemaiti, X.; Wang, X.; Li, Y.; Wang, Y.; Xiao, L.; Ma, Y.; Qi, K.; Zhang, Y.; Liu, J.; Li, J. Enhanced photocatalytic and antibacterial activities of S-scheme SnO2/Red phosphorus photocatalyst under visible light. Chemosphere 2022, 296, 134013. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Palanisamy, G.; Pragasan-L, A.; Albeshr, M.F.; Alrefaei, A.F.; Lee, J.; Liu, X. Photocatalytic degradation of organic pollutants and inactivation of pathogens under visible light via SnO2/rGO composites. Chemosphere 2023, 335, 139102. [Google Scholar]
- Fang, M.; Tan, X.; Liu, Z.; Hu, B.; Wang, X. Recent Progress on Metal-Enhanced Photocatalysis: A Review on the Mechanism. Research 2021, 2021, 9794329. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Banach, M. Continuous synthesis of photocatalytic nanoparticles of pure ZnO and ZnO modified with metal nanoparticles. J. Nanostruct. Chem. 2021, 11, 601–617. [Google Scholar] [CrossRef]
- Arzate-Quintana, C.; Leyva-Porras, C.; Favila-Pérez, M.A.; Castillo-González, A.R.; Quiñonez-Flores, C.M.; Faudoa-Arzate, A. Biofilm integrity and cytomorphology of Candida albicans after exposure to UV-light on ZnO thin films: SEM Analysis. Microsc. Microanal. 2021, 27 (Suppl. 1), 1896–1898. [Google Scholar] [CrossRef]
- Gryzińska, M.; Kot, B.; Dudzińska, E.; Biernasiuk, A.; Jakubczak, A.; Malm, A.; Andraszek, K. Changes in the level of DNA methylation in Candida albicans under the influence of physical and chemical factors. Int. J. Mol. Sci. 2023, 24, 15873. [Google Scholar] [CrossRef]
- Huo, Z.Y.; Kim, Y.J.; Suh, I.Y.; Lee, D.M.; Lee, J.H.; Du, Y.; Wang, S.; Yoon, H.J.; Kim, S.W. Triboelectrification induced self-powered microbial disinfection using nanowire-enhanced localized electric field. Nat. Commun. 2021, 12, 3693. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, T.; Yu, C.; Xie, X. Locally enhanced electric field treatment (LEEFT) for water disinfection. Front. Environ. Sci. Eng. 2020, 14, 78. [Google Scholar] [CrossRef]
- Freebairn, D.; Linton, D.; Harkin-Jones, E.; Jones, D.S.; Gilmore, B.F.; Gorman, S.P. Electrical methods of controlling bacterial adhesion and biofilm on device surfaces. Expert. Rev. Med. Devices 2013, 10, 85–103. [Google Scholar] [CrossRef]
- Miao, W.; He, W.; Fang, Z.; Guo, K.; Yang, Z. Internal electric field enhanced photocatalytic transfer hydrogenation in heterojunction: Modulations and applications. Mater. Today Energy 2023, 37, 101408. [Google Scholar] [CrossRef]
- Dai, B.; Guo, J.; Gao, C.; Yin, H.; Xie, Y.; Lin, Z. Recent Advances in Efficient Photocatalysis via Modulation of Electric and Magnetic Fields and Reactive Phase Control. Adv. Mater. 2023, 35, 2210914. [Google Scholar] [CrossRef]
- Ruelas-Casas, V.J.; Ramírez-Valdespino, C.A.; Quiñonez-Flores, C.M.; Castillo-González, A.R.; Ramos-Moctezuma, I.R.; González-Chávez, S.A.; Caballero-Hernández, D.E.; Arzate-Quintana, C. Morphology of Candida albicans Exposed to Electric Current Treatments and UV Radiation in Sabouraud Broth Analyzed with Scanning Electron Microscopy. Microsc. Microanal. 2024, 30, 822–823. [Google Scholar] [CrossRef]
- Ponja, S.D.; Williamson, B.A.D.; Sathasivam, S.; Scanlon, D.O.; Parkin, I.P.; Carmalt, C.J. Enhanced electrical properties of antimony doped tin oxide thin films deposited via aerosol assisted chemical vapour deposition. J. Mater. Chem. C 2018, 6, 7257–7266. [Google Scholar] [CrossRef]
- Melo-Solarte, D.S.; Betancur-Pérez, J.F.; Narváez-Solarte, W. Evaluación de la luz ultravioleta (UVA, UVB y UVC) como agente bactericida contra Escherichia coli (Migula) Castellani y Chalmers-ATCC®-25922. Boletín Científico Cent. Mus. Mus. Hist. Nat. 2023, 27, 117–126. [Google Scholar] [CrossRef]
- Gu, J.; Liu, S.; Ni, W.; Ren, W.; Haussener, S.; Hu, X. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 2022, 5, 268–276. [Google Scholar] [CrossRef]
- Li, H.; Lin, C.; Yang, Y.; Dong, C.; Min, Y.; Shi, X.; Wang, L.; Lu, S.; Zhang, K. Boosting Reactive Oxygen Species Generation Using Inter-Facet Edge Rich WO3 Arrays for Photoelectrochemical Conversion. Angew. Chem. 2022, 135, e202210804. [Google Scholar]
- Grigioni, I.; Dozzi, M.V.; Selli, E. Photoinduced electron transfer in WO3/BiVO4 heterojunction photoanodes: Effects of the WO3 layer thickness. J. Phys. Condens. Matter 2019, 32, 014001. [Google Scholar] [CrossRef]

| Without ferrites | CoFe2O4 | Co0.8Ni0.2Fe2O4 | |
|---|---|---|---|
| No physical treatment | 0% | 26.1% (*) | 30.4% (**) |
| 100V | 33.88% | 21.9% | 27.4% |
| UVC | 27.8% | 29.5% | 40.4% (**) |
| 2959G | 0% | 33.1% (**) | 35.8% (**) |
| 100V + 2959G | 33.8% | 32.5% | 26.7% |
| 100V + UVC | 38.1% | 29.6% | 28.9% |
| 100V + UVC + 2959G | 38.1% | 30.91% | 25.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grijalva-Castillo, M.C.; Saénz-Hernández, R.J.; Cobos-Márquez, A.A.; Herrera-Ojeda, F.A.; Díaz-Chávez, F.E.; Acosta-Galindo, I.R.; Leyva-Porras, C.; Castillo-González, A.R.; Favila-Pérez, M.A.; Quiñonez-Flores, C.M.; et al. Synergistic Disinfection of Photocatalytic Nanomaterials Exposed to UVC, Electricity and Magnetic Fields Against Candida albicans. Coatings 2025, 15, 968. https://doi.org/10.3390/coatings15080968
Grijalva-Castillo MC, Saénz-Hernández RJ, Cobos-Márquez AA, Herrera-Ojeda FA, Díaz-Chávez FE, Acosta-Galindo IR, Leyva-Porras C, Castillo-González AR, Favila-Pérez MA, Quiñonez-Flores CM, et al. Synergistic Disinfection of Photocatalytic Nanomaterials Exposed to UVC, Electricity and Magnetic Fields Against Candida albicans. Coatings. 2025; 15(8):968. https://doi.org/10.3390/coatings15080968
Chicago/Turabian StyleGrijalva-Castillo, María Cristina, Renee Joselin Saénz-Hernández, Adrián Alberto Cobos-Márquez, Francisco Alonso Herrera-Ojeda, Fernando Efraín Díaz-Chávez, Irving Ricardo Acosta-Galindo, César Leyva-Porras, Alva Rocío Castillo-González, María Alejandra Favila-Pérez, Celia María Quiñonez-Flores, and et al. 2025. "Synergistic Disinfection of Photocatalytic Nanomaterials Exposed to UVC, Electricity and Magnetic Fields Against Candida albicans" Coatings 15, no. 8: 968. https://doi.org/10.3390/coatings15080968
APA StyleGrijalva-Castillo, M. C., Saénz-Hernández, R. J., Cobos-Márquez, A. A., Herrera-Ojeda, F. A., Díaz-Chávez, F. E., Acosta-Galindo, I. R., Leyva-Porras, C., Castillo-González, A. R., Favila-Pérez, M. A., Quiñonez-Flores, C. M., Cisneros, J. C., & Arzate-Quintana, C. (2025). Synergistic Disinfection of Photocatalytic Nanomaterials Exposed to UVC, Electricity and Magnetic Fields Against Candida albicans. Coatings, 15(8), 968. https://doi.org/10.3390/coatings15080968








