Biocompatibility of Titanium Oxide Nanotubes Layer Formed on a Ti-6Al-4V Dental Implant Screw in hFOB Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Anodic Oxidation
2.2. Characterization
2.3. In Vitro Assay Conditions
2.3.1. Cell Cultures and Maintenance
2.3.2. Cell Proliferation and Viability Test by Flow Cytometry
3. Results
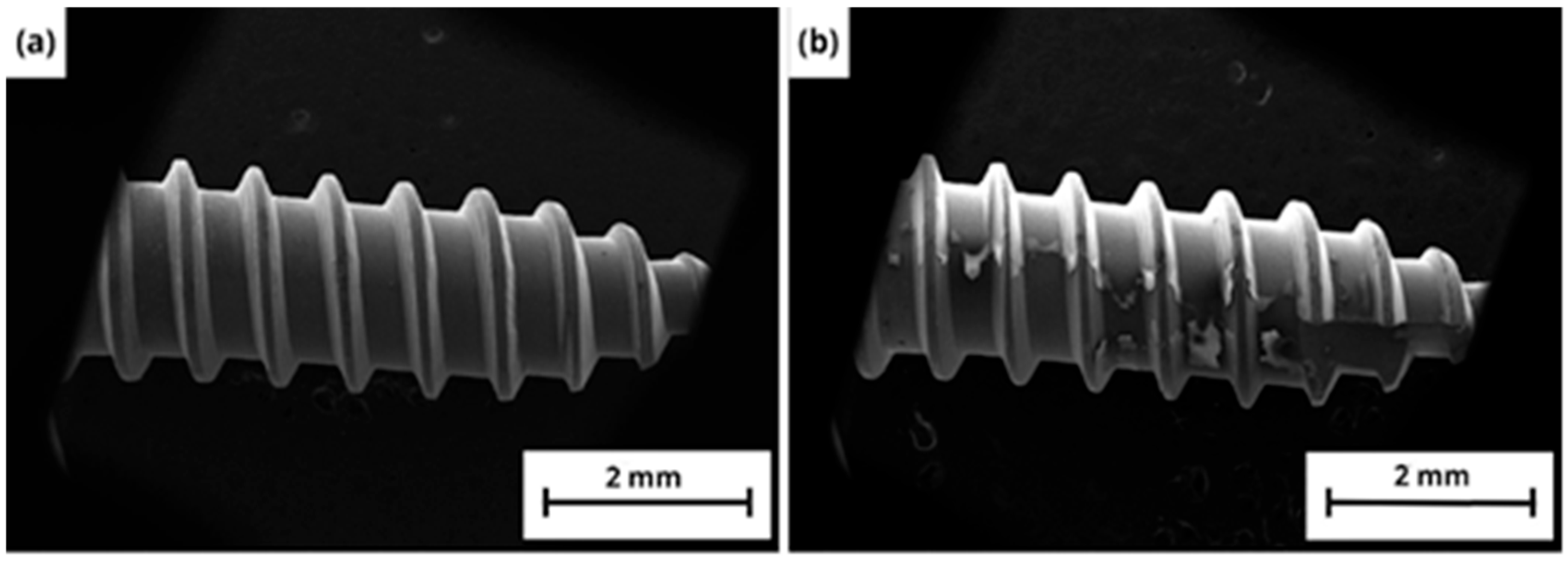
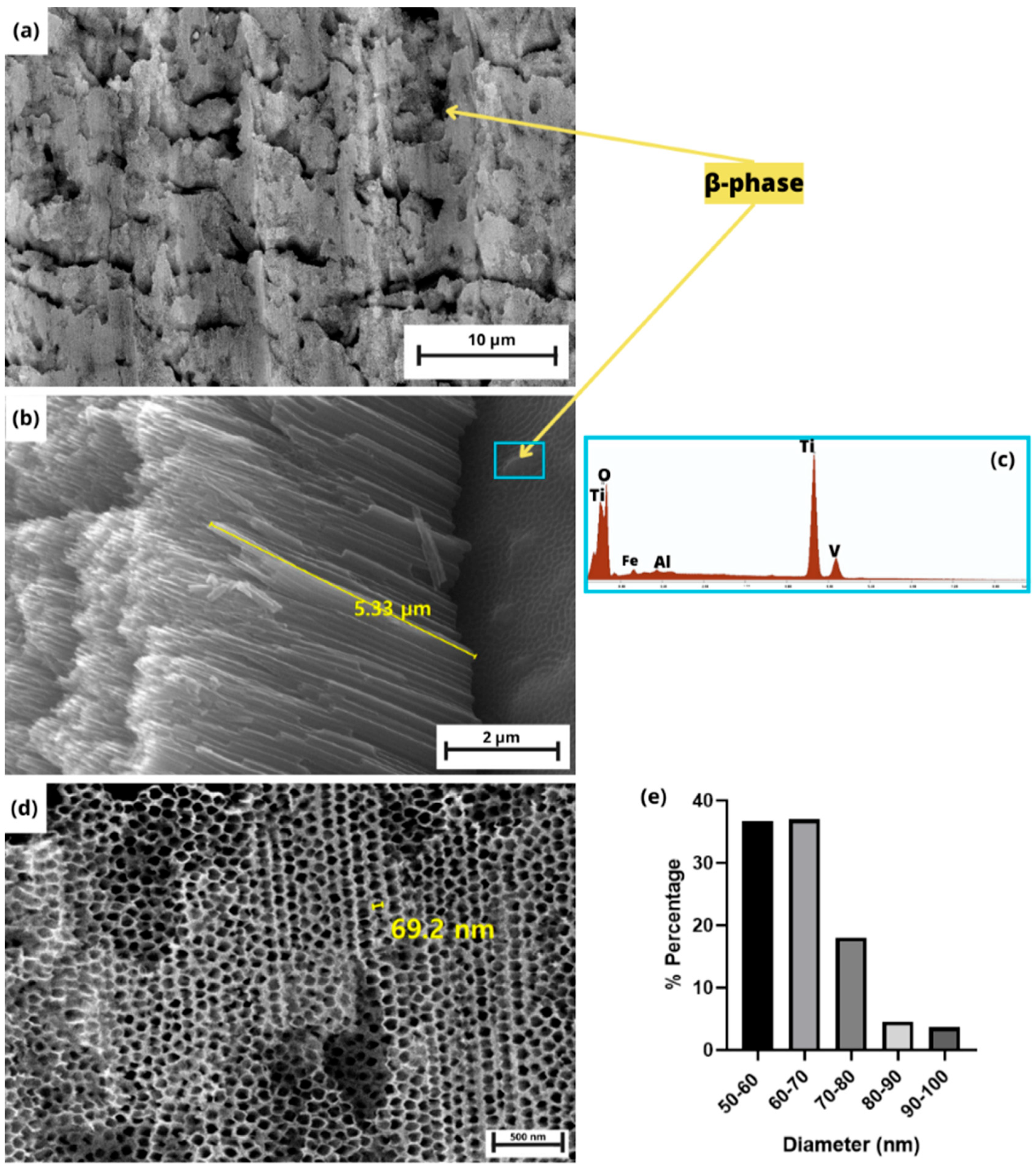
3.1. TNT Characterization
3.1.1. XRD Pattern
3.1.2. Morphology
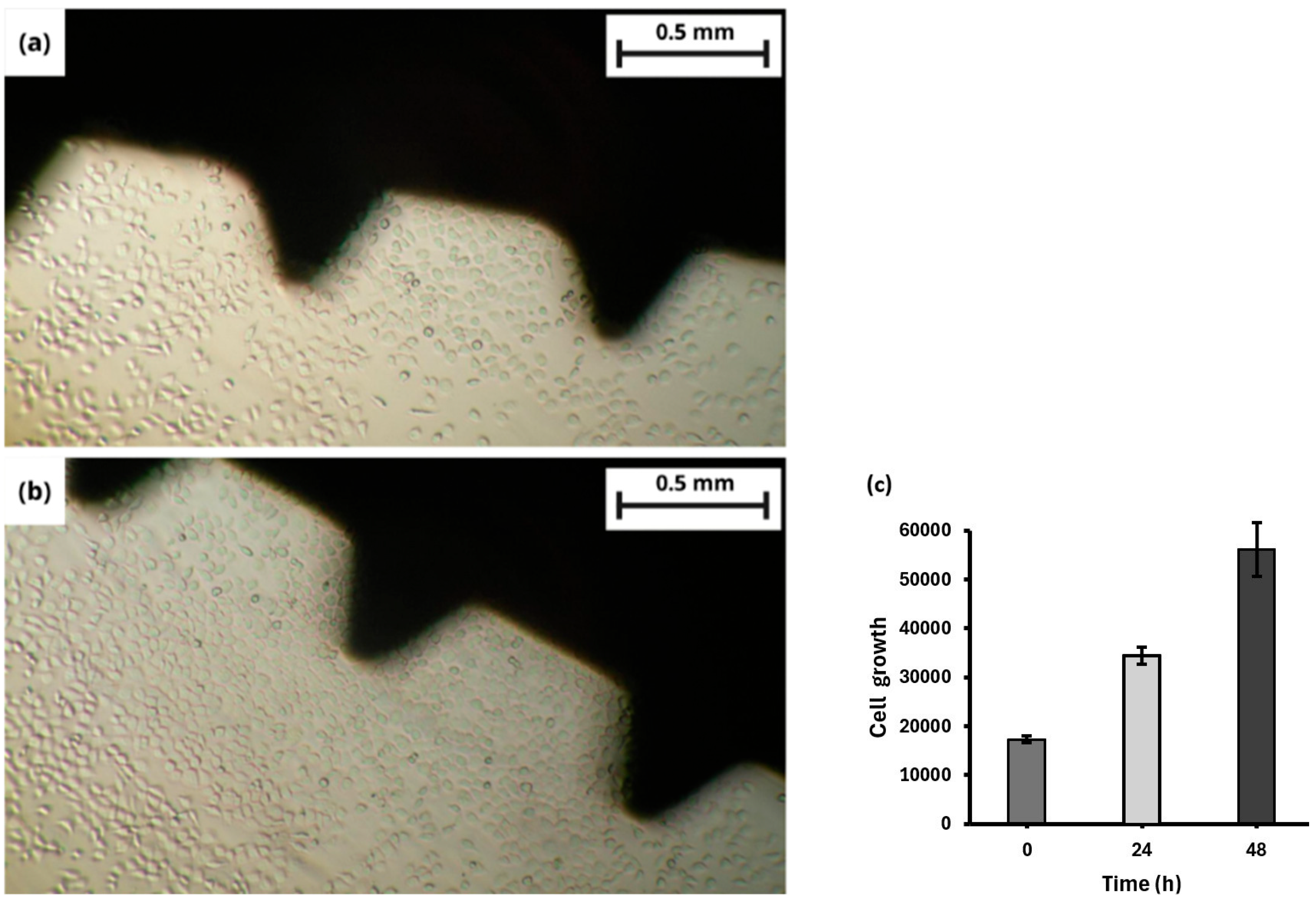
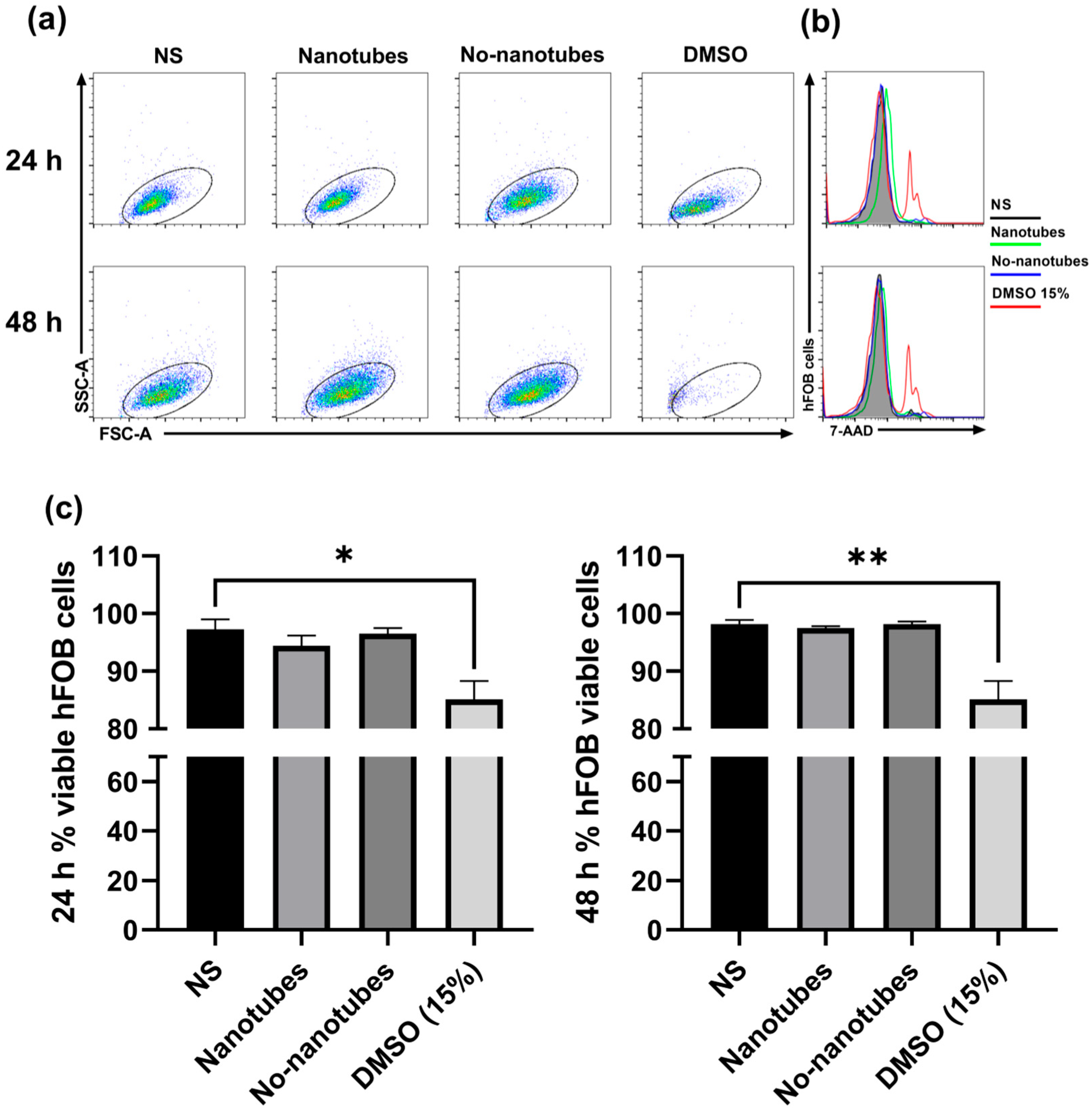
3.2. In Vitro Assay
Proliferation and Viability of hFOB Cells with TNTs on a Dental Screw
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polley, C.; Radlof, W.; Hauschulz, F.; Benz, C.; Sander, M.; Seitz, H. Morphological and mechanical characterisation of three-dimensional gyroid structures fabricated by electron beam melting for the use as a porous biomaterial. J. Mech. Behav. Biomed. Mater. 2022, 125, 104882. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef]
- Rho, J.Y.; Tsui, T.Y.; Pharr, G.M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials 1997, 18, 1325. [Google Scholar] [CrossRef]
- Joshi, M.G.; Advani, S.G.; Miller, F.; Santare, M.H. Analysis of a femoral hip prosthesis designed to reduce stress shielding. J. Biomech. 2000, 33, 1655–1662. [Google Scholar] [CrossRef]
- Choi, B.H.; Choi, Y.S.; Kang, D.G.; Kim, B.J.; Song, Y.H.; Cha, H.J. Cell behavior on extracellular matrix mimic materials based on mussel adhesive protein fused with functional peptides. Biomaterials 2010, 31, 8980–8988. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.G.; Alsolami, S.M.; Arossa, S.; Ramos, G.; Anieka, J.; Steckbauer, A.; Duarte, C.M.; Li, M. In situ monitoring reveals cellular environmental instabilities in human pluripotent stem cell culture. Commun. Biol. 2022, 5, 119. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone–implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, B.; Shen, X.; Lu, D.; He, W.; Zan, X.; Li, L.; Pan, Y. Enhanced vascularization and osseointegration under osteoporotic conditions through functional peptide coating on implant surfaces. Mater. Today Bio. 2024, 27, 101150. [Google Scholar] [CrossRef]
- Hasegawa, T.; Inufusa, A.; Imai, Y.; Mikawa, Y.; Lim, T.H.; An, H.S. Hydroxyapatitecoating of pedicle screws improves resistance against pull-out force in the osteoporotic canine lumbar spine model: A pilot study. Spine J. 2005, 5, 239–243. [Google Scholar] [CrossRef]
- Hussain, M.; Askari Rizvi, S.H.; Abbas, N.; Sajjad, U.; Shad, M.R.; Badshah, M.A.; Malik, A.I. Recent developments in coatings for orthopedic metallic implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Wang, K.; Jin, H.; Song, Q.; Huo, J.; Zhang, J.; Li, P. Titanium dioxide nanotubes as drug carriers for infection control and osteogenesis of bone implants. Drug Deliv. Transl. Res. 2021, 11, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Torres Avila, I.P.; Padilla Martinez, I.I.; Perez Hernandez, N.; Bañuelos Hernandez, A.E.; Velázquez, J.C.; Castrejón Flores, J.L.; Hernandez Sanchez, E. Surface modification of the Ti-6Al-4Valloy by anodic oxidation and its effect on osteoarticular cell proliferation. Coatings 2020, 10, 491. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Liang, C.; Li, H.; Guo, L.; Liu, S.; Wang, H. Surface Roughness and hydrophilicity of titanium after anodic oxidation. Rare Met. Mater. Eng. 2016, 45, 858–862. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Yao, C.; Webster, T.J. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesión. J. Biomed. Mater. Res. 2008, 84, 447–453. [Google Scholar] [CrossRef]
- Min, S.K.; Kang, H.K.; Jang, D.H.; Jung, S.Y.; Kim, O.B.; Min, B.M.; Yeo, I.S. Titanium surface coating with a laminin-derived functional peptide promotes bone cell adhesion. Biomed. Res. Int. 2013, 1, 638348. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, H.; Ding, X.; Yan, Q.; Wang, I.; Ma, W. Simulation of a anodizing current time curves and morphology evolution of TiO2 nanotubes anodized in electrolytes with different NH4F concentrations. Electrochim. Acta. 2015, 176, 1083–1091. [Google Scholar] [CrossRef]
- Luo, J.; Li, B.; Ajami, S.; Ma, S.; Zhou, F.; Liu, C. Growth of TiO2 nanotube on titanium substrate tio enhacnce its bio tribological performance and biocorrosion resistance. J. Bionic. Eng. 2019, 16, 1039–1051. [Google Scholar] [CrossRef]
- Luo, J.; Tamaddon, M.; Yan, C.; Ma, S.; Wang, X.; Zhou, F.; Liu, C. Improving the fretting biocorrosion of Ti6Al4V alloy by decorating structure optimized TiO2 nanotubes layer. J. Mater. Sci. Technol. 2020, 49, 47–55. [Google Scholar] [CrossRef]
- Torres Avila, I.P.; Souza, R.M.; Chino Ulloa, A.; Ruiz Trabolsi, P.A.; Tadeo Rosas, R.; Carrera Espinoza, R.; Hernández Sánchez, E. Effect of Anodization Time on the Adhesion Strength of Titanium Nanotubes Obtained on the Surface of the Ti-6Al-4V alloy by Anodic Oxidation. Crystals 2023, 13, 1059–1071. [Google Scholar] [CrossRef]
- Drobne, D.; Drašler, B.; Junkar, I.; Kulkarni, M.; Flašker, A.; Rugelj, N.; Resnik, M.; Iglič, A.; Schmuki, P.; Mozetič, M.; et al. Influence of various sterilization procedures on TiO2 nanotubes used for biomedical devices. Bioelectrochemistry 2016, 109, 79–86. [Google Scholar]
- Arenas, M.A.; Conde, A.; De Damborenea, J.J.; Domingo, C.; Hernandez-López, J.M.; Matykina, E. Morphologies of nanostructured TiO2 doped with F on Ti-6Al-4V alloy. Electrochim. Acta 2011, 56, 2221–2229. [Google Scholar]
- Ghicov, A.; Tsuchiya, H.; Macak, J.M. Titanium oxide nanotubes prepared in phosphate electrolytes. Electrochem. Commun. 2005, 7, 505–509. [Google Scholar] [CrossRef]
- Yin, B.; Dong, Y.; Cheng, H.; Xiong, L.; Liu, Y.; Zhang, Y.; Liu, Z.; Chen, R.; Gao, P.; Zheng, Z.; et al. Enhancing titanium dioxide nanotube array stability on dental implants through laser lithography-assited microline pattering. Acta Biomater. 2025, 43, 569–581. [Google Scholar] [CrossRef]
- Bloyce, A.; Morton, P.Y.; Bell, T. Surface engineering of titanium and titanium alloys. ASM Int. 1990, 4, 835. [Google Scholar]
- Tomastik, J.; Ctvrtlik, R. Nanoscratch test—A tool for evaluation of cohesive and adhesive properties of thin films and coatings. EDP Sci. 2013, 48, 00027. [Google Scholar] [CrossRef]
- Pereira, B.L.; Beilner, G.; Lepienski, C.M.; de Souza, G.B.; Kuromoto, N.K.; Szameitat, E.S.; Peng, A.N.; Lee, J.Y.; Claro, A.P.; Nugent, M.J. Scratch-resistant and well-adhered nanotube arrays produced via anodizing process on β-titanium alloy. Mater. Today Commun. 2021, 26, 101947. [Google Scholar] [CrossRef]
- Guohua, L.; Kaiying, W.; Nils, H.; Henrik, J. Progress on free-standing and flow-through TiO2 nanotube membranes. Sol. Energy Mater. Sol. Cells 2012, 98, 24–38. [Google Scholar]
- Mor, G.K.; Varghese, O.K.; Paulose, M. A review on highly ordered,vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Suhadolnik, L.; Bele, M.; Maver, U.; Rožanc, J.; Knez, Ž.; Marevci, M.K.; Finšgar, M. The synthesis, surface analysis, and celular response of Titanium oxynitride nanotubes arrays prepared on TiAl6V4 for potential biomedical applications. J. Mater. Res. Technol. 2023, 24, 4074–4090. [Google Scholar] [CrossRef]
- Zhao, L.; Mei, S.; Chu, P.K.; Zhang, Y.; Wu, Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 2010, 31, 5072–5082. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Sauter, A.; Xue, Y.; Birkeland, E.; Schoelermann, J.; Holst, B.; Cimpan, M.R. Label- freeimpedance flow cytometry for nanotoxicity screening. Sci. Rep. 2020, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Hachani, R.; Birchall, M.; Lowdell, M.; Kasparis, G.; Tung, L.D.; Manshian, B.B.; Soenen, S.; Gsell, W.; Himmelreich, U.; Gharagouzloo, C.; et al. Assessing cell-nanoparticle interactions by high content iron oxide nanoparticles as potential contrast agents for magnetic resonance imaging. Sci. Rep. 2017, 7, 7850. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrejón Flores, J.L.; Campos Juarez, Á.D.; Chino Ulloa, A.; Nava Palafox, F.; Cruz Ortiz, D.; Torres Avila, I.P. Biocompatibility of Titanium Oxide Nanotubes Layer Formed on a Ti-6Al-4V Dental Implant Screw in hFOB Cells In Vitro. Coatings 2025, 15, 715. https://doi.org/10.3390/coatings15060715
Castrejón Flores JL, Campos Juarez ÁD, Chino Ulloa A, Nava Palafox F, Cruz Ortiz D, Torres Avila IP. Biocompatibility of Titanium Oxide Nanotubes Layer Formed on a Ti-6Al-4V Dental Implant Screw in hFOB Cells In Vitro. Coatings. 2025; 15(6):715. https://doi.org/10.3390/coatings15060715
Chicago/Turabian StyleCastrejón Flores, José Luis, Ángel Daniel Campos Juarez, Alexis Chino Ulloa, Fernando Nava Palafox, David Cruz Ortiz, and Itzel Pamela Torres Avila. 2025. "Biocompatibility of Titanium Oxide Nanotubes Layer Formed on a Ti-6Al-4V Dental Implant Screw in hFOB Cells In Vitro" Coatings 15, no. 6: 715. https://doi.org/10.3390/coatings15060715
APA StyleCastrejón Flores, J. L., Campos Juarez, Á. D., Chino Ulloa, A., Nava Palafox, F., Cruz Ortiz, D., & Torres Avila, I. P. (2025). Biocompatibility of Titanium Oxide Nanotubes Layer Formed on a Ti-6Al-4V Dental Implant Screw in hFOB Cells In Vitro. Coatings, 15(6), 715. https://doi.org/10.3390/coatings15060715






